Integrin αDβ2, the most recently discovered member of the β2 subfamily of integrin adhesion receptors, is up-regulated on macrophage foam cells. Although other members of the subfamily have been subjects of extensive research, the recognition specificity and the molecular basis for αDβ2 ligand binding remain unknown. Based on the high extent of structural homology between αDβ2 and the major myeloid-cell-specific integrin αMβ2 (Mac-1), noted for its capacity to bind multiple ligands, we considered that the 2 integrins have similar recognition specificity. In this study, using recombinant and natural αDβ2-expressing cells, we demonstrate that αDβ2 supports adhesion and migration to many extracellular matrix proteins in a fashion similar to αMβ2. Consistent with these data, the recombinant αDI-domain of the receptor bound selected ligands. The binding was activation-dependent because the αDI-domain with its C-terminal α7 helix truncated, but not the form with the C-terminal part extended, bound ligands. When the αDI-domain segment Lys244-Lys260 (highly homologous to its αMI-domain counterpart Lys245-Arg261 responsible for αMβ2 multiligand-binding properties) was inserted into the mono-specific αLI-domain, the chimeric protein bound many ligands with affinities similar to those of wild-type αDI-domain. These results establish integrin αDβ2 as a multiligand receptor and indicate that the mechanism whereby αDβ2 exhibits broad ligand specificity resembles that used by αMβ2, the most promiscuous member of the integrin family.
Introduction
Integrins are ubiquitous α/β heterodimeric adhesion receptors that mediate cell-cell and cell-extracellular-matrix (ECM) interactions. The subfamily of β2 integrins comprises a monophyletic group of closely related glycoproteins critically involved in leukocyte adhesion and migration during the inflammatory immune responses. The group consists of 4 members, αLβ2 (LFA-1, CD11a/CD18), αMβ2 (Mac-1, CD11b/CD18), αXβ2 (p150,95, CD11c/CD18), and αDβ2 (CD11d/CD18). These integrins are assembled as a group on the basis of their common β2 subunit and their expression restricted to leukocytes. Whereas structure-function relationships of 3 integrins, αLβ2, αMβ2, and αXβ2, have been the subjects of intensive research, the ligand-binding specificity and pathophysiologic roles of the most recently discovered integrin αDβ21 are poorly understood. Like 2 other highly related β2 integrins, αMβ2 and αXβ2, integrin αDβ2 is expressed on myeloid cells such as monocytes and neutrophils. However, the pattern of expression of this integrin is unique among other myeloid cell-specific β2 integrins in that αDβ2 is expressed poorly on peripheral blood leukocytes but strongly on macrophage foam cells within atherosclerotic plaques.1,2 In contrast, the major myeloid-specific integrin αMβ2 is progressively up-regulated on circulating neutrophils and monocytes in response to chemotactic stimulation3,4 and is down-regulated in lesion-derived macrophages.5 The significance of the up-regulation of αDβ2 in monocyte-derived cells from atherosclerotic lesions is not known. The role of high levels of αDβ2 in these cells may become clear if the ligands with which this integrin interacts are defined. Previous studies demonstrated that αDβ2 is capable of binding vascular cell adhesion molecule 1 (VCAM-1)6,7 and intercellular adhesion molecule 3 (ICAM-3).1 VCAM-1 is present on endothelial cells and, thus, clearly absent from the extravascular compartment in which formation of foam cells occurs. ICAM-3 is present on lymphocytes that are detectable in atherosclerotic lesions (for a review, see Getz8 ). However, recruitment of monocytes into atherogenic foci and their retention within lesions would require cell interactions with their surroundings. Yet, essentially nothing is known about the interaction of αDβ2 with the ECM proteins.
It is well documented that the interactions of β2 integrins with ligands are mediated by their I-domains, the regions of about 180 amino acid residues inserted into the β-propeller of α subunits.9 The αDI-domain is highly homologous to the αMI-domain with 60% of its amino acid sequence being identical. A distinctive feature of integrin αMβ2 is its ability to bind numerous structurally diverse ligands, including many ECM proteins (reviewed in part by Yakubenko et al10 ). We have previously demonstrated that within the αMI-domain, the αM(Lys245-Arg261) segment contributes to the binding of many ligands.10 This segment forms the (βD-α5)-loop/α5-helix in the 3-dimensional structure of the αMI-domain11 and is the site of the major structure and sequence divergence between the αMI-domain and the αLI-domain, an integrin with a narrow ligand-binding specificity and that binds only ICAMs.12 We have noted that the αMI-domain region αM(Lys245-Arg261) and its homolog, Lys244-Lys260 in the αDI-domain, have 59% identical and 12% conserved residues (Figure 1). Furthermore, 4 residues in the αM sequence, Asp248, Tyr252, Asp254, and Pro257, which were shown to be critical for ligand binding by αMβ2,13,14 are present in the αDI-domain. Given these similarities, we hypothesized that αDβ2 and αMβ2 have overlapping recognition specificity and that the region in the αDI-domain homologous to αM(Lys245-Arg261) is involved in ligand binding.
In the present study, we have examined the molecular basis for ligand binding by integrin αDβ2. We have generated αDβ2-expressing cells and demonstrated that they are capable of binding many ECM proteins with specificity overlapping that of αMβ2, a finding that places αDβ2 into the category of multiligand receptors. Furthermore, we have shown that the mechanism whereby αDβ2 exhibits promiscuity in ligand binding is like that used by αMβ2.
Materials and methods
Proteins, peptides, and antibodies
Human fibrinogen was obtained from Enzyme Research Laboratories (South Bend, IN). Fibronectin was isolated from fresh human plasma by gelatin-agarose affinity chromatography.15 Vitronectin was isolated from outdated plasma treated with 8 M urea using heparin-agarose affinity chromatography.16 Plasminogen was purified from human plasma using lysine-agarose affinity chromatography followed by gel filtration as described.17 Recombinant cysteine-rich angiogenic inducer (Cyr61) was a generous gift from Dr S. Lam (University of Illinois, Chicago, IL). VCAM-1 and ICAM-3 were purchased from R&D Systems (Minneapolis, MN). The P2-C peptide (TMKIIPFNRLTIG), corresponding to the fibrinogen sequence γ383-395, was synthesized and purified as described.18 The monoclonal antibody (mAb) IB4 directed against the β2-integrin subunit and rat mAb 1/70, which recognizes mouse αM integrin subunit, were purified from the conditioned media of the hybridoma cell line obtained from American Tissue Culture Collection (ATCC; Manassas, VA) using protein A agarose (Amersham Biosciences, Piscataway, NJ). Polyclonal antibody 1950 directed against the β1-integrin subunit was purchased from Chemicon (Temecula, CA). Polyclonal antibody against the αDI-domain was generated by BioSource International (Camarillo, CA) using recombinant αDI-domain as an immunogen. The antibody was isolated from rabbit serum by precipitation with 35% ammonium sulfate and then purified by affinity chromatography using αDI-domain-Sepharose. The polyclonal antibody recognizes both human and mouse αDI-domains and has no cross-reactivity with the αM-integrin subunits (V.P.Y., unpublished data, May 2004). Purified rabbit, mouse, and rat IgG were purchased from Sigma (St Louis, MO).
Amino acid alignment of the αMI-domain sequence Lys245-Arg261 with other integrin α subunit I-domains. The αMI-domain sequence was aligned with human αX, αD, αL, α1, α2, α10, α11, and αE using the National Center for Biotechnology Informatics (NCBI) database. Numbers on the right indicate the homology between αM (assigned a value of 100%) and other α subunits expressed as the percentage of identical residues (shown in bold). Dots above the αDI-domain sequence indicate residues homologous between αD and αM. Residues identified as critical for ligand binding in the αMI-domain13,14 are underlined.
Amino acid alignment of the αMI-domain sequence Lys245-Arg261 with other integrin α subunit I-domains. The αMI-domain sequence was aligned with human αX, αD, αL, α1, α2, α10, α11, and αE using the National Center for Biotechnology Informatics (NCBI) database. Numbers on the right indicate the homology between αM (assigned a value of 100%) and other α subunits expressed as the percentage of identical residues (shown in bold). Dots above the αDI-domain sequence indicate residues homologous between αD and αM. Residues identified as critical for ligand binding in the αMI-domain13,14 are underlined.
Expression of recombinant I-domains and site-directed mutagenesis
Two recombinant αDI-domains, the “active” and “nonactive,” were expressed in Escherichia coli. The cDNA encoding the “active” αDI-domain (residues Pro128-Lys314) was generated by polymerase chain reaction (PCR) from a randomly primed cDNA library of the U937 monocytoid cell line. A PCR fragment was digested with NdeI and XhoI and inserted into the pET-15b vector (Novagen, Madison, WI). The αDI-domain as a His-tag fusion protein containing 6 His residues at its NH2 terminus was purified from a soluble fraction of E coli lysates using affinity chromatography on Ni-chelating agarose (Qiagen, Valencia, CA). To express the “nonactive” αDI-domain (residues Pro128-Ala323), the cDNA coding this region was amplified from the U937 cell library and inserted into the PGEX-4T-1 vector (Amersham Biosciences) using EcoRI and XhoI sites for expression in E coli BL-21(DE3) pLysS cells. The αDI-domain as a fusion with GST was purified from a soluble fraction of E coli lysates using affinity chromatography on glutathione agarose essentially as described.14 The GST component was removed by cleavage with thrombin as described. The “active” αLI-domain, originally described by Lu et al,19 was prepared using the previously created αL-PGEX4T-1 construct (Gly128-Tyr307)14 as a framework. In the new construct, 2 lysines, Lys287 and Lys294, were substituted to cysteines to create a disulfide bond and, thus, lock the protein in the active conformation. Mutations were introduced using the QuickChange site-directed mutagenesis kit from Stratagene (La Jolla, CA) according to the manufacturer's instructions. The αLI-domain as a fusion with GST was purified from a soluble fraction of E coli using affinity chromatography on glutathione agarose and its fusion part removed by thrombin.
To generate the αL(αDLys244-Lys260) I-domain chimera, the segment corresponding to the αLI-domain sequence Ala242-Asp251 was changed to the homologous segment of the αDI-domain Lys244-Lys260. A segment switch was created by oligonucleotide-directed mutagenesis using PCR. The construction of the chimera was based on the observation that oligonucleotide sequence encoding αD Lys244-Lys260 contains a unique restriction site for XbaI. Therefore, designed mutagenic primers contained nucleotide sequence for the αDLys244-Lys260 segment and additional unchanged αL bases: 5′-CCTCTAGAATACAGTGACGTCATCCCCCAGGCAGAGAAGAAAGACATCATCCGCTACATCATC (forward), 5′-GTATTCTAGAGGGTCTTTGTACTTCTCCCCATCCGTGATGATGATAAGCAC (reverse); the αD sequences are in italics and the restriction site for XbaI is underlined. The pGEX-4T-1 vector containing the cDNA encoding the “active” αLI-domain was modified by PCR using PfuTurbo DNA polymerase with the following cycling parameters: 95°C for 30 seconds, 55°C for 1 minute, and 68°C for 11.5 minutes. Following temperature cycling, the product was treated with DpnI to digest the parental DNA template. The linear product was purified and digested with XbaI to produce a cDNA fragment with cohesive ends. These fragments were ligated and transformed into BL-21(DE3)pLysS E coli-competent cells. The accuracy of the DNA sequence was verified by sequencing. The chimeric I-domain as fusion with GST was expressed and purified following the procedure described for the wild-type I-domains.
Cells and stable transfection of integrin subunit constructs
The IC-21 macrophage cell line was obtained from the ATCC (Rockville, MD). Cells were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA) supplemented with 10% FBS, 0.1 mg/mL streptomycin, 0.1 U/mL penicillin, and 2 mM l-glutamine. The αMβ2-expressing and mock-transfected HEK 293 cells10 were maintained in DMEM/F-12 (Gibco) supplemented with 10% FBS, 2 mM glutamine, 15 mM HEPES, 0.1 mg/mL streptomycin, and 0.1 U/mL penicillin. The cDNA encoding the β2-integrin subunit was generated as previously described.10 The full-length cDNA of the αD-integrin subunit was cloned from a human U937 monocytoid cell line cDNA library. The library was synthesized from total cellular RNA using SuperScriptII reverse transcriptase (Invitrogen) as described previously.20 The αD cDNA was cloned into pcDNA3.1/Neo(-) vector and the β2 cDNA was cloned into pcDNA3.1/Hygro(-) vector (Invitrogen). HEK 293 cells were stably transfected with pcDNA3.1 plasmids with inserted αD and β2 using LipofectAMINE 2000 reagent (Invitrogen). After 48 hours at 37°C in 5% CO2, cells were harvested and cultured in medium with 500 μg/mL G418 (Invitrogen) and 250 μg/mL Hygromycin (Invitrogen). After 14 days, surviving cells were collected, sorted, and analyzed using flow cytometry and immunoprecipitation.
Flow cytometry
Fluorescence-activated cell sorting (FACS) analyses were performed to assess the expression of receptors on the surface of the cells transfected with αDβ2 integrin. The cells were incubated with anti-αD polyclonal antibody and anti-β2 mAb IB4 and analyzed using a FACScan (Becton Dickinson, San Jose, CA) as described.10 Populations of cells expressing similar amounts of αMβ2 and αDβ2 were selected by FACS using a BD Biosciences (San Jose, CA) FACS Vantage Instrument.
Immunoprecipitation
Cells (5 × 106) were labeled with 100 μg Immunopure Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) in 200 μL PBS for 30 minutes at 22°C. The cells were solubilized with a lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM CaCl2, 1 mM PMSF, 100 μg/mL leupeptin, 10 mM benzamidine) for 30 minutes at 22°C. The lysates were incubated with 10 μg normal mouse IgG (Sigma) and 50 μL Zysorbin-G (Zymed, San Francisco, CA) for 2 hours at 4°C. After centrifugation, the supernatant was incubated with 10 μg mAb IB4 (anti-β2) for 2 hours at 4°C. The integrin-mAb complex was captured by incubating with 50 μL protein A-Sepharose (Amersham Biosciences) for 2 hours at 4°C. The immunoprecipitated proteins were eluted with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and analyzed by Western blotting. The Immobilon-P membranes (Millipore, New Bedford, MA) were incubated with streptavidin conjugated to horseradish peroxidase and developed using enhanced SuperSignal Chemiluminescent Substrate (Pierce).
Adhesion and migration assays
For adhesion assays, 96-wells tissue culture plates (Costar, Cambridge, MA) were coated with different concentrations of fibrinogen, fibronectin, vitronectin, plasminogen, Cyr61, and VCAM-1 for 3 hours at 37°C. The wells were post-coated with 0.5% PVA for 1 hour at 22°C. Cells in DMEM/F-12 were labeled with 10 μM calcein AM (Molecular Probes, Eugene, OR) and assays were performed as described previously.10,21
Cell migration assays with calcein-labeled IC-21 cells were performed under sterile conditions using uncoated Transwell inserts with a pore size of 8 μm and 6.5 mm in diameter (Costar, Corning, NY) as described.21 Briefly, the lower chambers contained 600 μL of 10 μg/mL vitronectin. Cells (150 μL) in DMEM/F-12 at a concentration of 2.5 × 106/mL were placed in the upper chamber and allowed to migrate for 18 hours at 37°C in 5% CO2.Two hours prior to the completion of the migration assay, calcein AM was added to the lower chamber to label cells. Assays were stopped by removing cells from the upper surface of the polycarbonate membrane. Cells migrating to the bottom of the filter were detected using a CytoFluorII fluorescence plate reader (Applied Biosystems, Farmington, MA).
Surface plasmon resonance studies
The interaction between the I-domains and various ligands was measured using surface plasmon resonance (SPR) using a Biacore 3000 instrument (Biacore, Uppsala, Sweden). Vitronectin, fibrinogen, fibronectin, Cyr61, plasminogen, VCAM-1, and ICAM-3 were covalently coupled via primary amines, at concentrations ranging between about 1000 and 2000 response units, to the dextran matrix of CM5 sensor chips. Different concentrations of the I-domains in HBS-P buffer (Biacore) supplemented with 1 mM MgCl2 were flowed over cells containing various ligands. All data were corrected for the response obtained using a blank reference flow cell that was activated with EDC/NHS and then blocked with ethanolamine. Steady-state experiments were performed by injecting the analytes at 10 μL/min for 3 minutes. The chip surface was regenerated using 2 M NaCl plus 50 mM NaOH. Data were analyzed using the BIAevaluation 3.1 program (BiaCore, Uppsala, Sweden).
Results
Analyses of ligand-binding properties of integrin αDβ2
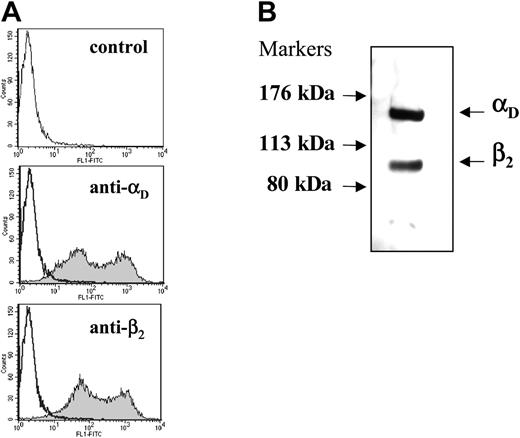
To search for potential αDβ2 ligands, we have generated a cell line expressing this integrin. HEK 293 cells were cotransfected with the wild-type αD and β2 and stable transfectants were established. FACS analyses using antibodies specific for the integrin subunits demonstrated similar levels of expression of αD and β2 (Figure 2A). Heterodimer association of the integrin was evaluated by immunoprecipitation of detergent-lysed surface-labeled cells (Figure 2B). Anti-β2-specific mAb IB4 immunoprecipitated both the αD (molecular weight [MW], 150 kDa) and β2 (MW, 100 kDa) subunits from cells, indicating that they are associated on the cell surface.
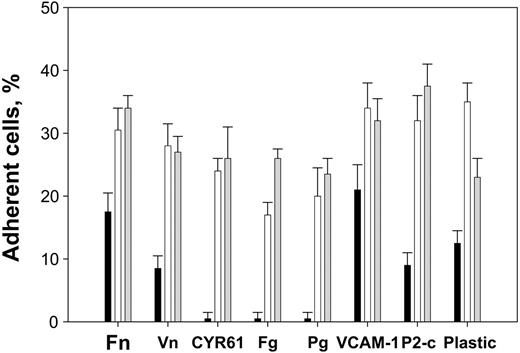
To test the possibility that the recognition specificity of αDβ2 overlaps with that of the related integrin αMβ2, we have investigated the ability of the αDβ2-expressing cells to adhere to several EMC proteins that represent well-characterized αMβ2 ligands. These included: (1) the ECM proteins fibronectin, vitronectin, and fibrinogen, and (2) the proteins Cyr61 and plasminogen, which are overexpressed or become ECM-associated during the inflammatory responses.22,23 Furthermore, cell adhesion to VCAM-1 was tested because it represents an established αDβ2 ligand.1 Additionally, cell adhesion to the fibrinogen P2-C peptide, which duplicates the binding site for αMβ2 in fibrinogen24 and contains the generic recognition information required for αMβ2 binding,25 was examined. Naked plastic, which typifies the ability of αMβ2 to stick to many unrelated proteins, was also tested. Figure 3 illustrates that the αDβ2-expressing HEK 293 cells adhered to wells coated with all selected ligands and that comparable levels of αDβ2- and αMβ2-expressing HEK 293 cells attached to each protein. In this figure, maximal cell adhesion to each ligand is shown as obtained from the dose-dependent curves, and the data are expressed as a percentage of added cells. αDβ2 supported efficient adhesion to vitronectin, fibronectin, Cyr61, plasminogen, and P2-C similar to αMβ2. αDβ2-mediated adhesion to fibrinogen was slightly lower than that mediated by αMβ2. By contrast, the αDβ2-expressing cells adhered better to uncoated plastic than the αMβ2-expressing cells. Because cells expressing similar levels of αDβ2 and αMβ2 were used in these experiments, the difference in adhesion may reflect a stronger affinity of αDβ2 to plastic. In control experiments, mock-transfected HEK 293 cells adhered poorly to fibrinogen, Cyr61, and plasminogen, as expected.18,26,27 These cells adhered to a lower extent to fibronectin,21 vitronectin, and VCAM-1, consistent with the presence of endogenous β1 integrins (see next paragraph).
Analysis of integrin expression and heterodimer formation in HEK 293 cells transfected with wild-type αDβ2. (A) The binding of anti-αD-specific polyclonal antibody and anti-β2-specific mAb IB4 to the αDβ2-expressing cells was analyzed by flow cytometry. Results are presented as histograms with the logarithm of fluorescence intensity on the abscissa and the cell number on the ordinate. Control cells incubated with Alexa 488-conjugated secondary antibody are shown in the top panel. (B) Immunoprecipitation of biotin-labeled αDβ2. Cells (5 × 105) were labeled with biotin, lysed, and immunoprecipitated with 10 μg anti-β2 mAb IB4. The immunoprecipitates were analyzed by Western blotting using streptavidin conjugated to horseradish peroxidase. The integrin subunits were detected using an enhanced chemiluminescent substrate and exposed to Kodak BioMax film.
Analysis of integrin expression and heterodimer formation in HEK 293 cells transfected with wild-type αDβ2. (A) The binding of anti-αD-specific polyclonal antibody and anti-β2-specific mAb IB4 to the αDβ2-expressing cells was analyzed by flow cytometry. Results are presented as histograms with the logarithm of fluorescence intensity on the abscissa and the cell number on the ordinate. Control cells incubated with Alexa 488-conjugated secondary antibody are shown in the top panel. (B) Immunoprecipitation of biotin-labeled αDβ2. Cells (5 × 105) were labeled with biotin, lysed, and immunoprecipitated with 10 μg anti-β2 mAb IB4. The immunoprecipitates were analyzed by Western blotting using streptavidin conjugated to horseradish peroxidase. The integrin subunits were detected using an enhanced chemiluminescent substrate and exposed to Kodak BioMax film.
Adhesion of αDβ2- and αMβ2-expressing HEK 293 cells to different ligands. HEK 293 cells expressing wild-type αDβ2 (□) and αMβ2 ( ), and mock-transfected cells (▪), were labeled with calcein and their adhesion to different ligands was tested. Aliquots (50 μL) of 5 × 105/mL cells in DMEM/F-12 were added to the wells coated with increasing concentrations of different ligands. After incubation for 30 minutes at 37°C, the nonadherent cells were removed by 2 washes with PBS and fluorescence was measured. The number of adherent cells was calculated by using the fluorescence of aliquots with a known number of labeled cells. Maximal adhesion to each substrate is shown as was determined from the dose-dependent curves of adhesion. Maximal adhesion to fibronectin (Fn), fibrinogen (Fg), plasminogen (Pg), Cyr61, vitronectin (Vn), P2-C, and VCAM-1 was observed at coating concentrations of 10 μg/mL, 2 μg/mL, 10 μg/mL, 2.5 μg/mL, 5 μg/mL, 50 μg/mL, and 5 μg/mL, respectively. Data are expressed as a percentage of added cells and are the mean ± SE of 3 to 6 individual experiments.
), and mock-transfected cells (▪), were labeled with calcein and their adhesion to different ligands was tested. Aliquots (50 μL) of 5 × 105/mL cells in DMEM/F-12 were added to the wells coated with increasing concentrations of different ligands. After incubation for 30 minutes at 37°C, the nonadherent cells were removed by 2 washes with PBS and fluorescence was measured. The number of adherent cells was calculated by using the fluorescence of aliquots with a known number of labeled cells. Maximal adhesion to each substrate is shown as was determined from the dose-dependent curves of adhesion. Maximal adhesion to fibronectin (Fn), fibrinogen (Fg), plasminogen (Pg), Cyr61, vitronectin (Vn), P2-C, and VCAM-1 was observed at coating concentrations of 10 μg/mL, 2 μg/mL, 10 μg/mL, 2.5 μg/mL, 5 μg/mL, 50 μg/mL, and 5 μg/mL, respectively. Data are expressed as a percentage of added cells and are the mean ± SE of 3 to 6 individual experiments.
Adhesion of αDβ2- and αMβ2-expressing HEK 293 cells to different ligands. HEK 293 cells expressing wild-type αDβ2 (□) and αMβ2 ( ), and mock-transfected cells (▪), were labeled with calcein and their adhesion to different ligands was tested. Aliquots (50 μL) of 5 × 105/mL cells in DMEM/F-12 were added to the wells coated with increasing concentrations of different ligands. After incubation for 30 minutes at 37°C, the nonadherent cells were removed by 2 washes with PBS and fluorescence was measured. The number of adherent cells was calculated by using the fluorescence of aliquots with a known number of labeled cells. Maximal adhesion to each substrate is shown as was determined from the dose-dependent curves of adhesion. Maximal adhesion to fibronectin (Fn), fibrinogen (Fg), plasminogen (Pg), Cyr61, vitronectin (Vn), P2-C, and VCAM-1 was observed at coating concentrations of 10 μg/mL, 2 μg/mL, 10 μg/mL, 2.5 μg/mL, 5 μg/mL, 50 μg/mL, and 5 μg/mL, respectively. Data are expressed as a percentage of added cells and are the mean ± SE of 3 to 6 individual experiments.
), and mock-transfected cells (▪), were labeled with calcein and their adhesion to different ligands was tested. Aliquots (50 μL) of 5 × 105/mL cells in DMEM/F-12 were added to the wells coated with increasing concentrations of different ligands. After incubation for 30 minutes at 37°C, the nonadherent cells were removed by 2 washes with PBS and fluorescence was measured. The number of adherent cells was calculated by using the fluorescence of aliquots with a known number of labeled cells. Maximal adhesion to each substrate is shown as was determined from the dose-dependent curves of adhesion. Maximal adhesion to fibronectin (Fn), fibrinogen (Fg), plasminogen (Pg), Cyr61, vitronectin (Vn), P2-C, and VCAM-1 was observed at coating concentrations of 10 μg/mL, 2 μg/mL, 10 μg/mL, 2.5 μg/mL, 5 μg/mL, 50 μg/mL, and 5 μg/mL, respectively. Data are expressed as a percentage of added cells and are the mean ± SE of 3 to 6 individual experiments.
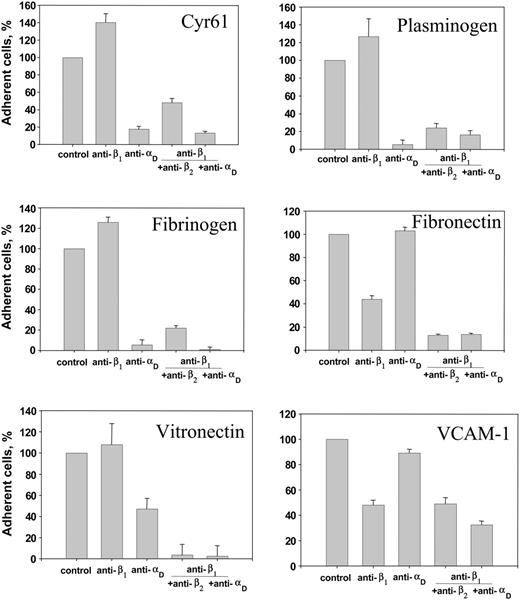
HEK 293 cells express other integrins, particularly those that belong to the β1 group21 and that are known to bind the ECM proteins. For example, integrin α5β1 binds fibronectin,28 α4β1 mediates adhesion to VCAM-1,29,30 and α6β1 was shown recently to bind Cyr61.31 To determine the contribution of β1 integrins to adhesion mediated by the αDβ2-expressing HEK 293 cells, we performed adhesion experiments in the presence of anti-β1, anti-αD, and anti-β2 function-blocking antibodies. As shown in Figure 4, no inhibition of adhesion by anti-β1 polyclonal antibody to Cyr61, fibrinogen, vitronectin, plasminogen, P2-C, and uncoated plastic (not shown) was detected, suggesting that cell attachment to these substrates was entirely αDβ2-dependent. In agreement with these results, anti-αD polyclonal antibody blocked cell adhesion to these proteins by about 90% (except vitronectin, to which it blocked adhesion by 55%). Nevertheless, the combination of anti-β1 and anti-αD (or anti-β1 plus anti-β2) antibodies completely blocked cell adhesion to vitronectin. In contrast, anti-β1 antibody inhibited adhesion to fibronectin and VCAM-1 by about 50% to 55%, indicating that endogenous α5β1 and α4β1, respectively, on the αDβ2-expressing cells contribute to adhesion. At the same time, anti-αD alone was a poor inhibitor of cell adhesion; it did not inhibit adhesion to fibronectin and produced only about 15% inhibition of cell adhesion to VCAM-1. However, the combination of anti-β1 and either anti-αD or anti-β2 antibodies effectively inhibited cell adhesion to these ligands, illustrating the contribution of αDβ2. Neither control rabbit nor mouse IgG inhibited cell adhesion (not shown). Taken together, these observations indicate that αDβ2 is capable of engaging many ECM proteins during cell adhesion and suggest that the recognition specificity of αDβ2 is similar to that exhibited by αMβ2.
Further evidence for the similarity of ligand specificity of integrins αDβ2 and αMβ2 was obtained in inhibition experiments using soluble peptide P2-C. We previously reported that P2-C inhibits αMβ2-mediated cell adhesion not only to the γC domain of fibrinogen, from which this sequence is derived, but also to other fibrinogen domains32 and to many unrelated proteins (Valeryi Lishko, unpublished data, June 2002).27,33 P2-C was also an effective inhibitor of αDβ2-mediated cell adhesion to vitronectin, fibrinogen, plasminogen, and VCAM-1 (Table 1). Whereas cell adhesion to fibronectin and Cyr61 was less sensitive to inhibition, it was blocked by a combination of P2-C and anti-β1 antibody. These results clearly show that within ECM proteins, αDβ2 binds sequences that contain recognition information resembling that of the αMβ2 recognition peptide P2-C.
Effect of fibrinogen peptide P2-C on adhesion of the αDβ2-expressing HEK 293 cells to different ligands
Ligands . | Vitronectin . | Plasminogen . | Fibrinogen . | Fibronectin . | VCAM-1 . | Cyr61 . |
|---|---|---|---|---|---|---|
| P2-C | 77 ± 3 | 68 ± 6 | 70 ± 6 | 42 ± 3 | 83 ± 3 | 20 ± 5 |
| P2-C + anti-βt | 84 ± 4 | 72 ± 3 | 88 ± 7 | 94 ± 2 | 96 ± 2 | 78 ± 3 |
Ligands . | Vitronectin . | Plasminogen . | Fibrinogen . | Fibronectin . | VCAM-1 . | Cyr61 . |
|---|---|---|---|---|---|---|
| P2-C | 77 ± 3 | 68 ± 6 | 70 ± 6 | 42 ± 3 | 83 ± 3 | 20 ± 5 |
| P2-C + anti-βt | 84 ± 4 | 72 ± 3 | 88 ± 7 | 94 ± 2 | 96 ± 2 | 78 ± 3 |
Wells were coated with different ligands at concentrations which produce maximal cell adhesion (as described in Figure 3). Calcein-labeled αDβ2-expressing HEK 293 cells were incubated with 200 μg/mL P2-C or with a mixture of P2-C and anti-βt antibody diluted 1:500 for 20 minutes at 22°C and then added to the coated wells. Adhesion was quantitated as described in “Materials and methods.” Results are expressed as a percentage of inhibition of adhesion, with adhesion in the absence of competing agents defined as 0% inhibition. The data shown are means ± SE of 3 individual experiments, each with triplicate measurements.
αDβ2 on macrophages mediates adhesion and migration to various ECM proteins
To extend our findings with transfected cells, we examined the ability of macrophage cell line IC-21 to bind various ECM proteins. As assessed by flow cytometry analyses (Figure 5A), unstimulated cultured cells express both integrins αMβ2 and αDβ2, with the latter receptor being expressed at a slightly higher level (1.3-fold). IC-21 cells adhered strongly and in a concentration-dependent manner to various ECM proteins (not shown). To determine a relative contribution of the integrins on macrophages to this adhesion process, we examined the effect of antibodies directed against anti-αM and anti-αD integrin subunits. Table 2 shows that individual antibodies exerted partial inhibition of adhesion. However, the combination of 2 antibodies effectively inhibited adhesion to selected ECM proteins by 45% to 65%. In parallel experiments, natural macrophages elicited by thioglycollate injection and isolated from the mouse peritoneal cavities efficiently adhered to the ECM proteins in an αDβ2-dependent manner and no inhibition of adhesion by control rabbit or rat IgG was detected (not shown).
Inhibition of IC-21 cell adhesion to various ECM proteins by anti-αD and anti-αM antibodies
Ligand . | Anti-αD . | Anti-αM . | Anti-αD + anti-αM . |
|---|---|---|---|
| Vitronectin | 27 ± 4 | 52 ± 9 | 63 ± 5 |
| Fibrinogen | 26 ± 4 | 29 ± 9 | 48 ± 8 |
| Fibronectin | 29 ± 8 | 22 ± 1 | 45 ± 4 |
| Plasminogen | 23 ± 9 | 39 ± 5 | 56 ± 7 |
| Cyr61 | 43 ± 9 | 48 ± 8 | 59 ± 8 |
Ligand . | Anti-αD . | Anti-αM . | Anti-αD + anti-αM . |
|---|---|---|---|
| Vitronectin | 27 ± 4 | 52 ± 9 | 63 ± 5 |
| Fibrinogen | 26 ± 4 | 29 ± 9 | 48 ± 8 |
| Fibronectin | 29 ± 8 | 22 ± 1 | 45 ± 4 |
| Plasminogen | 23 ± 9 | 39 ± 5 | 56 ± 7 |
| Cyr61 | 43 ± 9 | 48 ± 8 | 59 ± 8 |
Calcein-labeled IC-21 cells were preincubated with 20 μg/mL anti-αD polyclonal antibody or 20 μg/mL anti-αM mAb M1/70 for 20 minutes at 22°C and then added to wells coated with different ligands. Adhesion was quantitated as described in “Materials and methods.” Results are expressed as a percentage of inhibition of adhesion and are means ± SE from 3 separate adhesion assays, each with triplicate measurements.
Effect of function blocking antibodies on adhesion of the αDβ2-expressing HEK 293 cells. Calcein-labeled αDβ2-expressing HEK 293 cells were preincubated for 20 minutes at 22°C with anti-β1 polyclonal antibody 1950 (1:500 dilution), 20 μg/mL anti-αD polyclonal antibody, or with combinations of anti-β1 with either anti-αD or anti-β2 mAb IB4 (20 μg/mL). Cells were added to microtiter wells coated with various ligands at concentrations that produce maximal adhesion, and cell adhesion was determined as described in Figure 3. Data are expressed as a percentage of control (adhesion in the absence of antibodies) and are the mean ± SE of 3 individual experiments performed in triplicate in each experiment.
Effect of function blocking antibodies on adhesion of the αDβ2-expressing HEK 293 cells. Calcein-labeled αDβ2-expressing HEK 293 cells were preincubated for 20 minutes at 22°C with anti-β1 polyclonal antibody 1950 (1:500 dilution), 20 μg/mL anti-αD polyclonal antibody, or with combinations of anti-β1 with either anti-αD or anti-β2 mAb IB4 (20 μg/mL). Cells were added to microtiter wells coated with various ligands at concentrations that produce maximal adhesion, and cell adhesion was determined as described in Figure 3. Data are expressed as a percentage of control (adhesion in the absence of antibodies) and are the mean ± SE of 3 individual experiments performed in triplicate in each experiment.
Previous studies demonstrated that αMβ2 is capable of promoting leukocyte migration to several ECM proteins, including fibrinogen and fibronectin.21,34 To examine whether αDβ2 can support leukocyte migration, we have examined the ability of vitronectin, as a representative ECM protein, to mediate migration of IC-21 cells. As shown in Figure 5B, cells efficiently migrated toward vitronectin and this process depended on both αDβ2 and αMβ2 because anti-αD polyclonal antibody and anti-αM mAb M1/70 blocked the response.
Expression of αDβ2 and αMβ2 on the surface of IC-21 macrophage cell line and their role in macrophage migration. (A) The level of αDβ2 and αMβ2 expression was assessed by flow cytometry with mAb 1/70, which recognizes mouse αMβ2, and polyclonal anti-αD antibody, which recognizes both human and mouse αD integrin subunits. Control cells are shown as open histograms. (B) IC-21 cells were analyzed for their ability to migrate to 10 μg/mL vitronectin either in the absence or in the presence of blocking polyclonal anti-αD and anti-αM mAb M1/70. Cells were preincubated with 2.5 μg/mL of each antibody for 20 minutes before their addition to the upper chamber of Transwell plates. In control samples, cells were pretreated with 2.5 μg/mL of each rabbit or rat IgG. Cells were allowed to migrate toward vitronectin for 18 hours at 37°C and the extent of cell migration was assessed as described in “Materials and methods.”
Expression of αDβ2 and αMβ2 on the surface of IC-21 macrophage cell line and their role in macrophage migration. (A) The level of αDβ2 and αMβ2 expression was assessed by flow cytometry with mAb 1/70, which recognizes mouse αMβ2, and polyclonal anti-αD antibody, which recognizes both human and mouse αD integrin subunits. Control cells are shown as open histograms. (B) IC-21 cells were analyzed for their ability to migrate to 10 μg/mL vitronectin either in the absence or in the presence of blocking polyclonal anti-αD and anti-αM mAb M1/70. Cells were preincubated with 2.5 μg/mL of each antibody for 20 minutes before their addition to the upper chamber of Transwell plates. In control samples, cells were pretreated with 2.5 μg/mL of each rabbit or rat IgG. Cells were allowed to migrate toward vitronectin for 18 hours at 37°C and the extent of cell migration was assessed as described in “Materials and methods.”
The αDI-domain mediates ligand binding
We next sought a molecular basis for ligand recognition by αDβ2. Because the I-domain is responsible for ligand binding in other integrins, and in cell adhesion studies polyclonal antibodies raised against the recombinant αDI-domain inhibited cell adhesion, we examined the ability of the isolated αDI-domain to interact with various proteins. Furthermore, because previous studies demonstrated that the length of the C-terminal α7 helix regulates the activation state of the αMI-domain35 and based on the homology between the αMI- and αDI-domains, we have produced 2 recombinant proteins. The first, “active” αDI-domain, encompasses residues Pro128-Lys314 and the second, “nonactive,” spans the Pro128-Ala323 sequence. The αDI-domains were expressed in E coli and purified from soluble fractions of cell lysates (Figure 6). The capacity of αDI-domains to bind selected ECM proteins and other ligands was tested using SPR. The protein ligands were coupled to the chip, and the SPR profiles across a range of the αDI-domain concentrations flowed over protein surfaces were determined. A representative set of SPR sensograms for binding of the active αDI-domain to vitronectin immobilized on the CM5 chip is shown in Figure 7. The maximal responses achieved at equilibrium for each αDI-domain concentration (Figure 7 inset) were used to determine the dissociation constant (Kd). The active αDI-domain bound to all ligands tested, with Kd values in the range between about 0.3 to 8 μM (Table 3). It is noteworthy that the αDI-domain bound ICAM-3 and VCAM-1, its 2 established ligands.1,6 Among proteins tested, Cyr61 appears to exhibit the highest affinity for the active αDI-domain (Kd 0.29 ± 0.07 μM). No binding of the nonactive αDI-domain was detected.
Dissociation constants of I-domains for protein ligands
. | Kd, μM . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Ligand . | αD active . | αD nonactive . | αL (αD) chimera . | αL active . | |||
| Fibrinogen | 0.76 ± 0.63 | ND | 1.45 ± 0.57 | ND | |||
| Vitronectin | 1.03 ± 0.41 | ND | 2.32 ± 0.71 | ND | |||
| Fibronectin | 0.38 ± 0.14 | ND | 1.99 ± 0.54 | ND | |||
| Cyr61 | 0.29 ± 0.07 | ND | 0.96 ± 0.27 | ND | |||
| Plasminogen | 0.96 ± 0.34 | ND | 2.0 ± 0.41 | ND | |||
| VCAM-1 | 8.13 ± 0.91 | ND | 8.58 ± 0.56 | ND | |||
| ICAM-3 | 1.89 ± 1.3 | ND | 6.83 ± 0.72 | 1.01 ± 0.24 | |||
. | Kd, μM . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Ligand . | αD active . | αD nonactive . | αL (αD) chimera . | αL active . | |||
| Fibrinogen | 0.76 ± 0.63 | ND | 1.45 ± 0.57 | ND | |||
| Vitronectin | 1.03 ± 0.41 | ND | 2.32 ± 0.71 | ND | |||
| Fibronectin | 0.38 ± 0.14 | ND | 1.99 ± 0.54 | ND | |||
| Cyr61 | 0.29 ± 0.07 | ND | 0.96 ± 0.27 | ND | |||
| Plasminogen | 0.96 ± 0.34 | ND | 2.0 ± 0.41 | ND | |||
| VCAM-1 | 8.13 ± 0.91 | ND | 8.58 ± 0.56 | ND | |||
| ICAM-3 | 1.89 ± 1.3 | ND | 6.83 ± 0.72 | 1.01 ± 0.24 | |||
Different concentrations of I-domains ranging from 0.05 to 16 μM in HBS-P buffer supplemented with 1 mM MgCl2 were flowed over the flow cells with immobilized protein ligands. The Kd values were estimated from the equilibrium portions of sensograms using the BIA evaluation 3.1 program. Results are mean ± SE of 3 to 5 individual determinations. ND indicates no significant binding was detected.
SDS-PAGE of generated I-domains. The I-domains were isolated from soluble fractions of E coli lysates and purified using affinity chromatography, and their purity was assessed by SDS-PAGE on a 12.5% gel under reducing conditions followed by staining with Coomassie blue.
SDS-PAGE of generated I-domains. The I-domains were isolated from soluble fractions of E coli lysates and purified using affinity chromatography, and their purity was assessed by SDS-PAGE on a 12.5% gel under reducing conditions followed by staining with Coomassie blue.
The Lys244-Lys260 sequence in the αDI-domain is responsible for ligand recognition
We previously reported that the αMI-domain sequence Lys245-Arg261 is involved in binding of many unrelated ligands by αMβ2 and, thus, defines its multiligand binding properties.10 The high extent of homology between αM(Lys245-Arg261) and the αDI-domain sequence Lys244-Lys260 (Figure 1) suggests that the latter segment may be involved in recognition of multiple ligands by αDβ2. To investigate the role of this segment, we generated a chimeric protein in which the αD(Lys244-Lys260) was grafted into the corresponding position of the αLI-domain, the most closely related I-domain but one that does not bind many ligands. To exclude the possibility that this manipulation may cause activation of the chimeric protein enabling it to bind ligands with unusual specificity, the αLI-domain was expressed in the fixed active conformation. In this protein, originally described by Lu et al,19 Lys287 and Lys294 in the C-terminal part of the αLI-domain were substituted to Cys, which resulted in the protein, on the formation of the disulfide bond, being locked in a constitutively active conformation. The αD(Lys244-Lys260) segment was inserted into the framework of this active αLI-domain. The control αL- and chimeric I-domains were purified from E coli lysates and were homogeneous as assessed by SDS-PAGE (Figure 6). The ligand-binding properties of isolated proteins were compared by SPR. No binding of the control αLI-domain to all proteins tested, except its ligand ICAM-3,36 was detected. In contrast, the chimeric I-domain was capable of binding various ligands immobilized to the surfaces of CM5 chips. The Kd values of interactions between the chimera and different proteins, as determined from the equilibrium portions of the SPR sensograms, were found to be in the range of about 1 to 8 μM (Table 3). Thus, the insertion of the αDI-domain segment changed the specificity of the αLI-domain and converted it into the αDI-domain-binding protein. Although it did not impart the full binding activity, the affinities of ligands for the chimeric protein were comparable with those found with the wild-type αDI-domain. For example, Cyr61 was the most preferred ligand for the chimera. Thus, these data demonstrate that the Lys244-Lys260 segment within the αDI-domain is largely responsible for its multiligand-binding properties.
Analyses of the αDI-domain binding to vitronectin by SPR. Representative profiles of the SPR responses for αDI-domain binding (concentrations ranging from 0.05 to 3 μM) to vitronectin coupled to the CM5 chip. The “active” αDI-domain (Pro128-Lys314) in HBS-P buffer supplemented with 1 mM MgCl2 was used. RU indicates response units. The inset shows a dose-dependent saturable binding of the αDI-domain to vitronectin.
Analyses of the αDI-domain binding to vitronectin by SPR. Representative profiles of the SPR responses for αDI-domain binding (concentrations ranging from 0.05 to 3 μM) to vitronectin coupled to the CM5 chip. The “active” αDI-domain (Pro128-Lys314) in HBS-P buffer supplemented with 1 mM MgCl2 was used. RU indicates response units. The inset shows a dose-dependent saturable binding of the αDI-domain to vitronectin.
Discussion
In the present study, we have analyzed the ligand-binding properties of leukocyte integrin αDβ2. The following conclusions are drawn form these analyses: (1) αDβ2 is capable of binding proteins that are either the constituents of the ECM, such as fibronectin, or become ECM-associated during the inflammatory response, including fibrinogen, vitronectin, Cyr61, and plasminogen; (2) within αDβ2, the αDI-domain is responsible for its ligand binding function; and (3) the αDI-domain segment Lys244-Lys260 contributes to binding of multiple ligands. Thus, these studies identify the new ligands for αDβ2 and establish this integrin as a multiligand receptor. They also provide insights into the mechanism by which this integrin is capable of recognizing unrelated proteins.
The important finding of this study is that the recognition specificity of αDβ2 closely resembles that exhibited by the major leukocyte integrin αMβ2 (Mac-1). Among integrin family members, αMβ2 has the broadest specificity and has the capacity to bind multiple ligands that belong to different protein families. Protein ligands for αMβ2 include numerous ECM proteins (fibronectin, laminin, collagen, Cyr61), blood proteins (fibrinogen, kininogen, C3bi, factor X), proteases (elastase, plasminogen, MMP-9), and so forth. Based on the overlapping specificities of αMβ2 and αDβ2 in recognition of many ECM proteins, it is reasonable to assume that αDβ2 is capable of interacting with many other αMβ2 ligands. For example, the finding that αDβ2-expressing cells adhere to plastic, which represents a hallmark of αMβ2, lend further support to the idea that both integrins have very similar binding properties.
Ligand binding by the αDI-domain was found to be regulated by its conformation; whereas the αDI-domain with its C-terminus truncated (Pro128-Lys314) was active, the protein with an extended C-terminal end (Pro128-Ala323) manifested negligible ligand binding. Regulation of ligand binding by the C-terminal part has been described for the I-domains of other β2 integrins, including αLβ2, αMβ2, and αXβ2.19,35,37 The high-affinity form of the αMI-domain can be generated by unlocking the C-terminal Ile316 from a hydrophobic socket either by mutation or truncation the α7 C-terminal helix at Lys315.35 The fact that the αDI-domain contains an invariable Ile in the 311LKEKIFA317 sequence, which is highly homologous to the αM312LREKIFA318, and the observation that truncation of the αDI-domain at Lys314 converts it into the active form, suggest that the αDI-domain undergoes conformational changes similar to those of the αMI-domain. The I-domains of the αM, αL, and αX subunits have been crystallized in both “open” and “closed” conformations that correspond to the high- and low-affinity states, respectively.37-39 Although the 3-dimensional structure of the αDI-domain is not solved, its high homology to αM and αX I-domains and similar regulation of ligand binding by conformation of the C-terminus suggests that the αDI-domain can exist in the “open” and “closed” conformations. Thus, our findings with the I-domain of αDβ2 supports the general mechanism by which the β2 integrins are activated.
We have previously demonstrated the αM segment Lys245-Arg261 is involved in binding of many unrelated ligands and thus serves as a consensus recognition site.10 A homologous segment in the αDI-domain, Lys244-Lys,260 was found to fulfill a parallel function. Grafting this segment into the corresponding position of the αLI-domain imparted to the chimeric protein the ability to bind numerous ECM proteins and plastic. The binding activity of the chimeric I-domain, based on the estimated Kd, was only 2- to 3-fold lower than that of the wild-type αDI-domain, indicating that αD (Lys244-Lys260) is centrally involved in recognition of various ligands. Nevertheless, because grafting of the αD segment did not restore the binding function completely, other regions of the αDI-domain could contribute to binding. Taken together, the αDβ2 characteristics, including promiscuity in ligand binding, regulation of ligand binding by conformation of the C-terminal portion of the αDI-domain, and the role of the highly conserved αD(Lys244-Lys260) segment as a consensus docking site, closely recapitulate the properties of αMβ2.
Because the αDβ2-expressing cells adhered to the fibrinogen P2-C peptide and soluble P2-C inhibited cell adhesion to the ECM proteins, these observations are consistent with the conclusion that the structural features of ligands permissive for their binding by αDβ2 and αMβ2 are similar. The P2-C peptide duplicates sequence 383TMKIIPFNRLTIG395, which is the binding site for αMβ2 in the γC-domains of fibrinogen.18,24 Because P2-C inhibits αMβ2-mediated cell adhesion not only to fibrinogen but also to other ligands,27,33 it is believed that this peptide contains critical structural information required for αMβ2 binding. Furthermore, the results presented here suggest that recognition of multiple ligands by the αDI-domain may also depend on sequences typified by P2-C. However, despite the requirements for the “P2-C”-like recognition component and the overall similarity in the ligand repertoire, some proteins appear to be preferred αMβ2 ligands, whereas others are recognized better by αDβ2. For example, the capacity of fibrinogen to support αMβ2-mediated adhesion was higher than that of αDβ2 (Figure 3) and recombinant αMI-domain bound more strongly to fibrinogen than the αDI-domain (not shown). On the other hand, Cyr61 was superior to all other ligands in binding by the αDI-domain (Table 3) and its binding by the αDI-domain was about 2-fold higher than by the αMI-domain (not shown). At present, the critical determinants responsible for differential binding by αD and αM I-domains remain to be determined.
On αDβ2-expressing HEK293 cells, both αDβ2 and β1 integrins contribute to adhesion to selected ECM proteins. We found that cell adhesion to fibronectin, vitronectin, and VCAM-1 could be blocked only by the combination of anti-β1 and anti-αD (or anti-β2) antibodies. Indeed, fibronectin and VCAM-1 are well-characterized ligands for β1 integrins α5β1 and α4β1, respectively. Moreover, integrin αvβ1 on HEK293 cells can bind both vitronectin and fibronectin. Previously, we have demonstrated the same contribution of β1 integrins to adhesion of the αMβ2-expresing cells.21 In contrast, adhesion of the αDβ2-expressing cells to such proteins as fibrinogen, Cyr61, and plastic was entirely αDβ2 dependent with little contribution from β1 integrins. Although integrins α5β1 and α6β1 have been shown to bind fibrinogen and Cyr61, respectively,31,40 both proteins appear to be the preferred ligands for αDβ2 on the αDβ2-expressing cells.
The finding that αDβ2 and αMβ2 have analogous recognition specificity suggests that they may fulfill a common function. Our previous studies with integrin αMβ2 provided insights into how the αMβ2 ability to bind ECM proteins may control cell migration and adhesion. We demonstrated that the progressive increase of αMβ2 on the cell surface led to increased adhesion to fibronectin and, as a consequence, inhibited cell migration mediated by β1 integrins.21 Based on these studies, we proposed that owing to the nonselective nature of αMβ2 binding to ECM proteins and its ability to be progressively up-regulated on the surface of neutrophils, αMβ2 serves as a brake in neutrophil migration at the site of inflammation. Given the similarity in the ligand-binding profiles of αMβ2 and αDβ2, one is tempted to speculate that αDβ2 can perform similar functions. The observations that αDβ2 is strongly up-regulated within atherosclerotic plaques and that its levels are gradually increased on monocyte differentiation to macrophages in the presence of oxidized LDL,2 the major mediator of atherosclerosis, seem to fit this hypothesis. However, a marked distinction between αMβ2 and αDβ2 is that αMβ2 expression in neutrophils involves translocation of the integrin from pre-existing internal pools in response to chemotactic stimuli,41 whereas αDβ2 up-regulation requires de novo synthesis in the presence of atherogenic stimuli.2 Such a difference in up-regulation of 2 integrins seems to be consistent with a model in which αMβ2 is readily available in the acute inflammatory response, whereas αDβ2 may be required for chronic inflammation. A recent report suggests that not only the recruitment of monocytes, but also their retention within atherosclerotic lesions, contributes to plaque development.42 Whether integrin αDβ2 increases monocyte adhesiveness and thus contributes to their retention in the evolving plaque remains to be established.
In summary, these studies establish leukocyte integrin αDβ2 as a multiligand receptor capable of binding diverse ECM proteins with a specificity overlapping that of αMβ2, the most promiscuous member of the integrin family. Given the capacity of αMβ2 to initiate a multitude of cellular responses in neutrophils, one can expect that αDβ2 plays important roles in monocyte/macrophage biology.
Prepublished online as Blood First Edition Paper, October 20, 2005; DOI 10.1182/blood-2005-06-2509.
Supported by the National Institutes of Health (T.P.U.) and the American Heart Association (T.P.U. and V.P.Y.). The Biacore 3000 was purchased through a Shared Instrumentation Grant RR016789-01A1 from the National Institutes of Health.
V.P.Y. and T.P.U. designed research, V.P.Y. performed research, S.P.Y. contributed to analyses of Biacore data, and V.P.Y. and T.P.U. wrote the article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr S. Lam for a generous gift of Cyr61. We thank Tim Burke for critical reading of the manuscript and helpful suggestions. We acknowledge the Molecular Biotechnology Core Lab for making the Biacore 3000 available for these studies.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal