Dendritic-cell (DC) migration to secondary lymphoid organs is crucial for the initiation of adaptive immune responses. Although LPS up-regulates CCR7 on DCs, a second signal is required to enable them to migrate toward the chemokine CCL19 (MIP-3β). We found that the nitric oxide (NO) donor NOR4 provides a signal allowing LPS-stimulated DCs to migrate toward CCL19. NO affects DC migration through both the initial activation of the cGMP/cGMP kinase (cGMP/cGK) pathway and a long-term effect that reduced cGK activity via negative feedback. Indeed, migration of DCs toward CCL19, unlike migration toward CXCL12 (SDF-1α), required inhibition of cGK. LPS increased both cGK expression and cGK activity as measured by phosphorylation of the key cGK target vasodilator-stimulated phosphoprotein (VASP). Because cGK phosphorylation of VASP can disrupt focal adhesions and inhibit cell migration, LPS-induced VASP phosphorylation may prevent DCs from migrating without a second signal. Long-term NOR4 treatment inhibited the increase in cGK-dependent VASP phosphorylation, releasing this brake so that DCs can migrate. NO has been implicated in the regulation of autoimmunity through its effect on T cells. Our results suggest that NO regulation of DC migration and cytokine production may contribute to the protective effects of NO in autoimmune disorders.
Introduction
Dendritic cells (DCs) activate naive T cells in peripheral lymphoid tissues and play a pivotal role in initiating and instructing adaptive immune responses.1,2 At the site of infection where they take up antigens, DCs also contribute to innate immunity: They recognize and respond to common pathogen-associated molecular patterns (PAMPs) by releasing proinflammatory products that regulate other inflammatory cells.3 In a later phase, recognition of PAMPs triggers a complex maturation program that results in the increase in DC antigen-presenting ability and responsiveness to chemokines like CCL19/MIP-3β, which promote DC migration from peripheral tissues to secondary lymphoid organs.4,5
The outcome of an immune response depends not only on the pathogen that activates DCs but also on various inflammation-associated factors that modulate DC maturation and determine whether DCs polarize T-cell development into T helper 1 (Th1)–, Th2-, or regulatory T-cell subsets.6 Prostaglandin E2 (PGE2) and other inflammatory products that act through the cAMP/cAMP kinase (cAMP/cAK) signaling pathway can affect DC maturation and drive them toward a Th2-inducing program.7-9 These agents also regulate DC chemokine and chemokines receptor expression; they down-regulate receptors for inflammatory chemokines like CCR5 while increasing CCR7 or CXCR4 receptors for CCL19/MIP-3β and CXCL12/SDF-1α, respectively.10-12
Interestingly, inflammatory agents are required for mature DCs to be able to migrate toward certain chemokines. Even after monocyte-derived immature DCs (iDCs) are induced to mature and up-regulate CCR7, they remain unresponsive to CCL19 and do not migrate unless they are exposed to an inflammatory stimulus like PGE2 or ATP.12-15 The importance of inflammatory factors for DC migration to lymph nodes (LNs) has been underscored recently.16,17 EP4-deficient mice have Langerhans cells defective in their ability to migrate to LNs and impaired contact hypersensitivity immune responses.
Nitric oxide (NO) is a free radical gas produced during the development of inflammation by many cells, including macrophages, endothelial cells, and granulocytes.18 Although NO can be toxic and proinflammatory when produced in large amounts, it may also regulate adaptive immune responses.18,19 NO protects human and mouse DCs against apoptosis in models of sepsis.20 NO also inhibits IL-12 and regulates MHC distribution during the maturation of mouse DCs,21,22 but the role of NO in human DC maturation is still unclear.
Because NO acts principally through the cGMP/cGMP kinase (cGMP/cGK) signaling pathway, which often has effects similar to cAMP/cAK,23 we hypothesized that NO might also be an important regulator of DC migration. We found that the addition of the NO donor, NOR4, during DC maturation enables DCs to migrate toward CCL19. We investigated the mechanisms by which NO couples CCR7 to the signal transduction involved in migration and, in particular, the cytoskeletal protein vasodilator-stimulated phosphoprotein (VASP), a target of cGK involved in cytoskeletal rearrangements occurring during cell migration.24-26 Here we show that LPS stimulation induced a large increase in cGK expression and activity in DCs, leading to increased cGK-dependent VASP phosphorylation that impairs the ability of DCs to migrate. The presence of NO during DC maturation as a long-term effect decreased cGK-mediated phosphorylation of VASP. Thus, the enhanced ability to migrate induced by NO may be due in part to an increase in the amount of unphosphorylated VASP available to help form focal adhesions once DCs are stimulated by chemokines.
Materials and methods
Generation of iDCs and cell cultures
iDCs were generated from human CD14+ monocytes obtained from leukapheresis of healthy donors and treated with IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) for 5 days as described.27 After 5 days, cells had an iDC phenotype: CD14-, CD1a++, CD86+, HLA-DR++. In some experiments iDCs were seeded at 1.2 × 106 to 2 × 106 cells per 2 mL in 12-well plates in RPMI 1640 with 5% FBS with or without graded doses of Escherichia coli LPS (0.25 to 1 μg/mL) (Sigma, St Louis, MO) alone or with graded amounts of NOR4 (25 to 100 μM) (Calbiochem, San Diego, CA) or the vehicle DMSO (Sigma). In other experiments the cGMP analog, 8-bromo-cGMP (8B-cGMP), (Sigma) was used to mimic an increase in cGMP levels. The highly specific cGK inhibitor DT-3 (Calbiochem), a membrane-permeable peptide that is 20 000 times more selective for cGK (Ki = 25 nM) than cAK (Ki = 493 μM),28 was also used. In some experiments we used a specific cAK inhibitor derived from the endogenous protein kinase A inhibitor (PKI) consisting of the peptide sequence 14 to 22 of PKI (Ki = 36 nM) that has been myristoylated at the N terminus (myrPKI) to enhance cell permeability (Calbiochem).29
Fluorescence-activated cell sorting (FACS) analysis
DCs were stained with the following conjugated monoclonal antibodies (mAbs): CD1a-PE (SFcI19thy1A8) (Beckman Coulter, Miami, FL); CD14-FITC (MΦP9) (BD Bioscience, Lexington, KY); CD86-PE (IT2.2), CD83-FITC (HB15), CXCR4-PE (12G5), CCR5-FITC (2D7/CCR5), CD38-PE (HIT2) (BD Bioscience Pharmingen, San Diego, CA); CD54 (LB2/H616, Clark Laboratory, Seattle, WA). CCR7 was detected using mouse anti–human CCR7 (2H4) (BD Bioscience Pharmingen), followed by FITC-labeled rabbit antimouse specific for IgG and IgM (BioSource, Camarillo, CA). The intracellular detection of DC-LAMP was performed as described27 using mouse anti–human DC-LAMP (104.G4) (Immunotech Beckman Coulter, Somerset, NJ). Fluorescence acquisition was done on FACScan analyzer (Becton Dickinson, San Jose, CA) and data analysis with CellQuest software.
Cytokine detection
IL-12p70, IL-6, TNF-α, and IL-10 enzyme-linked immunosorbent assay (ELISA) was performed on supernatants collected from DCs 24 hours after LPS stimulation with or without graded doses of NOR4 or 8B-cGMP. IL-12p70 ELISA (Duo Set; R&D Systems, Minneapolis, MN) was performed according to the manufacturer's instruction. IL-6, TNF-α, and IL-10 were detected by ELISA in triplicate using a matched pair of cytokine-specific mAbs and recombinant cytokines as standards (BD Bioscience Pharmingen): capture anti–IL-6, MQ2-13A5; detection anti–IL-6, MQ2-39C3; capture anti–TNF-α, mAb11; detection anti–TNF-α, mAb11; capture anti–IL-10, JES3-19F1; detection anti–IL-10, JES3-12G8.
Migration assay
DC migration was measured in duplicate using a transwell system (24-well plates; 8.0-μm pore size; Costar, Corning, NY). A total of 600 μL RPMI medium with or without 200 ng/mL recombinant human CCL19/MIP-3β or CXCL12/SDF-1α (RDI, Flanders, NJ) was added to the lower chamber. Wells with medium only were used as a control for spontaneous migration. A total of 2.5 × 105 cells in 100 μL were added to the upper chamber and incubated at 37°C for 3 hours. Cells that migrated into the lower chamber were harvested, concentrated to a volume of 200 μL, and counted by flow cytometry acquiring events for a fixed time of 30 seconds. The counts fell within a linear range of the control titration curves obtained by testing increasing concentrations of cells. The mean number of spontaneously migrated cells was subtracted from the total number of cells that migrated in response to the chemokine. Values are given as the mean number of migrated cells plus or minus SEM.
Western blotting
iDCs were treated for 24 hours in the presence or absence 0.3 μg/mL LPS with or without 100 μM NOR4, and then 2 × 106 cells were stimulated or not for 2 or 15 minutes with 250 ng/mL human CCL19 or CXCL12 at 37°C. The reaction was stopped with ice-cold PBS, and then cells were spun down at 17 210g for 5 minutes at 4°C and lysed by sonication in lysis buffer (50 mM Tris-Cl pH 7.4, 150 mM NaCl, 2 mM EDTA,1 mM PMSF, 1% Triton X-100) with a protease inhibitor cocktail (Sigma). Proteins were assayed by BCA assay (Pierce, Rockford, IL). Lysates were resolved by an 8% to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and electrotransferred to PVDF membranes. Immunoblotting was performed with the monoclonal anti–phospho-VASP (Ser239) antibody (16C2) (Alexis, San Diego, CA).24 Normalization of phosphorylated VASP was performed either with the polyclonal anti-VASP (M4) (Alexis) or anti-p38 MAPK (C20) (Santa Cruz Biotechnology, Santa Cruz, CA). HRP-conjugated secondary antibodies were used (Jackson ImmunoResearch Labs, West Grove, PA); blots were developed with enhanced chemiluminescence (ECL) and analyzed with Image J 1.33u (National Institutes of Health [NIH], Bethesda, MD) software.
Measurement of intracellular cGMP
iDCs (2.5 × 106 cells) were incubated at 37°C for various periods (30 minutes, 1.5 hours, 5 hours, 24 hours) in the presence or absence of 0.3 μg/mL LPS with or without 100 μM NOR4. Reactions were terminated by adding ice-cold ethanol: 1N HCl, 99:1; lysates were stored at -70°C until cGMP determination. cGMP levels were determined by using the acetylation protocol cGMP EIA kit (American Qualex, San Clemente, CA) according to the manufacturer's instruction. cGMP levels were normalized by cell number.
RNA purification and real-time PCR
Total RNA from iDCs treated for 24 hours in the presence or absence of 0.3 μg/mL LPS with or without 30 to 100 μM NOR4 was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription (RT) of RNA samples (1 μg) was performed using oligo(dT)20 and the cloned AMV first-strand cDNA synthesis kit (Invitrogen). Specific primers for human cGKI, Iα, Iβ, and II (primers in Table S1; see the Supplemental Materials link at the top of the online article, at the Blood website) were used for quantitative real-time polymerase chain reaction (PCR) amplification in an ABI PRISM 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) using SYBR Green PCR Master Mix. Nonspecific amplification was excluded by performing a dissociation curve analysis and by analyzing real-time polymerase chain reaction (PCR) products on 3% agarose gels. Controls were included to exclude amplification of contaminating genomic DNA. cDNA extracted from lung for cGKI and from kidney for cGKII was used as positive controls. The relative expression of the target gene in different samples was normalized to the endogenous β-actin (primers in Table S1) and was calculated with the 2-ΔΔCT method.30
Results
NO reduces LPS-induced IL-12 production but does not block DC maturation
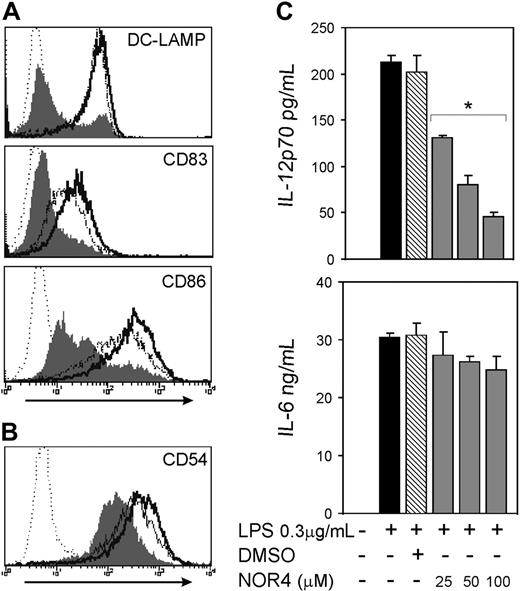
To test if increasing NO concentration affects maturation and migration of iDCs, we used NOR4, an NO donor that releases NO at a slow rate,31 thereby more closely mimicking the production of NO from cells in inflammatory sites. The maximal concentration of NOR4 used in this study (100 μM) produces NO in the range of plasma concentrations of NO during inflammation.32 At all concentrations used, NOR4 did not affect DC numbers and viability as measured by trypan blue exclusion and annexin V/PI staining (data not shown). Furthermore, NOR4 had no major effect on LPS-induced DC maturation, including little or no effect on induced expression of either DC-LAMP or CD83 or up-regulation of CD86 (Figure 1A). The adhesion molecule CD54 and the cell surface ectoenzyme CD38, which are up-regulated by LPS and play a role in DC migration,33,34 also were not substantially affected by the presence of NOR4 during DC stimulation with LPS (Figure 1B and data not shown).
The activation of the cAMP/cAK pathway in DCs (eg, through PGE2 or ATP) drives DCs toward a Th2 immune response via down-regulation of IL-12p70 production. We tested if NO had a similar effect. The addition of either NOR4 or 8B-cGMP in a dose-dependent fashion consistently inhibited LPS-induced production of IL-12p70 (Figure 1C) and TNF-α (Figure S1 and data not shown) without significantly affecting IL-6 production (Figures 1C and S1 and data not shown). NOR4 also reduced LPS-induced increases in IL-10 about 2-fold (P < .02; Figure S1). Therefore, while NO does not significantly affect DC maturation induced by LPS, it does selectively decrease the release of a set of cytokines produced by DCs.
NO reduces the LPS-induced release of IL-12p70 without affecting DC maturation. (A-B) Intracellular expression of DC-LAMP and surface expression of CD83 and CD86 (A) and CD54 (B) in DCs matured for 24 hours with 0.3 μg/mL LPS only (bold open histograms) or plus 100 μM NOR4 (open histograms). iDCs treated for 24 hours in medium only are represented by filled histograms. Isotype controls are indicated by dotted histograms. Data shown are representative of more than 10 independent experiments for DC-LAMP, CD83, and CD86 and 3 experiments for CD54. (C) iDCs were stimulated or not (□) for 24 hours with LPS (0.3 μg/mL) in the presence ( ) or absence (▪) of graded doses of NOR4 (25, 50, 100 μM) or the vehicle control DMSO (
) or absence (▪) of graded doses of NOR4 (25, 50, 100 μM) or the vehicle control DMSO ( ). Supernatants were collected and analyzed for IL-12p70 and IL-6 by ELISA. *P < .001 compared with control LPS-treated DCs (▪), unpaired Student t test. Data are from 1 representative experiment of 9 independent experiments performed using cells from different donors. A statistical analysis of the 9 experiments using the paired t test gave a value of P = .01, confirming that the inhibition of IL-12p70 production by NOR4 is statistically significant
). Supernatants were collected and analyzed for IL-12p70 and IL-6 by ELISA. *P < .001 compared with control LPS-treated DCs (▪), unpaired Student t test. Data are from 1 representative experiment of 9 independent experiments performed using cells from different donors. A statistical analysis of the 9 experiments using the paired t test gave a value of P = .01, confirming that the inhibition of IL-12p70 production by NOR4 is statistically significant
NO reduces the LPS-induced release of IL-12p70 without affecting DC maturation. (A-B) Intracellular expression of DC-LAMP and surface expression of CD83 and CD86 (A) and CD54 (B) in DCs matured for 24 hours with 0.3 μg/mL LPS only (bold open histograms) or plus 100 μM NOR4 (open histograms). iDCs treated for 24 hours in medium only are represented by filled histograms. Isotype controls are indicated by dotted histograms. Data shown are representative of more than 10 independent experiments for DC-LAMP, CD83, and CD86 and 3 experiments for CD54. (C) iDCs were stimulated or not (□) for 24 hours with LPS (0.3 μg/mL) in the presence ( ) or absence (▪) of graded doses of NOR4 (25, 50, 100 μM) or the vehicle control DMSO (
) or absence (▪) of graded doses of NOR4 (25, 50, 100 μM) or the vehicle control DMSO ( ). Supernatants were collected and analyzed for IL-12p70 and IL-6 by ELISA. *P < .001 compared with control LPS-treated DCs (▪), unpaired Student t test. Data are from 1 representative experiment of 9 independent experiments performed using cells from different donors. A statistical analysis of the 9 experiments using the paired t test gave a value of P = .01, confirming that the inhibition of IL-12p70 production by NOR4 is statistically significant
). Supernatants were collected and analyzed for IL-12p70 and IL-6 by ELISA. *P < .001 compared with control LPS-treated DCs (▪), unpaired Student t test. Data are from 1 representative experiment of 9 independent experiments performed using cells from different donors. A statistical analysis of the 9 experiments using the paired t test gave a value of P = .01, confirming that the inhibition of IL-12p70 production by NOR4 is statistically significant
NO induces DC migration toward CCL19/MIP-3β. (A) Surface CCR7 expression of iDCs matured or not for 24 hours with LPS (0.3 μg/mL) in the presence or absence of 100 μM NOR4 or DMSO. The mean fluorescence intensity (M) and percentages of positive cells (%) are indicated. Data shown are representative of more than 10 independent experiments. (B) iDCs matured or not for 24 hours with 0.3 μg/mL LPS in the presence or absence of 100 μM NOR4 or DMSO were tested for their migration toward CCL19. Data shown are mean of duplicate cultures ± SEM and are representative of 9 independent experiments. (C) iDCs were matured for 24 hours with graded doses of LPS with medium only (•), 100 μM NOR4 (♦), or 0.001% DMSO (▴) and tested for migration toward CCL19. Data are from 1 representative experiment of 2 performed using cells from different donors.
NO induces DC migration toward CCL19/MIP-3β. (A) Surface CCR7 expression of iDCs matured or not for 24 hours with LPS (0.3 μg/mL) in the presence or absence of 100 μM NOR4 or DMSO. The mean fluorescence intensity (M) and percentages of positive cells (%) are indicated. Data shown are representative of more than 10 independent experiments. (B) iDCs matured or not for 24 hours with 0.3 μg/mL LPS in the presence or absence of 100 μM NOR4 or DMSO were tested for their migration toward CCL19. Data shown are mean of duplicate cultures ± SEM and are representative of 9 independent experiments. (C) iDCs were matured for 24 hours with graded doses of LPS with medium only (•), 100 μM NOR4 (♦), or 0.001% DMSO (▴) and tested for migration toward CCL19. Data are from 1 representative experiment of 2 performed using cells from different donors.
NOR4 induces DC migration of LPS-matured DCs toward CCL19/MIP-3β
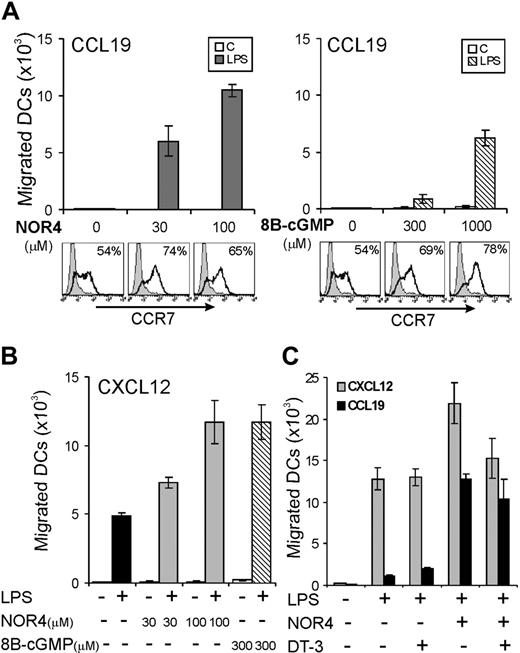
We next tested if NO affects migration of LPS-matured DCs toward CCL19/MIP-3β, as shown for other inflammatory factors.13,14 LPS alone induced a substantial increase in CCR7 expression (Figure 2A) but did not induce DCs to be responsive to CCL19 (Figure 2B). The addition of 100 μM NOR4 with LPS potently enabled DCs to respond to CCL19 and migrate (Figure 2B), and the effect of NOR4 was dose dependent (Figure 3B). LPS-induced up-regulation of CCR7 was only slightly enhanced by NOR4 (Figure 2A). Thus, NOR4-induced enhancement of migration toward CCL19 was not simply due to an increase in CCR7. The NOR4 effect on migration increased with graded doses of LPS (Figure 2C), suggesting a synergistic effect between NOR4 and LPS in sensitizing DCs to migrate. A substantial effect of NOR4 was evident with as little as 0.3 μg/mL LPS, a concentration that induces complete maturation of iDCs. Therefore, we used this concentration of LPS in the following experiments unless indicated otherwise.
We tested if NO could increase DC migration not only to CCL19 but also to other chemokines. Upon maturation iDCs down-regulate CCR5 and up-regulate CXCR4, a receptor for CXCL12/SDF-1α, a chemokine that regulates migration of DCs toward LNs.35 NOR4 enhanced LPS-induced down-regulation of CCR5 (data not shown) and up-regulation of CXCR4 in a dose-dependent fashion (Figure 3). LPS-treated DCs were already responsive to CXCL12; NOR4 also enhanced LPS-induced migration toward CXCL12 (Figure 3B). In this case the NOR4-dependent enhancement of migration toward CXCL12 was gradual as well as the increase in CXCR4 expression (Figure 3). In contrast, maximal up-regulation of CCR7 was evident at the lower dose of NOR4, while optimal migration toward CCL19 was only evident at higher doses (Figure 3). These data suggest that NOR4 promotes increased DC migration to CCL19 via an additional mechanism independent from altering receptor expression. NOR4 affected specifically chemokine-directed chemotaxis and had little or no effect on spontaneous migration in the absence of chemokines (data not shown). Furthermore, treatment of iDCs with graded doses of NOR4 alone did not induce increases in CCR7 or CXCR4 expression (not shown) or promote migration toward CCL19 or CXCL12 (Figure 3B). Therefore, the enhancement of DC migration by NO requires a maturation stimulus.
NO enhances LPS-induced expression of CXCR4 and migration toward CXCL12/SDF-1α. iDCs were treated or not for 24 hours with 0.3 μg/mL LPS in the absence or presence of graded doses of NOR4. (A) Surface expression of CXCR4 or CCR7. The mean fluorescence intensity (M) and percentages of positive cells (%) are indicated. (B) Cells treated with medium only (•) or 0.3 μg/mL LPS plus graded doses of NOR4 (▴) were tested for their chemotactic response toward CXCL12 or CCL19. The receptor levels after stimulation with LPS plus graded doses of NOR4 are shown in the inserts; the percentages of cells expressing CXCR4 (▪) and CCR7 (♦) are shown in the top and bottom panels, respectively. Data shown are representative of 3 independent experiments.
NO enhances LPS-induced expression of CXCR4 and migration toward CXCL12/SDF-1α. iDCs were treated or not for 24 hours with 0.3 μg/mL LPS in the absence or presence of graded doses of NOR4. (A) Surface expression of CXCR4 or CCR7. The mean fluorescence intensity (M) and percentages of positive cells (%) are indicated. (B) Cells treated with medium only (•) or 0.3 μg/mL LPS plus graded doses of NOR4 (▴) were tested for their chemotactic response toward CXCL12 or CCL19. The receptor levels after stimulation with LPS plus graded doses of NOR4 are shown in the inserts; the percentages of cells expressing CXCR4 (▪) and CCR7 (♦) are shown in the top and bottom panels, respectively. Data shown are representative of 3 independent experiments.
Moreover, NOR4 also enhanced the reciprocal regulation of CCR5 and CXCR4 induced by CD40 stimulation (D.G. and L.A.C., unpublished data, March 2005), suggesting that NO regulates chemokine receptor levels irrespective of the maturation stimulus.
The effects of NOR4 on both chemokine receptor expression and migration toward CCL19 and CXCL12 suggest that elevated levels of NO in inflammatory sites may drive DCs toward a more mature phenotype and facilitate migration of DCs toward LNs.
Role of cGMP and cGK in NO-regulated DC migration
Because NO acts mainly through the activation of the cGMP/cGK pathway, we next tested if DC migration toward either CCL19 or CXCL12 could be enhanced by the cell-permeable analog, 8B-cGMP. Unlike NOR4, which enhanced migration of LPS-treated DCs toward CCL19 at relatively low concentrations, 8B-cGMP affected DC migration only at high concentrations (Figure 4A). Although a modest increase in cGMP levels appeared to be sufficient to enhance CCR7 expression, enhanced migration toward CCL19 apparently requires a high intracellular level of cGMP and/or a cGMP-independent mechanism. Low concentrations of 8B-cGMP (300 μM) enhanced DC migration toward CXCL12 (Figure 4B), suggesting that smaller increases in cGMP are needed to enhance CXCL12-versus CCL19-dependent migration.
To further analyze the role of the cGMP/cGK pathway, we tested if a highly specific cGK inhibitor, the cell-permeable peptide DT-3,28 could affect NOR4-dependent DC migration. If NOR4 enhances migration mainly via activation of the cGMP/cGK pathway, then inhibition of cGK activity by DT-3 should inhibit migration. However, the cGK inhibitor was unable to block NOR4-induced DC migration toward CCL19 and only partially inhibited the enhanced DC migration toward CXCL12 (Figure 4C). Thus, while cGMP may contribute to the increased sensitivity to migration toward CCL19 induced by NOR4, a cGMP/cGK-independent pathway is also involved. Furthermore, the relative contribution of the cGMP/cGK pathway on DC migration seems to be different for CXCL12 versus CCL19.
cGK and cAK regulate the ability of LPS-treated DCs to migrate toward CCL19
We next tested if the cGMP/cGK pathway can directly modulate the chemokine signaling pathways activated through CCR7 or CXCR4. We added the specific cGK inhibitor DT-3 to DCs during the migration assay and, to our surprise, we found that preincubation of LPS-matured DCs with graded doses of DT-3 just prior the chemotactic assay sensitized DCs so they could migrate toward CCL19 (Figure 5A, top). Therefore, persistent cGK activity prevents mature DCs from migrating toward CCL19. These data again suggested that NO affects DC migration via a mechanism independent from the “activation” of the cGMP/cGK pathway. DT-3 had little or no effect on LPS-induced migration toward CXCL12 (Figure 5A, bottom). These data further support the hypothesis that the cGMP/cGK pathway plays a different role in regulating CCL19 versus CXCL12 migration.
NO induction of migration toward CCL19 is only partially dependent on the cGMP/cGK pathway. (A-B) iDCs were treated or not for 24 hours with 0.3 μg/mL LPS in the presence or absence of graded doses of NOR4 or 8B-cGMP. Surface expression of CCR7, percentages of positive cells (%) are indicated. Chemotactic responses toward CCL19 (A) or CXCL12 (B) are shown as the mean of duplicate cultures ± SEM. Data shown are representative of 3 independent experiments. (C) iDCs were treated or not for 24 hours with 0.3 μg/mL LPS in the presence or absence of 100 μM NOR4 with or without 500 nM DT-3 (cGK inhibitor) and tested for their chemotactic response toward CCL19 or CXCL12. Data shown are representative of 3 independent experiments.
NO induction of migration toward CCL19 is only partially dependent on the cGMP/cGK pathway. (A-B) iDCs were treated or not for 24 hours with 0.3 μg/mL LPS in the presence or absence of graded doses of NOR4 or 8B-cGMP. Surface expression of CCR7, percentages of positive cells (%) are indicated. Chemotactic responses toward CCL19 (A) or CXCL12 (B) are shown as the mean of duplicate cultures ± SEM. Data shown are representative of 3 independent experiments. (C) iDCs were treated or not for 24 hours with 0.3 μg/mL LPS in the presence or absence of 100 μM NOR4 with or without 500 nM DT-3 (cGK inhibitor) and tested for their chemotactic response toward CCL19 or CXCL12. Data shown are representative of 3 independent experiments.
Inhibition of either cGK or cAK allows LPS-matured DCs to migrate toward CCL19, while migration toward CXCL12 requires cAK activity. iDCs were treated (▪) or not (□) with 0.3 μg/mL LPS for 24 hours. Cells were harvested, incubated with graded doses of either the cGK inhibitor DT-3 (A) or the cAK inhibitor myrPKI (B), and then tested for their chemotactic response toward CCL19 or CXCL12. Data shown are representative of 3 experiments using different donors.
Inhibition of either cGK or cAK allows LPS-matured DCs to migrate toward CCL19, while migration toward CXCL12 requires cAK activity. iDCs were treated (▪) or not (□) with 0.3 μg/mL LPS for 24 hours. Cells were harvested, incubated with graded doses of either the cGK inhibitor DT-3 (A) or the cAK inhibitor myrPKI (B), and then tested for their chemotactic response toward CCL19 or CXCL12. Data shown are representative of 3 experiments using different donors.
Because NO also can activate the cAMP signaling pathway,36 we tested if a specific cAK inhibitor, permeable peptide myristoylated PKI (myrPKI), could affect DC migration. As observed for cGK inhibition, inhibition of cAK during the chemotactic assay also enabled LPS-treated DCs to migrate toward CCL19 (Figure 5B, top). However, LPS-induced sensitivity to migration toward CXCL12 was completely blocked by the cAK inhibitor (Figure 5B, bottom). The addition of DT-3 or myrPKI in the chemotactic assay did not affect chemokine-specific chemotaxis of DCs not treated with LPS, and random cell motility was not significantly affected in either immature or LPS-treated DCs (data not shown). Taken together these data suggest a differential regulation by cyclic nucleotides of DC migration toward CCL19 versus CXCL12. Both cGK and cAK activities potently inhibit migration signals induced by CCL19 interaction with CCR7. In contrast, cAK activity, but not cGK activity, is required to allow DC migration toward CXCL12.
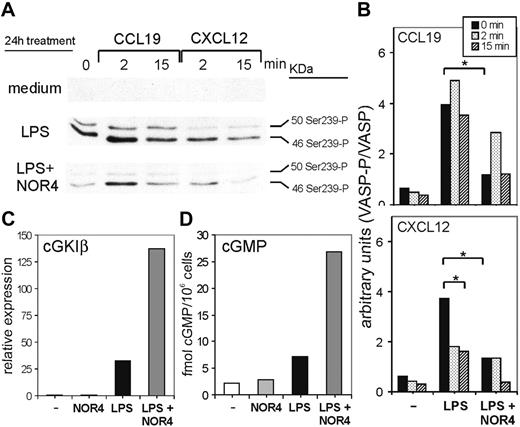
LPS induces cGK-dependent phosphorylation of VASP in DCs, which is inhibited by NOR4. iDCs were treated or not for 24 hours with 0.3 μg/mL LPS in the presence or absence of 100 μM NOR4. Cells from each group were then stimulated for the indicated times with either CCL19 or CXCL12. (A,B) Cell extracts were prepared and immunoblotted with anti–phospho-VASP antibody and then with anti-VASP antibody. (A) Antiphospho-VASP immunoblot of 1 representative experiment of 3 performed with cells from different donors. (B) Quantitative densitometric analysis of 3 different immunoblot experiments. The activated phospho-VASP expression was normalized to the total VASP. *P < .001, paired Student t test. (C) mRNA levels of cGKIβ determined by quantitative real-time RT-PCR. The relative expression of cGKIβ was normalized to the endogenous β-actin. Data shown are from 1 representative of 2 independent experiments giving the same results. (D) cGMP levels in cell extracts were measured with cGMP EIA assay. Data shown are representative of 2 independent experiments.
LPS induces cGK-dependent phosphorylation of VASP in DCs, which is inhibited by NOR4. iDCs were treated or not for 24 hours with 0.3 μg/mL LPS in the presence or absence of 100 μM NOR4. Cells from each group were then stimulated for the indicated times with either CCL19 or CXCL12. (A,B) Cell extracts were prepared and immunoblotted with anti–phospho-VASP antibody and then with anti-VASP antibody. (A) Antiphospho-VASP immunoblot of 1 representative experiment of 3 performed with cells from different donors. (B) Quantitative densitometric analysis of 3 different immunoblot experiments. The activated phospho-VASP expression was normalized to the total VASP. *P < .001, paired Student t test. (C) mRNA levels of cGKIβ determined by quantitative real-time RT-PCR. The relative expression of cGKIβ was normalized to the endogenous β-actin. Data shown are from 1 representative of 2 independent experiments giving the same results. (D) cGMP levels in cell extracts were measured with cGMP EIA assay. Data shown are representative of 2 independent experiments.
NO reduces cGK-mediated phosphorylation of VASP, which may prevent DCs from migrating toward CCL19
The data reported in the previous section suggest that cGK and cAK activities already expressed or induced by LPS prevent DCs from migrating toward CCL19. Thus, we reconsidered the possible mechanisms by which NOR4 facilitates the migration of LPS-triggered DCs (Figure 4A). The cGMP/cGK pathway appeared to be able to exert either a positive (as shown earlier) or a negative effect on DC migration. How might this occur? One possibility was that after the initial activation of the cGMP/cGK pathway by NOR4, long-term treatment with the NO donor reduces cGK activity so that in the absence of cGK a brake is released enabling DCs to migrate.
To test this hypothesis, we first examined if NO could reduce cGK activity constitutively expressed or induced after LPS treatment. To measure cGK activity, we selected the cGK target VASP, because VASP is involved in cytoskeletal rearrangements occurring during cell migration.26 Interestingly, cGK-dependent VASP phosphorylation induces detachment of VASP and other cytoskeletal proteins from focal adhesions, thus inhibiting cell migration.26,37 Thus, phosphorylated VASP might contribute to the inhibitory effect of cGK activity on DC migration. We measured cGK activity by using an mAb (VASP-16C2) that specifically detects phosphorylation of VASP on serine 239 phosphorylation, an established and useful monitor of cGK activity in intact cells.24,37 The basal level of cGK activity in iDCs was very low, but LPS stimulation alone induced a substantial increase in cGK-dependent VASP phosphorylation (Figure 6A). Thus, cGK activity is not constitutively expressed in DCs but is up-regulated by LPS. The addition of NOR4 in combination with LPS strongly inhibited VASP phosphorylation (Figure 6A, first lane, and Figure 6B), indicating that NOR4 blocks the increase in cGK activity induced by LPS. The overall levels of VASP protein did not change in any of the DC groups (Figure 6B and data not shown). Thus, the observed changes in VASP phosphorylation were not due to changes in expression of total VASP.
The experiments using the cGK inhibitors suggested that cGK activity might be differently affected by CCR7 versus CXCR4 signaling. To test this, we compared cGK-dependent VASP phosphorylation after stimulating iDCs, LPS-matured DCs, or LPS plus NOR4-matured DCs with CCL19 or CXCL12 (Figure 6A). Cells treated only with LPS upon CCL19 stimulation retained a high level of VASP phosphorylation, consistent with their inability to migrate toward CCL19 (Figures 2, 3, and 6A-B). Contrary to CCL19 stimulation, CXCL12 stimulation of LPS-treated DCs decreased VASP phosphorylation, again consistent with the ability of DCs to migrate toward CXCL12 (Figures 3 and 6A-B). For both chemokines NOR4 treatment during LPS maturation further reduced the level of VASP phosphorylation after stimulation, consistent with the ability of NO to induce migration toward CCL19 and enhance migration toward CXCL12 (Figure 6A-B).
How might long-term treatment with NOR4 reduce cGK activity in LPS-treated DCs? One possibility was that exposure to NO reduces cGK expression in DCs, as reported in other cell types.38,39 To determine which cGK isoforms are expressed in DCs, we used real-time PCR. Mammals have 2 cGK genes: one encodes for the soluble form cGKI, which produces 2 alternatively spliced isoforms, cGKIα and cGKIβ; the other encodes for membrane-associated cGKII.40 We detected almost no cGKI and very low levels of cGKII expression in iDCs (Figures 6C and S2), and cGKII expression was not significantly affected by any treatment (Figure S2). However, LPS induced a large increase in cGKI at 24 hours (Figures 6C and S2) that was entirely due to increases in cGKIβ (Figure S2). This induction of cGKIβ expression was consistent with the increased cGK-dependent VASP phosphorylation observed after LPS treatment. NOR4 alone had no effect on cGKI but together with LPS induced 4- to 5-fold increases in cGKI levels in DCs compared with LPS treatment only (Figures 6C and S2). Therefore, the observed decrease in cGK-dependent VASP phosphorylation induced by NOR4 was not due to a down-regulation in cGK mRNA expression.
A reduction in cGK activity after NOR4 treatment could also be mediated by a decrease in cGMP levels caused by either the down-regulation of guanylate cyclases, the cGMP-synthesizing enzymes, or by the up-regulation of cGMP-phosphodiesterases, the hydrolyzing enzymes. Similarly to cGK, an NO-induced long-term desensitization has also been described for guanylate cyclases.41 To test if long-term treatment of NOR4 down-regulates cGMP in DCs we examined changes in cGMP levels during treatment of DCs with LPS alone or with NOR4. Within 30 to 60 minutes NOR4 induced an initial increase in cGMP in DCs treated or not with LPS (Figure S3). After this initial peak cGMP levels decreased in DCs treated only with NOR4 but were sustained in DCs treated with both LPS and NOR4 (Figure S3), reaching 20-fold higher levels than DCs treated only with LPS for 24 hours (Figure 6D). Therefore, long-term treatment of DCs with LPS plus NOR4 leads to continuous release of NO and consequently a sustained increase in cGMP levels. NOR4 ability to reduce cGK-dependent VASP phosphorylation induced by LPS cannot be due to a decrease in cGMP levels.
Overall our data suggest that LPS increases both cGK expression and activity in DCs, leading to phosphorylation of VASP, which is in part responsible for preventing DCs from migrating toward CCL19. Long-term NOR4 treatment inhibits the increase in cGK-dependent phosphorylation of VASP, thereby releasing a brake so that DCs can migrate.
Discussion
NO regulates migration of human DCs toward the LN-directing chemokines CCL19 and CXCL12. Whereas LPS-stimulated monocyte-derived DCs do not migrate toward CCL19, NOR4 enables them to migrate (Figure 2B). NOR4 enhanced LPS-induced expression of the CCR7 and CXCR4 receptors; however, enhancement by NO of chemokine receptor expression alone could not account for why DCs were able to migrate. The effects of NO on DC chemokine receptor expression and migration parallel the effects of cAMP-increasing inflammatory stimuli10-12 ; both stimuli couple CCR7 to signal transduction pathways that allow migration, thus sensitizing DCs to migrate toward CCL19.
While chemokine receptor expression can be uncoupled from the ability of DCs to migrate,42,43 the mechanism responsible for this has not been elucidated. Surprisingly, the inhibition of cGK prior to the chemotactic assay enabled DCs to migrate toward CCL19 (Figure 5), suggesting that cGK activity constitutively expressed or induced by LPS might prevent DCs from migrating even though they express chemokine receptors. Consistent with this model, LPS induced DCs to express cGKI (Figure 6C) and sustained cGK activity as measured by phosphorylation of the key cGK target VASP (Figure 6A). VASP expression and regulation upon chemokine stimulation has not been previously reported in DCs. Besides being a useful tool to monitor cGK activity,24 VASP may also provide a possible explanation for the inhibitory role of cGK in DC migration. VASP binds cytoskeletal proteins zyxin and vinculin involved in focal adhesion formation; cGK-dependent VASP phosphorylation results in depletion of these cytoskeletal proteins from focal adhesions, destabilization of motility structures, and inhibition of migration.26,37 The substantial increase in cGK-dependent VASP phosphorylation induced by LPS was retained upon short-term CCR7 stimulation with CCL19. Thus, phosphorylation of VASP correlated with unresponsiveness to CCL19. High cGK activity and subsequent VASP phosphorylation may disrupt focal adhesion formation and DC migration (Figure 7).
VASP may not be the only cGK target in LPS-stimulated DCs. Two actin binding proteins, LIM and LASP-1, are direct substrates for cGK and cAK; upon phosphorylation their affinity for F-actin is reduced, causing their relocalization from membrane extensions to the cytosol and reducing cell migration.44,45 Furthermore, cAK- and cGK-dependent VASP phosphorylation can inhibit integrin αIIbβ3 activation, another important step for cell polarization and migration.46
The presence of NOR4 during DC maturation strongly reduced basal levels of cGK-dependent VASP phosphorylation, and this inhibition was maintained after either CCL19 or CXCL12 stimulation (Figure 6A-B). The absence of high levels of phosphorylated VASP correlated with the ability of DCs to migrate. Thus, NO may sensitize DCs so they can migrate through the inhibition of LPS-induced increases in cGK activity and VASP phosphorylation (Figure 7). cGK-associated signaling pathways are controlled by feedback regulation.41,47 One feedback regulatory mechanism is the suppression of cGKIα expression by long-term exposure to NO-releasing agents.38,39 However, NOR4 did not reduce cGK activity in DCs simply by inhibiting LPS-induced cGK expression, which was rather enhanced by NOR4 (Figure 6C). Also, DCs treated with both LPS and NOR4 had higher cGMP levels than DCs treated with only LPS. Thus, the lower cGK activity in the NOR4 plus LPS-treated cells was not due to decreased production or increased degradation of cGMP, although the possibility of reduced cGMP in cell microcompartments cannot be excluded. After the initial activation of the cGMP/cGK pathway, long-term treatment with NO may induce a negative feedback pathway that reduces cGK-dependent VASP phosphorylation in DCs (Figure 7). It remains to be determined whether this reduction is due to posttranslational regulation, a direct inhibition of cGK activity or another mechanism. Although NO acts mainly through the cGMP pathway, it can also directly modify lipids, nucleic acids, and proteins. For instance, the activity of many transcriptional factors or cysteine proteases is altered by direct nitrosylation from NO.48 We are currently investigating the mechanisms responsible for the reduced cGK activity induced by NOR4 in DCs.
NO promotes LPS-mediated DC migration toward CCL19. LPS alone induces expression of cGK and phosphorylation of VASP, thereby preventing DCs from responding to CCL19 and migrating. NO releases DCs from this inhibition via a feedback inhibition pathway that blocks cGK-dependent phosphorylation of VASP.
NO promotes LPS-mediated DC migration toward CCL19. LPS alone induces expression of cGK and phosphorylation of VASP, thereby preventing DCs from responding to CCL19 and migrating. NO releases DCs from this inhibition via a feedback inhibition pathway that blocks cGK-dependent phosphorylation of VASP.
NO also enhanced the migration of LPS-treated DCs toward CXCL12; consistent with some findings49 but in contrast to others,14 matured DCs were sensitive to CXCL12-dependent migration even in the absence of NO or other signals (Figure 3B). In contrast to CCL19 stimulation, short-term stimulation of LPS-treated DCs with CXCL12 reduced cGK-dependent VASP phosphorylation (Figure 6A-B), consistent with the ability of the DCs to migrate toward CXCL12. One explanation for the differential sensitivity of LPS-treated DCs to migrate toward CCL19 versus CXCL12 may be that CXCL12 alone, unlike CCL19, can directly reduce VASP phosphorylation and release DCs to migrate. The cAK inhibitor myrPKI selectively blocked DC chemotaxis toward CXCL12 but not to CCL19, underscoring another difference between CXCR4 and CCR7 signaling pathways: cAK activation is required for CXCL12-dependent migration but not for CCL19-dependent migration. Ligation of CXCR4 by CXCL12 activates the cAMP/cAK pathway in T cells.50
Cell migration is a complex phenomenon that involves cell polarization and requires a dynamic regulation of receptors, adhesion molecules, cytoskeletal proteins, and intracellular regulatory molecules, with coordinated formation and degradation of gradients of second messengers, like calcium or cAMP.51 These requirements may explain why the NO and cGMP/cGK pathway has been implicated in both positive and negative effects on cell migration. The activation of the cGMP/cGK pathway by NO positively regulates migration, possibly through the induction of chemokine receptor expression41 (Figure 4A). However, we also found that high levels of cGK activity in LPS-stimulated DCs may inhibit migration (Figures 5 and 6A-B). In addition to its role in VASP regulation, the cGK pathway can negatively affect migration in other ways. NO, via cGMP/cGK, inhibits smooth muscle cell migration by either activating a MAPK phosphatase, which inactivates MAPKs,52 or by activating a tyrosine phosphatase that decreases tyrosine phosphorylation of proteins involved in focal adhesion formation and actin polymerization.53,54 cGK reduces myosin light chain (MLC) phosphorylation, an important step for cell polarization and migration, either by phosphorylating and decreasing MLC kinase activity or by activating MLC phosphatase.55,56 Another major target of cGK is IP3 R-associated cGK substrate (IRAG), which when phosphorylated inhibits IP3-induced calcium release from intracellular stores.57 Thus, cGK may block another step in the signaling activated by chemokine receptor engagements, because DC migration toward CCL19 is dependent on CCL19-induced intracellular calcium mobilization.58 Further studies are required to determine the various mechanisms by which NO and the cGMP pathway may regulate DC movement.
This study is the first to define cGK expression and activity in DCs and the important role cGK plays in regulating DC migration toward LN-directing chemokines. An interesting possibility is that a negative feedback mechanism similar to that reported here for NO might be responsible for the observed effect of PGE2 on DC migration. Consistent with this possibility, we found that cAK sensitizes DCs to migrate toward CCL19 as well as cGK. Scandella et al found that PGE2 can induce a marked reduction in the expression of the PGE2 receptors EP2 and EP4 13 as one possible means of feedback control.
NOR4 did not affect the induction of DC maturation by LPS but did affect cytokine production by DCs. NO inhibited IL-12 p70, IL-10, and TNF-α production by mature DCs but did not affect IL-6 release. Using a protein array system to detect the expression of a range of cytokines and chemokines, we did not detect any other major changes by adding NOR4 to LPS-treated DCs (D.G. and E.A.C., unpublished data, November 2004). These results are consistent with previous work showing that inducible nitric oxide synthase (iNOS)–deficient mice make more IL-12 p40 than wild-type mice in response to LPS59 and with the recent observation that NO inhibits LPS-induced expression of IL-12 p35 and IL-12 p40 mRNA in murine macrophages and DCs through the inhibition of NF-κB activation.21 The cGMP analog, 8Br-cGMP, was able to mimic the effect of NOR4, suggesting that the cGMP-dependent pathway is involved in NO-dependent cytokine down-regulation. The cGMP pathway can inhibit NF-κB activation via I-κB stabilization.41
Several studies, including studies with iNOS-deficient mice, have implicated NO in the regulation specific immune responses.18,19,60 NO can inhibit T-cell proliferation, either in a cytokine-dependent or -independent manner.61-63 Mice deficient for iNOS develop significantly stronger Th1 immune responses and, unlike wild-type mice, are highly susceptible to Leishmania major infections.60 This defect has been attributed mainly to the absence of NO acting directly on T cells. However, our data suggest that iNOS deficiency could lead to T-cell skewing because of dysregulation of DCs as well as T cells. Several studies with iNOS-deficient mice and autoimmune models have implicated NO in the control of autoreactive T cells and prevention of the development of autoimmunity.64-70 The effect of NO on DC migration and cytokine production reported here might contribute to the observed protective effects of NO in autoimmune disorders.
Prepublished online as Blood First Edition Paper, October 25, 2005; DOI 10.1182/blood-2005-07-2901.
Supported by National Institutes of Health (NIH) grants AI44257, AI52203, and RR00166.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Joseph Beavo and Valeria Vasta for helpful discussions and Drs Andy Craxton, Grant Hughes, Asa Bengtsson, and Kevin Chen for helpful critical comments.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal