Abstract
Transplantation-associated stress can compromise the hematopoietic potential of hematopoietic stem cells (HSCs). As a consequence, HSCs may undergo “exhaustion” in serial transplant recipients, for which the cellular and molecular bases are not well understood. Hematopoietic exhaustion appears to be accelerated in the absence of p21Cip1/Waf1 (p21), a cyclin-dependent kinase inhibitor (CKI) in irradiated hosts. Our recent study demonstrated that unlike loss of p21, deletion of p18INK4C (p18), a distinct CKI, results in improved long-term engraftment, largely because of increased self-renewing divisions of HSCs in vivo. We show here that HSCs deficient in p18 sustained their competitiveness to wild-type HSCs from unmanipulated young mice, and retained multilineage differentiation potential after multiple rounds of serial bone marrow transfer over a period of more than 3 years. Further, p18 absence significantly decelerated hematopoietic exhaustion caused by p21 deficiency. Such an effect was shown to occur at the stem cell level, likely by a counteracting mechanism against the cellular senescence outcome. Our current study provides new insights into the distinct impacts of these cell-cycle regulators on HSC exhaustion and possibly HSC aging as well under proliferative stress, thereby offering potential pharmacologic targets for sustaining the durability of stressed HSCs in transplantation or elderly patients.
Introduction
Hematopoietic stem cells (HSCs) are the most important element for establishing the long-term engraftment of hematopoietic transplants in recipients. Thus, the sustained self-renewal potential of donor HSCs is critical for maintaining the long-term durability of the graft. While HSCs are thought to be capable of self-regeneration in vivo over a lifetime without an apparent limit under homeostatic conditions,1,2 it is well known that the repopulating ability of HSCs can be significantly compromised in transplant recipients.3-8 Studies by several laboratories have demonstrated that the functional HSC units reach only 4% to 10% of normal levels after each transplantation4,7-10 and as a consequence, serial bone marrow transfer can only sustain hematopoiesis for 4 to 6 rounds in irradiated mouse recipients. The limited repopulating ability of HSCs during serial transplantation has led to one view that the self-renewal ability of HSCs is intrinsically limited.11 But extrinsic factors such as the transplantation procedure4 and the irradiated bone marrow microenvironment7 are also likely involved. Since a substantial portion of stem cell transplantations still involve the use of total body irradiation (TBI) (though not with an ablative dose nowadays) as a host conditioning regime, it is of clinical relevance to define an effective approach for sustaining the self-renewal potential of transplanted HSCs in the irradiated recipients. Despite the importance of this issue, the potential exhaustion of donor HSCs in transplant recipients has not been resolved at the molecular level.
Given the limited success of in vitro HSC expansion to date, in vivo manipulations of HSCs appear to be important alternatives to enhance HSC therapy. In addition to the administration of hematopoietic growth factors2 and the alteration of homing receptors (such as CXCR4 and CD26)12,13 or microenvironmental elements (such as the stem-cell “niche”),14 direct manipulation of the intracellular self-renewal machinery is an important strategy currently being explored. Cell-cycle regulators have been demonstrated to be intrinsically involved in the self-renewal of adult stem cells.15 In particular, cyclin-dependent kinase inhibitors (CKIs) appear to have an important role in modulating the self-renewing potential of stem cells. Interestingly, different CKIs appear to affect self-renewal in distinct manners. For instance, in the absence of p21Cip1/Waf1 (p21 hereafter), the founding member of CKIs, the hematopoietic potential of HSCs was nearly exhausted after 2 rounds of bone marrow transplantation.10 In contrast, we have recently documented an engraftment advantage of the hematopoietic cells deficient in p18INK4C (p18 hereafter), a member of the INK4 family of CKIs.16 This advantage appears to be largely if not solely due to increased self-renewal of transplanted HSCs in vivo16 rather than a general increase in proliferation of the donor cells (H.S., Y. Song, H.Y., D. Shields, Y.Y., S. Cao, and T.C., manuscript in preparation).
Based on our previous results, we sought to examine whether the known exhaustion of transplanted HSCs in irradiated hosts can be significantly overcome or minimized by suppressing p18 expression during prolonged long-term engraftment. To this end, we have focused on the regenerative ability of transplanted p18-/- HSCs, in comparison with that of HSCs deficient in p21 or in both p18 and p21, following serial bone marrow transplantations over an extended time period. Our current study demonstrates that deleting p18 in HSCs is effective in sustaining the durability of transplanted HSCs beyond the lifetime of a mouse, and this deletion is able to substantially compensate for the deleterious effect of p21 deletion on long-term hematopoietic repopulation, likely due to the opposite effects of p18 and p21 on HSC senescence under stress conditions.
Materials and methods
Mice
p18+/- mice and p18-/-p21-/- double-knockout mice in C57BL/6;129/Sv background were kindly provided by David Franklin (Purdue University, West Lafayette, IN; p18+/- mice were originally created by Franklin et al in Yue Xiong's laboratory17 ). p18-/- and p18+/+ littermates were produced from p18+/- breeding pairs that were maintained in our laboratory. p21-/- mice and p21+/+ littermates were kindly provided by Andrew Stewart (University of Pittsburgh, PA; the p21+/- breeding mice in C57BL/6; 129/Sv background were purchased from the Jackson Laboratories [Bar Harbor, ME] and were originally created in Tyler Jacks' laboratory18 ). Female C57BL/6129/SvF1 recipients at the age of 2 months were purchased from the Jackson Laboratories. All the procedures involved in the mouse work were approved by the Institutional Animal Care and Use Committee of University of Pittsburgh.
Serial bone marrow transplantation
Bone marrow–nucleated cells (BMNCs; 2 × 106/host) from male p18+/+ or p18-/- donor mice at the age of 2 months were transplanted into lethally irradiated (10 Gy) female wild-type recipients (2 months old). The same procedure was applied in secondary, tertiary, and quaternary recipients at intervals as indicated in Figure 1A. Three animals in each group were killed after each serial bone marrow transplantation (sBMT) to measure the cobblestone area–forming cells (CAFCs) and colony-forming cells (CFCs) in bone marrow as previously described.10,16 Blood was collected 12 months after the 4th round of sBMT to measure the donor contribution with the semiquantitative polymerase chain reaction (PCR) for Y chromosome.10,16
Competitive bone marrow transplantation coupled with serial transfer
The competitive bone marrow transplantation (cBMT) procedure was detailed in our previous publication16 and is further illustrated in Figure 2A. Briefly, test cells (2 × 106 BMNCs/host) from p18-/- or p18-/-p21-/- male mice and an equal number of wild-type BMNCs (competitor cells) from male mice (wild-type littermates in primary cBMT and then 2-month-old wild-type C57BL/6129/SvF1 mice in subsequent rounds of cBMT) were cotransplanted into lethally-irradiated (10 Gy) female recipients. The competitor cells from unmanipulated marrow were repetitively used in each sequential cBMT as a standard measure for determining the repopulation ability of varied types of the original input cells over the course of the serial transfer. Blood was collected after each cBMT and the relative contribution of test cells (-/-) to the competitor cells (+/+) in the reconstituted host was quantified by the semiquantitative PCR as previously described.16 Some mice were killed after each cBMT for the genotypic analyses in bulk BMNCs, different cell lineages, and different hematopoietic compartments by the PCR described in “PCR for tracing donor contribution.”
Flow cytometric analysis and cell sorting
One of the well-characterized immunophenotypes for murine long-term repopulating HSCs, CD34-Lin-c-Kit+Sca-1+(CD34-LKS),21 was applied in our current study. The method used to quantify the frequency of CD34-LKS in bone marrow was previously described.16 To isolate the cells, Sca-1+ cells were enriched with the EasySep Kit (StemCell Technologies, Vancouver, BC, Canada) according to the manufacturer's protocol. The cells were stained with a mixture of biotinylated antibodies against mouse CD3, CD4, CD8, B220, Gr-1, Mac-1, and TER-119 (Caltag, Burlingame, CA), then costained with streptavidin–PE-Cy7, anti–Sca-1–PE, anti–c-Kit–APC, and anti-CD34–FITC (BD Pharmingen, San Diego, CA). Propidium iodide was used for dead cell exclusion. CD34-LKS cells were sorted into 96-well plates (Becton Dickinson, Franklin Lakes, NJ) at 1 cell/well using the MoFlo High-Speed Cell Sorter with subsystems of Cyclone Automated Cloner and Sort Master Droplet Control (DakoCytomation, Fort Collins, CO). For sorting the mature cells in different lineages, BMNCs were stained with a mixture of anti-CD3–PE, anti–Gr-1–PE-Cy7, anti–Mac-1–APC and anti-B220–PE–Texas Red (BD Pharmingen). Cells of different lineages including T (CD3+), B (B220+), and myeloid (Mac-1+) were sorted into separate tubes and then lysed for genotyping by PCR analysis.
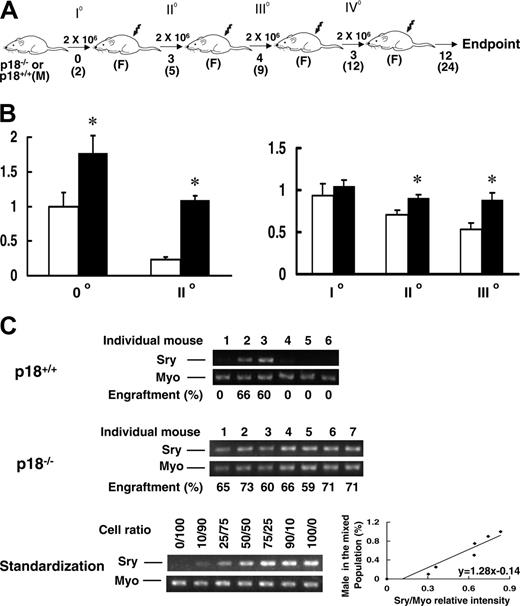
Continued presence of p18-/-HSC progeny without apparent exhaustion during sBMT. BMNCs were collected from p18-/- or p18+/+ male mice at 2 months of age and transplanted into lethally irradiated (10 Gy) female recipients (2 × 106 cells/recipient). After long-term engraftment was established in the primary recipients, the same dose of BMNCs were pooled from the primary recipients and retransplanted into lethally irradiated female recipients. The same procedure was repeated for additional 3 times (A). 0°, I°, II°, III°, and IV° indicate nonrecipient, first, second, third, and fourth sBMTs, respectively. M and F indicate male and female, respectively. Numbers under the arrows indicate the months after each sBMT, and numbers in the parentheses are the cellular age of original transplanted HSCs. In vitro hematopoietic activity was assayed with the CAFC assay and CFC assay as previously described.10 The frequencies of day-35 CAFCs (left) and CFCs (right) normalized to the nonrecipient wild-type control after different sBMTs are shown (B). *P < .05 (Student t test, n = 3). Error bars indicate SD. □ and ▪ indicate p18+/+ and p18-/- groups, respectively. To further assess the exhaustion of hematopoietic regeneration, peripheral blood was collected from the recipients 12 months after IV° sBMT and the PCR-based semiquantitative analysis for mouse Y chromosome–specific sequence (Sry) normalized to the housekeeping gene myogenin (Myo) was applied to calculate the contribution of the original male donor cells (C). The spleen- or blood-nucleated cells from male and female mice were mixed at different ratios to acquire the standardization for the semiquantitative analysis. According to the standardization generated simultaneously (bottom panel), the normalized percentages of donor cells in the blood are indicated under the PCR images (top, middle panels). Numbers above the gel images indicate individual p18-/- or p18+/+ transplant recipients, and engraftment level (%) in each recipient is indicated under the gel image.
Continued presence of p18-/-HSC progeny without apparent exhaustion during sBMT. BMNCs were collected from p18-/- or p18+/+ male mice at 2 months of age and transplanted into lethally irradiated (10 Gy) female recipients (2 × 106 cells/recipient). After long-term engraftment was established in the primary recipients, the same dose of BMNCs were pooled from the primary recipients and retransplanted into lethally irradiated female recipients. The same procedure was repeated for additional 3 times (A). 0°, I°, II°, III°, and IV° indicate nonrecipient, first, second, third, and fourth sBMTs, respectively. M and F indicate male and female, respectively. Numbers under the arrows indicate the months after each sBMT, and numbers in the parentheses are the cellular age of original transplanted HSCs. In vitro hematopoietic activity was assayed with the CAFC assay and CFC assay as previously described.10 The frequencies of day-35 CAFCs (left) and CFCs (right) normalized to the nonrecipient wild-type control after different sBMTs are shown (B). *P < .05 (Student t test, n = 3). Error bars indicate SD. □ and ▪ indicate p18+/+ and p18-/- groups, respectively. To further assess the exhaustion of hematopoietic regeneration, peripheral blood was collected from the recipients 12 months after IV° sBMT and the PCR-based semiquantitative analysis for mouse Y chromosome–specific sequence (Sry) normalized to the housekeeping gene myogenin (Myo) was applied to calculate the contribution of the original male donor cells (C). The spleen- or blood-nucleated cells from male and female mice were mixed at different ratios to acquire the standardization for the semiquantitative analysis. According to the standardization generated simultaneously (bottom panel), the normalized percentages of donor cells in the blood are indicated under the PCR images (top, middle panels). Numbers above the gel images indicate individual p18-/- or p18+/+ transplant recipients, and engraftment level (%) in each recipient is indicated under the gel image.
Clonal cell culture
Single CD34-LKS cells were deposited into 96-well plates (1 cell/well). Each well contained 100 μL of Iscove modified Dulbecco medium (IMDM) supplemented with 50 ng/mL Flt3 ligand (Flt3-L), 50 ng/mL stem cell factor (SCF), 10 ng/mL thrombopoietin (TPO) and 10 ng/mL interleukin-3 (IL-3) (PeproTech, Rocky Hill, NJ). The CFC assay with bulk BMNCs was performed according to the manufacturer's instructions (Stem Cell Technologies). Seven to 10 days after initiation of these clonal cultures, sufficient colonies were harvested under a microscope and lysed for subsequent PCR reaction.
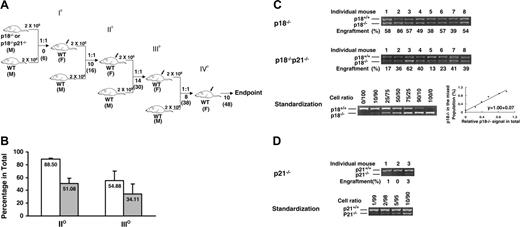
Sustained competitiveness of p18-/- HSCs after multiple rounds of cBMT. BMNCs from p18-/- or p18-/-p21-/- knockout mice were mixed with an equal number (2 × 106/each) of competitor cells (BMNCs from 2-month-old wild-type mice) and injected into lethally irradiated (10 Gy) recipient mice. Ten months after primary cBMT, 2 × 106 BMNCs from the recipients were rechallenged with an equal amount of the competitor cells and secondarily transplanted into lethally irradiated recipients. This procedure was sequentially repeated twice (donor cells were pooled from 3-5 animals, and there were 10 recipients/each group for each cBMT). The actual interval between each cBMT is shown (A). Some mice were kept longer than the interval. I°, II°, III°, and IV° indicate first, second, third, and fourth cBMTs, respectively. 1:1 indicates that equal amounts of test cells and competitor cells were cotransplanted into the recipients. M and F indicate male and female, respectively. Numbers under the arrows indicate the months after each cBMT, and numbers in the parentheses are the cellular age of original transplanted HSCs. Peripheral blood was collected from the recipients after long-term engraftment during each interval between sequential cBMTs, and PCR-based semiquantitative analysis as described in our previous study16 was performed to determine the contribution of p18-/- or p18-/-p21-/- to the overall hematopoietic reconstitution. The spleen- or blood-nucleated cells from wild-type and mutant mice were mixed at different ratios to obtain a standardization curve for the semiquantitative analysis. The average levels of engraftment in blood from p18-/- or p18-/-p21-/- origin 12 months after secondary and 6 months after tertiary cBMTs are shown (B). Error bars indicate SD. □ and ▪ indicate p18-/- and p18-/-p21-/- transplantation groups, respectively. Each group includes 8 animals, and the difference between p18-/- and p18-/-p21-/- is significant (P < .05) based on the Student t test. The representative results after tertiary cBMT are shown (C) along with the standardization generated simultaneously (bottom panel). Numbers above the gel images indicate individual animals, and the normalized percentages of test cells of the total in blood are shown below the images. As a control to confirm the accelerated exhaustion of hematopoietic repopulation in the absence of p21 as reported previously,10 the identical cBMT procedure (A) was used to test the repopulating ability of BMNCs from p21-/- mice at the age of 12 months (D). Eight to 12 months after the first cBMT, there was virtually no detectable p21-/- band in blood after 30 cycles of the PCR, and there were 2 blood samples at a barely detectable level (1%-3%) of the engraftment after 35 cycles of the PCR as representatively shown here. This experiment was intended to confirm a previous claim of HSC exhaustion due to p21 absence.10 It was not performed at the same time as other 2 groups but the competitor cells were from the same type of donor mice as described in “Materials and methods.” Therefore, the results between the p21-/- group and other groups, though imperfect, are still comparable since all these different test cells were directly assessed with the same type of competitor cells in the irradiated hosts. The singular effect of age would not substantially contribute to the exhaustion based on earlier studies by others showing no disadvantage of aged BMNCs in the competitive repopulation model.4,19,20
Sustained competitiveness of p18-/- HSCs after multiple rounds of cBMT. BMNCs from p18-/- or p18-/-p21-/- knockout mice were mixed with an equal number (2 × 106/each) of competitor cells (BMNCs from 2-month-old wild-type mice) and injected into lethally irradiated (10 Gy) recipient mice. Ten months after primary cBMT, 2 × 106 BMNCs from the recipients were rechallenged with an equal amount of the competitor cells and secondarily transplanted into lethally irradiated recipients. This procedure was sequentially repeated twice (donor cells were pooled from 3-5 animals, and there were 10 recipients/each group for each cBMT). The actual interval between each cBMT is shown (A). Some mice were kept longer than the interval. I°, II°, III°, and IV° indicate first, second, third, and fourth cBMTs, respectively. 1:1 indicates that equal amounts of test cells and competitor cells were cotransplanted into the recipients. M and F indicate male and female, respectively. Numbers under the arrows indicate the months after each cBMT, and numbers in the parentheses are the cellular age of original transplanted HSCs. Peripheral blood was collected from the recipients after long-term engraftment during each interval between sequential cBMTs, and PCR-based semiquantitative analysis as described in our previous study16 was performed to determine the contribution of p18-/- or p18-/-p21-/- to the overall hematopoietic reconstitution. The spleen- or blood-nucleated cells from wild-type and mutant mice were mixed at different ratios to obtain a standardization curve for the semiquantitative analysis. The average levels of engraftment in blood from p18-/- or p18-/-p21-/- origin 12 months after secondary and 6 months after tertiary cBMTs are shown (B). Error bars indicate SD. □ and ▪ indicate p18-/- and p18-/-p21-/- transplantation groups, respectively. Each group includes 8 animals, and the difference between p18-/- and p18-/-p21-/- is significant (P < .05) based on the Student t test. The representative results after tertiary cBMT are shown (C) along with the standardization generated simultaneously (bottom panel). Numbers above the gel images indicate individual animals, and the normalized percentages of test cells of the total in blood are shown below the images. As a control to confirm the accelerated exhaustion of hematopoietic repopulation in the absence of p21 as reported previously,10 the identical cBMT procedure (A) was used to test the repopulating ability of BMNCs from p21-/- mice at the age of 12 months (D). Eight to 12 months after the first cBMT, there was virtually no detectable p21-/- band in blood after 30 cycles of the PCR, and there were 2 blood samples at a barely detectable level (1%-3%) of the engraftment after 35 cycles of the PCR as representatively shown here. This experiment was intended to confirm a previous claim of HSC exhaustion due to p21 absence.10 It was not performed at the same time as other 2 groups but the competitor cells were from the same type of donor mice as described in “Materials and methods.” Therefore, the results between the p21-/- group and other groups, though imperfect, are still comparable since all these different test cells were directly assessed with the same type of competitor cells in the irradiated hosts. The singular effect of age would not substantially contribute to the exhaustion based on earlier studies by others showing no disadvantage of aged BMNCs in the competitive repopulation model.4,19,20
PCR for tracing donor contribution
Primer sequences for amplifying the p18 and p21 mutant/wild-type alleles were described in our previous publications.10,16 In addition to the genotyping PCR for p18, the following primer pair was used to confirm the absence of p21 in p18/p21 double-mutant cells: p21null-F (5′-GCG AGG ATC TCG TCG TGA C-3′), and p21 null-R (5′-TCA TCA ATT TAT GCA GAC-3′). In the PCR for quantifying the Y chromosome DNA (Sry), the following primers were used: Sry-F (5′-TGG GAC TGG TGACAATTG TC-3′), Sry-R (5′-GAG TACAGG TGT GCAGCT CT-3′), Myo-F (5′-TTA CGT CCA TCG TGG ACA GC-3′), and Myo-R (5′-TGG GCT GGG TGT TAG TCT TA-3′). Nucleated blood cells were lysed in 1 × PCR buffer containing 2.5 mM MgCl2 and 20 μg/mL proteinase K for 1 hour at 60°C, followed by an inactivation of the reaction for 20 minutes at 95°C. The parameters for thermal cycling of PCR were as follows: 30 seconds at 94°C, 30 seconds at 57°C, and 1 minute at 72°C (30 cycles for the semiquantitative analysis with bulk blood cells and 35 cycles for the single-colony analysis).
Results
In vitro activity of p18-/- hematopoietic cells from the serial transplant recipients
We first examined the exhausting effect of irradiated hosts on transplanted HSCs with the classical sBMT, in which no competitor cells were cotransplanted (Figure 1A). BMNCs from male p18+/+ or p18-/- mice were transplanted into lethally irradiated female mice and sBMT was sequentially performed for 4 rounds with an interval of 3 to 4 months. During each sBMT, the in vitro activity of both HSCs and hematopoietic progenitor cells (HPCs) from p18-/- recipient mice was significantly higher than that from p18+/+ recipient mice, as examined by the in vitro surrogate assays for HSCs and HPCs, namely CAFCs and CFCs, respectively (Figure 1B). Of note, CAFC frequency at day 35 in p18-/- recipient mice was 5 times higher than that in p18+/+ recipient mice after secondary sBMT. CFC frequency was maintained at a normal level (that of unmanipulated bone marrow) in p18-/- recipient mice, while it gradually declined in p18+/+ recipient mice during 3 rounds of sBMT. These results contrast with the accelerated hematopoietic exhaustion in the absence of p21 during sBMT, as shown in a previous study.10
Sustained in vivo hematopoiesis of p18-/- HSCs after multiple BMTs
Our next step was to determine the in vivo long-term repopulating ability (LTRA) of HSCs 12 months after quaternary sBMT, by quantifying the presence of HSC progeny in blood (Figure 1A). PCR-based semiquantitative analysis for the Y chromosome–specific sequence (Sry) was used to track cellular origin.10 Based on the standardization simultaneously generated under identical PCR conditions, we calculated the percentage of donor cell contribution to recipient hematopoiesis. As we expected, most recipients (4 of 6) of p18+/+ HSCs had undetectable levels of donor cells in their blood (Figure 1C). In contrast, the original p18-/- male donor–derived cells accounted for 66.7% on average (60%-73%) of whole mature blood cells in all recipients (n = 7). By an analysis with the single limiting dose, we estimated that there was only 1 HSC unit (which is able to yield detectable donor cells in the blood of a recipient animal) per 5 × 106 BMNCs in p18+/+ transplant recipients after 4 rounds of sBMT compared with 1 HSC per 1 to 2 × 105 BMNCs in normal unmanipulated marrow.22 The 100% engraftment rate in the p18-/- transplant group precluded us from directly estimating the frequency of functional units of HSCs in the p18-/- recipients. However, p18-/- HSCs at this later point could be estimated following serial cBMT for 3 times over 30 months, subsequent to our previous study (Figure 2A).16
In the cBMT experiment, equal numbers of p18-/- BMNCs and competitor cells (wild-type BMNCs freshly isolated from mice that did not receive transplants) were cotransplanted into lethally irradiated recipients in each cBMT. Because LTRA and HSC frequency in mouse wild-type BMNCs have been well documented, the fixed number (2 × 106) of unmanipulated competitor cells can serve as a standard measure for varied types of input cells to be compared (Figure 2A). Blood was collected 6 to 14 months after each cBMT and the relative contribution to hematopoiesis from each genotype (p18-/- versus wild-type) was quantified by the semiquantitative PCR assay.16 After 3 rounds of cBMT in 30 months, LTRA of p18-/- HSCs was still equivalent (54.9%) to that of the competitor cells (Figure 2B-C), which indicates there is no apparent exhaustion of hematopoietic potential compared with that of the wild-type transplanted cells at this time point.
An opposing effect of p18 absence over p21 absence on HSC exhaustion
To test whether p18 absence can counteract the known accelerated hematopoietic exhaustion caused by p21 absence,10 we directly assessed the long-term engraftment of hematopoietic cells from mice deficient in both p21 and p18 (p18-/-p21-/-, or “double-knockout”).23 LTRA of HSCs from p18-/-p21-/- mice was compared with that from p18-/- mice using the cBMT assay (Figure 2A).16 In contrast to a previous study showing early exhaustion of hematopoietic reconstitution in the absence of p21 after sBMT,10 p18-null was able to overcome the accelerated hematopoietic exhaustion caused by p21 deletion as demonstrated by 51.1% and 34.1% of reconstitution level in secondary and tertiary recipients of the p18-/-p21-/- cells, respectively (Figure 2B-C). As a confirmatory result to the previous report,10 we were also able to observe an accelerated hematopoietic exhaustion in a different experiment where p21-/- BMNCs were cotransplanted with the same competitor cells in the current study (Figure 2D). The fact that p18-/-p21-/- HSCs were able to sustain their LTRA after 3 rounds of cBMT suggests that the absence of p18 can significantly mitigate the negative impact of p21 deletion on the LTRA of HSCs. Therefore, these data provide direct evidence for the opposite effects of p18 and p21 on the hematopoietic potential of HSCs in the context of serial transplantation.
Multilineage differentiation of p18-/- or p18-/-p21-/- HSCs
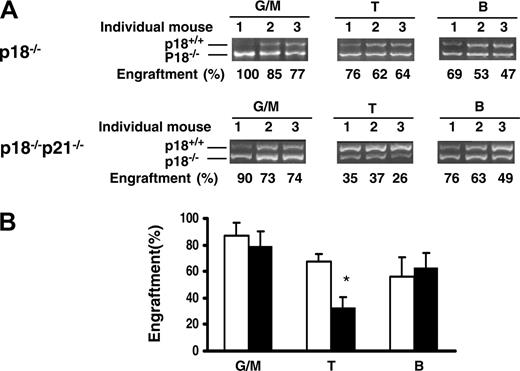
Multilineage analysis of bone marrow cells from tertiary recipients was performed to determine whether p18-/- or p18-/-p21-/- HSCs maintained their ability to give rise to both myeloid and lymphoid lineages after multiple cBMTs performed over a prolonged period of time. The immunophenotypically defined myeloid (granulocytes and monocytes) and lymphoid (T and B lymphocytes) cells were sorted for the determination of p18 genotype (p18-/- vs p18+/+). As shown in Figure 3, substantial engraftment levels of p18-/- cells were seen in both myeloid and lymphoid lineages, indicating the multilineage potential of transplanted p18-/- or p18-/-p21-/- HSCs for 30 months over multiple cBMTs (at the cellular age of 36 months). Notably, the relative contribution of p18-/- donor cells to competitor cells in myeloid lineage was higher than that observed in lymphoid lineage (Figure 3B). This result is in agreement with previous studies by others that showed favored myelopoiesis over lymphopoiesis in aged mice, which is indicative of ongoing aging of hematopoietic cells in vivo.24-26 However, the relative decrease of T lymphopoiesis was significantly improved in the p18-/- group compared with that in the p18-/-p21-/- group (P < .05), suggesting distinct roles of p18 and p21 in T lymphopoiesis in addition to their direct effects on HSCs.
Representation of p18-/-, p18-/-p21-/-, or p21-/- genotype in the HSC compartment
To further distinguish the contribution of test cells versus competitor cells in the stem cell compartment, we isolated HSCs with the primitive immunophenotype for murine HSCs, CD34-LKS,21 from the recipients of cBMT and determined their genotype at the single-cell level. It should be noted that this immunophenotype with LTRA does not seem to change in aged or mice that received transplants based on the data by others26 and our own (Feng Xu, H.S., and T.C., unpublished observations, July 2005). Single HSCs were sorted into 96-well plates (1 cell/well) and cultured in medium with appropriate cytokine stimuli to form colonies (more than 30 cells/colony) in 7 to 10 days, which allowed us to efficiently track the origin of individual HSCs that were initially isolated from the marrow. Sufficient numbers of colonies were randomly picked up for the genotyping PCR analysis on the marrow harvested at varied time points after cBMT (Table 1). Data demonstrated that the majority of CD34-LKS cells were of p18-/- or p18-/-p21-/- origin after tertiary or quaternary cBMT. Interestingly, the dominance of p18-/-p21-/- genotype was higher than that of p18-/- alone. In the p21-/- control group, p21-/- HSC progeny were barely detectable in blood after primary cBMT (Figure 2D), while 63.3% of CD34-LKS cells and 18.9% of CFCs remained of the p21-/- origin. When normalized to the bone marrow cellularity at the time of sacrifice, the absolute yield of CD34-LKS cells was in fact significantly lower in the p21-/- transplant group than in p18-/- recipients.
Representation of KO in stem/progenitor cell pools
. | . | Clonal analysis of stem/progenitor cells . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| cBMT*and clonal culture . | Time after cBMT, mo . | No. mice analyzed . | No. colonies assayed . | % KO . | Absolute yield of KO CD34– LKS cells† . | ||
| p18–/–‡ | |||||||
| II° | |||||||
| CD34–LKS | 12 | 3 | 109 | 92.7 | ND | ||
| III° | |||||||
| CD34–LKS | 8 | 3 | 93 | 76.3 | 3633 ± 356 | ||
| p18–/–p21–/– | |||||||
| II° | |||||||
| CD34–LKS | 16 | 3 | 106 | 99.1 | ND | ||
| CFC | 16 | 3 | 88 | 97.7 | – | ||
| III° | |||||||
| CD34–LKS | 6 | 2 | 91 | 90.1 | ND | ||
| CFC | 6 | 2 | 88 | 87.6 | – | ||
| IV° | |||||||
| CD34–LKS | 10 | 4 | 76 | 78.9 | ND | ||
| CFC | 10 | 4 | 37 | 40.5 | – | ||
| p21–/–§ | |||||||
| I° | |||||||
| CD34–LKS | 8 | 3 | 60 | 63.3 | 1452 ± 394 | ||
| CFC | 8 | 3 | 67 | 18.9 | – | ||
. | . | Clonal analysis of stem/progenitor cells . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| cBMT*and clonal culture . | Time after cBMT, mo . | No. mice analyzed . | No. colonies assayed . | % KO . | Absolute yield of KO CD34– LKS cells† . | ||
| p18–/–‡ | |||||||
| II° | |||||||
| CD34–LKS | 12 | 3 | 109 | 92.7 | ND | ||
| III° | |||||||
| CD34–LKS | 8 | 3 | 93 | 76.3 | 3633 ± 356 | ||
| p18–/–p21–/– | |||||||
| II° | |||||||
| CD34–LKS | 16 | 3 | 106 | 99.1 | ND | ||
| CFC | 16 | 3 | 88 | 97.7 | – | ||
| III° | |||||||
| CD34–LKS | 6 | 2 | 91 | 90.1 | ND | ||
| CFC | 6 | 2 | 88 | 87.6 | – | ||
| IV° | |||||||
| CD34–LKS | 10 | 4 | 76 | 78.9 | ND | ||
| CFC | 10 | 4 | 37 | 40.5 | – | ||
| p21–/–§ | |||||||
| I° | |||||||
| CD34–LKS | 8 | 3 | 60 | 63.3 | 1452 ± 394 | ||
| CFC | 8 | 3 | 67 | 18.9 | – | ||
Ko indicates knock-out; I°, primary; II°, secondary; III°, tertiary; IV°, quaternary; ND, not done; and –, not applicable.
The cBMT procedure was detailed in our previous publication16 and is further illustrated in Figure 2A. Briefly, test cells (2 × 106 BMNCs/host) from p18–/– or p18–/–p21–/– male mice and an equal number of wild-type BMNCs (competitor cells) from male mice (wild-type litter mates in primary cBMT and then 2-month-old wild-type C57BL/6129/SvF1 mice in subsequent rounds of cBMT) were cotransplanted into lethally irradiated (10 Gy) female recipients. Overall engraftment levels in blood are presented in Figure 2C-D
Absolute yield of KO CD34–LSK cells was calculated according to the bone marrow cellularity per harvest (including ilia, femurs, and tibias; n = 8), the frequency (by flow cytometry) of viable CD34–LKS (being able to respond to growth stimuli in vitro) and percentage of KO in CD34–LKS cells. P < .01 (t test, between p18–/– and p21–/– cBMT groups). The lower yield of CD34–LKS cells due to the absence of p21 must have been substantially underestimated since the p18–/– group had undergone 2 additional rounds of cBMT
The representations of p18–/– genotype after after secondary cBMT have been reported in our previous publication.16 All the mice after quaternary cBMT in the p18–/– group developed T-cell leukemia. Characterization of the leukemic cells and relation to HSC regeneration involving a range of analyses are to be reported in a different manuscript41
This experiment was done much later than the other 2 experiments (p18–/– and p18–/–p21–/–) to confirm the accelerated hematopoietic exhaustion due to p21 absence10 as rationalized in the legend of Figure 2D. The competitor cells in all these experiments were from the same type of unmanipulated bone marrow
Multilineage differentiation that favors myelopoiesis after an extended long-term engraftment. (A) The recipients were killed after tertiary cBMT and their BMNCs were collected and stained with lineage markers. Myeloid (Mac-1+), T (CD3+)–, and B (B220+)–cell lineages were sorted for semiquantitative PCR analysis as described in Figure 2C. The actual results are shown. Numbers above the gel images indicate individual animals, and the calculated percentages of donor cells are indicated below the PCR images. (B) Contribution of p18-/- or p18-/-p21-/- donor cells to different lineages in tertiary recipients (6 months after transplantation). Error bars indicate SD. □ and ▪ indicate p18-/- and p18-/-p21-/- transplantation groups, respectively. *P < .05 (n = 3, Student t test).
Multilineage differentiation that favors myelopoiesis after an extended long-term engraftment. (A) The recipients were killed after tertiary cBMT and their BMNCs were collected and stained with lineage markers. Myeloid (Mac-1+), T (CD3+)–, and B (B220+)–cell lineages were sorted for semiquantitative PCR analysis as described in Figure 2C. The actual results are shown. Numbers above the gel images indicate individual animals, and the calculated percentages of donor cells are indicated below the PCR images. (B) Contribution of p18-/- or p18-/-p21-/- donor cells to different lineages in tertiary recipients (6 months after transplantation). Error bars indicate SD. □ and ▪ indicate p18-/- and p18-/-p21-/- transplantation groups, respectively. *P < .05 (n = 3, Student t test).
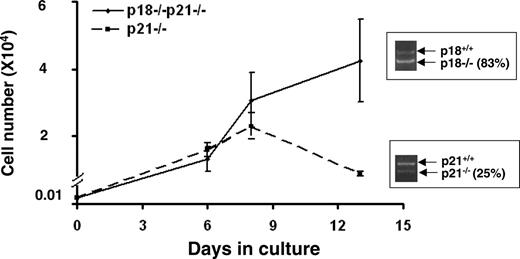
To further validate the different impacts of p21 and p18 on a per-cell basis, we cultured the CD34-LKS mixed populations isolated from either the p21-/- or p18-/-p21-/- cBMT group for 14 days and then checked the ratio of p21-/-- or p18-/-p21-/--differentiated cells versus wild-type competitor progeny by semiquantitative PCR. The CD34-LKS cells in both groups were able to differentiate into myeloid cells during the culture. Consistent with our in vivo findings, the initial higher representation (63.3%) at HSC level dropped to 25% of HSC progeny in the p21-/- group, whereas the representation remained (78.9% to 83%) in the p18-/-p21-/- group (Figure 4). Together, these data suggest that a progressive decrease of function per HSC is ultimately followed by a possible depletion of the HSC pool due to the absence of p21, and that such a process can be substantially overcome by deleting p18 at the stem cell level regardless of p21 absence.
Discussion
Our current study demonstrates that transplanted p18-/- HSCs sustain their self-renewing ability at a comparable level to unmanipulated wild-type HSCs after multiple serial transfers in both competitive and noncompetitive repopulation models. Furthermore, p18 absence is able to significantly counteract the accelerated exhaustion of hematopoietic repopulation resulting from p21 deficiency. Therefore, we have reported for the first time that hematopoietic exhaustion in serial transplantation models can be overcome by targeting a specific cell-cycle inhibitor, and different cell-cycle inhibitors can have opposite impacts on the outcome.
Both extrinsic and intrinsic factors may account for HSC exhaustion in transplant recipients. Regarding the extrinsic factors, at least 2 considerations have been given from previous studies by others. One is the failure to achieve a physiologic concentration of HSCs due to insufficient mitogens for their regeneration upon the dilution of original donor HSCs in new recipients.2 Another one is the physical disruption due to transplantation procedure or/and radiation damage (if TBI is used), which can result in homing defect27 or permanent loss of self-renewal potential6,7,28 of the transplanted HSCs. However, it is unclear how these extrinsic effects are transmitted onto the intrinsic properties of transplanted HSCs. In fact, the intrinsic mechanism may also affect HSC exhaustion in an autonomous manner. Because serial bone marrow transfer usually spans the lifetime of a mouse or longer besides its imposed transplantation stress, the resultant stem-cell exhaustion has been thought to be attributable to the replicative senescence of the transplanted HSCs, resembling aging of HSCs.11 This link is supported by the similarity of limited HSC self-replication in both serial transplant recipients and aged mice.19,29 While the aging effect might be cooperative with the extrinsic factors in leading to hematopoietic exhaustion, the hematopoietic exhaustion was distinguishable from the aged phenotype to a certain extent in the absence of p18. On one hand, we observed the disappearance of hematopoietic exhaustion in p18-/- groups compared with the wild-type cells after serial transplantation over 3 years, while on the other hand we observed a favored myeloid differentiation of HSCs that has been similarly observed in old mice and is now considered as a sign for HSC aging.24-26 Notably, however, the LTRA after each cBMT decreased compared with the previous one, and this reduction coincided with a relative increase in HSC frequency as defined by the immunophenotype (Table 1). We observed a dominant p18-/- phenotype in the HSC compartment, but the representation of p18-/- phenotype in blood did not correlate with the ratio in the stem cell pool after tertiary cBMT. The reverse relationship was more overt in the p18-/-p21-/- group, and the most dramatic in the p21-/- group (Table 1; Figure 2D). This suggests a decline in repopulation potential per HSCs rather than an actual loss of the phenotypic HSCs after transplantation. This observation is consistent with previous studies in aged hematopoietic tissue or transplants.30,31 The similarity between HSCs in transplant recipients and aged mice suggests that extensive cell divisions may pose replicative stress on HSCs and ultimately lead to premature cellular senescence. Interestingly, p18 absence is able to significantly counteract the process through a distinct mechanism, yet to be defined.
In vitro growth of LKS cells. CD34-LKS cells were isolated from the recipients of p21-/- primary cBMT (n = 3) or quaternary p18-/-p21-/- cBMT (n = 4). CD34-LKS cells (100) were sorted into 96-well plates and cultured in IMDM supplemented with 100 ng/mL SCF, 50 ng/mL Flt-3, and 25 ng/mL TPO. Cell numbers were counted at 3 time points during the culture. The growth curves were plotted based on the means ± SD of triplicate cultures. An aliquot of the differentiated cells were lysed for the semiquantitative PCR analysis as described in Figure 2. The inserts in the graph are the PCR results showing the altered genotypic representation (knockout vs wild-type). Based on the single-cell analysis (Table 1), the initial ratios of p21-/- and p18-/-p21-/- in the starting CD34-LKS cells were 63.3% and 78.9%, respectively. No differentiation block was observed in both groups based on the cell morphology under a microscope.
In vitro growth of LKS cells. CD34-LKS cells were isolated from the recipients of p21-/- primary cBMT (n = 3) or quaternary p18-/-p21-/- cBMT (n = 4). CD34-LKS cells (100) were sorted into 96-well plates and cultured in IMDM supplemented with 100 ng/mL SCF, 50 ng/mL Flt-3, and 25 ng/mL TPO. Cell numbers were counted at 3 time points during the culture. The growth curves were plotted based on the means ± SD of triplicate cultures. An aliquot of the differentiated cells were lysed for the semiquantitative PCR analysis as described in Figure 2. The inserts in the graph are the PCR results showing the altered genotypic representation (knockout vs wild-type). Based on the single-cell analysis (Table 1), the initial ratios of p21-/- and p18-/-p21-/- in the starting CD34-LKS cells were 63.3% and 78.9%, respectively. No differentiation block was observed in both groups based on the cell morphology under a microscope.
The molecular mechanisms that underlie HSC exhaustion are poorly understood. Given that replicative cellular senescence is likely involved, telomere length was hypothesized to be a critical cellular timer in mediating the senescent outcome. This hypothesis was supported by the observation of shortened telomere length after serial transplantation32 and early exhaustion of transplanted HSCs deficient in telomerase.33 However, overexpression of telomerase in HSCs was not sufficient to overcome hematopoietic exhaustion, although it could prevent telomere shortening during serial transplantation.34 In our study, we did not observe a difference in telomere length between p18+/+ and p18-/- sBMT groups (data not shown). Therefore, these studies suggest that telomere erosion is not a sole or indispensable mechanism for HSC exhaustion.
The p53 and retinoblastoma (Rb) pathways are considered to be the 2 major activating mechanisms for cellular senescence in many other cell types.35 p21 is a primary transcriptional target of p53 in mediating cell-cycle arrest, and p16 is a key INK4 protein inhibiting the activity of CDK4/6 in the Rb pathway. Both cell-cycle inhibitors were recently indicated to be involved in radiation-induced HSC senescence.36 However, because p21 is expressed at a higher level in quiescent HSCs,37,38 it is unlikely to play an indispensable role in HSC senescence. Instead, the Rb pathway might be a more dominant mechanism for HSC senescence but not HSC quiescence, as p16 and its alternative reading frame, p19ARF, were up-regulated in the HSCs from mice that had received serial transplants (H.Y. and T.C., unpublished data, July 2005). We therefore hypothesize that under the replicative stress caused by transplantation, HSCs may undergo senescence, and such a process is accelerated in the absence of p21 due to a higher cycling status of HSCs. In fact, given the increased number of HSCs and normal hematopoietic potential in p21-/- under homeostatic conditions10 and the unperturbed function of HSCs after ex vivo targeting of p21,39,40 we suggest that the senesent state of p21-/- HSCs in the serial recipients reflect a secondary effect of p21 absence on HSCs under the repetitive proliferative stress. In the absence of p18, the outcome can be substantially improved perhaps due to an antagonistic effect of p18 absence on the cellular senescence. But it remains to be determined with regard to how the effect of p18 deletion is mediated by other molecules to compensate for the consequence of p21 loss on hematopoietic exhaustion, and more importantly, how p18 interacts with p21 physiologically in HSCs. Nevertheless, our current study sets the stage regarding our current understanding of the distinct roles that p18 and p21 play in stem-cell regulation. An understanding of the different impacts that these 2 CKIs have in HSC kinetics offers molecular targets for possible pharmacologic intervention to improve stem-cell functioning in transplantation or elderly patients.
Prepublished online as Blood First Edition Paper, October 18, 2005; DOI 10.1182/blood-2005-02-0685.
Supported by National Institutes of Health (NIH) grants (DK02761, HL70561) and the Scholar Award from American Society of Hematology (2003).
H.Y. and Y.Y. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We specially thank Drs David Franklin and Andrew Stewart for providing the knockout mouse strains, as well as Drs Joel Greenberger and Byeong-chel Lee for helpful comments on this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal