Abstract
Immune responses to pathogens need to be maintained within appropriate levels to minimize tissue damage, whereas such controlled immunity may allow persistent infection of certain types of pathogens. Interleukin 10 (IL-10) plays an important role in such immune regulation. We previously showed that HSV-stimulated human plasmacytoid dendritic cells (pDCs) induced naive CD4+ T cells to differentiate into interferon γ (IFN-γ)/IL-10–producing T cells. Here we show that HSV-stimulated pDCs induce allogeneic naive CD4+ T cells to differentiate into cytotoxic regulatory T cells that poorly proliferate on restimulation and inhibit proliferation of coexisting naive CD4+ T cells. IL-3–stimulated pDCs or myeloid DCs did not induce such regulatory T cells. Both IFN-α and IL-10 were responsible for the induction of anergic and regulatory properties. High percentages of CD4+ T cells cocultured with HSV-stimulated pDCs, and to a lesser extent those cocultured with IL-3–stimulated pDCs, expressed granzyme B and perforin in an IL-10–dependent manner. CD4+ T cells cocultured with HSV-stimulated pDCs accordingly exhibited cytotoxic activity. The finding that virus-stimulated pDCs are capable of inducing CD4+ cytotoxic regulatory T cells suggests that this DC subset may play an important role in suppressing excessive inflammatory responses and also in inducing persistent viral infection.
Introduction
Maintaining the strength of immune responses to pathogens within appropriate levels is important in eliminating the pathogens without accompanying pathologic inflammatory responses. Such well-balanced immunity can be achieved by simultaneous secretion of an anti-inflammatory cytokine interleukin 10 (IL-10) together with proinflammatory cytokines. For example, without IL-10, mice infected with Toxoplasma gondii succumb to a lethal immune response.1 On the other hand, IL-10 has been shown to prevent complete eradication of Leishmania major after acute infection and to allow persistence after clinical cure.2,3 Thus, a subtle balance between hosts and pathogens is maintained by IL-10 during immunity to infections.
CD4+ T cells concurrently producing interferon γ (IFN-γ) and IL-10 have been repeatedly isolated from hosts suffering from chronic infections with different types of pathogens such as Mycobacterium tuberculosis,4 Borrelia,5 Leishmania,2 and hepatitis C virus,6 suggesting the physiologic importance of IFN-γ/IL-10–producing CD4+ T cells. These pathogens are characterized by the tendency to persist after clinical cure, which leaves a risk of reactivation of pathogens in an immunocompromised host. The IL-10 derived from CD4+ T cells may be involved in establishing persistence of pathogens.2,3,7 An important question is how the IFN-γ/IL-10–producing CD4+ T cells are induced during infections.
Differentiation of naive CD4+ T cells to effector T cells that produce particular sets of cytokines is critically influenced by the type of dendritic cells (DCs). It has been shown that either different subsets of DCs or DCs activated by different stimuli are capable of inducing divergent CD4+ T-cell responses,8 prototypes of which are Th1 and Th2 responses. In humans, 2 distinct types of DCs exist: myeloid DCs (mDCs) and plasmacytoid DCs (pDCs).9 Notably, pDCs are well equipped to protect a host from viral infection in that (1) they characteristically express Toll-like receptor 7 (TLR7) and TLR9, which recognize single-stranded RNA and unmethylated CpG DNA derived from viruses, respectively10 ; (2) at the DC precursor stage, pDCs produce vast amounts of type I IFNs (IFN-α/β), essential cytokines in antiviral immunity,11 in response to viruses10,12 ; and (3) pDCs are able to present viral antigens after infected with viruses.13,14 Thus, pDCs appear to play a key role in developing and modulating antiviral immune responses.
We previously showed that after producing large amounts of type I IFNs in response to HSV, pDC precursors survive and increase the expression of major histocompatibility complex (MHC) and costimulatory molecules, thus developing to DCs that prime naive CD4+ T cells to differentiate into IFN-γ/IL-10–producing T cells, in contrast to IL-3–stimulated pDCs, which preferentially induce Th2 type cells.15 This finding implies that virus-stimulated, type I IFN-producing pDCs may be responsible for the induction of the immunoregulatory IFN-γ/IL-10–producing CD4+ T cells during infection with viruses that persist after clinical cure.
Although type I IFNs play a crucial role in eliminating viruses through direct antiviral effects and indirect immune-enhancing effects on various cell types in the immune system,16 findings are emerging that type I IFNs can also negatively regulate immune responses. For example, failure to induce protective Th1-type immunity to a virulent isolate of M tuberculosis is associated with increased induction of type I IFNs.17 IFN-α together with IL-10 induces differentiation of IL-10– and IFN-γ–producing human type 1 T regulatory (Tr1) cells in vitro.18 Type I IFNs attenuate the generation of antigen-specific CD8+ T cells apparently through the induction of CD4+ Tr1 cells.19 Thus, type I IFNs produced by virus-stimulated pDCs may have a role in negatively modulating antiviral immune responses.
Several studies have indicated that perforin and granzymes play an immuoregulatory role. During viral infection, perforin not only performs a cytotoxic function to kill virus-infected cells but also down-regulates excessive antiviral immune responses.20 Furthermore, recent studies have shown that a particular treatment of naive CD4+ T cells, that is, anti-CD3 plus anti-CD46 stimulation, induces perforin/granzyme B-expressing regulatory T cells that kill autologous immune cells.21,22 However, antigen-presenting cells (APCs) responsible for the induction of perforin and granzymes in CD4+ T cells remain to be determined.
Here, we investigated whether type I IFN-producing, HSV-stimulated pDCs are capable of inducing CD4+ immunoregulatory T cells. We compared HSV-stimulated pDCs (HSV-pDCs) with IL-3–stimulated pDCs (IL-3–pDCs) and granulocyte-macrophage colony-stimulating factor (GM-CSF)–stimulated mDCs (GM-CSF–mDCs) to examine whether viral stimulation specifically endows pDCs with functions down-regulatory for CD4+ T cells. We show that HSV-pDCs, but not the other 2 types of DCs, induce anergic and regulatory CD4+ T cells in a type I IFN- and IL-10–dependent manner. HSV-pDCs, and to a lesser extent IL-3-pDCs, induce CD4+ T cells that express perforin and granzymes and have cytotoxic activity. These data suggest a novel aspect of immunoregulatory roles of type I IFN-producing pDCs in antiviral immunity.
Materials and methods
Isolation of DCs and T cells
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coat of healthy donors (kindly provided by Kyoto Red Cross Blood Center, Kyoto, Japan). CD4+CD11c-lin- cells and CD4+CD11c+lin- cells were isolated as pDC precursors and mDCs, respectively, as described,15 using a FACSAria cell sorter (BD Biosciences, San Jose, CA). Reanalysis of the sorted cells confirmed a purity of more than 98%. Naive CD4+ T cells were isolated by negative selection using the CD4+ T cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) from umbilical cord blood obtained with written informed consent.
DC/T-cell coculture
mDCs and pDC precursors were plated in round-bottomed 96-well plates at an initial density of 1 × 105 cells/mL in 100 μL RPMI 1640 (Sigma, St Louis, MO) supplemented with 10% fetal calf serum (FCS; ThermoTrace, Melbourne, Australia), 2 mM l-glutamine, penicillin G, streptomycin (Gibco BRL, Carlsbad, CA), and 10 mM HEPES (nacalai tesque, Kyoto, Japan; referred to as complete medium). Then, 50 ng/mL GM-CSF (a gift from DNAX Research Institute, Palo Alto, CA), 106 plaque-forming unit (PFU)/mL HSV-1 (KOS strain, attenuated with UV irradiation, a gift from Dr M. Yasukawa, Ehime University, Ehime, Japan, and Dr Tetsushi Yoshikawa, Fujta Health University, Aichi, Japan), 10 ng/mL IL-3 (PeproTech, London, United Kingdom), or 6 μg/mL oligodeoxynucleotide (ODN) 2216 (Hokkaido System Science, Sapporo, Japan), which has been shown to potently induce pDC precursors to produce IFN-α,23 was added. After 3 days, 5 × 104 naive CD4+ T cells were added in a final volume of 200 μL/well. T cells were cultured in the presence of 10 U/mL recombinant human IL-2 (a gift from Shionogi Osaka, Japan) and 10 ng/mL IL-15 (PeproTech), which have been shown to enhance proliferation of regulatory T cells without affecting their regulatory function.24 At day 8, cells were harvested and analyzed for their profile of cytokine production and proliferative capacity. In some experiments, 10 μg/mL mouse anti–human IFN-α/β receptor monoclonal antibody (mAb; PBL Biomedical Laboratories, Piscataway, NJ) or 10 μg/mL anti–human IL-10 mAb (eBioscience, San Diego, CA) was added at day 0 and day 4.
Analysis of intracellular cytokine, granzymes, and perforin by flow cytometry
Intracellular cytokine analysis was performed after 8 days of priming of T cells, as described.25 Fixed and permeabilized T cells were incubated with phycoerythrin (PE)–conjugated anti-IL-2 or anti-IL-10, and fluorescein isothiocyanate (FITC)–conjugated anti-IFN-γ mAbs. All the mAbs were purchased from BD PharMingen (San Diego, CA). For analysis of granzymes and perforin, T cells stimulated with DCs were stained with anti-CD25-PE (Immunotech, Marseille, France) before fixation, permeabilization, and incubation with FITC-conjugated anti–granzyme A (clone CB9), anti–granzyme B (clone GB11), or anti–perforin mAb (clone δG9). All the mAbs were purchased from BD PharMingen. Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed with CellQuest software (BD Biosciences). For the analysis of granzyme- and perforin-expressing cells, CD25+ cells were gated.
Proliferation of stimulated T cells
T cells stimulated with DCs were tested for their proliferative capacity following second allogeneic activation. Stimulated T cells (5 × 104 cells/well) were restimulated in triplicate with irradiated (4000 rad) allogeneic CD3-depleted PBMCs (1 × 105 cells/well) in a final volume of 200 μL complete medium in 96-well round-bottomed plates. After 4 days of culture, wells were pulsed for 16 hours with 1 μCi/well (0.037 MBq) [3H] thymidine (Amersham, Uppsala, Sweden). Cells were harvested and analyzed in a scintillation counter (TopCount, Packard Instrument, Meriden, CT).
Suppression of naive T-cell proliferation
T cells stimulated with DCs were tested for their ability to suppress the proliferation of naive T cells to allogeneic APCs. Naive CD4+ T cells from cord blood (5 × 104 cells/well) were cocultured in triplicate with irradiated, allogeneic CD3-depleted PBMCs (1 × 105 cells/well), in the absence or presence of stimulated T cells (5 × 104 cells/well) in a final volume of 200 μL in complete medium. Control cultures consisted of naive and stimulated T cells in the absence of allogeneic CD3-depleted PBMCs, and stimulated T cells plus allogeneic CD3-depleted PBMCs in the absence of naive T cells. After 4 days, wells were pulsed for 16 hours with 1 μCi/well (0.037 MBq) [3H] thymidine. Cells were harvested and analyzed in a scintillation counter.
Flow cytometric cytotoxicity assay
Flow cytometric cytotoxicity assay was performed to measure in vitro cellular cytotoxicity of CD4+ T cells in a manner previously described.22 CD4+ T cells stimulated with DCs were used as effector cells, and human cell lines (Daudi and U937) or activated T cells, which have been shown to be more susceptible to killing than resting T cells,21 were used as target cells. To prepare activated T cells, total T cells were isolated from PBMCs using CD3 MicroBeads (Miltenyi Biotec), and were stimulated with Dynabeads CD3/CD28 T-cell expander (Dynal, Oslo, Norway) and 50 U/mL IL-2 for 5 days according to the manufacturer's instructions. Daudi and U937 were labeled with 200 nM 5- (and 6-) CFSE (Molecular Probes, Eugene, OR) in PBS for 15 minutes at 37°C in a volume of 1 mL. The activated T cells were stained with CFSE and propidium iodide, and propidium iodide-negative viable cells were isolated using a FACSAria cell sorter. CFSE-labeled target cells were then washed twice in PBS and seeded to round-bottomed 96-well plates. A constant number (2.5 × 104 cells/well) of target cells were added along with effector cells at the indicated effector-to-target (E/T) ratios. Cell mixtures were incubated in a total volume of 100 μL complete medium for 4 hours at 37°C. In parallel, target cells were incubated alone to measure basal apoptosis. Immediately before analysis, 1 μg/mL 7-aminoactinomycin D (7-AAD; BD PharMingen) was added to each sample. To analyze the role of the perforin/granzyme pathway in killing, 4 mM EGTA (nacalai tesque) was added to inhibit calcium-dependent perforin polymerization. Samples were analyzed using a FACSCalibur flow cytometer. The percentage of specific lysis was calculated as follows: 100 × (% sample lysis - % basal lysis)/100 - % basal lysis.26
HSV-pDCs induce poor proliferation of naive CD4+ T cells. Allogeneic naive CD4+ T cells (5 × 104) were stimulated with 1 × 104, 5 × 103,or 2.5 × 103 of 5 different types of DCs, as indicated, in 200 μL for 8 days. Fold increases in T-cell numbers during culture are shown. The data shown are representative of more than 10 experiments. ♦ indicates HSV-pDC; ▪, ODN2216-pDC; ▴, IL-3-pDC;*, GM-CSF-mDC; •, HSV-mDC.
HSV-pDCs induce poor proliferation of naive CD4+ T cells. Allogeneic naive CD4+ T cells (5 × 104) were stimulated with 1 × 104, 5 × 103,or 2.5 × 103 of 5 different types of DCs, as indicated, in 200 μL for 8 days. Fold increases in T-cell numbers during culture are shown. The data shown are representative of more than 10 experiments. ♦ indicates HSV-pDC; ▪, ODN2216-pDC; ▴, IL-3-pDC;*, GM-CSF-mDC; •, HSV-mDC.
Statistical analysis
Data are presented as means plus or minus SD. Statistical comparisons were performed using unpaired 2-tailed Student t tests, with a P value below .05 taken to indicate significance.
Ethical principles
This study was approved by the institutional review board at the Graduate School of Medicine, Kyoto University, and abides by the tenets of the Declaration of Helsinki.
Results
Virus-stimulated pDCs induce poor proliferation of allogeneic naive CD4+ T cells
First we compared proliferation of allogeneic naive CD4+ T cells stimulated with 5 different types of DCs, specifically, HSV-pDCs, ODN2216-pDCs, IL-3–pDCs, GM-CSF–mDCs, and HSV-mDCs, during primary culture. We stimulated pDCs with HSV, ODN2216, or IL-3 for 3 days, and mDCs with GM-CSF or HSV for 3 days. Allogeneic naive CD4+ T cells were cocultured with these activated pDCs or mDCs at 3 different ratios for 8 days in the presence of 10 U/mL IL-2 and 10 ng/mL IL-15. During the primary culture, T cells stimulated with GM-CSF–mDCs vigorously proliferated, whereas those stimulated with HSV-pDCs or ODN2216-pDCs underwent markedly suppressed proliferation (Figure 1). Proliferation of T cells stimulated with IL-3–pDCs or HSV-mDCs was also poor, although the degree of suppression was less than that observed in T cells stimulated with HSV-pDCs or ODN2216-pDCs. Similar data were obtained at all 3 ratios between DCs and T cells. The poorer proliferation of T cells stimulated with HSV-mDCs than those stimulated with GM-SCF–mDCs is probably due to the absence of TLR9 in mDCs10 and the resultant absence of activation with HSV.27 Thus, IFN-α–producing HSV-pDCs and ODN2216-pDCs have a poor capacity to induce naive CD4+ T-cell proliferation. Because stimulation with viruses rather than ODNs is more physiologic, we used HSV-pDCs as IFN-α–producing pDCs in the following experiments.
Allogeneic naive CD4+ T cells cocultured with virus-stimulated pDCs produce both IFN-γ and IL-10 and less IL-2
We have previously shown that HSV-pDCs induce allogeneic naive CD4+ T cells to differentiate into IFN-γ– and IL-10–producing cells.15 This cytokine profile is similar to that of Tr1 cells,28 and thus we examined whether these T cells produce a smaller amount of IL-2 than CD4+ T cells that were stimulated with other types of DCs, which is another feature of regulatory T cells.28 As shown in Figure 2, HSV-pDCs induced a significant number of naive CD4+ T cells to differentiate into IFN-γ/IL-10 double-producing cells, but induced a lower number of IL-2–producing cells than GM-CSF–mDCs. IL-3-pDCs also induced a lower number of IL-2–producing cells than GM-CSF–mDCs, whereas IL-3–pDCs induced a lower number of IFN-γ/IL-10–producing cells than HSV-pDCs. GM-CSF–mDCs induced large numbers of IFN-γ– and IL-2–producing T cells, but not IL-10–producing T cells. These data indicate that HSV-pDCs induce IFN-γ+IL-10+IL-2lo CD4+ T cells that have a cytokine profile similar to Tr1 cells.
HSV-pDCs induce anergic and regulatory CD4+ T cells
A cardinal feature of regulatory T cells is a poor proliferative capacity, that is, anergy, in response to secondary stimulation. Thus, we examined proliferative activity of T cells stimulated with different DCs on restimulation. We cocultured naive CD4+ T cells with allogeneic HSV-pDCs, IL-3–pDCs, or GM-CSF–mDCs for 8 days. Thereafter, we restimulated the CD4+ T cells with T cell–depleted and irradiated PBMCs from the same donor as that of DCs for 5 days and measured [3H] thymidine incorporation during the last 16 hours. As shown in Figure 3A, CD4+ T cells originally cocultured with HSV-pDCs showed markedly reduced proliferation after restimulation. CD4+ T cells originally cocultured with IL-3–pDCs proliferated significantly more than T cells originally cocultured with HSV-pDCs (P < .05), but significantly less than T cells cocultured with GM-CSF–mDCs (P < .005). CD4+ T cells originally cocultured with GM-CSF–mDCs vigorously proliferated after restimulation. These data indicate that HSV-pDCs, and to a lesser extent IL-3–pDCs, induce CD4+ T-cell anergy.
Cytokine production by CD4+ T cells stimulated with HSV-pDCs, IL-3-pDCs, or GM-CSF–mDCs. Naive CD4+ T cells stimulated with allogeneic DCs for 8 days were restimulated with immobilized anti-CD3 and soluble anti-CD28 mAbs in the presence of brefeldin A. After fixation and permeabilization, intracellular cytokine staining was performed. The percentages in each quadrant are indicated on the plot. The data shown are representative of 4 experiments.
Cytokine production by CD4+ T cells stimulated with HSV-pDCs, IL-3-pDCs, or GM-CSF–mDCs. Naive CD4+ T cells stimulated with allogeneic DCs for 8 days were restimulated with immobilized anti-CD3 and soluble anti-CD28 mAbs in the presence of brefeldin A. After fixation and permeabilization, intracellular cytokine staining was performed. The percentages in each quadrant are indicated on the plot. The data shown are representative of 4 experiments.
HSV-pDCs induce anergic and regulatory CD4+ T cells. (A) Naive CD4+ T cells stimulated with allogeneic DCs for 8 days were restimulated with irradiated allogeneic CD3-depleted PBMCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. (B) Naive CD4+ T cells were stimulated with irradiated allogeneic CD3-depleted PBMCs in the absence or presence of CD4+ T cells that had been stimulated with one of the 3 types of DCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. Error bars indicate SD. The data shown are representative of 3 experiments.
HSV-pDCs induce anergic and regulatory CD4+ T cells. (A) Naive CD4+ T cells stimulated with allogeneic DCs for 8 days were restimulated with irradiated allogeneic CD3-depleted PBMCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. (B) Naive CD4+ T cells were stimulated with irradiated allogeneic CD3-depleted PBMCs in the absence or presence of CD4+ T cells that had been stimulated with one of the 3 types of DCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. Error bars indicate SD. The data shown are representative of 3 experiments.
Next, we examined whether the anergic CD4+ T cells have a regulatory activity, that is, whether they suppress proliferation of coexisting T cells. We cocultured naive CD4+ T cells and DC-primed CD4+ T cells from the same donor, in the presence of PBMCs from the same donor as that of DCs for 5 days, and measured [3H] thymidine incorporation during the last 16 hours. As shown in Figure 3B, naive CD4+ T cells stimulated with allogeneic PBMCs, in the absence of DC-stimulated CD4+ T cells, exhibited moderate proliferation. This proliferation was significantly suppressed by coexisting CD4+ T cells stimulated with HSV-pDCs (P < .005). In contrast, CD4+ T cells stimulated with IL-3–pDCs or GM-CSF–mDCs did not show such suppressive activity. This indicates that CD4+ T cells stimulated with HSV-pDCs, but not those stimulated with IL-3–pDCs or GM-CSF–mDCs, have regulatory as well as anergic properties.
The induction of anergic and regulatory properties of CD4+ T cells is dependent on type I IFNs and IL-10
It has been shown that polyclonal stimulation of naive CD4+ T cells in the presence of IFN-α and IL-10 induces anergic and regulatory T cells.18 Thus, we asked whether type I IFNs derived from HSV-pDCs and IL-10 derived from CD4+ T cells themselves are responsible for the induction of anergic and regulatory properties of the T cells. We added blocking anti-IFN-α/β receptor mAb or anti-IL-10 mAb to the coculture of HSV-pDCs and allogeneic naive CD4+ T cells, and restimulated the T cells with PBMCs from the same donor as that of DCs for 5 days, and measured [3H] thymidine incorporation during the last 16 hours. As shown in Figure 4A, the addition of blocking anti-IFN-α/β receptor mAb or anti-IL-10 mAb during primary culture diminished the induction of anergy. The addition of a mixture of anti-IFN-α/β receptor mAb and anti-IL-10 mAb did not show an additive effect. Thus, both pDC-derived type I IFNs and T cell-derived IL-1015 present during priming are responsible for the induction of CD4+ T-cell anergy by HSV-pDCs.
Next, we examined whether type I IFNs and IL-10 are also responsible for the induction of regulatory T cells. We stimulated allogeneic naive CD4+ T cells with HSV-pDCs in the presence or absence of anti-IFN-α/β receptor mAb or anti-IL-10 mAb. Thereafter, we cocultured naive CD4+ T cells and HSV-pDC–stimulated CD4+ T cells from the same donor, in the presence of PBMCs from the same donor as that of pDCs. Inhibition of type I IFNs or IL-10 during the priming of naive CD4+ T cells with HSV-pDCs significantly reduced the generation of regulatory T cells (Figure 4B). The addition of a mixture of anti-IFN-α/β receptor mAb and anti-IL-10 mAb did not show an additive effect. Thus, both pDC-derived type I IFNs and T cell–derived IL-10 present during priming are responsible for the induction of regulatory T cells.
The induction of anergic and regulatory properties of CD4+ T cells is dependent on type I IFNs and IL-10. (A) Naive CD4+ T cells were stimulated with allogeneic HSV-pDCs for 8 days in the presence of isotype-matched control mAb, anti-IFN-α/β receptor mAb, anti-IL-10 mAb, or a mixture of the both mAbs. Alternatively, T cells were stimulated with allogeneic GM-CSF–mDCs for comparison. The CD4+ T cells were harvested and restimulated with irradiated allogeneic CD3-depleted PBMCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. (B) Naive CD4+ T cells were stimulated with allogeneic HSV-pDCs for 8 days in the presence of isotype-matched control mAb, anti-IFN-α/β receptor mAb, anti-IL-10 mAb, or a mixture of the both mAbs. Naive CD4+ T cells were stimulated with irradiated allogeneic CD3-depleted PBMCs in the absence or presence of the CD4+ T cells that had been stimulated with HSV-pDCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. Error bars indicate SD. The data shown are representative of 3 experiments.
The induction of anergic and regulatory properties of CD4+ T cells is dependent on type I IFNs and IL-10. (A) Naive CD4+ T cells were stimulated with allogeneic HSV-pDCs for 8 days in the presence of isotype-matched control mAb, anti-IFN-α/β receptor mAb, anti-IL-10 mAb, or a mixture of the both mAbs. Alternatively, T cells were stimulated with allogeneic GM-CSF–mDCs for comparison. The CD4+ T cells were harvested and restimulated with irradiated allogeneic CD3-depleted PBMCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. (B) Naive CD4+ T cells were stimulated with allogeneic HSV-pDCs for 8 days in the presence of isotype-matched control mAb, anti-IFN-α/β receptor mAb, anti-IL-10 mAb, or a mixture of the both mAbs. Naive CD4+ T cells were stimulated with irradiated allogeneic CD3-depleted PBMCs in the absence or presence of the CD4+ T cells that had been stimulated with HSV-pDCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. Error bars indicate SD. The data shown are representative of 3 experiments.
HSV-pDCs and IL-3–pDCs induce granzymes and perforin in CD4+ T cells in a partially IL-10–dependent manner
It has recently been shown that anti-CD3/anti-CD46 stimulation induces CD4+ regulatory T cells that express granzymes and perforin.21,22 Because perforin has been shown to play an important role in down-regulating T-cell responses in chronic viral infection,20 we examined whether HSV-pDCs induce the expression of granzymes and perforin in naive CD4+ T cells. We cocultured allogeneic naive CD4+ T cells with HSV-pDCs, IL-3–pDCs, or GM-CSF–mDCs for 8 days in the presence or absence of anti-IFN-α/β receptor mAb or anti-IL-10 mAb, and examined the expression of granzyme A, granzyme B, and perforin by intracellular staining. Unstimulated naive CD4+ T cells did not express detectable levels of granzyme A, granzyme B, or perforin (data not shown). As shown in Figure 5, granzyme A was induced by stimulation with any DCs: HSV-pDCs, IL-3–pDCs, and GM-CSF–mDCs. HSV-pDCs, and to a lesser extent IL-3–pDCs, induced high levels of granzyme B in CD4+ T cells, whereas GM-CSF–mDCs induced only a low level of granzyme B. HSV-pDCs, and to a lesser extent IL-3–pDCs, induced moderate levels of perforin, whereas GM-CSF–mDCs did not induce a significant level of perforin. The induction of granzyme A or granzyme B by HSV-pDCs did not diminish in the presence of anti-IFN-α/β receptor mAb, whereas the induction of perforin moderately diminished. The induction of the 3 molecules by HSV-pDCs or IL-3–pDCs partially but substantially diminished in the presence of anti-IL-10 mAb. These data indicate that HSV-pDCs and to a lesser extent IL-3–pDCs, but not GM-CSF–mDCs, induce CD4+ T cells to express granzyme B and perforin in a partially IL-10–dependent manner. Type I IFNs may also be involved in the induction of perforin.
HSV-pDCs and IL-3–pDCs induce granzymes and perforin in CD4+ T cells in a partially IL-10–dependent manner. Naive CD4+ T cells were stimulated with allogeneic HSV-pDCs or IL-3–pDCs for 8 days in the presence of isotype-matched control mAb, anti-IFN-α/β receptor mAb, or anti-IL-10 mAb. Alternatively, T cells were stimulated with allogeneic GM-CSF–mDCs for comparison. Activated T cells were gated based on the expression of CD25, and expression of intracellular granzyme A, granzyme B, and perforin was analyzed by flow cytometry. (A) Histograms. Open histograms represent cells stained with isotype-matched control mAbs. (B) Percentages of cells expressing the 3 molecules, indicated with markers in panel A. The data shown are representative of 3 experiments.
HSV-pDCs and IL-3–pDCs induce granzymes and perforin in CD4+ T cells in a partially IL-10–dependent manner. Naive CD4+ T cells were stimulated with allogeneic HSV-pDCs or IL-3–pDCs for 8 days in the presence of isotype-matched control mAb, anti-IFN-α/β receptor mAb, or anti-IL-10 mAb. Alternatively, T cells were stimulated with allogeneic GM-CSF–mDCs for comparison. Activated T cells were gated based on the expression of CD25, and expression of intracellular granzyme A, granzyme B, and perforin was analyzed by flow cytometry. (A) Histograms. Open histograms represent cells stained with isotype-matched control mAbs. (B) Percentages of cells expressing the 3 molecules, indicated with markers in panel A. The data shown are representative of 3 experiments.
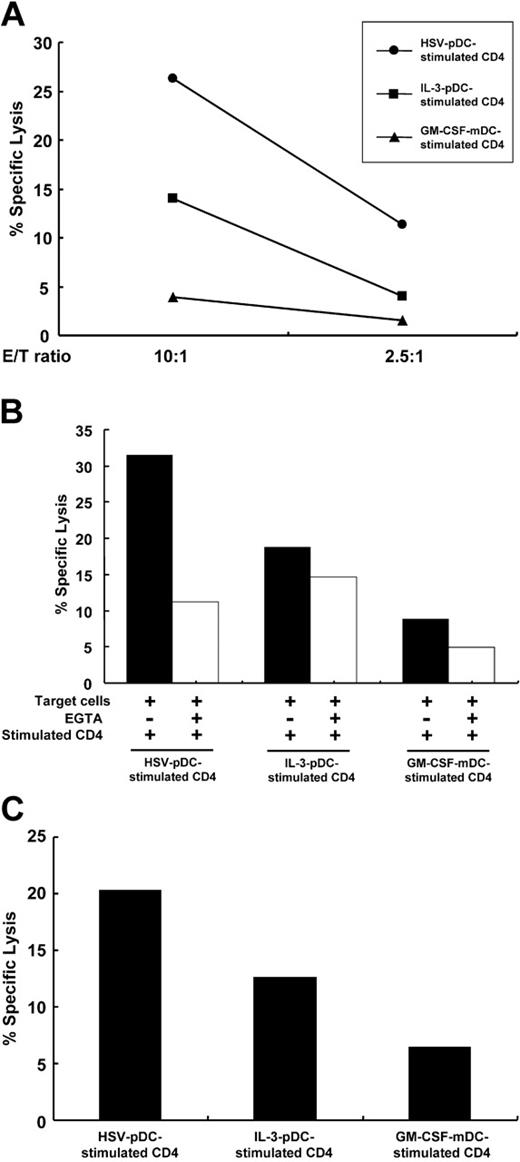
CD4+ T cells stimulated with HSV-pDCs exhibit perforin-dependent cytotoxicity
Finally, we examined whether CD4+ T cells stimulated with the 3 types of DCs have cytotoxic activity in accordance with the expression of granzymes and perforin. We used a Burkitt lymphoma cell line Daudi or a myelomonocytic cell line U937 as target cells of cytotoxicity assay. We stained the target cells with CFSE22 and cocultured the effector and target cells for 4 hours. Then the percentages of killed CFSE-labeled target cells were calculated by staining them with 7-AAD. As shown in Figure 6A, a large proportion of Daudi cocultured with HSV-pDC–stimulated CD4+ T cells underwent apoptosis. Daudi cocultured with IL-3/pDC-stimulated CD4+ T cells also underwent apoptosis, albeit to a lesser extent. The extent of apoptosis in both conditions was correlated with E/T ratios. In contrast, Daudi cocultured with GM-CSF/mDC-stimulated CD4+ T cells minimally underwent apoptosis. The apoptosis induced by HSV-pDC–stimulated CD4+ T cells was substantially diminished by the addition of EGTA, indicating that the death was perforin-dependent (Figure 6B). The apoptosis induced by IL-3-pDC–stimulated CD4+ T cells was marginally inhibited by EGTA. We obtained similar results using U937 as target cells (data not shown). As target cells, we also used activated T cells from the same donors as DC donors (allospecific targets), or activated T cells from donors different from DC and effector T-cell donors (third-party targets). Third-party target T cells (Figure 6C) as well as allospecific target T cells (data not shown) were substantially killed by HSV-pDC–stimulated CD4+ T cells and were also killed by IL-3–pDC–stimulated CD4+ T cells to a lesser extent, whereas the killing by GM-CSF–mDC–stimulated CD4+ T cells was minimal, as observed in the killing of unrelated tumor cell lines. These data indicate that in proportion to the degree of up-regulation of granzymes and perforin, CD4+ T cells stimulated with HSV-pDCs have the strongest perforin-dependent cytotoxic activity among the 3 CD4+ T cells stimulated with different DCs in an antigen-nonspecific manner. CD4+ T cells stimulated with IL-3–pDCs may also have a significant cytotoxicity.
Discussion
During immune responses to microbial pathogens, overwhelming pathologic immune reactions need to be avoided to minimize tissue damage. IFN-γ/IL-10–producing CD4+ T cells have been recognized in infections by several pathogens,2,4-6 and may play an important role in down-modulating pathologic immune reactions and may also be responsible for allowing persistent infection.2,3,7,29 Given that the direction of naive CD4+ T-cell differentiation is largely determined by the subset and activation state of DCs, it is important to elucidate which type of DCs induces naive CD4+ T cells to differentiate into immunoregulatory IFN-γ/IL-10–producing T cells during infection. Here we showed that the IFN-γ/IL-10–producing CD4+ T cells induced by HSV-pDCs acquire anergic and regulatory properties by the action of pDC-derived type I IFNs and T cell–derived IL-10. Interestingly, cytotoxic molecules perforin and granzyme B in CD4+ T cells are induced by pDCs in an IL-10–dependent manner. These results suggest an immunoregulatory role of type I IFN-producing pDCs through the induction of perforin/granzyme-expressing regulatory CD4+ T cells and present a possible mechanism by which pDCs induce coordinated antiviral immunity by preventing excessive immune responses.
CD4+ T cells stimulated with HSV-pDCs exhibit perforin-dependent cytotoxicity. (A) Naive CD4+ T cells stimulated with the 3 types of allogeneic DCs for 8 days (effector cells) were cocultured with CFSE-labeled Daudi target cells at an E/T ratio of 10:1 or 2.5:1 for 4 hours. Immediately before analysis, 7-AAD was added to each sample, and percentages of 7-AAD+ cells were analyzed by flow cytometry. Basal lysis of the target cells was 9.6%. The data shown are representative of 2 experiments. (B) Naive CD4+ T cells stimulated with the 3 types of allogeneic DCs for 8 days (effector cells) were cocultured with CFSE-labeled Daudi target cells at an E/T ratio of 30:1 for 4 hours in the absence or presence of EGTA. Percentages of 7-AAD+ cells were analyzed by flow cytometry as shown in panel A. Basal lysis of the target cells was 18%. Filled and open bars represent percent specific lysis induced in the absence or presence of EGTA, respectively. The data shown are representative of 3 experiments. (C) Naive CD4+ T cells stimulated with the 3 types of allogeneic DCs for 8 days (effector cells) were cocultured with CFSE-labeled activated T cells from third-party donors (target cells) at an E/T ratio of 30:1 for 4 hours. Percentages of 7-AAD+ cells were analyzed by flow cytometry as shown in panel A. Basal lysis of the target cells was 16%. The data shown are representative of 2 experiments.
CD4+ T cells stimulated with HSV-pDCs exhibit perforin-dependent cytotoxicity. (A) Naive CD4+ T cells stimulated with the 3 types of allogeneic DCs for 8 days (effector cells) were cocultured with CFSE-labeled Daudi target cells at an E/T ratio of 10:1 or 2.5:1 for 4 hours. Immediately before analysis, 7-AAD was added to each sample, and percentages of 7-AAD+ cells were analyzed by flow cytometry. Basal lysis of the target cells was 9.6%. The data shown are representative of 2 experiments. (B) Naive CD4+ T cells stimulated with the 3 types of allogeneic DCs for 8 days (effector cells) were cocultured with CFSE-labeled Daudi target cells at an E/T ratio of 30:1 for 4 hours in the absence or presence of EGTA. Percentages of 7-AAD+ cells were analyzed by flow cytometry as shown in panel A. Basal lysis of the target cells was 18%. Filled and open bars represent percent specific lysis induced in the absence or presence of EGTA, respectively. The data shown are representative of 3 experiments. (C) Naive CD4+ T cells stimulated with the 3 types of allogeneic DCs for 8 days (effector cells) were cocultured with CFSE-labeled activated T cells from third-party donors (target cells) at an E/T ratio of 30:1 for 4 hours. Percentages of 7-AAD+ cells were analyzed by flow cytometry as shown in panel A. Basal lysis of the target cells was 16%. The data shown are representative of 2 experiments.
Several studies have shown that pDCs are capable of inducing immunoregulatory T cells through various mechanisms. For example, pDC precursors induce CD4+ T-cell anergy, apparently due to the lack of costimulatory molecules.30 IL-3/CD40L-stimulated pDCs induce IL-10–producing CD8+ regulatory T cells.31 pDCs stimulated with CpG ODNs induce Foxp3-expressing CD4+CD25+ regulatory T cells.32 Mouse in vivo studies have shown that lung pDCs prevent asthmatic reactions to harmless inhaled antigens by inducing regulatory T cells.33 pDC precursors contained in the graft facilitate allogeneic hematopoietic stem cell engraftment.34 These findings indicate that pDC precursors that express low levels of costimulatory molecules or pDCs activated with particular stimulations are capable of inducing anergic or regulatory T cells. However, these studies do not ascribe the induction of T-cell suppression to type I IFNs. Here we show the importance of type I IFNs in inducing anergic and regulatory T cells by pDCs under the conditions where viral stimuli induce them to produce the cytokines. Although murine pDCs have been shown to induce anergic T cells through the expression of indoleamine 2,3-dioxygenase (IDO),35,36 the addition of an IDO inhibitor 1-methyl-d-tryptophan to the coculture of pDCs and naive CD4+ T cells did not inhibit the induction of regulatory T cells (unpublished data, September 2004), indicating that IDO is not involved in the system shown in this study.
Recent studies have been revealing an immunoregulatory role of type I IFNs through different mechanisms. First, IFN-α treatment of naive CD4+ T cells delays their entry into cell cycle early on T-cell receptor (TCR) triggering and sensitizes T cells to activation-induced cell death later after activation.37,38 These mechanisms may explain the markedly suppressed proliferation of naive CD4+ T cells stimulated with type I IFN-producing HSV-pDCs. The attenuated proliferation of CD4+ T cells stimulated with virus-activated pDCs may result in accumulation of IFN-γ/IL-10–producing regulatory CD4+ T cells at a chronic stage during viral infection.
Second, type I IFNs have been shown to induce CD4+ T cells that produce IL-10 together with IFN-γ. For example, polyclonal stimulation of human naive CD4+ T cells using anti-CD3 mAb in the presence of IFN-α induce IFN-γ/IL-10–producing CD4+ T cells that have regulatory activity.18,39 Type I IFN receptor-deficient mice generate reduced numbers of IL-10–producing CD4+ T cells, which correlates with enhancement of CD8+ T-cell responses, suggesting that type I IFNs suppress CD8+ T-cell responses through the induction of IL-10–producing CD4+ regulatory T cells.19 The present study suggests that type I IFN-producing pDCs may be the APCs responsible for the induction of type I IFN-dependent CD4+ regulatory T cells.
IFN-γ/IL-10–producing CD4+ T cells are identified in hosts suffering from chronic or persistent infections with various types of pathogens.2,4-6 Such T cells have been suggested to be responsible for permitting persistent infection.2 In addition, IL-10–producing CD4+ regulatory T cells generated in mice with retroviral infection have been shown to contribute to viral persistence.40 These IL-10–producing T cells may also play an important role in preventing excessive immune responses, as the absence of IL-10 leads to uncontrolled lethal immune responses.1 An important question is what types of DCs induce immunoregulatory IFN-γ/IL-10–producing CD4+ T cells frequently identified in infected hosts. In mice, it has been shown that IL-12/IL-10–producing CD8α+ DCs stimulated with heat-killed Listeria induce IFN-γ+IL-10+ regulatory CD4+ T cells that inhibit airway hyperreactivity.41 IL-12 has been shown to induce human CD4+ T cells to produce IL-10 together with IFN-γ.5,42 In humans, mDCs produce IL-12 but not IFN-α in response to bacterial stimuli, whereas pDCs produce IFN-α but not IL-12 in response to viral stimuli.10,15 Therefore, in humans, IL-12–producing mDCs may induce immunoregulatory IFN-γ/IL-10–producing CD4+ T cells in bacterial infections as shown in mice with CD8α+ DCs,41 whereas IFN-α–producing pDCs may induce such T cells in persistent infections with viruses such as HSV and hepatitis C virus.6
It has been shown that naturally occurring CD4+CD25+ regulatory T cells43,44 as well as IL-10–producing “adaptive” regulatory T cells in some cases19,41 highly express FOXP3 mRNA. Thus, we examined by real-time reverse transcription-polymerase chain reaction (RT-PCR) whether HSV-pDCs specifically induce the expression of FOXP3 mRNA in naive CD4+ T cells. Naive CD4+ T cells stimulated with HSV-pDCs, IL-3–pDCs, or GM-CSF–mDCs for 8 days expressed comparable levels of FOXP3 mRNA, which were about half of the FOXP3 levels in CD4+CD25+ T cells isolated from adult PBMCs (data not shown). Thus, FOXP3 mRNA is not specifically induced in CD4+ T cells stimulated with HSV-pDCs and does not appear to be important in regulatory activity of such T cells.
An increase in perforin-expressing CD4+ T cells has been observed in patients with chronic viral infections, suggesting a physiologic role of such T cells in antiviral immune responses.45 Because activated CD8+ T cells that cause tissue damage accumulate in perforin-deficient mice during chronic viral infection,20 perforin-mediated autologous cell killing appears to be important in avoiding pathologic immune reactions. Furthermore, stimulation of naive CD4+ T cells with anti-CD3 and anti-CD46 mAbs has been shown to induce them to differentiate into granzymes/perforin+ IL-10–producing regulatory T cells29 that kill allogeneic cells22 as well as autologous immune cells.21 These findings suggest that CD4+ T cells positive for granzymes/perforin play an important immunoregulatory role in chronic viral infections by killing pathogenic immune cells. However, the previous studies did not address the type of APCs responsible for inducing regulatory CD4+ cytotoxic T lymphocytes (CTLs). Here we propose that virus-stimulated pDCs are such APCs that keep antiviral immune responses at an appropriate level by inducing regulatory CD4+ CTLs. Because IL-3–stimulated pDCs are also capable of inducing CD4+ T cells positive for granzymes/perforin, pDCs may generate regulatory CD4+ CTLs in immune responses other than antiviral ones. The involvement of endogenous IL-10 in inducing the expression of granzymes and perforin was unexpected. IL-10 has been shown to enhance cytotoxic activity of natural killer cells and CTLs, although the expression of granzymes and perforin was not investigated in these studies.46,47 Thus, CD4+ T cells stimulated with pDCs may play an immunoregulatory role in chronic antiviral immune responses by expressing granzymes and perforin in an IL-10–dependent manner. The moderate reduction of perforin expression by anti-IFN-α/β receptor mAb also suggests the involvement of type I IFNs in the induction of perforin. Apparent lack of antigen specificity or allospecificity of the cytotoxicity in the previous21 and present studies, together with generalized and uncontrolled activation of T cells and macrophages in individuals with perforin gene defects,48 suggests importance of the perforin pathway in the maintenance of general immune homeostasis.
In summary, type I IFN-producing, virus-stimulated pDCs may play a dual role during the course of antiviral immune responses. Immediately after viral invasion, pDCs play a pivotal role in provoking antiviral innate immunity by producing a vast amount of type I IFNs. Thereafter, type I IFN-producing pDCs may down-modulate antiviral adaptive immune responses by inducing granzymes/perforin-positive, IFN-γ/IL-10+ CD4+ regulatory T cells. The present study adds another novel function to the versatile immune cell type, pDCs, that is, the induction of CD4+ cytotoxic regulatory T cells.
Prepublished online as Blood First Edition Paper, October 11, 2005; DOI 10.1182/blood-2005-04-1737.
Supported by a research grant from Takeda Science Foundation, Japan. K.K. preformed research and wrote the paper; N.K. designed research and wrote the paper; T.K. contributed vital experimental designs and techniques; and T.U. supervised the whole project.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Masaki Yasukawa and Tetsushi Yoshikawa for providing HSV-1 (KOS strain) and Keiko Fukunaga for her excellent technical assistance.


![Figure 3. HSV-pDCs induce anergic and regulatory CD4+ T cells. (A) Naive CD4+ T cells stimulated with allogeneic DCs for 8 days were restimulated with irradiated allogeneic CD3-depleted PBMCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. (B) Naive CD4+ T cells were stimulated with irradiated allogeneic CD3-depleted PBMCs in the absence or presence of CD4+ T cells that had been stimulated with one of the 3 types of DCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. Error bars indicate SD. The data shown are representative of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-04-1737/4/m_zh80030690490003.jpeg?Expires=1767778201&Signature=uxm1kOXFrEaszIjUmTJZnImudLVBA-Gi0PT7MRyqw8OJrXqrPYJgV3V~oH1lND73coUBl3siehMhuf8BrMFZkmPi9CjNz1uPy0gq9qmxQUERsFeCzVXWKkiwuNIeb3-t0o-axn9eHTIU9XOD1grrrHZNaLetnbBFaA7DpYBT1LayrV5ufGv7L-HS7H5Xop8WnpK9iZcOC4vkmRwtm~Zuervwqrqa36kbD~pm~EoXp-Sj0gkkf0Qvvjzko8eyTeKvUawY5JuKH6vaUsdMBTjmbh76a4ja42QOOYJCkF1EhJimNXa3352LmfQmGgST0TzvePcZDIe~B6TrrGSH4lf8fw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. The induction of anergic and regulatory properties of CD4+ T cells is dependent on type I IFNs and IL-10. (A) Naive CD4+ T cells were stimulated with allogeneic HSV-pDCs for 8 days in the presence of isotype-matched control mAb, anti-IFN-α/β receptor mAb, anti-IL-10 mAb, or a mixture of the both mAbs. Alternatively, T cells were stimulated with allogeneic GM-CSF–mDCs for comparison. The CD4+ T cells were harvested and restimulated with irradiated allogeneic CD3-depleted PBMCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. (B) Naive CD4+ T cells were stimulated with allogeneic HSV-pDCs for 8 days in the presence of isotype-matched control mAb, anti-IFN-α/β receptor mAb, anti-IL-10 mAb, or a mixture of the both mAbs. Naive CD4+ T cells were stimulated with irradiated allogeneic CD3-depleted PBMCs in the absence or presence of the CD4+ T cells that had been stimulated with HSV-pDCs. After 4 days, wells were pulsed for 16 hours with [3H] thymidine. Error bars indicate SD. The data shown are representative of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-04-1737/4/m_zh80030690490004.jpeg?Expires=1767778201&Signature=NT7mdrOVNV-Ri8VzFZl3Eoh1OrD6vBs-uhUYkYM1agVEwooRSdRbHsVa0uk48s2625h89dq-H8GqVZB1eTZ97DmZf26JXwQwUc9lob8inm4noq9hETZIzwSvYKhoXe1PHFb3~PMkp6f5WybDqYkAWcWUmPmAurD8zfUBGK5BWESZaAuhGtSS~e4ItsKFtpsEeT9LhNkyzdqKVp2M8RdFBNpJ1MN2G3dAnqXewXe1bVH9V-qgm6ay9Qdg6VRXXajtRi4b8JjoqPQEBe~RCLs3MeIWPwi4Y-AgLkoV~z4jEmZENCl2TVxNvTPQYru2BuvHAu~1m2c~eFHjUEOvz7V1yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal