Abstract
GILZ (glucocorticoid-induced leucine zipper), a gene induced by dexamethasone, is involved in control of T lymphocyte activation and apoptosis. In the present study, using Gilz transgenic mice (TG), which overexpress GILZ in the T-cell lineage, we demonstrate that Gilz is implicated in T helper-2 (Th-2) response development. After in vitro stimulation by CD3/CD28 antibodies, peripheral naive CD4+ T cells from TG mice secrete more Th-2 cytokines such as interleukin-4 (IL-4), IL-5, IL-13, and IL-10, and produce less Th-1 cytokines such as interferon-γ (IFN-γ) than wild-type mice (WT). CD4+ TG lymphocytes up-regulated Th-2 cytokine expression in the specific response to ovalbumin chicken egg (OVA) antigen immunization. Up-regulation correlated with increased expression of GATA-3 and signal transducer and activator of transcription 6 (Stat6), Th-2–specific transcription factors and decreased expression of T-bet, a transcription factor involved in Th-1 differentiation. Finally, in TG mice delayed-type hypersensitivity, a Th-1 response, was inhibited and bleomycin-induced pulmonary fibrosis, a Th-2 mediated disease, was more severe. These results indicate that Gilz contributes to CD4+ commitment toward a Th-2 phenotype and suggest this contribution may be another mechanism accounting for glucocorticoid immunomodulation.
Introduction
Because glucocorticoid hormones (GCHs) are potent modulators of immune response and inflammatory processes, they are widely used as immunosuppressive and anti-inflammatory agents in many types of acute and chronic inflammatory disorders, in autoimmune diseases, and in organ transplantation.1-3 GCHs play a role in the physiologic regulation of the immune response development.2-5 Besides modulating T lymphocyte activation/apoptosis, thus contributing to thymic selection, they also regulate cytokine production thus modulating T helper-1 (Th-1) and Th-2 differentiation.6-13 Differentiation toward Th-1 or Th-2 effectors depends upon a variety of stimuli such as antigen nature and dose and the strength and duration of signals through the T-cell receptor (TCR)/CD3 complex and microenvironment lymphokines, which activate signaling pathways.14-19 In fact, during immune response development interleukin-4 (IL-4) favors the Th-2 response, which is responsible for allergic and humoral-mediated immunity. Moreover, IL-4 receptor engagement, through signal transducer and activator of transcription 6 (Stat6), induces expression of GATA-3, the selectively expressed Th-2 effector transcription factor.20-23 Interferon-γ (IFN-γ) and IL-12 are crucial factors for the development of Th-1 responses and cell-mediated immunity; they induce the expression and maintain the activity of T-bet, which has been identified as a Th-1–specific transcription factor.24-26 GCHs inhibit the cellular immune response, such as delayed-type hypersensitivity (DTH), but do not affect or even enhance the humoral or allergic immune response.9-13 In fact, in vitro and in vivo GCH treatment inhibits CD4+ cell production of Th-1 cytokines, such as IL-2, IFN-γ, and tumor necrosis factor-β (TNF-β), but increases production of Th-2 cytokines, IL-4, IL-5, IL-10, and IL-13.9-13 How GCHs regulate the cytokine network and affect the Th-1/Th-2 balance and development remains to be elucidated. Gene transcription modulation mediates most GCH effects.27 GILZ (glucocorticoid-induced leucine zipper), which is rapidly induced by dexamethasone (DEX) treatment, encodes for a member of the leucine zipper family.28,29 It is found in normal T lymphocytes of thymus, spleen, and lymph nodes and, like GCHs, its overexpression promotes thymocyte apoptosis or protects T cells from cell death induced by TCR/CD3 triggering.30,31 GILZ overexpression also inhibits TCR/CD3-induced nuclear factor κB (NF-κB) activation and nuclear translocation, by directly interacting with p65/p52 molecules.32 It binds and inhibits RNA polymerase activating factor (Raf-1), with consequent inhibition of the downstream Raf-1 pathway including c-fos and activator protein 1 (AP-1).33 Moreover, GILZ directly binds and inhibits AP-1.34 Therefore, GILZ mimics some of the effects mediated by GCHs and appears to be involved in regulating T-cell activity. In the present investigation into GILZ as modulator of the Th-1/Th-2 balance, Gilz transgenic (TG) and wild-type (WT) mice provided naive CD4+ T cells, which were driven in vitro toward either the Th-1 or Th-2 subset. We observed GILZ overexpression down-regulated the Th-1 and up-regulated the Th-2 responses. These findings suggest GILZ contributes to the mediation of the GCH-induced shift toward Th-2 immune response.
Materials and methods
Mice
Six- to 10-week-old TG and WT mice were used and analyzed for genotype and GILZ expression as previously described.31 In each experiment TG mice were compared with WT littermates. Two independent transgenic lines were produced.31 Animal care was in compliance with regulations in Italy (D.M.116192), Europe (O.J. of E.C. L. 358/1 12/18/1986), and the US (Animal Welfare Assurance No. A5594-01, Department of Health and Human Services, Washington, DC).
Isolation and culture of CD4+ cells
Naive CD4+ T cells, from spleens of WT and TG mice were purified using CD4 isolation kit (Miltenyi Biotech, Bergish Gladbach, Germany). After selection, cells were labeled with fluorescein isothiocyanate (FITC)–conjugated anti-CD4 (L3T4; PharMingen, San Diego, CA) and phycoeryrhrin (PE)–conjugated anti-CD62L (MEL-14; PharMingen) antibodies (Abs) and sorted as CD4+CD62Lhigh on an Epics Altra Cell Sorter (Beckman Coulter, Barcelona, Spain). Purity of CD4+CD62Lhigh T cells was more than 98% as evaluated by flow cytometry. Purified naive CD4+CD62Lhigh T cells, cultured in Iscoves medium containing 10% fetal calf serum (FCS), were stimulated under default conditions (Th-0)35 with 1 μg/mL anti-CD3 (clone 145-2C11) and with 2 μg/mL anti-CD28 (clone 37-51) Abs (PharMingen). For Th-1 differentiation under skewing conditions,21 naive CD4+ T cells were cultured with 1 μg/mL anti-CD3 plus 2 μg/mL anti-CD28 in the presence of murine IL-12 (10 ng/mL; a generous gift from B. Hubbard, Genetics Institute, Cambridge, MA), IFN-γ (5 μg/mL; PharMingen) and anti–mouse IL-4 mAb (10 μg/mL; clone 11B11). To generate Th-2 under skewing conditions,21 naive CD4+ T cells were cultured with 1 μg/mL anti-CD3 plus 2 μg/mL anti-CD28, 10 ng/mL IL-4 (PEPROTECH EC, London, United Kingdom), 3 μg/mL anti–IL-12 (hybridoma supernatant, clone TOSH) and 10 μg/mL anti–IFN-γ mAbs (clone H22; generous gift from R. D. Schreiber, Washington University, St Louis, MO). On day 7 after priming, cells were restimulated for 6 hours with 1 μg/mL anti-CD3 without adding cytokines or Abs. For nonskewing conditions naive CD4+ T cells were cultured with anti-CD3/CD28 in presence of anti–IFN-γ and anti–IL-12 (Th-2) or anti–IL-4 (Th-1) Abs.36
Enzyme-linked immunosorbent assay (ELISA)
Supernatant was collected and cytokine contents were evaluated by sandwich ELISA. All the Abs specific for IFN-γ, IL-4, and IL-10 and IL-2 were purchased from PharMingen, and the assays were performed following manufacturer's instructions. IL-13 and IL-5 were assayed using ELISA kits as recommended by the manufacturer (RD Systems, Minneapolis, MN). The sensitivity limit was about 15 pg/mL for IL-4 and IL-5, 150 pg/mL for IL-10, 70 pg/mL for IFN-γ, 7 pg/mL for IL-13, and 3 U/mL for IL-2.
Intracellular cytokine staining
To evaluate cytoplasmic IFN-γ and IL-4, we generated Th-1 and Th-2 cells in vitro and then restimulated them with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 1 μM ionomycin for 5 hours in the presence of brefeldin A (1 μg/mL during the final 1.5 hours) (Sigma-Aldrich, Milan, Italy), as previously described.37 Anti-CD4–FITC–, anti–IFN-γ–PE– and anti–IL-4–PE–conjugated Abs were purchased from PharMingen. Isotype-matched irrelevant Abs were used as controls. IFN-γ and IL-4 expression was analyzed by flow cytometer (Beckman Coulter EPICS XL-MClL).
Western blot
Proteins were separated on SDS-polyacrylamide gel. Blots were incubated with GATA-3 (HG3-31) or T-bet (C-19) monoclonal Abs (Santa Cruz Biotechnology, DBA, Milan, Italy), or anti–mouse phospho–extracellular signal–regulated kinase (ERK)–1/2, or anti–mouse ERK-1/2, anti–mouse phospho-Stat6 (tyr 641), or anti–mouse Stat6 (Cell Signaling, Celbio, Milan, Italy), or rabbit polyclonal anti–mouse GILZ,28 and then with horseradish peroxidase (HRP)–conjugated anti–mouse or anti–rabbit immunoglobulin G (IgG; Pierce, Rockford, IL). Anti–β-tubulin Ab (Sigma-Aldrich) was used as control. Proteins were visualized by enhanced chemiluminescence (SuperSignal; Pierce).
In vivo immunization
Dendritic cells, purified as previously described,38 were pulsed overnight with ovalbumin chicken egg (OVA; 50 μg/mL) and injected at the dose of 5 × 105 cells into the hind footpads of each mouse. Draining lymph nodes were harvested 5 days later and CD4+ T cells were restimulated in vitro with OVA (100 μg/mL) using irradiated spleen cells as feeder. Culture supernatants were assayed for cytokine production after 72 hours of incubation.
Reverse transcriptase–polymerase chain reaction (RT-PCR) and real-time PCR
Total RNA was prepared using Trizol from Gibco-BRL (Life Technologies, Paisley, Scotland). RT-PCR was done using Moloney murine leukemia virus (M-MLV) RT (Invitrogen, San Diego, CA). The primers for GATA-3 were: sense 5′-GAAGGCATCCGACCCGAAAC-3′, antisense 5′-ACCCATGGCGGTGACCATGC-3′; GAPDH: sense 5′-CCCACTAACATCAATGGGG-3′, antisense 5′-CCTTCCACAATGCCAAAGTT-3′; GILZ: sense 5′-GTCCCAAGGACATGCCCATCTG-3′, antisense 5′-ACAGTCATTGTCAGGTGAAGCTG-3′; IL-13: sense 5′-CATGGTATGGAGTGTGGACC-3′, antisense 5′-CCAAAGCTGAGGCATCTCCCTT-3′; and Stat6: sense 5′-CTCTATGTTGACTTTCCACA-3, antisense 5′-ATTTCCACCAGGCTTTCACA-3′. Semiquantitative RT-PCR was performed using GAPDH as an internal control to normalize gene expression for PCR templates. PCR product was analyzed using a Kodak image station DC 120 and 1D image analysis software (Eastman Kodak, Rochester, NY).
For real-time PCR, sense primer for GATA-3 was 5′-ACCGGGTTCGGATGTAAGTC-3′, and antisense 5′-AGGCATTGCAAAGGTAGTGC-3′. For T-bet, sense was 5′-ACCACCTGTTGTGGTCCAAG-3′, and antisense 5′-CACCAAGACCACATCCACAA-3′. For GAPDH, sense was 5′-GCCTTCCGTGTTCCTACCC-3′, and antisense 5′-CAGTGGGCCCTCAGATGC-3′. PCR was done in CHROMO 4 (MJ Research Bio Rad, Milan, Italy) using DyNAmo HS SYBR GREEN qPCR kit (Finnzymes; Celbio). Gene expression was quantitated relative to the expression of GAPDH. The value of the relative expression for the WT naive samples are defined as 100%, and all other values are plotted compared with them.
siRNA preparation and transfection
Stat6-specific siRNA duplex was designed as previously described.39 Double-stranded nucleotide siRNA (sense, 5′-GUGAGGUCCUGUUCAGUGGGtt-3′; and antisense, 5′-CCCACUGAACAGGACCUCACtt-3′) were synthesized and annealed by manufacturer (MWG-Biotech AG, Germany). For siRNA transfection we used the highly efficient DOTAP reagent40 (Roche Diagnostic, Mannheim, Germany) previously used in normal cells, including naive CD4+ and CD8+ T cells.41,42 We also evaluated the percentage of transfection efficiency by Fluorescein-siRNA Transfection Control (New England, Beverly, MA) showing that RNA uptake occurred in a detectable range of 30% to 40% of the cells. Nucleotide siRNA (6.7 μg/mL) was added to CD4+ T cells (1 × 106 /mL) 1 hour before and 18 hours after anti-CD3/CD28 activation. The treatment had no toxic effect because cell viability, by trypan blue dye exclusion, was more than 95%.
The BW5147 T-cell line was transfected by electroporation (300 mA, 1500 μF) with 20μg pcDNA3 or pcDNA3 containing GILZ cDNA. After 24 hours, protein and RNA were extracted and analyzed for GILZ, GATA-3, and IL-13 expression by Western blot and semiquantitative RT-PCR, respectively.
Apoptosis evaluation and immunofluorescence staining for nuclear translocation
Luciferase assay
Naive CD4+ T cells (10 × 106) were electroporated (230 V, 75 Ohm, and 1500 μF) with 40 μg pGL3-TK-kBPD44 or 40 μg pAP-1-Luc plasmid (Stratagene, M-Medical, Milan, Italy). pRL-TK reporter plasmid, (1 μg; Promega, Milan, Italy) encoding Renilla luciferase was used as control. Cells were seeded in 48-well plates at a density of 1 × 106 cells/mL. The next day, cells were stimulated with 1 μg/mL anti-CD3 and 2 μg/mL anti-CD28 Abs. Luciferase assays used the dual luciferase reporter assay kit (Promega).
Bleomycin-induced pulmonary fibrosis
To induce pulmonary fibrosis, bleomycin sulfate (Sigma) or saline was given to mice in 3 weekly intratracheal instillations (1 U/kg per dose; total, 3 U/kg) under ketamine anesthesia.45 At 3 weeks after the last bleomycin dose, mice were killed by pentobarbital overdose (250 mg/kg), both lungs were removed, and histology, lung water content, and myeloperoxidase (MPO) activity were analyzed as previously described.46
Histologic examination
Lung biopsy samples were fixed for 1 week in 10% (wt/vol) PBS-buffered formaldehyde solution at room temperature, dehydrated using graded ethanol, and embedded in Paraplast (Sherwood Medical, Mahwah, NJ). Sections were stained with hematoxylin and eosin. All sections were studied by light microscopy (Dialux 22; Leitz, Wetzlar, Germany). Cells were stuck on slides coated with poly-l-lysine and mounted in buffered glycerol for fluorescence microscopic analysis. Photographs were taken on a Leitz Dialux 20 microscope with a SPOT-RT 2.2.1 COLOR camera (Diagnostic Instruments, Sterling Heights, MI) and SPOT software, v.3.4.5, for Windows.
DTH response
Mice were immunized by subcutaneous inoculations at the base of tail with OVA (150 μg) emulsified 1:1 in complete Freund adjuvant (CFA; Sigma-Aldrich) in a total volume of 100 μL.37 At day 15 mice were challenged with 300 μg OVA in 50 μL PBS in the left hind footpad. The right hind footpad was injected with a vehicle control (50 μL PBS). Responses were quantified 24 hours after challenge by measuring the difference in footpad thickness with a digital micrometer (Mitutoyo, Tokyo, Japan).
Statistical analysis
Data are presented as the mean plus or minus standard error (SEM) of N observation. For the in vivo studies, N represents the number of animals studied. In the histology experiments, the figures are representative of at least 3 experiments. Data sets were examined by 1- or 2-way analysis of variance; individual group means were compared with Student unpaired t test. P values of less than .05 were considered significant.
Results
TG CD4+ T lymphocytes produce more Th-2 lymphokines
In TG mice, murine Gilz is controlled by the T-cell–specific human CD2 promoter and enhancer that allow GILZ overexpression in all thymocyte subsets and in mature peripheral CD4+ and CD8+ T cells.31 We investigated production of Th-1 and Th-2 cytokines by splenic CD4+ naive T cells from TG and WT mice, using well-established experimental protocols.21,35,36 Lymphoid organ size and percentages of T and B cells and of the CD4/CD8 subset overlapped in TG and WT mice (not shown).
Freshly isolated naive CD4+CD62Lhigh lymphocytes from TG and WT littermates were activated in vitro under default conditions with anti-CD3/anti-CD28 Abs (Th-0)35 for different times; cell lysates were analyzed for GILZ expression and supernatants tested for cytokine production. Western blot showed that the basal levels of GILZ protein, higher in TG than WT cells, increased in both groups 3 hours after activation and the differences between TG and WT cells were maintained (Figure 1A).
Supernatant analysis by ELISA assay showed that TG lymphocytes produced more IL-4, IL-5, IL-13, and IL-10 than WT CD4+ lymphocytes. IFN-γ production was slightly but significantly lower, at 24 hours, in TG cells; IL-2 production was similar in both groups (Figure 1B).
No detectable cytokine production was found in unstimulated CD4+ lymphocytes, and activation levels, as evaluated by IL-2R staining, were comparable in both groups (not shown). As GILZ rescues T cells from activation-induced cell death (AICD),28 we evaluated the AICD of CD4+ cells differentiated for 7 days under default conditions and restimulated for 24 hours by anti-CD3 Ab by measuring apoptosis. Concurring with previously published data,31 apoptosis was lower in TG than WT cells. In particular, apoptosis percentage, evaluated by propidium iodide assay,43 was 45% ± 4% in TG and 68% ± 3% in WT (mean of 3 different experiments ± SEM), thus indicating a significantly (P < .01) lower apoptosis in TG.
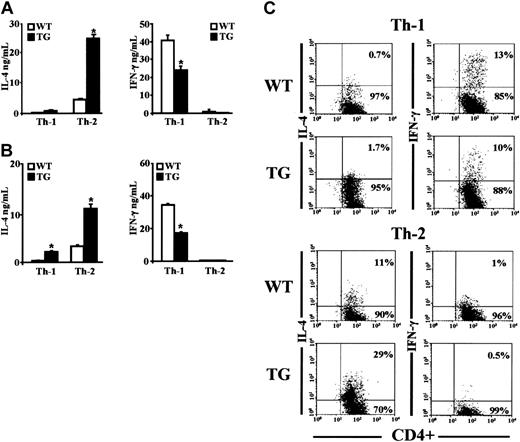
We differentiated CD4+ TG and WT cells in vitro under skewing conditions (ie, in the presence of IL-4, anti–IL-12, and anti–IFN-γ Abs [Th-2 conditions] or IL-12, IFN-γ, and anti–IL-4 Ab [Th-1 conditions]).16,19,21,35,36 To obtain a fully differentiated phenotype, cells were cultured for 7 days, washed, and triggered for 6 hours with anti-CD3 mAbs, and supernatants analyzed for IL-4 and IFN-γ content. Under Th-2 culture conditions cells produced IL-4 but not IFN-γ (Figure 2A). Th-2 cells from TG mice produced more IL-4 than those from WT mice (Figure 2A, left panel). Under Th-1 conditions, cells produced IFN-γ but not IL-4. Th-1 cells from TG mice produced significantly less IFN-γ than Th-1 cells from WT mice (Figure 2A, right panel).
Lymphokine production in CD4+ cells cultured under Th-0 conditions. Naive CD4+ CD62Lhigh T cells were isolated by cell sorter from the spleens of wild-type (WT) and transgenic (TG) littermate mice and stimulated with anti-CD3/anti-CD28 Abs. (A) Protein extracts from freshly naive CD4+ T cells (0 hours) and activated T cells (3 hours) were processed for immunoblot analysis using anti-GILZ Ab. The same extracts were reprobed with anti–β-tubulin Ab. (B) The culture supernatants were collected at 24 and 48 hours and the cytokine concentrations were determined by ELISA. Bars represent the mean values ± 1 SEM of 3 independent experiments. *P < .05 represents significant differences of TG vs WT mice.
Lymphokine production in CD4+ cells cultured under Th-0 conditions. Naive CD4+ CD62Lhigh T cells were isolated by cell sorter from the spleens of wild-type (WT) and transgenic (TG) littermate mice and stimulated with anti-CD3/anti-CD28 Abs. (A) Protein extracts from freshly naive CD4+ T cells (0 hours) and activated T cells (3 hours) were processed for immunoblot analysis using anti-GILZ Ab. The same extracts were reprobed with anti–β-tubulin Ab. (B) The culture supernatants were collected at 24 and 48 hours and the cytokine concentrations were determined by ELISA. Bars represent the mean values ± 1 SEM of 3 independent experiments. *P < .05 represents significant differences of TG vs WT mice.
Lymphokine production in CD4+ cells cultured under Th-1 and Th-2 polarizing conditions. (A) Naive CD4+CD62Lhigh T cells, isolated from spleens of TG and WT littermate mice, were differentiated under Th-1 and Th-2 skewing conditions (described in “Materials and methods”). After 7 days, the cells were washed and restimulated by anti-CD3 Ab for 6 hours. (B) Naive T cells were cultured for 7 days under nonskewing conditions with anti-CD3/anti-CD28 Ab in the presence of anti–IL-4 antibody for Th-1 or anti–IFN-γ and anti–IL-12 Abs for Th-2. Cells were washed and restimulated with anti-CD3 antibody for 6 hours. The amounts of cytokines secreted into the supernatants were measured by ELISA. Averages ± 1 SEM of 6 independent experiments are shown. *P < .05, TG vs WT. (C) Naive CD4+CD62Lhigh T cells from WT and TG littermates were differentiated for 7 days under Th-1 and Th-2 skewing conditions and restimulated with PMA (50 ng/mL) and inomycin (1 μM) for 5 hours. Intracellular cytokine staining was performed using anti-CD4–FITC antibody together with either anti–IFN-γ–PE or anti–IL-4–PE Abs. Control isotypes are omitted for clarity. Data are representative of 4 independent experiments. Numbers represent the percent of cells positive (upper right corner) or negative (lower right corner) for IL-4 or IFN-γ.
Lymphokine production in CD4+ cells cultured under Th-1 and Th-2 polarizing conditions. (A) Naive CD4+CD62Lhigh T cells, isolated from spleens of TG and WT littermate mice, were differentiated under Th-1 and Th-2 skewing conditions (described in “Materials and methods”). After 7 days, the cells were washed and restimulated by anti-CD3 Ab for 6 hours. (B) Naive T cells were cultured for 7 days under nonskewing conditions with anti-CD3/anti-CD28 Ab in the presence of anti–IL-4 antibody for Th-1 or anti–IFN-γ and anti–IL-12 Abs for Th-2. Cells were washed and restimulated with anti-CD3 antibody for 6 hours. The amounts of cytokines secreted into the supernatants were measured by ELISA. Averages ± 1 SEM of 6 independent experiments are shown. *P < .05, TG vs WT. (C) Naive CD4+CD62Lhigh T cells from WT and TG littermates were differentiated for 7 days under Th-1 and Th-2 skewing conditions and restimulated with PMA (50 ng/mL) and inomycin (1 μM) for 5 hours. Intracellular cytokine staining was performed using anti-CD4–FITC antibody together with either anti–IFN-γ–PE or anti–IL-4–PE Abs. Control isotypes are omitted for clarity. Data are representative of 4 independent experiments. Numbers represent the percent of cells positive (upper right corner) or negative (lower right corner) for IL-4 or IFN-γ.
In vitro polarization experiments under nonskewing conditions (ie, without exogenous cytokines, but with antibodies neutralizing the cytokine responsible for the development of the opposite phenotype)36 showed again that TG Th-2 cells produced more IL-4 than WT Th-2 cells, and TG Th-1 cells produced less IFN-γ than WT Th-1 cells (Figure 2B). Despite the Th-1 culture conditions, TG Th-1 effectors produced a detectable amount of IL-4 (Figure 2B, left panel). Low IFN-γ production by TG Th-1 effectors may be due to increased IL-4 production, as an excess of neutralizing anti–IL-4 Ab (15 μg/mL) brought TG IFN-γ levels up to that of WT effectors (data not shown).
Intracellular staining to detect the frequency of IL-4-and IFN-γ–producing cells of fully differentiated TG and WT Th-1 and Th-2 effectors, generated under skewing conditions, showed more IL-4–producing cells and fewer IFN-γ–producing cells in TG than in WT mice. The results of a representative experiment are shown in Figure 2C. No IL-4– or IFN-γ–positive cells were found in freshly isolated CD4+CD62Lhigh TG and WT cells (data not shown). These results suggest that the Gilz transgene commits CD4+ lymphocyte toward a Th-2 phenotype.
OVA-immunized TG mice produce more Th-2 cytokines
We next investigated the TG mice lymphokine response in vivo upon antigen immunization. TG and WT mice received injections of dendritic cells pulsed with OVA in the footpads. CD4+ cells were separated from draining lymph nodes 5 days later and were restimulated in vitro for 72 hours with OVA antigen presented by irradiated syngeneic spleen cells. Figure 3 shows OVA-primed TG cells secreted significantly more IL-4 than WT cells, which further increased after in vitro OVA rechallenge. Secretion of IL-5, IL-10, and IL-13 displayed the same profile. OVA-restimulated TG cells produced less IFN-γ than WT cells (Figure 3), suggesting the Gilz transgene shifts the balance of Th-1/Th-2 effectors toward a Th-2 phenotype.
TG CD4+ lymphocytes express more GATA-3 and less T-bet transcription factors
GATA-3 controls coordinated expression of IL-4, IL-5, and IL-13 by interacting with an intergenic site in the Th-2 cytokine locus containing the IL-4, IL-5, and IL-13 gene clusters.47,48 The correlation between GATA-3 and IL-10 production is indirectly suggested by knockout (KO) mice phenotype.22 We investigated whether GILZ-induced Th-2 lymphokine up-regulation correlates with increased GATA-3 expression. As shown in Figure 4A, unstimulated CD4+ naive T cells constitutively expressed a low but detectable level of GATA-3. When the cells were cultured under default conditions, GATA-3 expression increased in a time-dependent manner, reaching a higher level in TG than in WT cells at day 1. After 7 days of culture, when cells were washed and restimulated with anti-CD3 Ab for 6 hours, increased GATA-3 expression persisted, with expression in TG cells still slightly higher than in WT cells (Figure 4A). Although the increase in GATA-3 expression was not dramatic, it was consistently reproducible in repeated experiments. T-bet expression increased over time but was lower in TG than in WT cells. Consequently, the GATA-3/T-bet ratio was higher in TG than in WT cells (Figure 4A), confirming previous observation that in the mixed-cell population, generated under default conditions, the ratio of these transcription factors reflects the Th-1/Th-2 balance.49 We used real-time quantitative PCR to measure expression of mRNA for GATA-3 and T-bet. Figure 4B shows that, although anti-CD3/CD28 increased GATA-3 and T-bet expression by days 1 and 2 in WT and TG cells, significantly higher GATA-3 and lower T-bet expressions were observed in TG cells.
Up-regulation of Th-2 cytokines in OVA-challenged TG mice. TG and WT mice received intradermal injections into the hind footpads with OVA-pulsed DCs. CD4+ T cells from draining lymph nodes that were harvested 5 days later were cultured for 72 hours in the presence of either OVA (100 μg/mL) or medium. Cytokine content of supernatants was assessed in ELISA assays. Bars represent the mean values ± 1 SEM from 3 independent experiments. *P < .05, TG vs WT.
Up-regulation of Th-2 cytokines in OVA-challenged TG mice. TG and WT mice received intradermal injections into the hind footpads with OVA-pulsed DCs. CD4+ T cells from draining lymph nodes that were harvested 5 days later were cultured for 72 hours in the presence of either OVA (100 μg/mL) or medium. Cytokine content of supernatants was assessed in ELISA assays. Bars represent the mean values ± 1 SEM from 3 independent experiments. *P < .05, TG vs WT.
Next, we compared GATA-3 and T-bet expression in freshly isolated CD4+ T cells from regional lymph nodes of TG and WT mice that had received injections of OVA. GATA-3 expression was higher and T-bet lower in CD4+ TG T cells than in CD4+ WT cells (Figure 4C). This last finding provides evidence that CD4+ T cells had already been committed to produce Th-2 cytokines before the in vitro restimulation.
Expression of GATA-3 and T-bet. (A) Naive TG and WT CD4+CD62Lhigh T cells were cultured with anti-CD3/anti-CD28 Abs for the times indicated. On day 7, cells were washed and restimulated for 6 hours with anti-CD3 Ab. Protein extracts from freshly naive T cells (day 0) and cultured cells (days 1 and 7) were processed for immunoblot analysis using anti–GATA-3 Ab. The same extracts were re-probed with anti–T-bet and anti–β-tubulin Abs. Quantitative analysis of GATA-3/β-tubulin, T-bet/β-tubulin, and GATA-3/T-bet ratios are shown. (B) GATA-3 and T-bet expression, evaluated by real-time quantitative PCR, in CD4+CD62Lhigh freshly isolated or cultured with anti-CD3/anti-CD28 Abs for the indicated times. Relative expression is shown. Data are the mean values ± 1 SEM. *P < .05, TG vs WT. (C) GATA-3 and T-bet expression (as evaluated by Western blot analysis) in freshly isolated CD4+ T cells from regional lymph nodes of OVA-immunized mice or control mice. Western blot with β-tubulin was performed to ensure that equivalent amounts of proteins were loaded in each lane. Data are representative of 2 independent experiments. (D) The BW5147 cell line was transfected with pcDNA3-GILZ or pcDNA3 empty vector. Western blot analyses of GILZ and β-tubulin proteins are shown with the histogram of the GILZ/β-tubulin ratio (Di). mRNA expression was measured by semi-quantitative RT-PCR for GILZ (Dii), GATA-3 (Diii), and IL-13 (Div). RT-PCR data were normalized by 1D image analysis software to GAPDH expression and presented as mean ± 1 SEM of duplicate samples from 3 independent experiments. *P < .05 GILZ-transfected vs empty-vector–transfected cells.
Expression of GATA-3 and T-bet. (A) Naive TG and WT CD4+CD62Lhigh T cells were cultured with anti-CD3/anti-CD28 Abs for the times indicated. On day 7, cells were washed and restimulated for 6 hours with anti-CD3 Ab. Protein extracts from freshly naive T cells (day 0) and cultured cells (days 1 and 7) were processed for immunoblot analysis using anti–GATA-3 Ab. The same extracts were re-probed with anti–T-bet and anti–β-tubulin Abs. Quantitative analysis of GATA-3/β-tubulin, T-bet/β-tubulin, and GATA-3/T-bet ratios are shown. (B) GATA-3 and T-bet expression, evaluated by real-time quantitative PCR, in CD4+CD62Lhigh freshly isolated or cultured with anti-CD3/anti-CD28 Abs for the indicated times. Relative expression is shown. Data are the mean values ± 1 SEM. *P < .05, TG vs WT. (C) GATA-3 and T-bet expression (as evaluated by Western blot analysis) in freshly isolated CD4+ T cells from regional lymph nodes of OVA-immunized mice or control mice. Western blot with β-tubulin was performed to ensure that equivalent amounts of proteins were loaded in each lane. Data are representative of 2 independent experiments. (D) The BW5147 cell line was transfected with pcDNA3-GILZ or pcDNA3 empty vector. Western blot analyses of GILZ and β-tubulin proteins are shown with the histogram of the GILZ/β-tubulin ratio (Di). mRNA expression was measured by semi-quantitative RT-PCR for GILZ (Dii), GATA-3 (Diii), and IL-13 (Div). RT-PCR data were normalized by 1D image analysis software to GAPDH expression and presented as mean ± 1 SEM of duplicate samples from 3 independent experiments. *P < .05 GILZ-transfected vs empty-vector–transfected cells.
We also tested the effect of GILZ overexpression on GATA-3 and Th-2 lymphokine up-regulation by using a GILZ-transfected T-cell line, the BW5147 thymoma, an IL-13–producing cell line. GILZ overexpression (Figure 4Di-ii) up-regulated GATA-3 (Figure 4Diii) and IL-13 (Figure 4Div) mRNA compared with an empty vector-transfected control, further suggesting GILZ modulates GATA-3 and IL-13 expression. These results indicate that in TG CD4+ cells, GILZ contributes to GATA-3 up-regulation and to Th-2 lymphokine production.
TG CD4+ lymphocytes express more Stat6 transcription factor and less NF-κB transcriptional activity
Stat6, activated by IL-4, has been found to be critical for the function and development of Th-2 cells.50 Therefore, we tested Stat6 expression and phosphorylation in CD4+ cells at early times after CD3/CD28 activation. Figure 5A shows that Stat6 protein level was higher in freshly isolated CD4+ TG than WT cells, and that anti-CD3/CD28 activation up-regulated Stat6 expression in TG cells. Stat6 phosphorylation displayed the same profile. Anti–IL-4 Ab, although reverted the increased Stat6 phosphorylation, did not affect protein expression (Figure 5A).
We also evaluated Stat6 mRNA levels in fresh CD4+ cells from TG and WT mice. Results, expressed as Stat6/GAPDH ratio, indicate that, similar to that of protein, levels of Stat6 mRNA were significantly higher in TG compared with WT mice (Figure 5A). To address whether the Stat6 up-regulation was responsible for the increased GATA-3 expression in TG cells, we treated the cells with the Stat6 specific siRNA39 prior to anti-CD3/anti-CD28 activation. The depletion of Stat6 resulted in a decreased expression of GATA-3 (Figure 5B). These results suggest that GILZ can regulate Stat6 and that Stat6 levels, other than its phosphorylation, may contribute to Th2 differentiation.
Expression and phosphorylation of Stat6. (A) Naive TG and WT CD4+ CD62Lhigh T cells were cultured with anti-CD3/anti-CD28 Abs in the presence or in absence of anti–IL-4 Ab (10 μg/mL) for the indicated times. Protein extracts were processed for immunoblot analysis using anti-Stat6 Ab (top lane) or anti–phospho-Stat6 (pStat6) (middle lane). Quantitative analysis of Stat6/β-tubulin and pStat6/β-tubulin ratio is shown. Data shown are representative of 2 independent experiments. Quantitative analysis of Stat6/GAPDH, as evaluated by RT-PCR, is shown; bars represent the mean values ± 1 SEM of 2 independent experiments. **P < .01, TG vs WT. (B) CD4+ cells were transfected with siRNAs specific for Stat6 and, 1 hour later, activated by anti-CD3/anti-CD28 Abs for 24 hours. Western blot was analyzed by specific Abs for Stat6 and, after stripping for GATA-3 and β-tubulin. Quantitative analysis of Stat6/β-tubulin and GATA-3/β-tubulin is shown.
Expression and phosphorylation of Stat6. (A) Naive TG and WT CD4+ CD62Lhigh T cells were cultured with anti-CD3/anti-CD28 Abs in the presence or in absence of anti–IL-4 Ab (10 μg/mL) for the indicated times. Protein extracts were processed for immunoblot analysis using anti-Stat6 Ab (top lane) or anti–phospho-Stat6 (pStat6) (middle lane). Quantitative analysis of Stat6/β-tubulin and pStat6/β-tubulin ratio is shown. Data shown are representative of 2 independent experiments. Quantitative analysis of Stat6/GAPDH, as evaluated by RT-PCR, is shown; bars represent the mean values ± 1 SEM of 2 independent experiments. **P < .01, TG vs WT. (B) CD4+ cells were transfected with siRNAs specific for Stat6 and, 1 hour later, activated by anti-CD3/anti-CD28 Abs for 24 hours. Western blot was analyzed by specific Abs for Stat6 and, after stripping for GATA-3 and β-tubulin. Quantitative analysis of Stat6/β-tubulin and GATA-3/β-tubulin is shown.
As GILZ interacts with TCR-activated transcription factors,32,33 known to affect Th differentiation, we investigated whether GILZ interferes with NF-κB function in TG cells. We used promoter-driven expression of luciferase activity in CD4+ naive cells transfected with the reporter plasmid pGL3-TK-kBPD, containing 2 palindromic NF-κB sites, and stimulated with anti-CD3/anti-CD28 Abs for 2, 6, and 24 hours. Luciferase activity was significantly less in CD4+ TG than in CD4+ WT cells (Figure 6A). The minor NF-κB transcriptional activity correlated with the inhibition of NF-κB nuclear translocation in TG CD4+ cells (Figure 6B), suggesting that GILZ affects NF-κB activity.
To investigate the effect of GILZ on AP-1 activity,33 CD4+ TG and WT cells were transfected with the AP1-Luc reporter plasmid. Activation of AP-1–dependent transcription by anti-CD3/anti-CD28 was comparable in WT and TG cells (Figure 6C), and ERK activation followed the same behavior (Figure 6D), suggesting that, in this experimental model, GILZ did not interfere with mitogen-activated protein kinase (MAPK) activity.
GILZ overexpression increases bleomycin-induced lung injury response and decreases OVA-induced DTH response
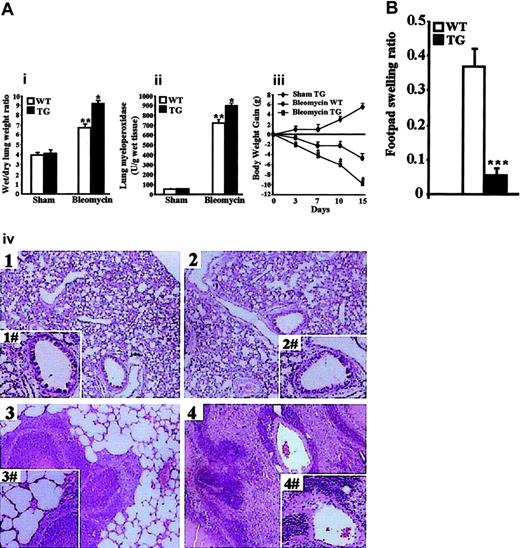
In vivo administration of bleomycin elicits a Th-2 response with IL-4 up-regulation, which activates mononuclear cells and leads to neutrophil infiltration, edema, and collagen synthesis in the lung tissues.45,51,52 After bleomycin treatment, inflammatory response and loss in body weight were evident in TG and WT mice. Figure 7Ai-ii shows the wet/dry lung weight ratio and lung MPO were higher in TG than in WT mice, indicating edema formation and neuthrophil infiltration were significantly greater in TG mice. Loss in body weight was significantly greater in TG mice. (Figure 7Aiii; only sham TG is shown in the figure). Histologic examination of lung sections revealed significant tissue damage, with edema and neutrophil infiltration in all bleomycin-treated mice (Figure 7Aiv, bottom panels). However, TG mice showed more severe lung injury with greater inflammatory cell infiltration and edema (Figure 7Aiv, bottom right panel vs bottom left panel). No histological abnormalities and inflammatory cell infiltration were observed in the sham groups (Figure 7Aiv, top panels). These data indicate the bleomycin elicits a more intense Th-2 inflammatory response and a more severe disease in TG than in WT mice.
In vivo administration of OVA antigen in CFA elicits a Th-1–dependent DTH response.53 We compared the DTH response in TG and WT mice. WT and TG mice were primed by intradermal injection with OVA in CFA and challenged on day 15 by injection of PBS or PBS containing OVA in the left and right hind footpad, respectively. Footpad swelling, measured 24 hours later, was significantly less in TG than in WT mice (Figure 7B), indicating less intense DTH response in TG mice.
Together, these results showing the inhibition of the Th-1–mediated DTH response and the worsening of the Th-2–mediated lung injury in TG mice suggest GILZ transgene contributes to in vivo CD4+ cell commitment toward Th-2 phenotype.
Discussion
Evidence from naive CD4+ TG lymphocyte differentiation in vitro, under skewing and nonskewing conditions, and in vivo in OVA-stimulated mice, converges to demonstrate that GILZ drives CD4+ lymphocytes toward a Th-2 phenotype.
Differentiated CD4+ TG lymphocytes, with their increased frequency of IL-4–producing cells as indicated by IL-4 intracellular staining, produced more IL-4, the lymphokine favoring Th-2 response, than littermate WT mice. Confirming that an up-regulated Th-2 response induces down-regulation of Th-1 response,21,36 CD4+ TG cells produced less IFN-γ, the lymphokine involved in Th-1 response. Our observations support the hypothesis that CD4+ TG cell overproduction of IL-4 affects Th-1 development. In the first place, in the in vitro nonskewing Th-1 differentiation assay, Th-1 TG cells showed diminished IFN-γ production and, despite the Th-1 environment, IL-4 production, while Th-1 WT cells displayed IFN-γ production and no IL-4 production. Second, excess neutralizing anti–IL-4 Ab reported TG IFN-γ levels at that of WT effectors. These data suggest that GILZ overexpression, regardless of the culture conditions (Th-1 or Th-2 conditions) induces an increased frequency of IL-4–producing cells and regulates Th-1/Th-2 effector commitment. In fact, TG mice developed more severe bleomycin-induced pulmonary fibrosis, a Th-2–dependent experimental disease, and weaker DTH, a Th-1–dependent reaction.
TG CD4+ lymphocytes display less NF-κB transcriptional activity. CD4+ cells from TG and WT mice were transfected with pRL-TK in combination with either (A) pGL3-TK-kBPD or (C) AP-1–Luc reporter plasmid. After 24 hours, cells were stimulated with anti-CD3/anti-CD28 Abs for the indicated times and luciferase activity was assessed. Results are expressed as fold induction (mean ± 1 SEM of 3 independent experiments) of the sample incubated with anti-CD3/anti-CD28 Abs versus the corresponding untreated sample, with the control value being 1. *P < .05; **P < .01. (B) Immunofluorescence analysis of NF-κB in TG and WT CD4+ cells activated with anti-CD3/anti-CD28 Abs for 6 hours, and stained with anti-p65 Ab by paraformaldehyde-saponin procedure. Images were captured with a Leitz Dialux 20 microscope using a 100×/1.25 NA oil objective. (D) ERK phosphorylation was evaluated in CD4+ cells stimulated for 15 or 30 minutes with anti-CD3/anti-CD28 Abs. Whole-cell lysates were probed with anti-phospho–ERK-1/2 (pERK-1/2) or with anti–ERK-1/2 Abs.
TG CD4+ lymphocytes display less NF-κB transcriptional activity. CD4+ cells from TG and WT mice were transfected with pRL-TK in combination with either (A) pGL3-TK-kBPD or (C) AP-1–Luc reporter plasmid. After 24 hours, cells were stimulated with anti-CD3/anti-CD28 Abs for the indicated times and luciferase activity was assessed. Results are expressed as fold induction (mean ± 1 SEM of 3 independent experiments) of the sample incubated with anti-CD3/anti-CD28 Abs versus the corresponding untreated sample, with the control value being 1. *P < .05; **P < .01. (B) Immunofluorescence analysis of NF-κB in TG and WT CD4+ cells activated with anti-CD3/anti-CD28 Abs for 6 hours, and stained with anti-p65 Ab by paraformaldehyde-saponin procedure. Images were captured with a Leitz Dialux 20 microscope using a 100×/1.25 NA oil objective. (D) ERK phosphorylation was evaluated in CD4+ cells stimulated for 15 or 30 minutes with anti-CD3/anti-CD28 Abs. Whole-cell lysates were probed with anti-phospho–ERK-1/2 (pERK-1/2) or with anti–ERK-1/2 Abs.
Even though information has been acquired about the processes underlying Th-1/Th-2 polarization, not all the molecular mechanisms have been clarified.17 It is known, for example, that microenvironment IL-4, through Stat6 activation, is required for Th-2 development. Nevertheless, IL-4 production could occur in vivo in Stat6-deficient mice, or Th-2 response develops in mice with defects in IL-4 signaling.50,54 The transcription factor GATA-3 is selectively expressed by Th-2 effectors and enforced expression of GATA-3 induces Th-2 differentiation while inhibiting T-bet expression and Th-1 differentiation,20-23 but its action on Th1 down-regulation is independent from IL-4 and involves repression of the IL-12 signal.55 In investigating the way GILZ induces Th-2 polarization, we found that GATA-3 and Stat6 expression was higher in TG than in WT cells because CD4+ TG cells, whether differentiated in vitro or freshly isolated from OVA-challenged mice, expressed more GATA-3 and less T-bet, and freshly isolated CD4+ TG cells expressed more Stat6 and, as consequence, displayed upon activation higher level of phosphorylated Stat6. The reduction of GATA-3 protein in the Stat6 siRNA-treated TG cells strongly suggests that GILZ modulates GATA-3 indirectly via Stat6 up-regulation. It has been previously reported that GILZ may act as a transcription regulator by interacting with a number of transcription factors, thus regulating gene expression.28,32,34 Results here suggest that GILZ increases Stat6 expression and that the levels of Stat6 may be relevant for Th2 differentiation. As T-bet plays a role in Th-1 cell differentiation, the ratio of GATA-3 to T-bet determines the Th-1/Th-2 balance.24-26,49 Our findings suggest that, by modulating GATA-3/T-bet expression, the GILZ transgene favors Th-2 commitment.
More severe bleomycin-induced lung injury and diminished DTH response in TG mice. (A) TG and WT mice were given bleomycin as described in “Materials and Methods.” (Ai) Lung water content, calculated by subtracting dry weight from wet weight. (Aii) MPO activity was evaluated 3 weeks after the last bleomycin injection. **P < .01 column 3 vs columns 1 and 2 (sham group); *P < .05 column 4 vs column 3 in panel Ai-Aii graphs. (Aiii), body weight gain of sham TG vs bleomycin-treated WT and TG mice. Data are expressed as mean ± 1 SEM from 6 mice for each group. *P < .05 bleomycin-treated TG vs bleomycin-treated WT. (Aiv), histologic evaluation by light microscopy of hematoxylin/eosin-stained lung section of sham WT (top left panel), sham TG (top right panel), bleomycin-treated WT (bottom left panel), and bleomycin-treated TG (bottom right panel) shows more interstitial injury and cellular infiltration in TG than in WT mice. Original magnification: × 125; insets, × 500. Images were captured with a Leitz Dialux 22 microscope with a 100×/1.25 NA oil objective (inserts were taken with a 200×/2.5 NA objective) and Axiocam Image System camera and software. The figure is representative of at least 3 independent experiments. (B) Ratios of footpad swelling of OVA-injected over PBS-injected footpads in WT mice (n = 7) and TG mice (n = 6) 24 hours after rechallenge OVA injection. Data are the mean values ± 1 SEM. ***P < .001.
More severe bleomycin-induced lung injury and diminished DTH response in TG mice. (A) TG and WT mice were given bleomycin as described in “Materials and Methods.” (Ai) Lung water content, calculated by subtracting dry weight from wet weight. (Aii) MPO activity was evaluated 3 weeks after the last bleomycin injection. **P < .01 column 3 vs columns 1 and 2 (sham group); *P < .05 column 4 vs column 3 in panel Ai-Aii graphs. (Aiii), body weight gain of sham TG vs bleomycin-treated WT and TG mice. Data are expressed as mean ± 1 SEM from 6 mice for each group. *P < .05 bleomycin-treated TG vs bleomycin-treated WT. (Aiv), histologic evaluation by light microscopy of hematoxylin/eosin-stained lung section of sham WT (top left panel), sham TG (top right panel), bleomycin-treated WT (bottom left panel), and bleomycin-treated TG (bottom right panel) shows more interstitial injury and cellular infiltration in TG than in WT mice. Original magnification: × 125; insets, × 500. Images were captured with a Leitz Dialux 22 microscope with a 100×/1.25 NA oil objective (inserts were taken with a 200×/2.5 NA objective) and Axiocam Image System camera and software. The figure is representative of at least 3 independent experiments. (B) Ratios of footpad swelling of OVA-injected over PBS-injected footpads in WT mice (n = 7) and TG mice (n = 6) 24 hours after rechallenge OVA injection. Data are the mean values ± 1 SEM. ***P < .001.
Although the cytokine environment is the major factor influencing CD4+ T-cell differentiation, signals delivered by TCR and costimulatory molecules influence Th-1/Th-2 development.15 A combination of TCR and CD28 signaling activates multiple transcription factors, including AP-1, which impacts on T-cell activation. Interestingly, through direct binding to AP-133 or to Raf-1, GILZ overexpressed in 3DO clones or in the COS cell line inhibits MAPK-ERK (MEK)–ERK and AP-1 transactivation.34 We here demonstrated that TG and WT CD4+ cells displayed similar levels of ERK phosphorylation and AP-1 transactivation, suggesting that in this experimental model, GILZ does not affect the MAPK pathway. While the in vitro transfection model allows the analysis of GILZ interaction with a single overexpressed transduction pathway, in normal T cells all pathways affected by GILZ may cross-talk each other with homeostatic adjustments, resulting in a different outcome. On the other hand, evidence of the ERK role in modulating IL-4 gene expression is contradictory. Inhibition is reported to decrease IL-4 production,56 but inhibition of ERK-1/ERK-2 is said to elevate levels of IL-4, IL-5, and IL-13, the Th-2 cytokines.57 Furthermore, inhibition of the ERK signaling pathway during agonist peptide priming of naive CD4+ cells alters AP-1 composition, inhibits c-fos protein, and causes early IL-4 gene expression and Th-2 differentiation.58
TCR and CD28 signaling also activate NF-κB, another transcription factor involved in T-cell activation. Remarkably, GILZ inhibits NF-κB nuclear translocation and transcriptional activity. When overexpressed in transfected T-cell lines or up-regulated in DEX-treated thymocytes and peripheral T lymphocytes, GILZ directly binds to p65 and p52 and inhibits NF-κB activation,32 and we here demonstrated that p65 nuclear translocation and NF-κB activation is inhibited in TG cells. The role of NF-κB in Th differentiation is not yet fully understood. NF-κB transactivation has been associated with a preferential switch toward the Th-1 phenotype, because c-Rel and Rel-B KO mice have a deficient IFN-γ response.59,60 Moreover, transgenic mice, which overexpress the dominant-negative form of I-κB, with inhibited NF-κB signaling pathway, develop normal Th-2 responses but impaired Th-1 response.61 On the other hand, p50 NF-κB is critical for Th-2 differentiation because CD4+ cells from P50-/- mice do not express GATA-3 and do not produce Th-2 cytokines,62 suggesting that different NF-κB members modulate the Th-2 phenotype although their specific roles have not been defined. GILZ, depending on expression levels, may interact with Raf or NF-κB, respectively (Barbara Di Marco, Michela Massetti, Antonio Macchiarulo, Stefano Bruscoli, G. M., and C. R. manuscript in preparation), and we suggest that in transgenic CD4+ cells, GILZ, by interacting with NF-κB, may contribute to Th differentiation. Moreover, GILZ could participate in Th-2 differentiation and control Th-1/Th-2 balance through modulation of more than 1 signal and transcription factor. More studies, including backcrossing of TG with other genetically manipulated mice, will clarify the relative impact of each molecular mechanism.
GILZ-driven Th-2 differentiation may be one of the molecular mechanisms underlying to GCH-driven Th differentiation. In fact, GCHs modulate cytokine and cytokine receptor expression, regulate survival of T cells, and impact on T-cell proliferation in response to TCR triggering.1-6 GCH treatment of human naive CD4+ T cells stimulates IL-4 and IL-10 production and suppresses IFN-γ production.63 Several different mouse experimental models indicate GCHs promote the Th-2 response by up-regulating IL-4, IL-10, and IL-13, and inhibit Th-1 responses by blocking production of IL-12, IFN-γ, and TNF-α.9,64 In mice DEX preferentially suppresses IFN-γ but not IL-4, and favors Th-2 development.10,65 Despite these findings that the effects of GCHs on T lymphocytes depend on the concomitant presence of signals such as those delivered by TCRs, coaccessory molecules, and cytokines, and thus on the consequent activation state of the cell, the mechanism of GCHs on Th cell differentiation is not yet fully understood, although the final outcome of an immune response is known to require cross-talk between GCHs and environment cytokines.66 As Th differentiation is based on the intrinsic Th-1/Th-2 balance rather than the absolute amount of 1 of these T-cell subpopulations, it is plausible to hypothesize that the combined effect of GCHs on several cytokines may drive an adaptive response to environmental changes. GCH-induced GILZ up-regulation may mediate this event, at least in part.
Understanding the mechanisms responsible for GCH's effects on Th differentiation could open the way for a new pharmacologic approach. Targeting GILZ, for example, could inhibit a Th-2 response and spare a Th-1 response, where this response would be therapeutically helpful.
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2005-05-2183.
Supported by Associazione Italiana Ricerca sul Cancro (AIRC), Milan, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal