Following T-cell receptor and CD28 signaling, CD8+ T cells express a receptor for CD83, a molecule up-regulated on functionally mature dendritic cells. Although this expression pattern suggests that CD83 is involved in adaptive immunity, little is known about its function in the periphery, and the existence of its ligand on T cells is controversial. We demonstrate that the engagement of the CD83 ligand (CD83L) preferentially enriches and significantly amplifies the number of antigen-specific CD8+ T cells. Coengagement of the T-cell receptor, CD28, and CD83L supports priming of naive CD8+ T cells that retain antigen specificity and cytotoxic function for more than 6 months. Therefore, engagement of the CD83L provides a unique signal to activated CD8+ T cells that could be exploited to generate long-lived antigen-specific cytotoxic T cells for the treatment of cancer and infection.
Introduction
Maturation of human cytotoxic T cells is driven by a series of signals that induces differentiation of naive T cells into central memory, effector memory, and terminal effector cells.1 Many of these signals are delivered by cell-surface molecules expressed on professional antigen presenting cells (APCs) to counterreceptors expressed on CD8+ T cells. Following engagement of the T-cell receptor by major histocompatibility complex (MHC) class I molecules, naive CD8+ T cells are poised to receive 1 or more costimulatory signals that will induce their expansion and differentiation. In contrast to the constitutive expression of MHC on unstimulated APCs, most costimulatory molecules are only up-regulated following activation.2,3 Costimulatory molecules, including the B7 family (CD80, CD86, inducible costimulator [ICOS] ligand) and tumor necrosis factor (TNF) family (4-1BB ligand and OX40 ligand) members are induced following activation of macrophages, B cells, and dendritic cell (DCs).2,3 Likewise, except for the constitutive expression of CD28 on resting T cells, costimulatory counterreceptors are not expressed on naive T cells and require activation for their expression.4 4-1BB, OX40, or ICOS are not expressed on naive T cells and require several days to achieve their peak expression level, which diminishes as effector CD8+ T cells differentiate into memory cells. The delayed appearance of these costimulatory counterreceptors on CD8+ T cells supports the hypothesis that they are not involved in priming but rather function to sustain an ongoing response by supporting the survival of newly generated effector cells.4
In an effort to identify factors that support the optimal generation of T-cell immunity, we sought molecules that contribute to the priming of naive CD8+ human T cells, drive their continuous antigen-specific expansion, maintain their cytotoxic function, and support their long-term survival. One molecule, CD83, has several characteristics that made it an attractive candidate.5,6 First, CD83 is highly expressed on mature DCs, but is not detectable on APCs that do not prime naive T cells such as immature DCs, resting B cells, and monocytes. Second, the expression of CD83 in situ is maximal in the interfollicular T-cell regions of lymph node, tonsil, and spleen, suggesting that its function might be associated with either T-cell expansion and/or survival.5,7 Little is known about the function of human CD83, and the existence of its ligand on T cells is controversial.8-10 The CD83-deficient mouse demonstrated a specific block in CD4+CD8- thymocyte development without increased CD4-CD8- or CD4-CD8+ thymocytes.11 This defect appeared to be secondary to the loss of CD83 expression on the thymic epithelium. Although the function of peripheral CD4+ T cells from CD83-deficient mice appeared to be normal, the function of CD8+ T cells was not detailed in the study.11
In the present report, we show that CD83L is induced by CD28-mediated costimulation on both CD8+ and CD4+ human T cells. Using an artificial APCs expressing CD83, we also demonstrate, for the first time, that engagement with CD83 delivers a significant signal specifically supporting the expansion of newly primed naive CD8+ T cells. This interaction enhances the in vitro generation of cytotoxic T lymphocytes (CTLs) specific for viral antigens such as HIV, where the precursor frequency is very low. Furthermore, engagement with CD83 enables the long-term survival of antigen-specific T-cell cultures by inducing proliferation and inhibiting apoptosis. The observation that CD83 delivers a unique signal important to establishing T-cell immunity may prove useful in the therapy of cancer and infectious disease.
Materials and methods
cDNAs
Full-length human CD83 cDNA was cloned from mature DCs by reverse transcriptase-polymerase chain reaction (RT-PCR) and the sequence was verified by DNA sequencing. Partial cDNA encoding the Fcγ portion of human immunoglobulin G1 (IgG1) was cloned from a human fetus liver cDNA library (Clontech, Palo Alto, CA).
Cell culture
Jurkat, HPB-ALL, U937, and K562 cells were cultured in RPMI 1640 with 10% fetal calf serum (FCS; Invitrogen, Carlsbad, CA) and gentamycin (30 μg/mL; Invitrogen). Chinese hamster ovary (CHO) cells were grown in DMEM/F12 (1:1; Invitrogen) supplemented with 10% newborn calf serum (Sigma, St Louis, MO). CHO cells stably expressing human CD83 (CHO/CD83) were established by retroviral infection and subsequent flow cytometry-guided sorting.
Purification and activation of T cells
CD3+ T cells were purified from peripheral-blood mononuclear cells (PBMCs) using a negative selection kit (Miltenyi Biotec, Auburn, CA). CD4+ and CD8+ T cells were purified by a positive isolation kit (Dynal, Lake Success, NY). Where indicated, CD8+ T cells were separated into CD45RA+ and CD45RO+ fractions using FITC-conjugated mAbs (Caltag, Burlingame, CA) and anti-FITC monoclonal antibody (mAb)-conjugated magnetic beads (Miltenyi Biotec, Auburn, CA). Purified T cells were stimulated with anti-CD3 (150 ng/mL; Research Diagnostics, Flanders, NJ) in the presence or absence of anti-CD28 (500 ng/mL; Research Diagnostics) for the indicated time periods.
Generation of DCs
Immature DCs were generated from purified monocytes using interleukin-4 (IL-4, 10 ng/mL; PeproTech, Rocky Hill, NJ) and granulocyte-macrophage colony-stimulating factor (GM-CSF, 50 ng/mL; Immunex, Thousand Oaks, CA) in RPMI 1640 supplemented with 10% FCS. Maturation of DCs was induced by double-stranded (ds) RNA (25 μg/mL; Sigma) and TNF-α (50 ng/mL; PeproTech).12
Production of dodecameric soluble CD83 protein
The extracellular domain of human CD83 cDNA (amino acids 1-143) and the Fcγ portion of human IgG1 (hinge, CH2, and CH3) were PCR amplified and fused using standard techniques. The IgA tailpiece of 18 amino acids was further fused to the C-terminus (Figure 1) and the resultant CD83-Fcγ-IgA tailpiece fusion construct was subcloned into a pMX/puromycin retroviral vector13 (gift from Dr Kitamura, University of Tokyo, Japan). All DNA constructs were verified by DNA sequencing. The retroviral DNA vector was transfected into 293GPG packaging cells14 (gift from Dr Dranoff, Dana-Farber Cancer Institute, Boston, MA; and Dr Mulligan, Children's Hospital, Boston, MA) using Lipofectamine Plus (Invitrogen) and replication-defective viral supernatant was used to infect CHO cells. After selection with puromycin (6 μg/mL), CHO clones secreting dodecameric soluble CD83 protein were selected by enzyme-linked immunosorbent assay (ELISA) and Western blot analysis.
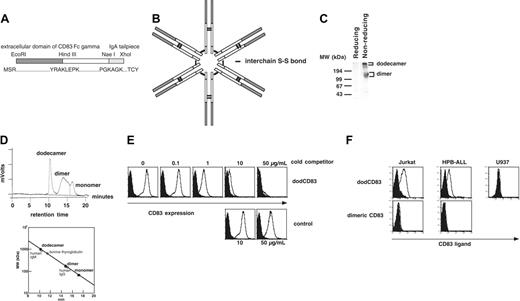
Construction, structure, and verification of dodecameric form of soluble CD83. (A) A dodecameric form of soluble CD83 (dodCD83) was constructed by fusing the CD83 extracellular portion, Fcγ portion of human IgG1, and human IgA tailpiece. The primary structure of dodCD83 is shown as a monomeric form. (B) The predicted structure of dodCD83. (C) With or without reducing treatment by dithiothreitol, purified dodCD83 protein was subjected to SDS-PAGE, transferred to a PVDF membrane, and blotted with goat anti-human Ig (H+L) Ab. (D) Purified dodCD83 (100 μg) was loaded onto a size-exclusion HPLC column and fractionated based on size. Purified human IgM, IgG, or gel filtration standard was also run and used as a reference protein. (E) CHO/CD83 cells were preincubated with graded amounts of dodCD83 or control protein and subsequently stained with PE-labeled anti-CD83 mAb. (F) T-cell leukemic lines Jurkat and HPB-ALL and monocytic cell line U937 cells were analyzed for CD83L expression using dimeric and dodecameric of soluble CD83. Cells were incubated with either dodCD83 (open curve) or control dodecameric protein (filled curve), followed by incubation with PE-conjugated secondary antibody.
Construction, structure, and verification of dodecameric form of soluble CD83. (A) A dodecameric form of soluble CD83 (dodCD83) was constructed by fusing the CD83 extracellular portion, Fcγ portion of human IgG1, and human IgA tailpiece. The primary structure of dodCD83 is shown as a monomeric form. (B) The predicted structure of dodCD83. (C) With or without reducing treatment by dithiothreitol, purified dodCD83 protein was subjected to SDS-PAGE, transferred to a PVDF membrane, and blotted with goat anti-human Ig (H+L) Ab. (D) Purified dodCD83 (100 μg) was loaded onto a size-exclusion HPLC column and fractionated based on size. Purified human IgM, IgG, or gel filtration standard was also run and used as a reference protein. (E) CHO/CD83 cells were preincubated with graded amounts of dodCD83 or control protein and subsequently stained with PE-labeled anti-CD83 mAb. (F) T-cell leukemic lines Jurkat and HPB-ALL and monocytic cell line U937 cells were analyzed for CD83L expression using dimeric and dodecameric of soluble CD83. Cells were incubated with either dodCD83 (open curve) or control dodecameric protein (filled curve), followed by incubation with PE-conjugated secondary antibody.
Using a protein G column (Mab Trap Kit; Amersham Pharmacia Biotech, Piscataway, NJ), soluble CD83 protein was purified from protein-free media conditioned with transfectant CHO cells (PF-CHO; HyClone Laboratories, Logan, UT). Dimeric soluble CD83 protein without the IgA tail was produced similarly. Control dodecameric fusion protein encoding the partial extracellular domain of CD83 (amino acids 1-83) or mouse Ig κ signal-HA tag fused to Fcγ and IgA tailpiece was produced in a similar way and was purified from cell lysates or conditioned medium, respectively.
HPLC analysis
Purified soluble CD83, human IgM, human IgG protein (Caltag) and gel filtration standard (BioRad, Hercules, CA) (100 μg each) were each loaded onto a size exclusion high-performance liquid chromatography (HPLC) column (Superose 12 HR10/30; Amersham Pharmacia Biotech) and eluted with PBS in order to determine their size.
Flow cytometry analysis
mAbs recognizing the following antigens were used: CD16 (3G8), CD32 (C1KM5), CD64 (10.1), CD80 (DAL1), CD83 (HB15e), CD86 (BU36), and mouse isotype controls from Caltag; CD8 (RPA-T8), human leukocyte antigen (HLA)-A2 (BB7.2), CD80 (L307.4), and CD83 (HB15) from BD Pharmigen (San Diego, CA); and CD80 (DAL-1) and CD83 (HB15) from Ancell (Bayport, MN). Human IgG1 was purchased from Calbiochem (La Jolla, CA). Blocking mAbs for CD80 (C4) and CD86 (1F9) were made in-house. In order to detect CD83L, cells were incubated with blocking mAbs against Fcγ receptors and then with goat γ-globulin (Sigma). dodCD83 (50 μg/mL) was incubated at room temperature. Washed cells were labeled with goat anti-human PE-conjugated F(ab′)2 fragments (Jackson Immunoresearch Laboratories, West Grove, PA) in the presence of goat serum (Sigma).
Establishment of K562-derived APCs
cDNAs encoding HLA-A*0201 (A2), CD80, and CD83 were subcloned into an expression vector, pMX.13 Expression vectors were transfected onto 293GPG14 and replication-defective virus supernatants were harvested and immediately used or frozen at -80°C. Each infection was performed under conditions so that at least 105 independent transduced cells were obtained. K562 cells were infected with virus supernatants in the presence of polybrene (8 μg/mL; Sigma), and positive cells were isolated by mAb staining and flow cytometry-guided sorting. K562 cells were first transduced with A2 to generate APC/A2. Subsequently, APC/A2 was transduced with either CD80 or CD83 to produce APC/A2/CD80 and APC/A2/CD83. Finally, APC/A2/CD80 was transduced with CD83 to produce APC/A2/CD80/CD83. Each transduction was performed with the same virus stock in order to generate cell lines expressing similar levels of particular transduced molecules. Polyclonal cell lines consisting of at least 105 independent clones were used to prevent cloning induced variations. Although the expression of transduced molecules on K562 was stable after culture for 2 months, only freshly thawed cells of early passages were used.
Production of class I peptide-specific CD8+ T-cells
Purified HLA-A2-positive CD8+ T cells were plated in 24-well plates at 2 × 106 cells/well in RPMI 1640 with 10% human AB serum. K562-derived cells were pulsed with synthetic peptides, 27AAGIGILTV35 of MART1, 58GILGFVFTL66 of the influenza virus matrix antigen, or 476ILKEPVHGV485 peptide of the RTpol (Pol) of HIV (New England Peptides, Fitchburg, MA) for 6 to 10 hours at room temperature. These cells were then irradiated, washed, and added to the responder cells at a responder-stimulator ratio of 20:1. Supplementation with 10 IU/mL IL-2 (Chiron, Emeryville, CA) and 10 ng/mL IL-15 (Peprotech and R&D Systems, Minneapolis, MN) was performed every 3 to 4 days between stimulations.
Tetramer assays
HLA-A*0201 tetramers were produced as described.15 T cells were incubated with PE-conjugated tetramers (10 μg/mL) and subsequently with anti-CD8 mAb conjugated to PC5 (Immunotech, Marseille, France). Where indicated, cells were pulsed with BrdU and counterstained with tetramer and FITC-conjugated Annexin V or FITC-conjugated anti-BrdU mAb to enumerate apoptotic and proliferating cells (R&D Systems and BD Pharmingen). HLA-A*0201 tetramer against the peptide, 11LLFGYPVYV19 of the HTLV-1 TAX protein, was used as a negative control.
Cytotoxicity assays
The standard cytotoxicity assay was performed as described.16
IFN-γ enzyme-linked immunospot assay (ELISPOT)
PVDF plates (Millipore, Bedford, MA) were coated with capture mAb (1D1K; MABTECH, Mariemont, OH). T cells were incubated with T2 cells in the presence of peptide (10 μg/mL) for 20 hours at 37°C. Plates were washed and incubated with biotin-conjugated detection mAb (7-B6-1; MABTECH). Horseradish peroxidase (HRP)-conjugated SA (DAKO, Carpenteria, CA) was added, and interferon (IFN)-γ spots were developed.
Statistical analysis
Data were analyzed as the total number and percentage of CD8+ tetramer-staining T cells generated by stimulation with peptide-pulsed transfectants. The 1-sided Student t test was used to assess whether CD80 and/or CD83 expression resulted in a significant increase. The impact of CD83 expression over time was examined using a repeated-measures mixed model analysis of variance including treatment with CD83, time, and an interaction between treatment and time.
Results
A dodecameric, but not dimeric, form of soluble CD83 protein detects CD83L on T-cell leukemic cell lines
In order to detect CD83L, we produced dimeric8 and dodecameric forms of soluble CD83 (dodCD83). The dodCD83 construct was produced by fusing the human IgA tailpiece to the dimeric CD83 C-terminus, human CD83-IgG fusion construct (Figure 1A). Compared with dimeric soluble IgG fusion proteins, dodecameric forms have been shown to have a very slow rate of dissociation, suggesting a highly avid interaction.17,18 Transfected CHO cells secreted dodCD83 with the predicted structure shown in Figure 1B.19 Purified dodCD83 was subjected to SDS-PAGE analysis and immunoblotted with goat anti-human Ig Ab. Under nonreducing conditions, 2 major bands were noted, 1 at 1000 kDa and another at 150 to 160 kDa, representing the dodecameric and dimeric forms of soluble CD83, respectively (Figure 1C). As expected, under reducing conditions, neither the dodecameric nor dimeric form was detectable by Western blotting. The monomeric form of CD83 was only detected with a higher concentration of goat anti-human Ig Ab under reducing conditions (data not shown).
We then performed a gel filtration analysis to estimate the size of dodCD83. Purified dodCD83, human IgM, human IgG protein and gel filtration standard was passed over a size-exclusion HPLC column. As shown in Figure 1D, purified dodCD83 appears to have a molecular weight (MW) of 950 to 1000 kDs since it was eluted immediately before human IgM.
In order to verify the specificity and the proper folding of dodCD83, we performed a competition assay with anti-CD83 mAb (Figure 1E). CHO cells expressing CD83 (CHO/CD83) were premixed with graded amounts of dodCD83 or control IgG/A protein and subsequently stained with PE-labeled anti-CD83 mAb. dodCD83 (50 μg/mL) almost completely abrogated anti-CD83 staining of CHO/CD83, whereas control protein did not. Although anti-CD83 mAb was able to detect CD83 protein by flow cytometric analysis, it failed to detect CD83 protein by immunoblot analysis following SDS-PAGE, suggesting that it does not recognize the denatured CD83 protein. Since anti-CD83 mAb specifically recognized dodCD83, it is likely that dodCD83 was properly folded.
Using dodCD83, we studied the expression of CD83L on human T-cell leukemic cell lines. Although a dimeric form of soluble CD83 did not stain the cell lines, dodCD83 clearly stains CD83L on the cell surface of Jurkat and HPB-ALL (Figure 1F). This staining is not an artifact mediated by Fcγ binding since these cell lines do not express any Fcγ receptors (data not shown). On the other hand, U937 was not stained with dodCD83 despite the fact that it expresses Fcγ receptors. This strongly supports that CD83L staining is specific and not due to an Fcγ receptor-mediated artifact.
CD28 costimulation is required to induce the expression of CD83L on human T cells
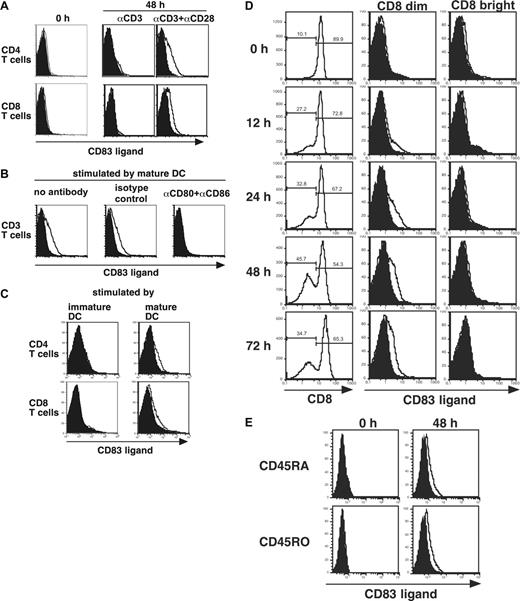
Using primary human T cells, we examined whether T-cell receptor (TCR) and CD28 signals could induce the expression of CD83L. There was no detectable expression of CD83L on resting CD4+ or CD8+ T cells (Figure 2A). Human peripheral CD4+ and CD8+ T cells were stimulated with anti-CD3 mAb and anti-CD28 mAb, and expression of CD83L was analyzed with dodCD83. Faint but distinct CD83L expression was reproducibly induced on both CD4+ and CD8+ T cells. CD83L was not detectable following stimulation with anti-CD3 mAb alone, suggesting that CD28-mediated costimulatory signal is critical for the up-regulation of CD83L (Figure 2A).
We next examined whether mature DC could induce the expression of CD83L. Allogeneic CD3+ T cells, cocultured with mature DCs, expressed CD83L after 48 hours (Figure 2B). CD28 costimulation was necessary for the induction of CD83L, since blockade with anti-CD80 and anti-CD86 mAbs completely abrogated CD83L expression induced by allogeneic DCs. As shown in Figure 2C, CD83L expression was detected when CD4+ and CD8+ T cells were stimulated by mature (high B7 expression), but not immature (low B7 expression), DCs. These results support the hypothesis that CD28-mediated costimulation is necessary for the induction of CD83L on activated CD4+ and CD8+ T cells.
Activated CD8 dim, but not CD8 bright, T cells up-regulate CD83L expression after activation
Treatment with anti-CD3 plus anti-CD28 antibodies induced the cell-surface expression of CD83L on only a subpopulation of activated T cells. It is well known that T-cell activation induces significant alterations in the expression of cell-surface molecules. In the case of CD8+ T cells, CD8 expression has been reported to rapidly decrease following antigenic stimulation.20 Importantly, it has been shown that only those CD8+ T cells that receive both antigen-specific and costimulatory signals down-regulate CD8 expression, and TCR signal alone is not enough to decrease the expression level of CD8.21 Since the induction of CD83L requires both CD3- and CD28-mediated signals (Figure 2A-B), we analyzed the relationship between the expression level of CD8 and CD83L on activated CD8+ T cells. We incubated CD8+ T cells with anti-CD3 and anti-CD28, and analyzed the CD83L expression on 2 distinct fractions, CD8 dim and CD8 bright T cells (Figure 2D). CD83L expression became detectable on CD8+ dim T cells 12 hours after activation. Maximum induction was seen at 48 hours in all donors tested, and CD83L expression was present at 72 hours in surviving CD8 dim cells. The CD83L expression was not observed on CD8 bright T cells at any time point examined. We then isolated CD45RA and CD45RO CD8+ T cells that were activated with anti-CD3 and anti-CD28 for 48 hours. CD83L expression was induced on both fractions to a similar degree (Figure 2E).
Engagement of CD83L enhances proliferation of memory and newly primed antigen-specific CD8+ T cells
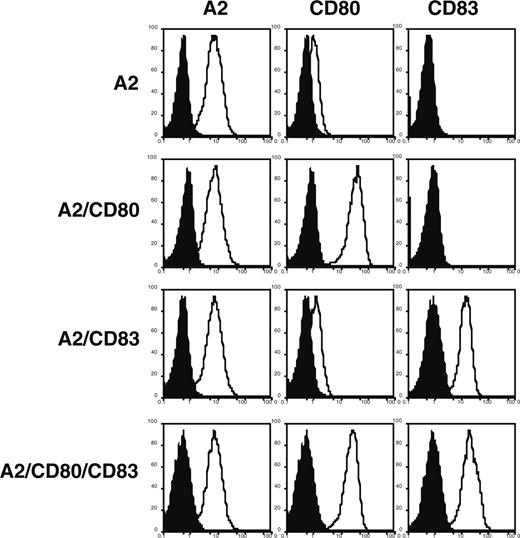
We next analyzed the functional consequences of CD83 engagement. Since all known monoclonal antibodies to CD83 are “silent” (ie, are neither inhibitory nor stimulatory),9 we prepared artificial APCs in order to isolate the contributions of each molecule to the induction of antigen-specific T-cell proliferation. We selected K562 as the APC backbone, since it expresses the adhesion molecules ICAM-1 and LFA-3, but not the HLA class I and class II molecules or the costimulatory molecules CD86, 4-1BB ligand, OX40 ligand, or ICOS ligand (M.O.B., L.M.N., and N.H.; unpublished data, May 2005).22 Four APCs (APC/A2, APC/A2/CD80, APC/A2/CD83, and APC/A2/CD80/CD83) were developed by introducing A2, CD80, and/or CD83 into K562 (Figure 3).
Using these APCs, we examined the effect of CD83 on the priming and expansion of HLA class I-restricted peptide-specific CD8+ T cells. We used 2 HLA-A*0201-restricted peptides that are known to stimulate antigen-specific CD8+ T cells in healthy donors, 58GILGFVFTL66 derived from the influenza (Flu) virus matrix antigen, and 27AAGIGILTV35 derived from the melanocyte differentiation antigen, MART1. Since most healthy donors have been previously exposed to influenza, stimulation with the HLA-A2-restricted influenza antigen induces a recall response by CD8+ memory T cells. In contrast, despite a relatively high precursor (p) CTL frequency in healthy donors, MART1-specific CD8+ T cells are phenotypically and functionally naive23,24 (N.H., M.O.B., and L.M.N.; unpublished data, May 2005). Therefore, stimulation of CD8+ T cells from healthy donors with MART1-specific antigenic epitopes will be considered a model for the priming of naive T cells.
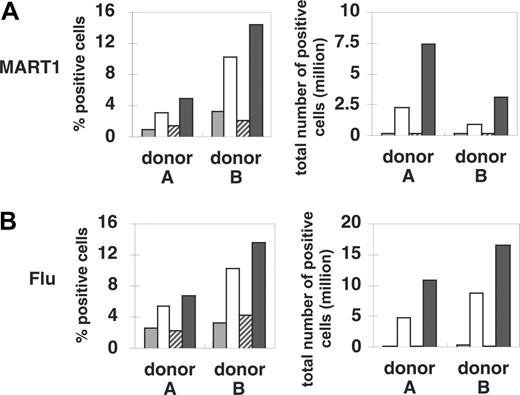
Purified CD8+ T cells from 4 HLA-A2-positive healthy donors were stimulated 3 times on a weekly basis by peptide-pulsed transfectants APC/A2, APC/A2/CD80, APC/A2/CD83, or APC/A2/CD80/CD83. The MART1 tetramer positivity of purified CD8+ T cells before stimulations was 0.02% to 0.09%. Between the stimulations, IL-2 and IL-15 were added to the culture. Stimulated T cells were counted and stained with HLA/peptide tetramer complexes to determine the percentage of peptide-specific T cells (Figure 4, left panel). The total number of peptide-specific T cells was calculated and is shown on the right in Figure 4. Three rounds of stimulation with peptide-pulsed APC/A2 resulted in detectable (0.6%-3.3%) MART1-specific T cells (Figure 4A, left panel). However, these cultures underwent little expansion, resulting in a relatively small number of MART1 T cells in total (Figure 4A, right panel). Compared with APC/A2, stimulation with MART1 peptide-pulsed APC/A2/CD80 induced a significant increase in the percentage of tetramer-positive cells (by 2.1-7.2 percentage points, P = .004; Figure 4A, left panel). The total number of MART1-specific T cells also increased (by 4.7-8.2 × 106, P = .005; Figure 4A, right panel). In contrast, stimulation with MART1 peptide-pulsed APC/A2/CD83 did not increase the percentage (P = .48; Figure 4A, right panel) or increase the total number (P = .30; Figure 4A, right panel) of antigen-specific T cells when compared with stimulation with APC/A2. However, as shown on the left of Figure 4A, if the triple transfectant APC/A2/CD80/CD83 was used as the stimulating APC, the highest percentage of MART1-specific T cells was consistently obtained in every donor tested when compared with APC/A2 (by 3.9-11.1 percentage points, P = .009) and when compared with APC/A2/CD80 (by 1.5-4.2 percentage points, P = .012). Furthermore, peptide-pulsed APC/A2/CD80/CD83 consistently generated the largest number of MART1-specific T cells when compared with APC/A2 (by 10.7-15.9 million, P = .0007) or when compared with APC/A2/CD80 (by 7.7-3.9 million, P = .003). Since peptide-pulsed APC/A2/CD83 did not promote enrichment or an increased number of MART1-specific T cells, the increased immunogenicity of APC/A2/CD80/CD83 compared with APC/A2/CD83 cannot be ascribed to an additive effect of CD83. Instead, CD83 function appears to be costimulation dependent, consistent with our data on CD83L expression (Figure 2). The fact that APC/A2/CD83 was unable to generate antigen-specific T cells in the presence of IL-2 and IL-15 suggests that neither of these cytokines was able to replace the CD28 signal. These results show that CD83 can augment the CD80-dependent proliferation of antigen-specific CD8+ T cells, although CD83 expression itself does not independently confer a growth advantage to antigen-specific CD8+ T cells. Similar results were consistently obtained using Flu peptide-pulsed APCs as shown in Figure 4B.
Engagement of CD3 and CD28 induces CD83L expression on the surface of T lymphocytes. Peripheral T cells were stimulated and tested for CD83L expression by staining with either dodCD83 (open curve) or control dodecameric protein (filled curve). (A) Purified CD4+ and CD8+ T cells demonstrated CD83L expression when stimulated by anti-CD3 and anti-CD28, but not when stimulated with anti-CD3 alone. (B) CD83L expression is demonstrated on CD3+ T cells stimulated with allogeneic mature DCs. Expression is abrogated by incubation with anti-CD80 and anti-CD86 mAbs but not with isotype controls. (C) Purified CD4+ and CD8+ T cells demonstrated CD83L expression when stimulated with allogeneic mature DCs, but not when stimulated with immature DCs. (D) Purified CD8+ T cells were optimally stimulated with anti-CD3 and anti-CD28 for the indicated time periods and analyzed for CD83 counterreceptor expression using dodCD83. The CD8 dim and CD8 bright populations were defined by electronic gating after staining with anti-CD8 mAb. (E) CD45RA and CD45RO CD8+ T cells were optimally stimulated with anti-CD3 and anti-CD28 for 48 hours and analyzed for CD83 counterreceptor expression using dodCD83. Between each experiment, flow cytometry settings were held constant to allow for direct comparison. Since DC-stimulated T cells have higher autofluorescence than mAb-activated T cells, the control staining was shifted slightly to the right for DC-stimulated T cells. Also, with increasing time of stimulation, greater autofluorescence of activated T cells was observed. Figures are representative of multiple donors: in panel A 1 of 4 is shown, and in panels B-E 1 of 2 is shown.
Engagement of CD3 and CD28 induces CD83L expression on the surface of T lymphocytes. Peripheral T cells were stimulated and tested for CD83L expression by staining with either dodCD83 (open curve) or control dodecameric protein (filled curve). (A) Purified CD4+ and CD8+ T cells demonstrated CD83L expression when stimulated by anti-CD3 and anti-CD28, but not when stimulated with anti-CD3 alone. (B) CD83L expression is demonstrated on CD3+ T cells stimulated with allogeneic mature DCs. Expression is abrogated by incubation with anti-CD80 and anti-CD86 mAbs but not with isotype controls. (C) Purified CD4+ and CD8+ T cells demonstrated CD83L expression when stimulated with allogeneic mature DCs, but not when stimulated with immature DCs. (D) Purified CD8+ T cells were optimally stimulated with anti-CD3 and anti-CD28 for the indicated time periods and analyzed for CD83 counterreceptor expression using dodCD83. The CD8 dim and CD8 bright populations were defined by electronic gating after staining with anti-CD8 mAb. (E) CD45RA and CD45RO CD8+ T cells were optimally stimulated with anti-CD3 and anti-CD28 for 48 hours and analyzed for CD83 counterreceptor expression using dodCD83. Between each experiment, flow cytometry settings were held constant to allow for direct comparison. Since DC-stimulated T cells have higher autofluorescence than mAb-activated T cells, the control staining was shifted slightly to the right for DC-stimulated T cells. Also, with increasing time of stimulation, greater autofluorescence of activated T cells was observed. Figures are representative of multiple donors: in panel A 1 of 4 is shown, and in panels B-E 1 of 2 is shown.
Expression profiles of A2, CD80, and CD83 molecules on K562-derived stable cell lines coexpressing A2 with either CD80, CD83, or both CD80 and CD83. K562-derived stable transfectants were analyzed by flow cytometry with specific antibody (open curve) and an isotype control (filled curve) coupled to the appropriate chromophore. Note that there is a dim expression of endogenous CD80 on K562 cells.
Expression profiles of A2, CD80, and CD83 molecules on K562-derived stable cell lines coexpressing A2 with either CD80, CD83, or both CD80 and CD83. K562-derived stable transfectants were analyzed by flow cytometry with specific antibody (open curve) and an isotype control (filled curve) coupled to the appropriate chromophore. Note that there is a dim expression of endogenous CD80 on K562 cells.
MART1-specific CD8+ T cells generated by APC/A2/CD80/CD83 possess potent effector functions
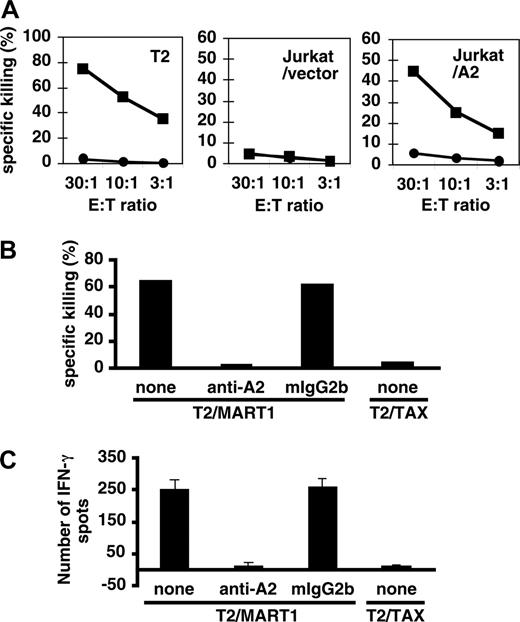
MART1-specific CTLs were established by repetitively stimulating purified HLA-A2+ CD8+ T cells with peptide-pulsed APC/A2/CD80/CD83 as described in “Materials and methods.” Antigen-specific cytotoxicity was demonstrated with the standard killing assay. T2 cells pulsed with MART1 but not control peptide were specifically killed (Figure 5A, left panel). In addition, peptide-pulsed Jurkat cells (HLA-A2-negative) were killed only when they were transduced with A2 (Figure 5A, center and right panels). HLA-A2-specific mAb, but not isotype-matched control mAb, inhibited this killing, suggesting that cytotoxicity was HLA-A2 restricted (Figure 5B). Furthermore, an enzyme-linked immunospot assay (ELISPOT) revealed that these MART1-specific CTLs were able to specifically secrete IFN-γ in response to MART1 peptide but not to control peptide (Figure 5C). These results show that MART1-specific CTLs generated by APC/A2/CD80/CD83 had potent antigen-specific effector functions. Antigen-specific CTLs generated by APC/A2/CD80 and APC/A2/CD80/CD83 did not show significant differences in effector function.
APC/A2/CD80/CD83 can generate antigen-specific T-cell lines to the foreign, virally derived antigen HIV pol, whereas APC/A2/CD80 cannot
Professional APCs such as mature DCs and CD40-activated B cells are known to express CD83 and possess the potential to prime CD8+ T cells of low precursor frequency to recognize foreign, virally derived antigen.25-27 In contrast, there have been no reports of artificial APCs that can prime naive CD8+ T cells of low precursor frequency against an antigen such as HIV pol.28 We therefore hypothesized that CD83 may be a critical molecule in enhancing the ability of antigen-presenting cells to prime naive T cells. To determine whether APC/A2/CD80/CD83 could successfully establish HIV pol-specific CD8+ T cells in vitro, we isolated CD8+ T cells from 6 HLA-A2-positive healthy donors and repeatedly stimulated with either APC/A2/CD80 or APC/A2/CD80/CD83 pulsed with HIV pol peptide. No tetramer-positive CD8+ T cells were detected after 5 or 6 stimulations regardless of APCs. While T-cell lines stimulated by APC/A2/CD80/CD83 gradually grew, expansion ceased in T cells stimulated by APC/A2/CD80. Following 8 rounds of stimulation, there were no surviving T cells stimulated by peptide-pulsed APC/A2/CD80 and tetramer analysis was not possible. In contrast, 5 (83%) of 6 cultures stimulated by APC/A2/CD80/CD83 survived (90% confidence interval [CI]: 42%-90%). Antigen specificity was shown in 3 lines (50%) by HIV pol-specific tetramer staining (90% CI: 15%-85%; Table 1) and HIV pol-specific cytotoxicity (data not shown).
CD83 is critical for establishing HIV pol-specific CD8+ T cells from naive donors
. | Tetramer analysis . |
|---|---|
| Stimulators | HIV pol positivity, % |
| Donor 1 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | Died |
| Donor 2 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 0.02 |
| Donor 3 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 1.89 |
| Donor 4 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 0.02 |
| Donor 5 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 0.58 |
| Donor 6 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 1.58 |
. | Tetramer analysis . |
|---|---|
| Stimulators | HIV pol positivity, % |
| Donor 1 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | Died |
| Donor 2 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 0.02 |
| Donor 3 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 1.89 |
| Donor 4 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 0.02 |
| Donor 5 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 0.58 |
| Donor 6 | |
| A2/CD80 | Died |
| A2/CD80/CD83 | 1.58 |
Purified A2-positive CD8+ T cells from healthy donors were repeatedly stimulated with either A2/CD80 or A2/CD80/CD83 pulsed with HIV pol peptide in a weekly manner. IL-2 and IL-15 were added to the cultures between the stimulations every 3 days. After 8 rounds of stimulation, tetramer assays were performed to determine the pol-specific expansion of CD8+ T cells.
CD83L signaling enhances the expansion of antigen-specific CD8+ T cells. HLA-A2+ CD8+ T cells were stimulated by MART1 (A) or Flu (B) peptide-pulsed APCs (APC/A2, APC/A2/CD80, APC/A2/CD83, or APC/A2/CD80/CD83). After 3 rounds of antigen stimulation and IL-2/IL-15 addition in between the stimulations, the cultures were stained with the relevant tetramer to determine percentage of peptide-specific T cells. The number of peptide-specific T cells was determined by calculating the product of the total number of T cells and the percentage of tetramer-staining cells. Representative results from 4 different donors are presented. (A) Compared with APC/A2, large increases in the percentage and number of antigen-specific CD8+ T cells were observed when T cells were stimulated by MART1-pulsed APC/A2/CD80 (percentage, P = .017; number, P = .002) and APC/A2/CD80/CD83 (percentage, P = .009; number, P < .001), but not by APC/A2/CD83 (percentage, P = .48; number, P = .30). When compared with APC/A2/CD80, APC/A2/CD80/CD83 generates a significant increase in the percentage and number of MART1-specific T cells (percentage, P = .012; number, P = .003).  indicates A2; □, A2/CD80;
indicates A2; □, A2/CD80;  , A2/CD83; ▪, A2/CD80/CD83. (B) Likewise, when APCs are pulsed with Flu peptide, the percentage and total number of antigen-specific T cells was increased when T cells were stimulated by APC/A2/CD80 (percentage, P = .009; number, P = .01) and APC/A2/CD80/CD83 (percentage, P = .009; number, P = .023), but not by or APC/A2/CD83 (percentage, P = .32; number, P = .32). When compared with APC/A2/CD80, APC/A2/CD80/CD83 generates a significant increase in the percentage and number of Flu-specific T cells (percentage, P = .01; number, P = .035).
, A2/CD83; ▪, A2/CD80/CD83. (B) Likewise, when APCs are pulsed with Flu peptide, the percentage and total number of antigen-specific T cells was increased when T cells were stimulated by APC/A2/CD80 (percentage, P = .009; number, P = .01) and APC/A2/CD80/CD83 (percentage, P = .009; number, P = .023), but not by or APC/A2/CD83 (percentage, P = .32; number, P = .32). When compared with APC/A2/CD80, APC/A2/CD80/CD83 generates a significant increase in the percentage and number of Flu-specific T cells (percentage, P = .01; number, P = .035).  indicates A2; □, A2/CD80;
indicates A2; □, A2/CD80;  , A2/CD83; ▪, A2/CD80/CD83.
, A2/CD83; ▪, A2/CD80/CD83.
CD83L signaling enhances the expansion of antigen-specific CD8+ T cells. HLA-A2+ CD8+ T cells were stimulated by MART1 (A) or Flu (B) peptide-pulsed APCs (APC/A2, APC/A2/CD80, APC/A2/CD83, or APC/A2/CD80/CD83). After 3 rounds of antigen stimulation and IL-2/IL-15 addition in between the stimulations, the cultures were stained with the relevant tetramer to determine percentage of peptide-specific T cells. The number of peptide-specific T cells was determined by calculating the product of the total number of T cells and the percentage of tetramer-staining cells. Representative results from 4 different donors are presented. (A) Compared with APC/A2, large increases in the percentage and number of antigen-specific CD8+ T cells were observed when T cells were stimulated by MART1-pulsed APC/A2/CD80 (percentage, P = .017; number, P = .002) and APC/A2/CD80/CD83 (percentage, P = .009; number, P < .001), but not by APC/A2/CD83 (percentage, P = .48; number, P = .30). When compared with APC/A2/CD80, APC/A2/CD80/CD83 generates a significant increase in the percentage and number of MART1-specific T cells (percentage, P = .012; number, P = .003).  indicates A2; □, A2/CD80;
indicates A2; □, A2/CD80;  , A2/CD83; ▪, A2/CD80/CD83. (B) Likewise, when APCs are pulsed with Flu peptide, the percentage and total number of antigen-specific T cells was increased when T cells were stimulated by APC/A2/CD80 (percentage, P = .009; number, P = .01) and APC/A2/CD80/CD83 (percentage, P = .009; number, P = .023), but not by or APC/A2/CD83 (percentage, P = .32; number, P = .32). When compared with APC/A2/CD80, APC/A2/CD80/CD83 generates a significant increase in the percentage and number of Flu-specific T cells (percentage, P = .01; number, P = .035).
, A2/CD83; ▪, A2/CD80/CD83. (B) Likewise, when APCs are pulsed with Flu peptide, the percentage and total number of antigen-specific T cells was increased when T cells were stimulated by APC/A2/CD80 (percentage, P = .009; number, P = .01) and APC/A2/CD80/CD83 (percentage, P = .009; number, P = .023), but not by or APC/A2/CD83 (percentage, P = .32; number, P = .32). When compared with APC/A2/CD80, APC/A2/CD80/CD83 generates a significant increase in the percentage and number of Flu-specific T cells (percentage, P = .01; number, P = .035).  indicates A2; □, A2/CD80;
indicates A2; □, A2/CD80;  , A2/CD83; ▪, A2/CD80/CD83.
, A2/CD83; ▪, A2/CD80/CD83.
Effector function of MART1-specific CD8+ T cells. (A) MART1-specific cytotoxic CD8+ T cells generated by APC/A2/CD80/CD83 killed target cells in an HLA-A2-specific manner. Target cells (T2 and Jurkat cells transduced with vector or A2) were pulsed with MART1 peptide (▪) or control peptide (•). 11LLFGYPVYV19 peptide of the TAX of HTLV-I was used as a negative control peptide. (B) Blocking experiments were performed using HLA-A2-specific mAb (BB7.2) and isotype control (mIgG2b). Peptide-pulsed T2 cells were used at an E/T ratio of 30:1. (C) MART1-specific cytotoxic CD8+ T cells generated by APC/A2/C80/CD83 secreted IFN-γ in an antigen-specific manner. Error bars represent standard deviation (SD).
Effector function of MART1-specific CD8+ T cells. (A) MART1-specific cytotoxic CD8+ T cells generated by APC/A2/CD80/CD83 killed target cells in an HLA-A2-specific manner. Target cells (T2 and Jurkat cells transduced with vector or A2) were pulsed with MART1 peptide (▪) or control peptide (•). 11LLFGYPVYV19 peptide of the TAX of HTLV-I was used as a negative control peptide. (B) Blocking experiments were performed using HLA-A2-specific mAb (BB7.2) and isotype control (mIgG2b). Peptide-pulsed T2 cells were used at an E/T ratio of 30:1. (C) MART1-specific cytotoxic CD8+ T cells generated by APC/A2/C80/CD83 secreted IFN-γ in an antigen-specific manner. Error bars represent standard deviation (SD).
Engagement of CD83L inhibits apoptosis and enhances proliferation, enabling the expansion of antigen-specific CD8+ T cells beyond 6 weeks
We further investigated immunologic functions of CD83 using APCs with or without CD83. Purified CD8+ T cells from HLA-A2-positive healthy donors were stimulated at weekly intervals by MART1 peptide-pulsed APC/A2/CD80 as described in “Materials and methods.” After 5 cycles of stimulation, MART1-specific CD8+ T-cell lines were split and subsequently stimulated with either peptide-pulsed APC/A2/CD80 or peptide-pulsed APC/A2/CD80/CD83. As shown in Figure 6A, APC/A2/CD80-stimulated MART1 cells did not increase in number. In contrast, MART1-specific CD8+ T cells stimulated with peptide-pulsed APC/A2/CD80/CD83 expanded with 2 additional rounds of stimulation. In a repeated-measures mixed model analysis of variance including treatment with CD83, time, and an interaction between treatment and time, all factors were statistically significant. The P value for a significant impact of CD83 was .006.
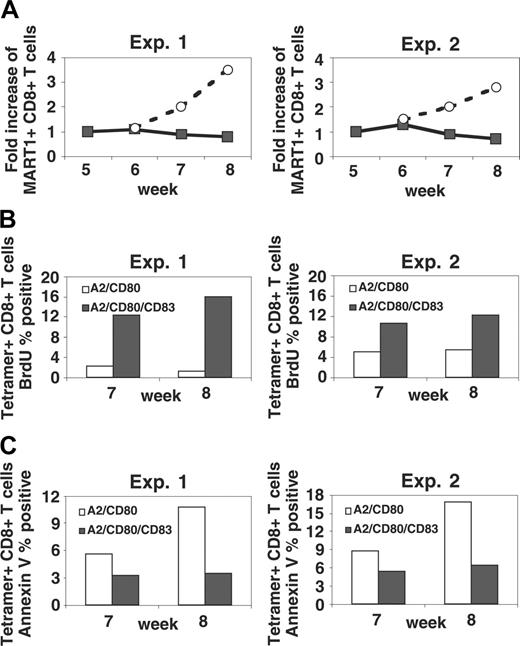
It was not clear if the ability of CD83 to contribute to the expansion of antigen-specific T cells was due to increased proliferation, inhibition of apoptosis, or both. To address this question, tetramer-stained T cells were counterstained with either FITC-conjugated anti-BrdU mAb to identify proliferating fractions or with FITC-conjugated Annexin V to identify apoptotic cells. As shown in Figure 6B, tetramer-positive CD8+ T cells stimulated by CD83+ APC/A2/CD80/CD83 showed a higher ratio of proliferating cells compared with those stimulated by CD83- APC/A2/CD80. Additionally, stimulation with APC/A2/CD80/CD83 dramatically suppressed the emergence of an apoptotic fraction compared with T cells stimulated with APC/A2/CD80 (Figure 6C). These results suggest that the observed expansion represents a combination of increased proliferation as well as an inhibition of apoptosis.
CD83-expressing APCs are capable of supporting functionally competent antigen-specific CD8+ T cells for more than 6 months
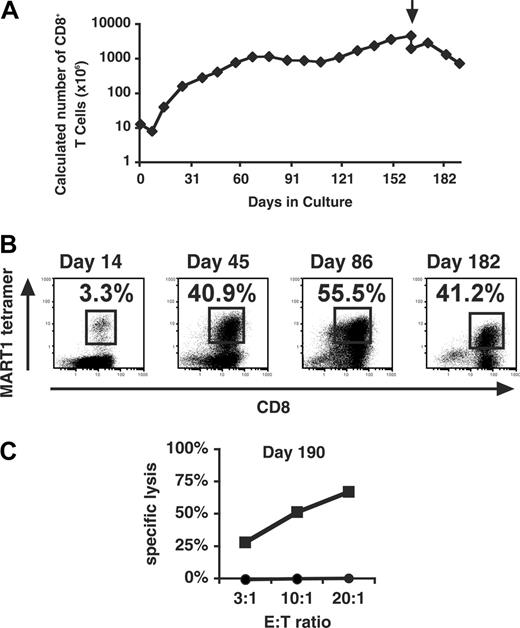
As shown in Figure 6A, antigen-specific T cells stimulated by APC/A2/CD80 ceased growing and died prior to week 12, consistent with the well-described fact that CD8+ T cells do not survive in the long term following stimulation with immobilized anti-CD3 and anti-CD28 mAbs unless they are cloned (data not shown).29 Our data suggested that CD83 signal might contribute to the long-term expansion and/or support of antigen-specific CD8+ T cells. Therefore, we examined whether stimulation every 7 to 14 days with MART1 peptide-pulsed APC/A2/CD80/CD83 could induce the long-term survival of MART1-specific CD8+ T cells. Between the stimulations, cultures were supplemented with IL-2 and IL-15. As shown in Figure 7A, logarithmic growth of the CD8+ T-cell line was induced during the initial culture period. After 60 days, expansion slowed, although cultures have been maintained for more than 6 months. After the second stimulation, the percentage of MART1-specific T cells reached 3.3%, and by day 45 increased to 40.9% (Figure 7B). Despite this prolonged culture period, antigen specificity was maintained throughout as shown by MART1-specific tetramer analysis. On day 190, functional competence was demonstrated by the ability of the T-cell line to specifically lyse antigen-pulsed targets in a cytotoxicity assay (Figure 7C).
Stimulation with APCs that express CD83 enhances proliferation and inhibits apoptosis, permitting continued expansion of MART1-specific T cells beyond 6 weeks. (A) Following 5 rounds of stimulation with MART1 peptide-pulsed APC/A2/CD80, T-cell lines noted to cease expansion were split and subsequently stimulated with either peptide-pulsed APC/A2/CD80 (▪) or peptide-pulsed APC/A2/CD80/CD83 (○). Between the stimulations, IL-2 and IL-15 were added to the culture. Those T cells stimulated by peptide-pulsed APC/A2/CD80/CD83 demonstrated significant antigen-specific expansion, while those stimulated by peptide-pulsed APC/A2/CD80 did not (P = .006). (B) An increase in MART1-specific T-cell proliferation was determined by tetramer staining and BrdU incorporation. This was consistently observed in both donors tested at weeks 7 and 8. (C) Apoptosis was inhibited as determined by Annexin V staining. This was consistently observed in both donors tested at weeks 7 and 8. The proliferating (B) and apoptotic fractions (C) are shown as a percentage of MART1 tetramer-positive cells. Two experiments with different donors were performed.
Stimulation with APCs that express CD83 enhances proliferation and inhibits apoptosis, permitting continued expansion of MART1-specific T cells beyond 6 weeks. (A) Following 5 rounds of stimulation with MART1 peptide-pulsed APC/A2/CD80, T-cell lines noted to cease expansion were split and subsequently stimulated with either peptide-pulsed APC/A2/CD80 (▪) or peptide-pulsed APC/A2/CD80/CD83 (○). Between the stimulations, IL-2 and IL-15 were added to the culture. Those T cells stimulated by peptide-pulsed APC/A2/CD80/CD83 demonstrated significant antigen-specific expansion, while those stimulated by peptide-pulsed APC/A2/CD80 did not (P = .006). (B) An increase in MART1-specific T-cell proliferation was determined by tetramer staining and BrdU incorporation. This was consistently observed in both donors tested at weeks 7 and 8. (C) Apoptosis was inhibited as determined by Annexin V staining. This was consistently observed in both donors tested at weeks 7 and 8. The proliferating (B) and apoptotic fractions (C) are shown as a percentage of MART1 tetramer-positive cells. Two experiments with different donors were performed.
Discussion
A role for CD83 in the generation of antigen-specific immunity is strongly suggested by its restricted expression on professional APCs, most notably mature DCs. This report supports the conclusion that CD83 is important in: (1) priming naive CD8+ T cells; (2) driving their antigen-specific expansion; and (3) supporting their long-term survival and function.
In contrast to others,8,9 we have shown that activated T cells express CD83L. While our findings that activated CD8+ T cells express CD83L are in agreement with a previous report, we show, for the first time, that CD83 is also expressed by CD4+ T cells upon activation.10 These discrepancies may be explained by the fact that we used a dodecameric form of soluble CD83 instead of monomeric or dimeric forms, which, in our experimental conditions, do not stain T cells. It is important to note that the dodecameric form of soluble IgG/A fusion proteins has a much slower rate of dissociation compared with the dimeric form.17,18 Therefore, it is likely that the interaction between CD83 and its ligand is not avid enough to be detected by dimeric or monomeric form of soluble CD83. In fact, the expression level of CD83L detected by dodecameric soluble CD83 was rather low.
We show that engagement of CD83:CD83L preferentially enriches and significantly increases the number of antigen-specific CD8+ T cells. Even with high-affinity dodecameric soluble CD83, CD83L is not detectable on resting T cells. Since its expression was observed on activated T cells following stimulation through CD3/CD28 but not CD3 alone, we would expect that CD83L functions only when T cells are stimulated by APCs that coexpress a B7 family member. In fact, we find that, in our T-cell cultures, CD80 must be coexpressed with CD83 in order to demonstrate that CD83 can mediate a doubling in the number of generated antigen-specific T cells. Therefore, following primary CD3 and secondary CD28 stimulations, a fraction of antigen-specific T cells may increase the expression of tightly regulated CD83L, respond to the CD83-mediated signal, and selectively expand. This is likely physiologic as, in vivo, CD83 expression is restricted to B7-expressing cells such as mature DCs, activated B cells, and thymic epithelial cells. We also find that CD83L provides a survival signal to antigen-specific T cells. Like CD83L, costimulatory molecules such as 4-1BB, OX40, or ICOS are up-regulated by CD28 signaling and are known to deliver survival signals.4 However, while these costimulatory molecules are able to provide CD28-independent costimulation to T cells, CD83L is completely “silent” in the absence of CD28 costimulation. Taken all together, CD83 itself does not appear to function simply as a costimulatory molecule, but rather as an enhancer of costimulation mediated through CD28. The mechanism by which the CD83L signal achieves these effects awaits its molecular identification.
CD83L engagement supports sustained growth of antigen-specific CD8+ T cells. MART1-specific CD8+ T cells were generated by stimulation with MART1-pulsed APC/A2/CD80/CD83 every 7 to 14 days. The percentage increase or decrease in the number of cells was determined at each stimulation, and a fraction was subsequently stimulated and maintained in culture. Antigen specificity was demonstrated every 3 or 4 rounds of stimulation by tetramer analysis. (A) The predicted total number of CD8+ T cells generated over a 191-day culture period is shown. Arrow denotes removal of debris by Ficoll density gradient. (B) PE-conjugated MART1 tetramer staining versus CD8 staining is shown at 4 time points during the culture period. (C) Effector function was determined by cytotoxicity assay on day 190 using peptide-pulsed T2 cells as targets (▪ indicates MART1 peptide; •, HIV pol as control peptide).
CD83L engagement supports sustained growth of antigen-specific CD8+ T cells. MART1-specific CD8+ T cells were generated by stimulation with MART1-pulsed APC/A2/CD80/CD83 every 7 to 14 days. The percentage increase or decrease in the number of cells was determined at each stimulation, and a fraction was subsequently stimulated and maintained in culture. Antigen specificity was demonstrated every 3 or 4 rounds of stimulation by tetramer analysis. (A) The predicted total number of CD8+ T cells generated over a 191-day culture period is shown. Arrow denotes removal of debris by Ficoll density gradient. (B) PE-conjugated MART1 tetramer staining versus CD8 staining is shown at 4 time points during the culture period. (C) Effector function was determined by cytotoxicity assay on day 190 using peptide-pulsed T2 cells as targets (▪ indicates MART1 peptide; •, HIV pol as control peptide).
Using APCs expressing CD83+CD80+, but not CD83-CD80+, we were able to generate CD8+ T cells specific for the foreign antigen HIV pol from naive donors in vitro. “Professional” APCs, such as mature DCs and activated B cells, are capable of priming naive T cells specific for such foreign, virally derived antigens in vitro.25-27 Since these APCs are known to express CD83, it is possible that CD83 expression may be required to establish antigen-specific T-cell lines from naive donors who have never been exposed to the foreign antigen. This could be due to a process that occurs during priming or, alternatively, could simply be a consequence of the CD83+ APCs' enhanced ability to induce the expansion and long-term survival of CTLs. Intriguingly, although a number of artificial APCs have been previously reported, they do not express CD83 and, to our knowledge, have not been shown to be able to establish CD8+ T-cell lines that are specific for foreign, virally derived antigens such as HIV.28,30-35
Strategies for the generation of cytotoxic T cells for adoptive immunotherapy require the use of autologous APCs, such as DCs,36 CD40 activated B cells,27 or Epstein-Barr virus (EBV)-transformed B cells.37 Autologous APCs are prepared from a limited supply of patient material in skilled, specialized facilities where rigorous regulatory release criteria must be satisfied.36 The need for individualized therapies significantly increases the cost and time for product preparation. K562/A2/CD80/CD83, on the other hand, could be used as a standardized, “off-the-shelf” reagent for all HLA-A*0201-positive patients. The creation of APCs expressing other HLA alleles is currently in progress and would cover a sizable population of individuals. Given these properties and the fact that CD83 may be a critical component of an artificial APCs, we plan to translate these findings to the treatment of cancer and infectious diseases such as HIV.
Prepublished online as Blood First Edition Paper, October 20, 2005; DOI 10.1182/blood-2005-05-2073.
Supported by National Institutes of Health (NIH) grants HL54785-08 (N.H.), CA87720 (M.O.B.), CA92625-04 (L.M.N.), and AI56299 and CA84500 (G.J.F.); the Dr Mildred Scheel Stiftung der Deutschen Krebshilfe (S.A.), and a grant from the Cancer Research Institute (L.M.N.).
N.H. and M.O.B. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank G. Dranoff, R. Mulligan, and T. Kitamura for providing us with valuable reagents. We are grateful to R. Sweet for suggesting the use of IgA tailpiece construct. We thank A. Murray, A. Berezovskaya, J. Daley, and S. Lazo-Kallanian for excellent technical help.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal