In recent years, mesenchymal stem cells (MSCs) have been shown to inhibit T-lymphocyte proliferation induced by alloantigens or mitogens. However, no substantial information is available regarding their effect on natural killer (NK) cells. Here we show that MSCs sharply inhibit IL-2-induced proliferation of resting NK cells, whereas they only partially affect the proliferation of activated NK cells. In addition, we show that IL-2-activated NK cells (but not freshly isolated NK cells) efficiently lyse autologous and allogeneic MSCs. The activating NK receptors NKp30, NKG2D, and DNAM-1 represented the major receptors responsible for the induction of NK-mediated cytotoxicity against MSCs. Accordingly, MSCs expressed the known ligands for these activating NK receptors—ULBPs, PVR, and Nectin-2. Moreover, NK-mediated lysis was inhibited when IFN-γ-exposed MSCs were used as target cells as a consequence of the up-regulation of HLA class I molecules at the MSC surface. The interaction between NK cells and MSCs resulted not only in the lysis of MSCs but also in cytokine production by NK cells. These results should be taken into account when evaluating the possible use of MSCs in novel therapeutic strategies designed to improve engraftment or to suppress graft-versus-host disease (GVHD) in bone marrow transplantation.
Introduction
Mesenchymal stem cells (MSCs) are known for their characteristic of being multipotent stem cells, capable of forming bone, cartilage, and other mesenchymal tissues.1,2 In particular, in vitro experiments demonstrated that clonal MSCs can differentiate into different lineages including not only osteoblasts, chondrocytes, and adipocytes but also muscle cells,3 cardiomyocytes,4,5 and neural precursors.6 Moreover, MSCs are a component of the bone marrow stroma that have been shown to support hemopoiesis by providing suitable cytokines and growth factors.7,8 More recently, another function has been ascribed to MSCs. Thus, as shown by different groups, MSCs can inhibit T-cell responses induced by mitogens or alloantigens.9-11 The mechanisms underlying such immunosuppressive activity are only in part understood. Recent studies suggest that soluble factors produced by MSCs represent key mediators of MSC-mediated inhibition,9,12,13 though cell contact might also be involved.14-17
Little information is available regarding the cellular interactions between natural killer (NK) cells, major effector cells of innate immunity, and MSCs. NK cells are known to display strong cytolytic activity against tumor- or virus-infected cells.18,19 Their function is regulated by a series of surface receptors transducing inhibitory or activating signals. Inhibitory receptors are represented mainly by killer immunoglobulin-like receptors (KIRs)20 that are specific for allotypic determinants shared by different HLA class I alleles and CD94/NKG2A specific for HLA-E21 (a nonclassic MHC class 1 molecule), whose levels of expression are directly proportional to HLA class I surface density.22 In normal conditions, the expression of classic HLA class I or HLA-E molecules on the surfaces of normal autologous cells prevents activation of NK cells because of the interaction with these inhibitory receptors. The down-regulation of HLA class I expression or even the loss of single HLA class I alleles at the surface of tumor- or virus-infected cells can result in a lack of inhibitory interactions and can lead to NK-cell activation.23 Under these conditions, target cells become susceptible to NK-mediated killing. Different receptors and coreceptors are responsible for NK-cell activation on interaction with target cells.24 The natural cytotoxicity receptors (NCRs) NKp46, NKp30, and NKp44 represent crucial receptors for NK-cell triggering25 and mediate cytotoxic activity and cytokine production. Thus far, the NCR ligands expressed on target cells have not been identified. Other important receptors involved in NK-cell activation are NKG2D26 and DNAM-1,27 the ligands of which are represented by MICA/B and ULBPs for NKG2D28-30 and the poliovirus receptor (PVR) and Nectin-2 for DNAM-1.31 A series of coreceptors capable of supporting NCR-mediated NK-cell triggering has been described. These include 2B4,32 NTBA,33 NKp80,34 and CD59.35 CD48 represents the known ligand of 2B4,36 whereas NTBA mediates homophilic interactions.37
In this study we analyzed the effect of the interaction between MSC and NK cells. We show that MSCs can inhibit the IL-2-induced proliferation of resting, unactivated NK cells, whereas they had only a partial inhibitory effect on proliferating NK cells. More important, activated NK cells could efficiently lyse MSCs. This information may have relevant implications in novel bone marrow (BM) transplantation approaches in which MSCs are infused to optimize engraftment or to suppress graft-versus-host disease (GVHD).38-40
Materials and methods
Isolation and culture of MSCs
After obtaining ethics committee approval and informed consent, human MSCs were obtained from discarded bone tissues from 15 pediatric patients undergoing surgery to correct major scoliosis. Bone marrow cell suspensions were plated at a concentration of 1 × 106 cells/mL in 25-cm2 tissue-culture flasks in Mesencult basal medium supplemented with MSC stimulatory supplements (both from StemCell Technologies, Vancouver, BC, Canada) and incubated at 37°C in a 5% CO2 humidified atmosphere. After 24 hours, nonadherent cells were removed and fresh medium was added. Half the medium volume was replaced twice a week. When the cultures nearly reached confluence, cells were detached by treatment with trypsin/EDTA solution (BioWhittaker, Cambrex, Verviers, Belgium) and replated at 5 × 105 cells per 75-cm2 tissue-culture flask. MSCs were used in the experiments only after 2 to 3 expansion passages to ensure depletion of monocytes/macrophages. In some experiments, MSCs were cultured for 48 hours in the presence of 100 U/mL IFN-γ (PeproTech, London, United Kingdom) before their use.
Isolation and culture of NK cells
NK cells were isolated from the peripheral blood of healthy donors (some were also donors of MSCs) using the RosetteSep method (StemCell Technologies). Purified NK cells were cultured on irradiated feeder cells in the presence of 1.5 ng/mL phytohemagglutinin (Life Technologies, Paisley, Scotland) and 100 U/mL recombinant IL-2 (Proleukin; Chiron, Emeryville, CA) to obtain long-term activated polyclonal or, by limiting dilution, clonal NK cells. Alternatively, NK cells were used in cytotoxicity assays immediately after separation or on short-term activation (20 hours-7 days) with 100 U/mL IL-2 or 2 ng/mL IL-12 (PeproTech).
Monoclonal antibodies and cytofluorimetric analysis
The following monoclonal antibodies (mAbs), produced in our laboratory, were used in this study: JT3A (IgG2a, anti-CD3), c127 (IgG1, anti-CD16), c218 (IgG1, anti-CD56), BAB281 and KL247 (IgG1 and IgM, respectively, anti-NKp46), Z231 and KS38 (IgG1 and IgM, respectively, anti-NKp44), A76 and F252 (IgG1 and IgM, respectively, anti-NKp30), BAT221 (IgG1, anti-NKG2D), GN18 and F5 (IgG3 and IgM, respectively, anti-DNAM-1), MA127 (IgG1, anti-NTBA), GL183 (IgG1, anti-KIR2DL2/3/S2, anti-CD158b1/b2/j), EB6b (IgG1, anti-KIR2DL1/S1, anti-CD158a/h), Z27 (IgG1, anti-KIR3DL1, anti-CD158e), AZ158 (IgG2a, anti-KIR3DL1/2, anti-CD158e/k), Z270 and Z199 (IgG1 and IgG2b, respectively, anti-NKG2A), Y9 (IgM, anti-CD94), L95 (IgG1, anti-PVR), L14 (IgG2a, anti-Nectin-2), BAM195 (IgG1, anti-MICA), and A6-136 (IgM, anti-HLA class I).20,24,30,31,41
Anti-CD105-PE (N1-3A1, IgG1), anti-CD29 (4B7R, IgG1), anti-CD106 (1.G11B1, IgG1), and anti-CD166 (3A6, IgG1) were purchased from Ancell (Bayport, MN). M295 (IgG1, anti-ULBP1), M310 (IgG1, anti-ULBP2), M551 (IgG1, anti-ULBP3), and M475 (IgG1, anti-ULBP4) were kindly provided by Amgen (Seattle, WA). Anti-CD34 (8G12, IgG1), anti-CD45 and anti-CD45-PerCP (2D1, IgG1), and anti-CD14 (MΦP9, IgG2b) were purchased from Becton Dickinson (Mountain View, CA). Anti-CD56-PC5 (N901, IgG1), anti-CD48 (J4-57, IgG1), and anti-CD54-PE (84H10, IgG1) were provided by Immunotech-Coulter (Marseille, France). To evaluate the surface expression of HLA class I and HLA class II molecules, W632 and D1-12 (kind gift from R. Accolla) mAbs were used. The anti-CD56-PE (B159, IgG1) and, for intracellular cytokine staining, the anti-huIFN-γ-PE (B27, IgG1) mAbs were from BD Biosciences PharMingen (San Diego, CA).
To analyze the surface markers of MSCs, cell samples were first stained with the appropriate mAbs at 4°C for 30 minutes, washed, and incubated with PE-conjugated AffiniPure F(ab′)2 goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Then, after washing, cells were resuspended in RPMI 1640 medium supplemented with 5% FCS and analyzed on a FACScalibur by using the Cell Quest program (both from Becton Dickinson). To compare the surface densities of various molecules among different MSC populations, we calculated the mean ratio fluorescence intensity (MRFI)—the ratio between the mean fluorescence intensity (MFI) of cells stained with the selected mAb and that of cells stained with the second reagent only (negative control).
Long-term activated polyclonal and clonal NK cells were analyzed for the expression of activating and inhibitory receptors by single- and double-fluorescence analyses. Briefly, cells were incubated with appropriate mAbs followed by FITC- or PE-conjugated isotype-specific second reagents (Southern Biotechnology Associates, Birmingham, AL).
Proliferation assays by CFSE dilution and 3H-thymidine uptake
IL-2-induced proliferation of resting NK cells was evaluated by the 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution method. Briefly, freshly isolated NK cells were labeled with 5 μM CFSE (Molecular Probes, Eugene, OR) in serum-free RPMI 1640 for 7 minutes at 37°C. Labeled cells were washed twice with RPMI 1640 containing 10% FCS and then cultured in the presence of 100 U/mL IL-2 with or without irradiated MSCs (at different NK/MSC ratios ranging from 1:1 to 8:1). In some experiments, resting NK cells were induced to proliferate in the presence of 100 ng/mL IL-15 (PeproTech). Short-term activated NK cells were harvested at day 7 or 10 of culture and were stained with anti-CD45-PerCP and anti-CD56-PE mAbs. NK-cell proliferation was evaluated as CFSE fluorescence by triple-fluorescence cytofluorimetric analysis of CD45+CD56+ lymphocytes. Proliferation of long-term activated NK cells was evaluated by 3H-thymidine uptake. After culture for 3 or 7 days in the presence of IL-2 (100 U/mL) with or without irradiated MSCs (at different NK/MSC ratios ranging from 1:1 to 16:1), cells were pulsed for 18 hours with 3H-thymidine at 1 μCi (0.037 MBq)/well (Amersham Biosciences, Buckinghamshire, United Kingdom) and then harvested, and 3H-thymidine incorporation was measured using the Plate Chameleon Multilabel Counter (Hidex, Turku, Finland).
Cytolytic assays
Polyclonal (either short-term or long-term activated) and clonal NK cells were tested for cytolytic activity in a 4-hour chromium 51 (51Cr) release assay, as previously described.41 Autologous or allogeneic MSCs were used as targets. Briefly, MSCs were detached with trypsin/EDTA (ethylenedi-aminetetraacetic acid) solution, labeled with 100 μg 51Cr/106 cells, and plated at 5000 cells/well in 96-well V-bottom microplates. To test the lytic potential of NK cells against MSCs, effectors were plated at different effector-to-target (E/T) ratios. For mAb-mediated blocking experiments, NK cells were preincubated with mAbs specific to the various activating or inhibitory receptors (of IgM isotype, if available) at the concentration of 10 μg/mL and, after washing, were used in the cytolytic assay at a 10:1 or 5:1 E/T ratio. For masking of HLA class I molecules, MSCs were preincubated with saturating amounts of the anti-HLA class I A6136 mAb.
To characterize the signaling pattern of activating and inhibitory receptors of polyclonal and clonal NK cells, redirected-killing assays were performed. To this purpose, the murine mastocytoma cell line P815 was used as target in the presence of mAbs of IgG isotype at the concentration of 0.5 μg/mL.
Cytokine production
To evaluate IFN-γ production by NK cells after interaction with MSCs, coculture experiments were performed. Polyclonal activated NK cells were mixed with or without target MSCs in medium with 100 U/mL IL-2 at an E/T ratio of 8:1 in V-bottom, 96-well plates. In some experiments, MSCs were used after 48-hour exposure to IFN-γ. To inhibit cytokine secretion, monensin-containing GolgiStop (Becton Dickinson) was added at the beginning of cocultures. After 5 hours, cells were harvested, and surface and intracellular stainings were performed. Briefly, cells were first labeled with anti-CD56-PC5 mAb for 20 minutes at 4°C, then washed, fixed, and permeabilized with Cytofix/Cytoperm solution (Becton Dickinson). Afterward, for intracellular cytokine staining, cells were incubated with anti-IFN-γ-PE mAb for 30 minutes at 4°C, then washed and resuspended in PBS 2% FCS for cytofluorimetric analysis.
Results
MSCs prevent the IL-2-induced proliferation of resting NK cells but have only a partial inhibitory effect on proliferating NK cells
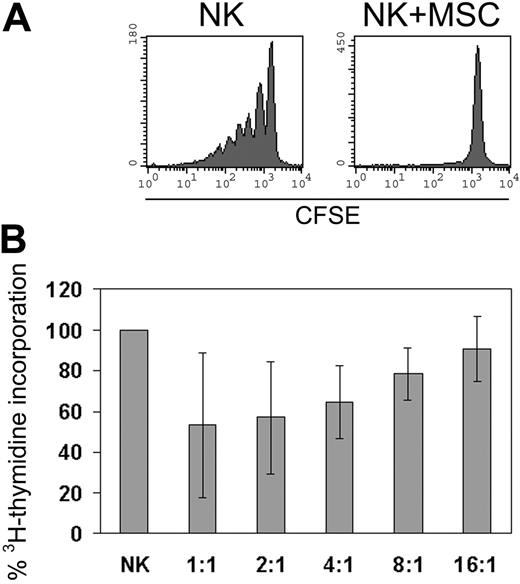
MSCs have been shown to sharply suppress T-cell proliferation of resting and activated T cells.9,42 To assess whether MSCs could exert a similar inhibitory effect on NK cells, we cultured allogeneic MSCs with resting, unactivated NK cells in the presence of exogenous IL-2 (100 U/mL) or with proliferating NK cells that had been cultured in IL-2 for more than 7 days. As shown in Figure 1A, MSCs prevented the proliferation of resting NK cells. It is of note that the inhibition of NK-cell proliferation occurred at all NK/MSC ratios tested (ranging from 1:1 to 8:1; data not shown). On the other hand, as shown in Figure 1B, only partial inhibition of proliferation of activated NK cells could be detected, and the extent of the inhibitory effect was dependent on the NK/MSC ratio. We further evaluated whether MSCs could also inhibit NK-cell proliferation induced by IL-15. Although not shown, MSCs had a similar suppressive effect on fresh NK cells when cultured in the presence of 100 ng/mL IL-15.
MSC-induced inhibition of NK-cell proliferation. NK cells were cultured alone or with allogeneic irradiated MSCs in the presence of 100 U/mL IL-2. (A) Results of 1 of 8 representative experiments in which the proliferation of resting NK cells was evaluated using the CFSE dilution method. CFSE fluorescence of NK cells (identified as CD45+CD56+ lymphocytes) was analyzed after 7 days of culture alone or with MSCs (NK/MSC ratio, 8:1). (B) Proliferative response of IL-2-cultured proliferating NK cells analyzed at day 7 of culture in the absence or presence of MSCs. Data are presented as percentage of 3H-thymidine incorporation by NK cells cultured in the presence of MSCs (at different NK/MSC ratios) with respect to NK cells cultured alone (100%). Error bars represent mean ± SD of 6 independent experiments.
MSC-induced inhibition of NK-cell proliferation. NK cells were cultured alone or with allogeneic irradiated MSCs in the presence of 100 U/mL IL-2. (A) Results of 1 of 8 representative experiments in which the proliferation of resting NK cells was evaluated using the CFSE dilution method. CFSE fluorescence of NK cells (identified as CD45+CD56+ lymphocytes) was analyzed after 7 days of culture alone or with MSCs (NK/MSC ratio, 8:1). (B) Proliferative response of IL-2-cultured proliferating NK cells analyzed at day 7 of culture in the absence or presence of MSCs. Data are presented as percentage of 3H-thymidine incorporation by NK cells cultured in the presence of MSCs (at different NK/MSC ratios) with respect to NK cells cultured alone (100%). Error bars represent mean ± SD of 6 independent experiments.
Surface expression on MSCs of ligands recognized by different activating NK receptors
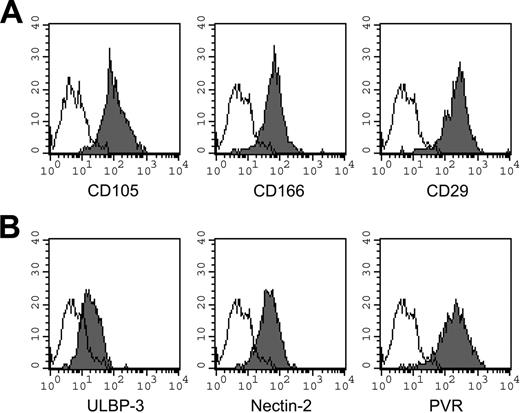
To analyze possible interactions between NK cells and MSCs, we first assessed MSCs for the expression of ligands recognized by activating NK receptors. We performed cytofluorimetric analysis using mAbs specific for known ligands of activating receptors and checked, in parallel, the presence of typical markers of MSCs. Table 1 shows the level of expression of the ligands of DNAM-1 and NKG2D triggering receptors and of HLA class I molecules in the 15 MSC populations analyzed in this study. Remarkably, MSCs expressed PVR and Nectin-2 (both ligands of DNAM-1) as well as ULBPs and MICA (both ligands of NKG2D). In particular, among the ligands of NKG2D, ULBP3 was expressed in all 15 MSC populations analyzed. Moreover, in no instance were CD48 (ligand of 2B4) and NTBA (mediating homophilic interactions) molecules expressed by MSCs. In agreement with previous reports,1,2 MSCs did not express CD34, CD45, or HLA-DR (data not shown), whereas they did express low/intermediate levels of HLA class I molecules. Figure 2 shows the cytofluorimetric analysis of MSCs derived from a representative donor (donor 2). Figure 2A shows the expression profiles of CD105, CD166, and CD29 (typical MSC markers). Figure 2B shows the surface expression of ULBP3, Nectin-2, and PVR molecules.
Surface expression of the ligands of DNAM-1 and NKG2D activating receptors and of HLA class I molecules in different MSC cell populations
. | DNAM-1 ligands . | . | NKG2D ligands . | . | |
|---|---|---|---|---|---|
| Donor . | Nectin-2 MRFI* . | PVR MRFI . | MICA/B and ULBPs†(MRFI) . | HLA class I MRFI . | |
| 1 | 8 | 24 | ULBP3 (3) | 69 | |
| 2 | 7 | 16 | ULBP3 (3) | 31 | |
| 3 | 3 | 8 | ULBP3 (2) | 10 | |
| 4 | 9 | 21 | ULBP3 (2) | 50 | |
| 5 | 7 | 35 | ULBP1 (7), 2 (2), 3 (2), 4 (3) | 47 | |
| 6 | 5 | 16 | ULBP1 (2), 2 (3), 3 (4), 4 (3) | 40 | |
| 7 | 12 | 33 | ULBP2 (3), 3 (4) | 61 | |
| 8 | 7 | 18 | MICA (2), ULBP1 (2), 2 (2), 3 (2) | 25 | |
| 9 | 4 | 6 | MICA (2), ULBP2 (2), 3 (2) | 8 | |
| 10 | 7 | 17 | MICA (2), ULBP1 (2), 3 (2) | 17 | |
| 11 | 6 | 10 | ULBP1 (2), 3 (2) | 20 | |
| 12 | 4 | 6 | ULBP3 (2) | 14 | |
| 13 | 8 | 15 | MICA (2), ULBP3 (3) | 30 | |
| 14 | 6 | 12 | ULBP3 (2) | 14 | |
| 15 | 6 | 10 | MICA (2), ULBP3 (3) | 38 | |
. | DNAM-1 ligands . | . | NKG2D ligands . | . | |
|---|---|---|---|---|---|
| Donor . | Nectin-2 MRFI* . | PVR MRFI . | MICA/B and ULBPs†(MRFI) . | HLA class I MRFI . | |
| 1 | 8 | 24 | ULBP3 (3) | 69 | |
| 2 | 7 | 16 | ULBP3 (3) | 31 | |
| 3 | 3 | 8 | ULBP3 (2) | 10 | |
| 4 | 9 | 21 | ULBP3 (2) | 50 | |
| 5 | 7 | 35 | ULBP1 (7), 2 (2), 3 (2), 4 (3) | 47 | |
| 6 | 5 | 16 | ULBP1 (2), 2 (3), 3 (4), 4 (3) | 40 | |
| 7 | 12 | 33 | ULBP2 (3), 3 (4) | 61 | |
| 8 | 7 | 18 | MICA (2), ULBP1 (2), 2 (2), 3 (2) | 25 | |
| 9 | 4 | 6 | MICA (2), ULBP2 (2), 3 (2) | 8 | |
| 10 | 7 | 17 | MICA (2), ULBP1 (2), 3 (2) | 17 | |
| 11 | 6 | 10 | ULBP1 (2), 3 (2) | 20 | |
| 12 | 4 | 6 | ULBP3 (2) | 14 | |
| 13 | 8 | 15 | MICA (2), ULBP3 (3) | 30 | |
| 14 | 6 | 12 | ULBP3 (2) | 14 | |
| 15 | 6 | 10 | MICA (2), ULBP3 (3) | 38 | |
Concerning MRFI, see “Materials and methods”
Only the expressed molecules among MICA and ULBP1-4 are indicated
IL-2-activated, but not resting, NK cells lyse MSCs
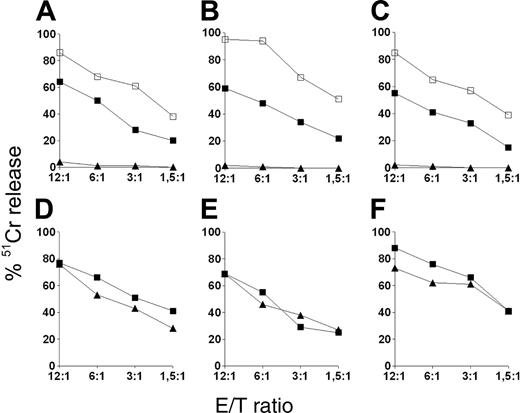
The finding that MSCs expressed ligands for activating NK receptors, together with the low expression of HLA class I molecules, suggested the possibility that MSCs could be susceptible to NK cell-mediated lysis. To test this hypothesis, we analyzed freshly isolated NK cells and NK-cell populations that had been exposed to IL-2 for a short (20-hour) or a long (7-day) interval for their ability to kill allogeneic MSC target cells. In agreement with a previous report,43 freshly isolated NK cells did not lyse MSCs, even when used at high E/T ratios (Figure 3A-C). However, the same NK cells, when exposed to IL-2 for 20 hours only, displayed strong cytolytic activity. For example, approximately 50% specific lysis was achieved at a 6:1 NK/MSC ratio. NK cells cultured in IL-2 for 7 days displayed even higher cytolytic activity. Although not shown, short-term culture of freshly isolated NK cells in the presence of IL-12 did not induce any significant cytolysis of MSCs, whereas low cytolytic activity could be detected only after 72 hours of culture. Having demonstrated the susceptibility of MSCs to lysis of allogeneic effector NK cells, we asked whether lysis also occurred in an autologous setting. To this aim, we performed a series of cytotoxicity experiments in which different MSC populations were used as target and autologous or allogeneic NK cells were used as effector. It is of note that these experiments were performed using long-term IL-2-activated NK cells because of the low numbers of NK cells isolated from the blood samples of pediatric donors. As shown in Figure 3D-F, MSCs were susceptible to NK-mediated lysis independently of whether autologous or allogeneic NK cells were used.
Informative surface markers expressed by MSCs. Phenotypic analysis of MSCs derived from a representative donor (donor 2). (A) Expression of typical markers of MSCs. (B) Expression profiles of known ligands for activating NK receptors. Results are expressed as MFI in arbitrary units (x-axis) compared with number of cells (y-axis). Open histograms represent controls using the second reagent only. Similar data were obtained in 15 different donors.
Informative surface markers expressed by MSCs. Phenotypic analysis of MSCs derived from a representative donor (donor 2). (A) Expression of typical markers of MSCs. (B) Expression profiles of known ligands for activating NK receptors. Results are expressed as MFI in arbitrary units (x-axis) compared with number of cells (y-axis). Open histograms represent controls using the second reagent only. Similar data were obtained in 15 different donors.
Analysis of the activating or inhibitory molecular interactions involved in the lysis of autologous and allogeneic MSCs
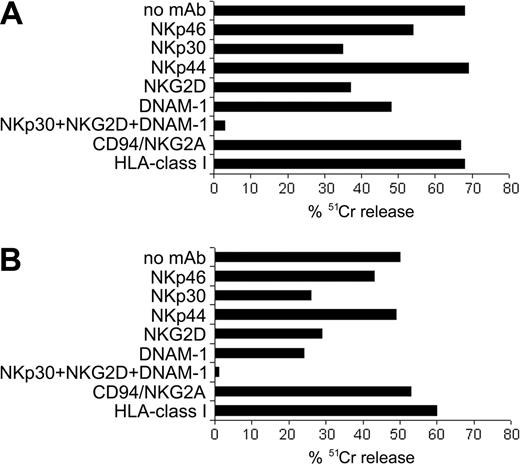
To define which receptor-ligand interactions were involved in the NK-mediated killing of MSCs, we used the mAb-mediated masking strategy in cytotoxicity assays. Figure 4 shows the results of 2 representative experiments. In Figure 4A, NK cells derived from an allogeneic donor were used as effector cells against 51Cr-labeled MSCs. mAb-mediated masking of NKp30, NKG2D, and DNAM-1 resulted in the inhibition of lysis. On the other hand, blocking of NKp46 resulted in low inhibition, whereas NKp44 did not appear to exert any substantial role. Consistent results were obtained in 10 independent experiments using different MSCs and different allogeneic NK-cell populations. Figure 4B shows a cytotoxicity assay in which NK cells were tested against autologous MSCs. In addition, in this autologous setting, the major activating NK receptors involved were NKp30, NKG2D, and DNAM-1. Similar results were obtained in 5 independent experiments using autologous combinations. Notably, in allogeneic and autologous NK/MSC combinations, the simultaneous masking of these receptors virtually abrogated cell lysis. Remarkably, autologous MSCs are as susceptible to lysis as allogeneic MSCs. These data suggest that interactions involving HLA class I-specific inhibitory receptors, possibly occurring between NK cells and MSCs, were not sufficient to protect MSCs from lysis. In fact, mAb-mediated masking of HLA class I molecules on target cells did not result in any significant increase of lysis (Figure 4). This is in agreement with the low levels of HLA class I molecules on the surface of MSCs. In addition, mAb-mediated blocking of the HLA-E-specific inhibitory receptor CD94/NKG2A did not result in increased cytotoxic activity. Given that the levels of surface HLA-E usually parallel the HLA class I surface density, our results suggest that MSCs are characterized by low or absent expression of HLA-E molecules.
NK-mediated lysis of MSCs. Results of cytotoxicity assays in which NK cells were used as effector cells against 51Cr-labeled MSCs at different E/T ratios. (A-C) NK cells derived from 3 different donors were tested as freshly isolated cells (▴) or after 20-hour (▪) or 7-day (□) culture in 100 U/mL IL-2 for cytolytic activity against 3 different allogeneic MSC populations. (D-F) Results of 3 independent experiments in which different MSC populations were used as targets while either autologous (squares) or allogeneic (triangles) IL-2-activated NK cells were used as effectors. Data are expressed as percentage of lysis.
NK-mediated lysis of MSCs. Results of cytotoxicity assays in which NK cells were used as effector cells against 51Cr-labeled MSCs at different E/T ratios. (A-C) NK cells derived from 3 different donors were tested as freshly isolated cells (▴) or after 20-hour (▪) or 7-day (□) culture in 100 U/mL IL-2 for cytolytic activity against 3 different allogeneic MSC populations. (D-F) Results of 3 independent experiments in which different MSC populations were used as targets while either autologous (squares) or allogeneic (triangles) IL-2-activated NK cells were used as effectors. Data are expressed as percentage of lysis.
Decreased susceptibility of MSCs to NK-cell-mediated lysis after exposure to IFN-γ: role of HLA class I molecules
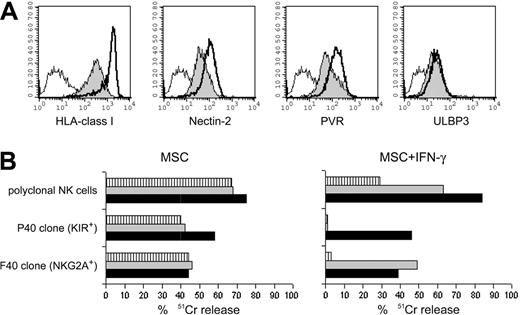
During infection or, more generally, during inflammatory responses, cells are exposed to proinflammatory cytokines, such as IFN-γ. IFN-γ has been shown to up-regulate the surface density of HLA class I and HLA class II molecules on MSCs.44,45 To test whether IFN-γ could modify the surface expression of ligands recognized by inhibitory or activating NK receptors (with a possible effect on the susceptibility of MSCs to NK-cell-mediated lysis), we cultured 5 different MSC populations with IFN-γ (100 U/mL). After 48 hours, IFN-γ-treated or untreated MSCs were analyzed by cytofluorometry to assess the expression of informative surface molecules. In agreement with previous data, IFN-γ induced increases in the levels of HLA class I (Figure 5A) and HLA class II (not shown) molecules in all MSC populations tested. Moreover, the expression of ICAM-1 was augmented in IFN-γ-treated MSCs (not shown). On the other hand, IFN-γ treatment did not significantly modify the expression of adhesion molecules (with the exception of ICAM-1). Interestingly, PVR and Nectin-2—the ligands of DNAM-1—but not those of NKG2D (such as ULBP3), were up-regulated (Figure 5A). We next tested the susceptibility of IFN-γ-treated MSCs to NK cell-mediated cytotoxic activity. MSCs were found to be less susceptible to lysis. Figure 5B shows a representative cytotoxicity assay in which NK cells were used against autologous MSCs that had been cultured in the presence of IFN-γ or in medium alone. Polyclonal NK cells efficiently lysed untreated MSCs at a 10:1 E/T ratio. Monoclonal antibody-mediated masking of HLA class I molecules or CD94/NKG2A receptor could not induce increases in lysis. On the other hand, the same NK cells were poorly cytolytic against IFN-γ-treated MSCs. In this case, mAb-mediated blocking of HLA class I or CD94/NKG2A resulted in sharp increases of cytotoxicity. These results suggest that not only classic HLA class I but also HLA-E molecules were up-regulated by IFN-γ in MSCs. This was clearly shown by the analysis of NK-cell clones. Indeed, as shown in Figure 5, KIR+ (KIR2DL2/3+) CD94/NKG2A- NK-cell clone P40 and KIR- CD94/NKG2A+ NK-cell clone F40 efficiently lysed untreated, but not IFN-γ-treated, autologous MSCs. In the P40 clone, lysis could be restored by mAb-mediated masking of HLA class I molecules. On the other hand, mAb-mediated masking of HLA class I molecules or the HLA-E-specific CD94/NKG2A receptor restored the cytolytic activity of the F40 NK-cell clone. Taken together, these data indicate that in IFN-γ-treated MSCs, the up-regulation of ligands recognized by triggering NK receptors is offset by the simultaneous up-regulation of HLA class I molecules, resulting in the inhibition of NK-cell function on interaction with inhibitory receptors.
Involvement of different activating and inhibitory NK receptors in the lysis of MSCs. Results of 2 mAb-mediated blocking experiments. 51Cr-labeled MSCs were used as target cells, and activated polyclonal NK cells were used as effectors. NK cells were preincubated with saturating amounts of mAb to the indicated receptors, washed, and added to the cytolytic assay. To mask HLA class I molecules, MSCs were preincubated with saturating amounts of anti-HLA class I A6136 mAb before addition to the assay. (A) Cytolytic assay in which effector NK cells were tested against allogeneic MSCs. (B) Experiment performed using autologous target MSCs. Data are expressed as percentage of lysis. The E/T ratio used was 10:1.
Involvement of different activating and inhibitory NK receptors in the lysis of MSCs. Results of 2 mAb-mediated blocking experiments. 51Cr-labeled MSCs were used as target cells, and activated polyclonal NK cells were used as effectors. NK cells were preincubated with saturating amounts of mAb to the indicated receptors, washed, and added to the cytolytic assay. To mask HLA class I molecules, MSCs were preincubated with saturating amounts of anti-HLA class I A6136 mAb before addition to the assay. (A) Cytolytic assay in which effector NK cells were tested against allogeneic MSCs. (B) Experiment performed using autologous target MSCs. Data are expressed as percentage of lysis. The E/T ratio used was 10:1.
Effect of IFN-γ-induced up-regulation of HLA class I expression on the NK-mediated lysis of autologous MSCs. (A) Analysis of the surface density of HLA class I, Nectin-2, PVR, and ULBP3 molecules on IFN-γ-treated and untreated MSCs derived from donor 15. Filled profiles represent the antigen expression on untreated MSCs, whereas thick lines refer to MSCs exposed to 100 U/mL IFN-γ for 48 hours. Open profiles represent the negative control. (B) Percentage of lysis of untreated or IFN-γ-treated MSCs. Source of autologous effector cells were polyclonal NK cells or the KIR2DL2/3+ NKG2A- F40 clone or the KIR-- NKG2A+ P40 clone. The E/T ratio was 6:1 for polyclonal NK cells and 10:1 for the NK-cell clones. Cytolytic assays were performed in the absence of mAbs ( ) or in the presence of anti-CD94/NKG2A (
) or in the presence of anti-CD94/NKG2A ( ) or anti-HLA class I (▪) mAb. Data are representative of experiments performed by using MSC and autologous polyclonal or clonal NK cells derived from 5 different donors.
) or anti-HLA class I (▪) mAb. Data are representative of experiments performed by using MSC and autologous polyclonal or clonal NK cells derived from 5 different donors.
Effect of IFN-γ-induced up-regulation of HLA class I expression on the NK-mediated lysis of autologous MSCs. (A) Analysis of the surface density of HLA class I, Nectin-2, PVR, and ULBP3 molecules on IFN-γ-treated and untreated MSCs derived from donor 15. Filled profiles represent the antigen expression on untreated MSCs, whereas thick lines refer to MSCs exposed to 100 U/mL IFN-γ for 48 hours. Open profiles represent the negative control. (B) Percentage of lysis of untreated or IFN-γ-treated MSCs. Source of autologous effector cells were polyclonal NK cells or the KIR2DL2/3+ NKG2A- F40 clone or the KIR-- NKG2A+ P40 clone. The E/T ratio was 6:1 for polyclonal NK cells and 10:1 for the NK-cell clones. Cytolytic assays were performed in the absence of mAbs ( ) or in the presence of anti-CD94/NKG2A (
) or in the presence of anti-CD94/NKG2A ( ) or anti-HLA class I (▪) mAb. Data are representative of experiments performed by using MSC and autologous polyclonal or clonal NK cells derived from 5 different donors.
) or anti-HLA class I (▪) mAb. Data are representative of experiments performed by using MSC and autologous polyclonal or clonal NK cells derived from 5 different donors.
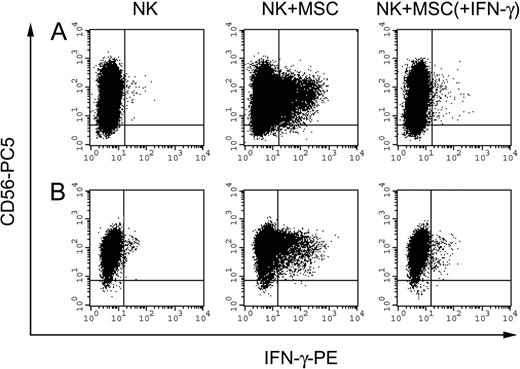
IFN-γ production by NK cells on interaction with MSCs. IFN-γ production by polyclonal activated NK cells after interaction with MSCs. Panels A and B represent 2 independent experiments performed in allogeneic and autologous settings, respectively. NK cells were incubated alone or in the presence of MSCs at an E/T ratio of 8:1. MSCs were untreated or treated with 100 U/mL IFN-γ (identified as MSC + IFN-γ) for 48 hours before their use in the experiment. After 5 hours of culture in the presence of 100 U/mL IL-2 and monensin-containing GolgiStop, cells were collected, and double staining was performed. To detect the frequency of IFN-γ-positive cells, NK cells were stained with anti-CD56-PC5 and anti-IFN-γ-PE mAbs and were analyzed by flow cytometry. Events (5 × 104) were acquired and analyzed by gating on low FSC, low SSC cells.
IFN-γ production by NK cells on interaction with MSCs. IFN-γ production by polyclonal activated NK cells after interaction with MSCs. Panels A and B represent 2 independent experiments performed in allogeneic and autologous settings, respectively. NK cells were incubated alone or in the presence of MSCs at an E/T ratio of 8:1. MSCs were untreated or treated with 100 U/mL IFN-γ (identified as MSC + IFN-γ) for 48 hours before their use in the experiment. After 5 hours of culture in the presence of 100 U/mL IL-2 and monensin-containing GolgiStop, cells were collected, and double staining was performed. To detect the frequency of IFN-γ-positive cells, NK cells were stained with anti-CD56-PC5 and anti-IFN-γ-PE mAbs and were analyzed by flow cytometry. Events (5 × 104) were acquired and analyzed by gating on low FSC, low SSC cells.
Cytokine production by NK cells on interaction with MSCs
To analyze whether NK cells could produce IFN-γ on interaction with MSCs, NK cells were incubated with autologous or allogeneic MSCs at different NK/MSC ratios in the presence of the monensin-containing GolgiStop. After 5 hours, cells were harvested, fixed, permeabilized, and stained with an anti-human IFN-γ mAb. Figure 6 shows 2 representative experiments (of 6 performed, 2 in autologous and 4 in allogeneic combinations). Control NK cells cultured alone were negative for intracellular staining (left dot plots), whereas NK cells incubated with allogeneic (Figure 6A) or autologous (Figure 6B) MSCs were positive for IFN-γ production (central dot plots). These results indicate that NK cells can produce IFN-γ on interaction with MSCs. Moreover, no IFN-γ production was detectable when IFN-γ-treated MSCs were used as stimulators (right dot plots), thus indicating that up-regulation of HLA class I molecules on the surfaces of MSCs can inhibit not only NK-mediated cytolytic activity but also cytokine production.
Discussion
Our data provide information on the result of the interaction between NK cells and MSCs. We show that MSCs are susceptible to NK-mediated lysis and that different NK receptor-ligand interactions contribute to NK-cell activation and MSC lysis. In addition, proliferative responses of resting NK cells to IL-2 or IL-15 were blocked even by low numbers of MSCs. In the case of activated NK cells, only a partial decrease in proliferative capacity could be detected. The mechanism(s) by which MSCs would inhibit NK-cell proliferation has not yet been identified. It is conceivable that inhibition may reflect the production of soluble factors, as has been shown for MSC-T-cell interaction. The role of TGF-β, IL-10, PGE2, and IDO are being investigated in our laboratory. A previous study reported that MSCs were resistant to allospecific CTLs capable of lysing PHA-blasts from the same MSC donor.43 This finding is compatible with the low surface expression of HLA class I molecules. On the other hand, the surface expression of low levels of HLA class I molecules favors the NK-mediated lysis of MSCs, as described in the present study. Moreover, MSCs expressed different ligands recognized by activating NK receptors, including PVR and Nectin-2 (DNAM-1 ligands) and ULBPs (NKG2D ligands). Accordingly, mAb-mediated blocking of NKG2D and DNAM-1 resulted in the partial inhibition of lysis. In addition, receptor-blocking experiments using specific mAbs revealed that lysis of MSCs was also dependent on NKp30 and NKp46, though the cellular ligands of these receptors have not been identified so far. Remarkably, NK cells could lyse not only allogeneic but also autologous MSCs. This finding is reminiscent of previous data regarding the susceptibility of immature DC (iDC) to lysis by autologous NK cells.46 NK-mediated lysis of iDC is thought to represent a mechanism of quality control, allowing positive selection of those DCs undergoing full maturation and thus capable of promoting optimal T-cell priming.47,48 In the case of iDC, however, NK-cell activation involved primarily the NKp30 receptor, whereas the lysis of MSCs is mediated by different receptor-ligand interactions. The fact that NK cells can lyse autologous MSCs may prompt one to ask why MSCs are not killed by NK cells in vivo. This may reflect differences between MSCs cultured in vitro and those existing in vivo. The latter represent an infrequent cell type that could perhaps be localized in niches, thus escaping the NK-mediated attack, or could express higher levels of HLA class I molecules, or lack ligands for activating receptors. This would render MSCs resistant to NK-mediated killing. Moreover, in vivo NK cells might not reach, under normal conditions, an activation state sufficient for killing MSCs. In this context, resting, nonactivated NK cells failed to kill MSCs in vitro. Whatever the explanation, cytolytic interactions between NK cells and MSCs might occur with BM transplantation in which cytokines capable of inducing NK-cell activation can be released. Indeed, our present data clearly show that even short-term exposure to IL-2 can induce strong NK cytotoxicity against MSCs. Thus, our data suggest that, in an inflammatory environment, NK cells may become potentially capable of lysing MSCs. Notably, MSCs are the precursors of stromal BM cells and are thought to favor hematopoietic-cell engraftment while preventing GVHD.49 Accordingly, MSCs, obtained under culture conditions similar to that of the present study, have been used in experimental protocols together with CD34+ hematopoietic-cell precursors.39,40 However, under these conditions, NK cells generated from donor CD34+ cells beyond 2 to 3 weeks after transplantation should not cause any relevant damage to MSCs because, during the same time interval, the latter might have undergone differentiation to BM stromal cells and other tissues. The adoptive transfer of NK cells has been proposed in the therapy of acute myeloid leukemias in allogeneic bone marrow transplantation to eradicate leukemic cells.50-52 The adoptive infusion of activated NK cells could potentially kill MSCs if they are infused shortly before or simultaneously with NK cells. Note, however, that this effect could be, at least in part, counteracted by the effect of IFN-γ. Indeed this cytokine, which is released by NK cells and other cell types, induced the up-regulation of HLA class I molecules in MSCs, thus rendering these cells resistant to NK-mediated lysis. It would be of interest to analyze the final outcome of the in vivo interactions between NK cells and MSCs in suitable murine models, in experimental settings reproducing conditions that occur in BM transplantation-associated adoptive immunotherapy involving donor NK cells and MSCs.
In conclusion, our results provide relevant information on the possible effect of NK cells on MSCs. Whether these interactions are relevant in physiologic conditions remains to be determined; however, our data offer an interesting clue regarding possible interferences occurring when the two cell types are used in combination in approaches of adoptive immunotherapy.
Prepublished online as Blood First Edition Paper, October 20, 2005; DOI 10.1182/blood-2005-07-2775.
Supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), Istituto Superiore di Sanità (ISS), Ministero della Salute, Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), Fondazione Compagnia di San Paolo, Turin, Italy; European Union FP6, LSHB-CT-2004-503319-Allostem (the European Commission is not liable for any use that may be made of the information contained); and Fondo per gli Investimenti della Ricerca di Base (FIRB)-MIUR progetto cod.RBNE017B4C.
G.M.S. designed and performed the research and wrote the paper; A.C. performed research; S.B. provided selected samples; M.C.M. provided financial support and analyzed the data; and L.M. designed the research, analyzed the data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal