Because Berkeley sickle cell mice are used as an animal model for human sickle cell disease, we investigated the progression of the histopathology in these animals over 6 months and compared these findings to those published in humans with sickle cell disease. The murine study groups were composed of wild-type mixed C57Bl/6-SV129 (control) mice and sickle cell (SS) mice (α-/-, β-/-, transgene +) of both sexes and between 1 and 6 months of age. SS mice were similar to humans with sickle cell disease in having erythrocytic sickling, vascular ectasia, intravascular hemolysis, exuberant hematopoiesis, cardiomegaly, glomerulosclerosis, visceral congestion, hemorrhages, multiorgan infarcts, pyknotic neurons, and progressive siderosis. Cerebral perfusion studies demonstrated increased blood-brain barrier permeability in SS mice. SS mice differed from humans with sickle cell disease in having splenomegaly, splenic hematopoiesis, more severe hepatic infarcts, less severe pulmonary manifestations, no significant vascular intimal hyperplasia, and only a trend toward vascular medial hypertrophy. Early retinal degeneration caused by a homozygous mutation (rd1) independent from that causing sickle hemoglobin was an incidental finding in some Berkeley mice. While our study reinforces the fundamental strength of this model, the notable differences warrant careful consideration when drawing parallels to human sickle cell disease.
Introduction
Several murine models have been developed to mimic human sickle cell (SS) disease. Of these, the Berkeley SS model has targeted deletions of murine α and β globins (α-/-, β-/-) with a transgene containing human α, βs, Aγ, Gγ, and β globins; thus, these mice express human sickle hemoglobin almost exclusively.1,2 Berkeley SS mice have several hematologic as well as histopathologic similarities and differences compared with human sickle cell disease. Published similarities include erythrocytic sickling, intravascular hemolysis, reticulocytosis, severe anemia (hematocrit, 10%-30%), leukocytosis, elevations of inflammatory cytokines, defects in urine concentrating ability, multiorgan infarcts, glomerulosclerosis, and pulmonary congestion.1-5 Published differences include lower-than-normal mean corpuscular hemoglobin concentrations, suggesting a partial thalassemic phenotype, and splenomegaly with exuberant splenic hematopoiesis in SS mice, rather than the higher-than-normal mean corpuscular hemoglobin concentrations and progressive splenic infarcts and atrophy with exuberant bone marrow hematopoiesis typical of human sickle cell disease.2 In addition, the erythrocytes of Berkeley SS mice, like those of all murine strains, have red-cell volumes that are considerably smaller than those of human erythrocytes.
Relatively little attention has been given to comparison of the natural progression of chronic organ injury in this model with that in human sickle cell disease. Potential strengths of murine models of sickle cell disease include the ability to intervene therapeutically and to crossbreed the mice with other mice harboring targeted genetic alterations for assessment of the impact of the therapeutic interventions and/or genetic alterations on the hematologic abnormalities and chronic organ injury in the SS mice. To achieve these goals, however, it is necessary to have a detailed understanding of the rate of development and severity of visceral pathology in SS mice, stratified for sex and age. This study was initiated to improve our understanding of the natural progression of organ pathology in Berkeley SS mice for both comparison with human sickle cell disease and as a baseline for assessing the effects of therapeutic interventions and/or genetic alterations.
Materials and methods
Transgenic and control mice
The Berkeley sickle mice with genotype Tg(Hu-miniLCR α1 Gγ Aγδ βs) Hba0 Hba0 Hbb0 Hbb0 and the hemizygous mice with genotype Tg(HuminiLCR α1 Gγ Aγ δ βs) Hba0 Hba0 Hbb0 Hbb+ were littermates of similar background. All of the hemizygous mice carried one transgene. These mice were supplied by Dr Mohandas Narla of The New York Blood Center and were housed and bred at the Medical College of Wisconsin Animal Resource Center. Control mice, interbred progeny of a cross between C57Bl/6 and 129S mice (2 of the 5 parental strains of the Berkeley mice), were housed and bred at Rockefeller University. Age and sex distributions by genotype for the mice studied for organ weights are detailed in Table 1 and those for histopathology in Table 2.
Organ weights as actual weights and as percentages of body weights in control and sickle mice
. | Mean or percentage . | . | P . | . | . | |||
|---|---|---|---|---|---|---|---|---|
. | SS; n = 14 . | WT; n = 10 . | Sickle vs WT . | Male vs female . | Increasing age . | |||
| Age, mo | 3.5 | 3.4 | .84 | NA | NA | |||
| Body weight, g | 21.0 | 23.2 | .23 | NA | NA | |||
| Male, % of total | 50 | 50 | > .99 | NA | NA | |||
| Actual organ weight, g | ||||||||
| Spleen | 1.2 | 0.11 | < .001* | .26* | .01* | |||
| Heart | 0.18 | 0.14 | .02* | .02* | .02* | |||
| Brain | 0.41 | 0.37 | .03* | .77* | .04* | |||
| Liver | 1.5 | 1.4 | .28* | < .001* | < .001* | |||
| Kidneys | 0.40 | 0.37 | .42* | < .001* | < .001* | |||
| Body weight, % of total | ||||||||
| Spleen | 5.7 | 0.47 | NA | NA | NA | |||
| Heart | 0.85 | 0.63 | NA | NA | NA | |||
| Brain | 2.0 | 1.7 | NA | NA | NA | |||
| Liver | 6.9 | 5.8 | NA | NA | NA | |||
| Kidneys | 1.9 | 1.6 | NA | NA | NA | |||
. | Mean or percentage . | . | P . | . | . | |||
|---|---|---|---|---|---|---|---|---|
. | SS; n = 14 . | WT; n = 10 . | Sickle vs WT . | Male vs female . | Increasing age . | |||
| Age, mo | 3.5 | 3.4 | .84 | NA | NA | |||
| Body weight, g | 21.0 | 23.2 | .23 | NA | NA | |||
| Male, % of total | 50 | 50 | > .99 | NA | NA | |||
| Actual organ weight, g | ||||||||
| Spleen | 1.2 | 0.11 | < .001* | .26* | .01* | |||
| Heart | 0.18 | 0.14 | .02* | .02* | .02* | |||
| Brain | 0.41 | 0.37 | .03* | .77* | .04* | |||
| Liver | 1.5 | 1.4 | .28* | < .001* | < .001* | |||
| Kidneys | 0.40 | 0.37 | .42* | < .001* | < .001* | |||
| Body weight, % of total | ||||||||
| Spleen | 5.7 | 0.47 | NA | NA | NA | |||
| Heart | 0.85 | 0.63 | NA | NA | NA | |||
| Brain | 2.0 | 1.7 | NA | NA | NA | |||
| Liver | 6.9 | 5.8 | NA | NA | NA | |||
| Kidneys | 1.9 | 1.6 | NA | NA | NA | |||
NA indicates not applicable.
P values for multiple regression with 3 factors
Number of mice by genotype, sex, and age for histopathology studies
. | Control . | . | Sickle . | . | ||
|---|---|---|---|---|---|---|
| Age, mo . | M . | F . | M . | F . | ||
| 1 | 2 | 2 | 2 | 1 | ||
| 2 | 0 | 0 | 1 | 3 | ||
| 3 | 3 | 3 | 0 | 2 | ||
| 4 | 0 | 0 | 1 | 1 | ||
| 5 | 1 | 1 | 2 | 0 | ||
| 6 | 2 | 2 | 1 | 1 | ||
. | Control . | . | Sickle . | . | ||
|---|---|---|---|---|---|---|
| Age, mo . | M . | F . | M . | F . | ||
| 1 | 2 | 2 | 2 | 1 | ||
| 2 | 0 | 0 | 1 | 3 | ||
| 3 | 3 | 3 | 0 | 2 | ||
| 4 | 0 | 0 | 1 | 1 | ||
| 5 | 1 | 1 | 2 | 0 | ||
| 6 | 2 | 2 | 1 | 1 | ||
M indicates male; F, female.
Ten of the control mice used for organ weights also were studied for histopathology.
Hematologic parameters
After administering anesthesia (Avertin, ∼0.5 mg/gm body weight), phlebotomy was performed on 16 SS mice (age, 2-14 months; mean, 6.5 months) of both sexes via the retro-orbital venous plexus or the left ventricle. Blood was collected into heparinized saline (4%-5% dilutional effects), and then the hematocrit (hct), mean red-cell volume (MCV), and mean red-cell hemoglobin concentration (MCHC) were determined using an automated veterinary blood counter (Heska; Fort Collins, CO). The reticulocyte count was determined by flow cytometry using thiazole orange, based on the percentage of thiazole orange-positive cells within the erythrocyte gate.
Organ weights
Control and SS mice were weighed, anesthetized, euthanized, and sent to the University of South Alabama for dissection. The spleen, liver, heart, kidneys, and brain were removed and weighed (Table 1). Organ weight data are reported as both actual measured weights and as percentages of body weight.
Histopathology
At specified ages, animals were weighed, anesthetized, and either prepared as indicated in “Organ weights” or perfusion-fixed with 10 mL phosphate-buffered saline (PBS) at 37°C, followed by 10 mL 4% paraformaldehyde in PBS. Perfusion was continued until clear fluid was obtained from the right atrium in all animals. The animals were partially dissected to expose intracranial, thoracic, and abdominal viscera; immersion-fixed in 4% paraformaldehyde (at least 7 days at 4°C); and sent to the University of South Alabama for dissection. Tissue was harvested by removal of the calvarium for extraction of the brain and brainstem, enucleation of the eyes, Rokitansky evisceration, and representative samplings of ribs, sternum, thoracolumbar vertebrae, and spinal cord. Tissues sampled for microscopic studies included skeletal muscle, cerebral hemispheres, brainstem, cerebellum, eyes, thymus, lungs, heart, spleen, liver, pancreas, kidneys, adrenals, stomach, small and large intestines, and bone with marrow from ribs and vertebrae. The animals were then sent to the Henry Ford Hospital (Detroit, MI), where the internal carotids of 2 control and 2 SS mice (both sexes, age 5 or 6 months old) were isolated under a surgical microscope and cross-sectioned (8 μm) for routine processing.
All 15 of the SS animals and 6 of the control animals analyzed for histopathology were perfusion-fixed. Organs from 10 control animals used for the weight study also were included for some parts of the histopathology study after determining that the method of preparation did not affect the results. These animals were not included in analyses judged potentially to be affected by the perfusion fixation (perivenous pallor in the liver, intravascular sickling, and pulmonary capillary congestion and hemorrhage) or affected by delayed fixation (microscopic acute hepatic infarcts and estimation of splenic hematopoiesis).
Tissues were embedded in paraffin, sectioned at 5 to 7 microns, and stained with hematoxylin and eosin (H&E). At least 3 sections per tissue block of viscera were stained with H&E for light microscopy. Additional sections were made for special stains, including Gomori modified Prussian blue stain for iron, Masson trichrome stain for fibrous connective tissue (infarcts) and degenerating neurons, and Verhoff-von Giesson stain for elastic fibers, each with appropriate controls. The murine microscopic studies were masked to age, sex, and genotype. Criteria for scoring of morphologic features are summarized in Table 3.
Scoring criteria for morphologic findings
Score . | Degree . | Distribution . | Magnification . | Field involved, % . | Glomerular size, U . |
|---|---|---|---|---|---|
| 0 | Absent | Normal | — | 0 | 6 |
| 1 | Minimal | 1-2 cells | 400 × | 10-30 | 7 |
| 2 | Mild | Focal cluster | 400 × | 30-50 | 8 |
| 3 | Mild-moderate | Few clusters | 100 × | 50 | 9 |
| 4 | Moderate | Many clusters | 100 × | 50-70 | 10 |
| 5 | Moderate-severe | Every field | 25 × | 70-90 | 11 |
| 6 | Severe/abundant | Most cells | 25 × | 90-100 | 12 |
Score . | Degree . | Distribution . | Magnification . | Field involved, % . | Glomerular size, U . |
|---|---|---|---|---|---|
| 0 | Absent | Normal | — | 0 | 6 |
| 1 | Minimal | 1-2 cells | 400 × | 10-30 | 7 |
| 2 | Mild | Focal cluster | 400 × | 30-50 | 8 |
| 3 | Mild-moderate | Few clusters | 100 × | 50 | 9 |
| 4 | Moderate | Many clusters | 100 × | 50-70 | 10 |
| 5 | Moderate-severe | Every field | 25 × | 70-90 | 11 |
| 6 | Severe/abundant | Most cells | 25 × | 90-100 | 12 |
— indicates not applicable.
Measurements were made using an ocular micrometer at 40 × for islet diameter, glomerular size, renal cortical thickness, and vascular diameter, or a vernier caliper (mm) for spleen diameter. Mean micrometer measurements indicate the average of 10 randomly selected areas. Photomicrographs were made using a Zeiss Axioskop (Zeiss, Thornwood, NY) and formatted using Adobe Photoshop (Adobe Systems, San Jose, CA). The specific features selected for grading on H&E stains are summarized in Table 4.
Tissues and morphologic features graded
Tissue . | Features graded . |
|---|---|
| Brain | Pyknotic Purkinje cells; iron pigment |
| Eyes | Retinal degeneration; iron pigment; infarcts |
| Lungs | Infarcts; thickness of intima and media of pulmonary arteries; mononuclear infiltrates; iron pigment; hemorrhages; vascular congestion; dilated capillaries |
| Kidneys | Glomerular diameter; cysts and infarcts; thickness of vascular intima and media on 10 × near renal sinus; iron pigment; mononuclear infiltrates |
| Liver | Calcification; infarcts; iron pigment; mononuclear infiltrates; pericentral venous pallor; steatosis |
| Spleen | Diameter of spleen (mm); increased white pulp (hematopoietic cells); luminal diameter (ectasia) of blood vessel in the white pulp; iron pigment; infarcts |
| Heart | Infarcts (recent/remote); vascular wall thickness; iron pigment; thickness of left and right ventricular free walls (mm) |
| Pancreas | Greatest islet diameter |
| Bone marrow | Cellularity, erythroid hyperplasia, iron pigment |
| Intravascular erythrocytes | Percentage of cells demonstrating sickle morphology |
| Carotid arteries | Abnormalities of the endothelium, intima, media, and adventitia |
Tissue . | Features graded . |
|---|---|
| Brain | Pyknotic Purkinje cells; iron pigment |
| Eyes | Retinal degeneration; iron pigment; infarcts |
| Lungs | Infarcts; thickness of intima and media of pulmonary arteries; mononuclear infiltrates; iron pigment; hemorrhages; vascular congestion; dilated capillaries |
| Kidneys | Glomerular diameter; cysts and infarcts; thickness of vascular intima and media on 10 × near renal sinus; iron pigment; mononuclear infiltrates |
| Liver | Calcification; infarcts; iron pigment; mononuclear infiltrates; pericentral venous pallor; steatosis |
| Spleen | Diameter of spleen (mm); increased white pulp (hematopoietic cells); luminal diameter (ectasia) of blood vessel in the white pulp; iron pigment; infarcts |
| Heart | Infarcts (recent/remote); vascular wall thickness; iron pigment; thickness of left and right ventricular free walls (mm) |
| Pancreas | Greatest islet diameter |
| Bone marrow | Cellularity, erythroid hyperplasia, iron pigment |
| Intravascular erythrocytes | Percentage of cells demonstrating sickle morphology |
| Carotid arteries | Abnormalities of the endothelium, intima, media, and adventitia |
Eyes from SS mice were either serially cross-sectioned or dissected so as to study the retina. For the latter, after removal of the vitreous, retinas were separated carefully from the retinal pigment epithelium and choroid and fixed in 2% paraformaldehyde in 0.1 M cacodylate buffer overnight at 4°C. Retinas were washed in 0.1 M cacodylate buffer with 5% sucrose and then analyzed for adenosine diphosphatase (ADPase) activity, a marker of viable blood vessels, by histochemistry, as previously published.6
After the ADPase incubation, retinas were opened with relaxing cuts and fixed flat in 25% Karnovsky fixative, pH 7.4, at 4°C overnight or longer. The tissue was then washed, dehydrated, and infiltrated and embedded in glycol methacrylate (JB-4, Polysciences; Warrington, PA), as published previously.6 Using dark-field illumination, the blood vessels in the whole block were photographed and mapped. Sections (2.5 μm) were cut on a dry glass knife with a Sorvall NT2 microtome (Sorvall, Newtown, CT), and the sections were stained with periodic acid-Schiff and hematoxylin or thionin and ammonium sulfide to stain the cell nuclei blue and develop the ADPase activity.6
Cerebral vasculature
After isoflurane anesthesia, fluorescein isothiocyanate (FITC) dextran (2 × 106 molecular weight; Sigma, St Louis, MO; 0.1 mL of 25 mg/mL in saline) was administered intravenously via the saphenous vein to 1 control and 1 SS mouse to delineate the vascular tree as previously described. Five minutes later, the mice were euthanized and the brains rapidly removed and placed in 4% paraformaldehyde at 4°C for 72 hours, followed by storage in PBS at 4°C. Tissue was then sent to the Henry Ford Hospital, where coronal sections (100 μm) were cut on a vibratome and analyzed using a Bio-Rad MRC 1024 (argon and krypton) laser-scanning confocal imaging system mounted onto a Zeiss microscope (Bio-Rad; Cambridge, MA).7 Areas of interest were scanned with a 10 ×/0.22 or 40 ×/1.0 oil-immersion objective lens in 512 × 512 pixel (1042 × 1042 μm for 10 × or 260.6 × 260.6 μm for 40 ×) format in the x-y direction using a 4 × frame-scan average.
DNA analysis for rd1 mutation
Statistical analysis
Data for each feature were summarized for sickle cell and for control animals, and then the groups were compared. Means were calculated for variables measured on a continuous scale or on a grading scale with outcomes spread over a large part of the 0-to-6 range. These mean values were compared between groups by t tests. When most of the outcomes in either group equaled 0, outcomes with scores of 1 or greater were characterized as positive outcomes, and the proportions of each group with a positive outcome were compared using exact chi-square tests. Means of the positive values also were tabulated. As indicated by visual inspection of the data, effects of age and sex were sought by chi-square tests, Mantel-Haenszel tests, or linear regression analyses. The splenic white pulp was analyzed as measured, and also as proportional to the diameter of the spleen and to the square of the spleen diameter, as possible estimates of splenic volume, assuming that the spleen is approximately cylindrical.
Results
The hematologic data on the SS mice were similar to previously published data. Comparison of 16 SS mice with 5 controls showed severe anemia (hematocrit, 21% ± 7%; mean ± SD; controls, 42.1% ± 2.9%), reduced MCV and MCHC (42 ± 2 fl and 31 ± 2 fl, respectively; controls 46 ± 1 and 33.4 ± 0.4, respectively), and markedly elevated reticulocyte count (48% ± 8%; controls, 4.9% ± 0.2%).
Organ weights
The spleens of SS mice were more than 10-fold greater in weight than those of control mice when expressed as either actual organ weights or as percentages of body weights (P < .001). Similarly, the brains and hearts of SS mice also were significantly heavier than those of control mice even after controlling for age and sex. The liver and kidney weights in both control and SS mice were strongly positively associated with both male sex and increasing age; after controlling for these variables, there was no significant difference in these organ weights between SS and control mice.
Histopathology
The perfusion fixation was judged to be of high quality since fewer than 20% of mice showed morphologic evidence of hepatic pericentral venous pallor, which was used as an indication of cellular alterations due to agonal changes (Table 5).
Pathologic findings in control and SS mice
. | Genotype . | . | . | |
|---|---|---|---|---|
. | Control; n = 16 . | Sickle; n = 15 . | P . | |
| Spleen | ||||
| Gross infarcts, % positive | 0 | 27 | .04 | |
| Micros infarct, % positive | 0 | 40 | .007 | |
| Diameter, mm | 2.2 | 5.7 | < .001 | |
| Iron, all ages | 1.9 | 4.4 | < .001 | |
| 2 y or younger | 0 | 2.7 | NA | |
| Older than 2 y | 2.6 | 5.9 | NA | |
| Hematopoiesis | 0.3; n = 6 | 5.9 | < .001 | |
| Vascular ectasia, all ages | 0.8 | 4.4 | < .001 | |
| 2 y or younger | 0.5 | 3.7 | NA | |
| Older than 2 y | 0.9 | 5.0 | NA | |
| Liver | ||||
| Mononuclear, % positive | 6 | 67 | < .001 | |
| Gross infarcts, % positive | 0 | 27 | .04 | |
| Micros infarcts, % positive | 0; n = 6 | 100 | < .001 | |
| Iron, % positive | 0 | 100 | < .001 | |
| Iron, mean of positive scores | NA | 4.1 | NA | |
| Calcification, % positive | 0 | 60 | < .001 | |
| Perivenous pallor, % positive | 6 | 20 | NS | |
| Kidney | ||||
| Cortical width, μm | 25.8 | 42.1 | < .001 | |
| Gross infarcts, % positive | 0 | 20 | .10 | |
| Micros infarcts, % positive | 0 | 67 | < .001 | |
| Iron, % positive* | 6 | 93 | < .001 | |
| Iron, mean of positive scores | 1.0 | 4.1 | NA | |
| Glomerular size, U | 2.7 | 4.5 | < .001 | |
| Arterial wall, μm | 1.7 | 2.1 | NS | |
| Mononuclear infiltrates, % positive | 0 | 40 | .007 | |
| Lungs | ||||
| Lymphs, % positive | 12.5 | 20 | NS | |
| Iron, all ages, % positive | 0 | 80 | < .001 | |
| 2 y or younger | 0 | 57 | NA | |
| Older than 2 y | 0 | 100 | NA | |
| Iron, all ages, mean of positive scores | NA | 1.75 | NA | |
| Capillary congestion, % positive | 0; n = 6 | 93 | .001 | |
| Pulmonary artery wall, μm | 1.8 | 2.7 | .002 | |
| Hemorrhage, % positive | 0; n = 6 | 60 | .02 | |
| Heart | ||||
| Micros infarcts, % positive | 0 | 33 | .02 | |
| Iron, % positive | 6 | 33 | .08 | |
| Iron, mean of positive scores | 1.0 | 1.2 | NA | |
| Blood vessel diameter, μm† | 3.7; n = 15 | 4.9 | .02 | |
| Blood vessel wall thickness, % greater than 1 μm | 12.5 | 80 | < .001 | |
| Brain and eye | ||||
| Pyknotic Purkinje cells, % | 0 | 93 | < .001 | |
| Retinal degeneration, % | 0 | 100 | < .001 | |
| Bone marrow | ||||
| Iron, % | 17 | 67 | .02 | |
| Iron, mean of positive scores | 1.5 | 1.3 | NA | |
| Cellularity score | 5.1 | 4.2 | < .001 | |
| Erythroid hyperplasia score | 0.88 | 4.8 | < .001 | |
. | Genotype . | . | . | |
|---|---|---|---|---|
. | Control; n = 16 . | Sickle; n = 15 . | P . | |
| Spleen | ||||
| Gross infarcts, % positive | 0 | 27 | .04 | |
| Micros infarct, % positive | 0 | 40 | .007 | |
| Diameter, mm | 2.2 | 5.7 | < .001 | |
| Iron, all ages | 1.9 | 4.4 | < .001 | |
| 2 y or younger | 0 | 2.7 | NA | |
| Older than 2 y | 2.6 | 5.9 | NA | |
| Hematopoiesis | 0.3; n = 6 | 5.9 | < .001 | |
| Vascular ectasia, all ages | 0.8 | 4.4 | < .001 | |
| 2 y or younger | 0.5 | 3.7 | NA | |
| Older than 2 y | 0.9 | 5.0 | NA | |
| Liver | ||||
| Mononuclear, % positive | 6 | 67 | < .001 | |
| Gross infarcts, % positive | 0 | 27 | .04 | |
| Micros infarcts, % positive | 0; n = 6 | 100 | < .001 | |
| Iron, % positive | 0 | 100 | < .001 | |
| Iron, mean of positive scores | NA | 4.1 | NA | |
| Calcification, % positive | 0 | 60 | < .001 | |
| Perivenous pallor, % positive | 6 | 20 | NS | |
| Kidney | ||||
| Cortical width, μm | 25.8 | 42.1 | < .001 | |
| Gross infarcts, % positive | 0 | 20 | .10 | |
| Micros infarcts, % positive | 0 | 67 | < .001 | |
| Iron, % positive* | 6 | 93 | < .001 | |
| Iron, mean of positive scores | 1.0 | 4.1 | NA | |
| Glomerular size, U | 2.7 | 4.5 | < .001 | |
| Arterial wall, μm | 1.7 | 2.1 | NS | |
| Mononuclear infiltrates, % positive | 0 | 40 | .007 | |
| Lungs | ||||
| Lymphs, % positive | 12.5 | 20 | NS | |
| Iron, all ages, % positive | 0 | 80 | < .001 | |
| 2 y or younger | 0 | 57 | NA | |
| Older than 2 y | 0 | 100 | NA | |
| Iron, all ages, mean of positive scores | NA | 1.75 | NA | |
| Capillary congestion, % positive | 0; n = 6 | 93 | .001 | |
| Pulmonary artery wall, μm | 1.8 | 2.7 | .002 | |
| Hemorrhage, % positive | 0; n = 6 | 60 | .02 | |
| Heart | ||||
| Micros infarcts, % positive | 0 | 33 | .02 | |
| Iron, % positive | 6 | 33 | .08 | |
| Iron, mean of positive scores | 1.0 | 1.2 | NA | |
| Blood vessel diameter, μm† | 3.7; n = 15 | 4.9 | .02 | |
| Blood vessel wall thickness, % greater than 1 μm | 12.5 | 80 | < .001 | |
| Brain and eye | ||||
| Pyknotic Purkinje cells, % | 0 | 93 | < .001 | |
| Retinal degeneration, % | 0 | 100 | < .001 | |
| Bone marrow | ||||
| Iron, % | 17 | 67 | .02 | |
| Iron, mean of positive scores | 1.5 | 1.3 | NA | |
| Cellularity score | 5.1 | 4.2 | < .001 | |
| Erythroid hyperplasia score | 0.88 | 4.8 | < .001 | |
Measurements are mean values of all grading scores unless otherwise designated as percentage of animals with positive scores and corresponding mean of the positive scores.
NA indicates not applicable; NS, not significant.
Among SS animals, iron scores in the kidney increased by .85 for each month of age (P < .001)
Among SS animals, sex and age were independent factors associated with cardiac vascular ectasia (coefficient = 1.5 for male sex [P = .03] and .44 for each month of age [P = .03])
Despite the effective perfusion, SS mice frequently had residual sickle erythrocytes in the visceral blood vessels, and this limited the masking of the specimens.
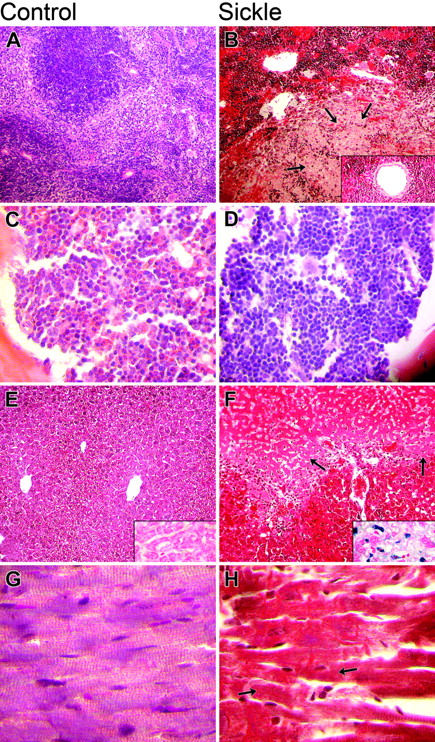
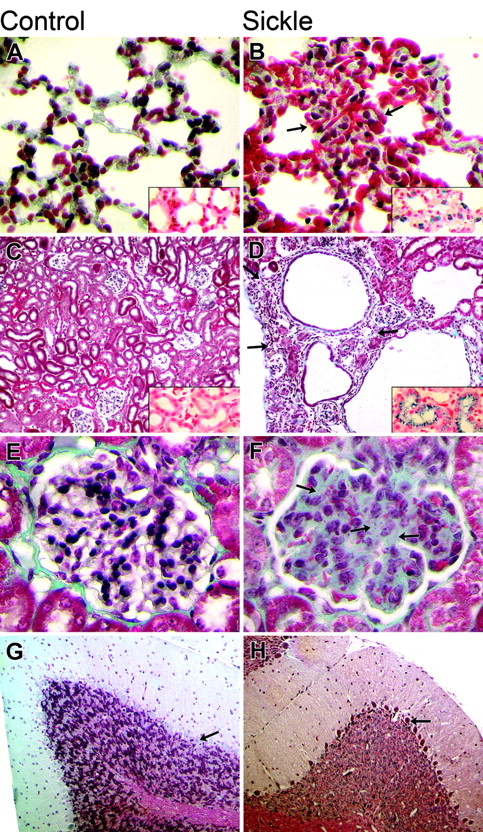
The major histopathologic findings of significant differences between the SS and control groups, along with their statistical significance, are shown in Table 5, and representative sections of spleen, bone marrow, liver, and heart are shown in Figure 1. Representative sections of lung, kidney, glomerulus, and cerebellum are shown in Figure 2.
In brief, the spleens of SS mice demonstrated splenomegaly (with maximum splenic diameter achieved in 2-3 months), expansion of splenic hematopoiesis, splenic infarcts, progressive iron deposition with age, and age-related vascular ectasia. Sections of bone marrow from vertebrae, sternums, and ribs of SS mice demonstrated somewhat less overall cellularity, thinner boney trabeculae, more clumping of hematopoietic cells, and marked erythroid hyperplasia when compared with the controls. The livers of SS mice demonstrated mononuclear-cell infiltration, both gross and microscopic infarcts, iron deposition, and calcifications, but no steatosis. The hearts of SS animals demonstrated increased diameters of blood vessels, microscopic infarcts, and increased iron deposition. The lungs of SS mice demonstrated hemorrhage, progressive iron deposition, capillary congestion, and increased wall thickness of pulmonary arteries. The kidneys of SS mice demonstrated increased cortical width, both gross and microscopic infarcts, iron deposition, enlarged glomeruli with the appearance of increased mesangium, and mononuclear-cell infiltration. In the cerebellum of more than 90% of SS mice, Purkinje cells were frequently pyknotic, but no pyknotic Purkinje cells were present in the controls.
Comparison of histopathology in control and sickle mice. The columns show microscopic findings in control (A, C, E, G) and sickle (B, D, F, H) mice. The 4 rows (top to bottom) show findings in the spleen (A, B), bone marrow (C, D), liver (E, F), and heart (G, H). (A, B) Sickle mice had severe splenic sinusoidal congestion by sickle erythrocytes, increased hematopoietic cells, infarcts (arrows), and vascular ectasia (inset) (H&E original magnification, 100 ×; inset, 400 ×). (C, D) Erythroid hyperplasia was more severe in the bone marrow of sickle mice (H&E original magnification, 100 ×). (E, F) Hepatic findings in sickle mice included more sinusoidal congestion, infarct, and siderosis (inset) (H&E original magnification, 100 ×; insets iron stain, 400 ×). (G, H) Cardiac findings in sickle mice included infarcts, which, when acute, appeared as loss of normal striations (arrows).
Comparison of histopathology in control and sickle mice. The columns show microscopic findings in control (A, C, E, G) and sickle (B, D, F, H) mice. The 4 rows (top to bottom) show findings in the spleen (A, B), bone marrow (C, D), liver (E, F), and heart (G, H). (A, B) Sickle mice had severe splenic sinusoidal congestion by sickle erythrocytes, increased hematopoietic cells, infarcts (arrows), and vascular ectasia (inset) (H&E original magnification, 100 ×; inset, 400 ×). (C, D) Erythroid hyperplasia was more severe in the bone marrow of sickle mice (H&E original magnification, 100 ×). (E, F) Hepatic findings in sickle mice included more sinusoidal congestion, infarct, and siderosis (inset) (H&E original magnification, 100 ×; insets iron stain, 400 ×). (G, H) Cardiac findings in sickle mice included infarcts, which, when acute, appeared as loss of normal striations (arrows).
Comparison of histopathology in control and sickle mice. The columns show microscopic findings in control (A, C, E, G) and sickle (B, D, F, H) mice. The 4 rows (top to bottom) show findings in the lung (A, B), kidney (C, D), glomerulus (E, F), and cerebellum (G, H). (A, B) Pulmonary findings in the sickle mice included more severe vascular congestion (arrows) and siderosis (inset) (H&E original magnification, 100 ×; inset iron stain, 100 ×). (C, D) Renal findings included infarcts (arrows) and siderosis (inset) in the sickle mice (Trichrome stain original magnification, 100 ×; inset iron stain, 100 ×). (E, F) Glomerular changes in the sickle mice included mesangial hyperplasia (arrows). (Trichrome stain original magnification, 400 ×). (G, H) Cerebellar findings included pyknotic Purkinje cell in the sickle mice (arrows) (Trichrome stain original magnification, 100 ×).
Comparison of histopathology in control and sickle mice. The columns show microscopic findings in control (A, C, E, G) and sickle (B, D, F, H) mice. The 4 rows (top to bottom) show findings in the lung (A, B), kidney (C, D), glomerulus (E, F), and cerebellum (G, H). (A, B) Pulmonary findings in the sickle mice included more severe vascular congestion (arrows) and siderosis (inset) (H&E original magnification, 100 ×; inset iron stain, 100 ×). (C, D) Renal findings included infarcts (arrows) and siderosis (inset) in the sickle mice (Trichrome stain original magnification, 100 ×; inset iron stain, 100 ×). (E, F) Glomerular changes in the sickle mice included mesangial hyperplasia (arrows). (Trichrome stain original magnification, 400 ×). (G, H) Cerebellar findings included pyknotic Purkinje cell in the sickle mice (arrows) (Trichrome stain original magnification, 100 ×).
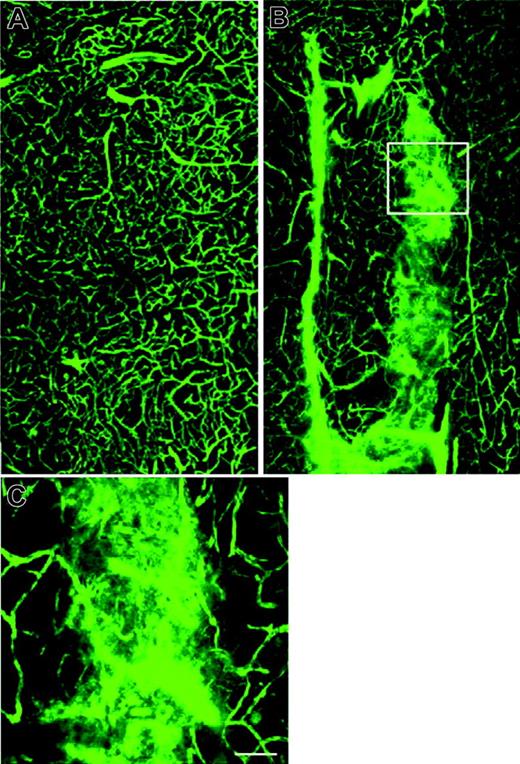
Scanning electron microscopic analysis of control mouse cerebral vessels perfused with the fluorescent plasma marker FITC-dextran demonstrated normal-sized and normally perfused microvessels (Figure 3A).
The SS mouse, however, exhibited irregularly dilated cerebral microvessels with diffuse extravascular leakage of FITC-dextran (n = 2; Figure 3B,C), indicating abnormal vascular permeability.
SS mice between the ages of 1 and 6 months also were notable for not having many of the histologic findings that have been described in humans of variable ages with sickle cell disease, including bone marrow infarcts, splenic atrophy, sickle retinopathy, intimal thickening within systemic vasculature, pulmonary vascular plexiform lesions, pulmonary fat or bone marrow emboli, thromboemboli, renal papillary necrosis, pulmonary infarcts, or disruption of vascular elastic lamina. Moreover, no significant pathologic changes were identified in sections of carotid arteries, cortical bone, pancreas, stomach, small intestines, large intestines, or thymus.
The only histopathologic feature that showed an association with sex was the measurement of cardiac vascular diameters, which was significantly greater in male than in female SS mice. Significant associations with age were observed for ectasia of cardiac vascular diameters and siderosis in the kidneys and, using 2 months of age as the cutoff, with ectasia of splenic arteries and siderosis in spleen and lungs (Table 5).
SS mice had extremely thin retinas with only a single layer of variably attenuated retinal blood vessels and pigmented lesions (Figure 4B).
With bright-field illumination, it was apparent that the pigmented lesions were associated with the remaining retinal vasculature (Figure 4D). Sectioning of these retinas demonstrated that there was no outer nuclear layer (photoreceptors), and the pigmented lesions were retinal pigment epithelial cells (RPEs) that had migrated into the retina and ensheathed surviving blood vessels. The histopathology suggested that the retinal degeneration in SS mice might be due to homozygous mutations in a retinal phosphodiesterase gene (rd1 mutation) (Pde6brd1), which has previously been described in one of the Berkeley parental strains (FVB).9,10 This hypothesis was confirmed by analysis of genomic DNA, which demonstrated homozygous rd1 mutations in the SS mice with retinal lesions. Hemizygous mice also had retinal degeneration, and these also were homozygous for the rd1 mutation.
Cerebral microvessels perfused with FITC-dextran. The plasma-demarcated capillary networks show a broad array of twists, turns, and junctions in the striatum from a control mouse (A). Irregularly dilated cerebral microvessels with leakage of FITC-dextran was detected in the striatum of an SS mouse (B, C). Panel C is a high magnification of boxed area in panel B (Bar = 40 μm).
Cerebral microvessels perfused with FITC-dextran. The plasma-demarcated capillary networks show a broad array of twists, turns, and junctions in the striatum from a control mouse (A). Irregularly dilated cerebral microvessels with leakage of FITC-dextran was detected in the striatum of an SS mouse (B, C). Panel C is a high magnification of boxed area in panel B (Bar = 40 μm).
Retinas from 1-year-old Berkeley SS mice that were heterozygous or homozygous for the rd1 mutation. (A, C, E) Heterozygous for the rd1 mutation; (B, D, F) homozygous for the rd1 mutation. Retinas were incubated for enzyme histochemical demonstration of ADPase activity and then flat-embedded in JB-4. The ADPase activity in viable blood vessels appears white with dark-field illumination (A, B) and black with bright-field illumination (C, D). (A) The normal mouse retina has a spoke wheel pattern of blood vessels that emanates from the optic nerve head (center). (B) The Berkeley mouse that was homozygous for rd1 had a much attenuated retinal vasculature and was missing the dense, deep capillary system. (C) There was no pigment present in the retina of the mouse heterozygous for rd1 with bright-field illumination. (D) The Berkeley rd1/rd1 mouse had many areas that were pigmented, suggesting that the retinal pigment epithelial (RPE) cells had migrated into the sensory retina (arrow). (E) When the normal-appearing retina in panels A and C was sectioned, the 2 nuclear layers of retina were apparent, and photoreceptor outer segments were present below the outer nuclear layer (bottom). The retinal vasculature in the inner retina appeared normal, and a vessel is seen connecting the superficial and deep capillary systems of the retina in this field. (F) The Berkeley rd1/rd1 mouse had no outer nuclear layer or photoreceptor outer or inner segments (bottom), a disorganized inner nuclear layer (bottom), and RPE cells (arrow) had migrated into the retina and ensheathed a dilated blood vessel.
Retinas from 1-year-old Berkeley SS mice that were heterozygous or homozygous for the rd1 mutation. (A, C, E) Heterozygous for the rd1 mutation; (B, D, F) homozygous for the rd1 mutation. Retinas were incubated for enzyme histochemical demonstration of ADPase activity and then flat-embedded in JB-4. The ADPase activity in viable blood vessels appears white with dark-field illumination (A, B) and black with bright-field illumination (C, D). (A) The normal mouse retina has a spoke wheel pattern of blood vessels that emanates from the optic nerve head (center). (B) The Berkeley mouse that was homozygous for rd1 had a much attenuated retinal vasculature and was missing the dense, deep capillary system. (C) There was no pigment present in the retina of the mouse heterozygous for rd1 with bright-field illumination. (D) The Berkeley rd1/rd1 mouse had many areas that were pigmented, suggesting that the retinal pigment epithelial (RPE) cells had migrated into the sensory retina (arrow). (E) When the normal-appearing retina in panels A and C was sectioned, the 2 nuclear layers of retina were apparent, and photoreceptor outer segments were present below the outer nuclear layer (bottom). The retinal vasculature in the inner retina appeared normal, and a vessel is seen connecting the superficial and deep capillary systems of the retina in this field. (F) The Berkeley rd1/rd1 mouse had no outer nuclear layer or photoreceptor outer or inner segments (bottom), a disorganized inner nuclear layer (bottom), and RPE cells (arrow) had migrated into the retina and ensheathed a dilated blood vessel.
Discussion
Berkeley SS mice demonstrate a wide spectrum of hematologic and histopathologic findings that are very similar to those found in humans with sickle cell disease, and yet there clearly are notable differences. Since even a minor difference in hematologic parameters can profoundly affect the severity of human sickle cell disease, in particular, erythrocyte MCHC and levels of fetal hemoglobin, it is important to compare the hematologic manifestation of SS mice to humans with sickle cell disease. SS mice have marked sickling, but the erythrocytes demonstrate a mild β-thalassemia phenotype, with reduced MCHC, as a result of a decreased rate of β globin synthesis relative to α globin.2,4 Since erythrocyte sickling increases as the intraerythrocytic hemoglobin concentration increases, the reduction in MCHC in SS mice would be expected to offer some protection from erythrocyte sickling, and thus perhaps from vaso-occlusion and organ damage. Despite this, erythrocyte sickling is severe in SS mice, and erythrocyte survival is very short.3 Moreover, in humans, HbS-β0 thalassemia is a clinically severe form of sickle cell disease, albeit with fewer cerebral infarcts, despite its association with reduced MCHC.11
Humans with sickle cell disease express variable amounts of fetal hemoglobin, and higher fetal hemoglobin levels are associated with less severe manifestations of the disease. Berkeley SS mice express exceedingly low levels of hemoglobin F after birth,2 and this low level would be expected to counterbalance some of the protection afforded by the reduced hemoglobin concentration. In fact, Fabry et al4 produced a series of mice expressing sickle hemoglobin with nearly balanced (βs/α) globin chain synthesis (0.93 ratio) in conjunction with 3 different levels of hemoglobin F (low, 3%; moderate, 20%; and high, 40%). The NY1KO mice with moderate (20%) hemoglobin F levels most closely resemble the Berkeley mice with regard to level of anemia, reticulocytosis, urine concentrating deficit, and severity of histopathologic findings. The NY1KO mice with very low levels of hemoglobin F had much more severe organ pathology and markedly reduced survival compared to either the Berkeley mice or the NY1KO mice with 20% hemoglobin F.12 These data suggest that in the absence of either a thalassemic phenotype or high levels of hemoglobin F, murine mouse models of sickle cell disease exhibit greater severity of disease than humans with sickle cell disease. Thus, either a mild β thalassemic phenotype, as in the Berkeley mice, or an increased hemoglobin F level, as in the NY1KO mice, appears to be required for survival beyond several months of age.
Hemizygous Berkeley mice, which lack mouse α globin and are heterozygous for mouse β globin, segregate into 2 subpopulations containing approximately 26% and 42% hemoglobin S due to the presence of either 1 or 2 copies of the human α1 γ δ βs transgene, respectively.13 Paradoxically, mice with only one copy of the transgene appear to be more sensitive to hypoxia and have more severe renal involvement than mice with 2 copies of the transgene, even though they have less hemoglobin S.13 Noguchi et al14 ascribed these observations to the low oxygen affinity (P50) of the chimeric human α murine β hemoglobin, which is more abundantly expressed in mice with only a single transgene. We also examined the pathology of hemizygous Berkeley mice with one copy of the human transgene (data not shown). The histopathologic abnormalities in these animals were generally intermediate between those of control and sickle mice, except for the kidney, where we confirmed the severe pathology previously reported by Noguchi et al14 and Diwan et al,15 as well as the cerebellum, where the Purkinje-cell abnormalities of the hemizygous mice were as severe as in the SS mice (data not shown). We also found increased splenic hematopoiesis without associated enlargement of the spleens in the hemizygous mice. It is important to emphasize, however, that the phenotype derived from the combination of a high O2 affinity hemoglobin and hemoglobin S does not correspond to a well-defined human category. Therefore, the hemizygous mice should not be considered a model of human sickle cell trait or human sickle cell disease.
Histopathologic findings in SS mice that are similar to those in human sickle cell disease include evidence of intravascular hemolytic anemia (cardiomegaly, renal tubular and hepatic reticuloendothelial siderosis, and exuberant hematopoiesis), vaso-occlusion (intravascular sickle cells, pyknotic Purkinje cells, multifocal visceral infarcts, and hemorrhages), vascular changes (pulmonary congestion and increased diameter of blood vessels in spleen and heart), pulmonary hemorrhages, siderosis (heart, lungs, kidneys, and spleen), and glomerular hypertrophy.16
Our findings in 1- to 6-month-old SS mice differ from those in human sickle cell disease patients of variable ages in several features. In the spleen, SS mice show splenomegaly rather than atrophy. Whereas splenomegaly in SS mice appears within the first month and is associated with markedly expanded hematopoiesis that persists into adulthood, the initial splenic hypertrophy in human sickle cell disease (until about 2 years of age) is usually followed by progressive siderofibrosis and atrophy with virtual asplenia in adulthood.17,18 Although both murine and human sickle cell disease feature exuberant hematopoiesis, the sites of the hematopoiesis differ. In sickle mice the spleen is the primary hematopoietic organ, which expands to accommodate the increased demand. In human sickle cell disease the bone marrow is the primary hematopoietic organ and is more limited in its capacity for expansion within its compartment of bone, making the marrow more susceptible to compression and ischemic injury. Additionally, in human sickle cell disease, marrow expansion occurs in nonhematopoietic fatty marrow,4 which then cannot only become the site of infection, infarction, and/or bone marrow embolism, but may also weaken the bone, predisposing it to pathologic fracture.19,20
Previous studies have shown similarities in the severity of renal structural and functional changes in SS mice compared with humans with sickle cell disease, including early nephromegaly, glomerular hypertrophy, cortical fibrosis with cyst formation, chronic interstitial nephritis, renal tubular siderosis, and concentrating defects.4,21-25 Further studies will be required to establish whether the glomerular lesions are identical to the mesangioproliferative glomerulopathy found in sickle cell disease patients. In this study, several important dissimilarities were noted, including the absence of papillary necrosis and more severe injury to the renal cortex in SS mice than in humans with sickle cell disease.
Hepatic pathology in SS mice is similar to that in humans with sickle cell disease with regard to congestion, siderosis, infarcts, and hepatomegaly.4 SS mice are dissimilar in having calcifications and a greater number of, and larger, infarcts. In addition the SS mice do not have viral-like hepatitis, or cholestasis, which are relatively common in human sickle cell disease.26-28
Cardiac pathology in SS mice is similar to that in humans with sickle cell disease with regard to cardiomegaly and infarcts in the absence of major coronary artery disease, suggesting vaso-occlusion of the small intracardiac vessels contributes to the pathogenesis of the ischemic injury.29-31
Pulmonary pathology in SS mice is similar to that in humans with sickle cell disease in intravascular sickle cell congestion, hemorrhages, siderosis, and pulmonary medial hypertrophy, but dissimilar in showing much less severe inflammatory and ischemic changes, with no evidence of pneumonia, vascular intimal hyperplasia, plexiform lesions, thromboemboli, fat or bone marrow emboli, edema, interstitial pneumonitis, mucous plugs, or infarcts.32-34 The marked intravascular sickle cell congestion that we observed may be due to the increased expression of endothelial-cell adhesion molecules in the lungs of Berkeley sickle cell mice reported previously by Belcher et al.35
Systemic vascular pathology in these relatively young SS mice is similar to that in young humans with sickle cell disease in showing congestion of small vessels by sickle cells and ectasia, but dissimilar in showing no significant intimal or medial hyperplasia, mural thrombi, perivascular fibrosis, or abnormalities of the elastic lamina (Figure 4D).36-38
Central nervous system pathology in SS mice is similar to that in humans with sickle cell disease in showing evidence of ischemic injury. These ischemic changes in the mice were pyknotic neurons (Purkinje cells) but in humans are reported as clinically apparent or silent strokes.11 The findings are also dissimilar in showing no large vessel vasculopathy, thrombotic/embolic infarcts, or hemorrhagic strokes.39-43 It is particularly striking that no cerebral infarctions were seen, because such infarcts occur in children with sickle cell disease.37-39 The circle of Willis is frequently the site of vascular abnormalities in children with stroke, and thus it is possible that the unusual rheologic characteristics of the circle of Willis contribute to the development of large vessel disease.40 The circle of Willis is variably developed in mice, depending on the genetic background, and while this might be expected to enhance the likelihood of stroke because of reduced collateral circulation, it also may affect rheologic factors that make the cerebral circulation vulnerable to stenosis and thrombosis in humans with sickle cell disease.
Pathology of the bone marrow in SS mice is similar to that in humans with sickle cell disease in demonstrating erythroid hyperplasia and thinning of boney trabeculae, but dissimilar in the absence of thrombosis, perivascular fibrosis, osteomyelitis, or infarcts.18,38,44
Finally, SS mice differ from humans with sickle cell disease in showing early rapid retinal degeneration with nearly complete loss of the outer retinal layers, rather than the retinopathy typical of humans with sickle cell disease.45-49 This was a striking incidental finding, which also has been reported in the FVB mouse strain (one of the parental strains of the Berkeley SS mouse) and is most likely caused by the homozygous mutation we identified in a retinal phosphodiesterase gene (rd1 genotype, PDEGF; 180072; 4p16.3).8,9,10 In unpublished studies by one of us (G.A.L., August 2005), approximately 25% of SS mice and 50% of hemizygous mice derived from another subcolony of Berkeley mice did not demonstrate retinal degeneration; these mice were heterozygous for the rd1 mutation. Thus, by chance, our subcolony appears to have developed a higher frequency of the rd1 mutation.
Overall, our findings demonstrate that the Berkeley SS mouse is likely to be a good model of human sickle cell disease for studies of chronic hemolytic anemia; small-vessel vaso-occlusion by sickle cells; chronic tissue hypoxia of heart, liver, kidneys, and brain; increased hematopoiesis; vascular ectasia; and sickle nephropathy. It is perhaps less well suited for studies of chronic sickle lung disease, the vasculopathy typical in humans with sickle cell disease, bone marrow infarcts, bone marrow or fat pulmonary emboli, retinopathy, renal papillary necrosis, and splenic atrophy.
We conclude that Berkeley SS mice have marked similarities to humans with sickle cell disease in many, but not all, organs. The differences, which probably reflect fundamental differences in hematopoiesis and organ physiology between the species, warrant careful consideration when using the Berkeley SS mouse as a model for human sickle cell disease.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-07-2839.
Supported in part by contract N01-HB-07086 (E.A.M.); grants HL44612 (C.A.H.), HL70981 (C.A.H.), HL45922 (G.A.L.), and HL19278 (B.S.C.) from the National Heart, Lung and Blood Institute; NS43324 from the National Institute of Neurological Disorders and Stroke; and funds from Stony Brook University.
Presented in part in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 6, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to acknowledge Sandra Holzhauer and Terri Besch for their expert technical assistance with the mouse perfusions and phlebotomy; Weiming Yang for the polymerase chain reaction analysis of rd1; and Suzanne Rivera for outstanding secretarial assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal