Abstract
When T cells are primed by dendritic cells (DCs) to initiate antigen-specific immune responses screening for matching antigen receptor-MHC/peptide pairs takes place in DC-T-cell conjugates. For an immune response DC-T-cell conjugates formed during priming events need to dissolve. Although detailed knowledge on molecules involved in the conjugate formation is available, dissolving of them has not been considered to be an active process. Here, we identify CYTIP (cytohesin-interacting protein) to mediate DC-T-cell deattachment. CYTIP, which is induced during maturation of DCs, shortly accumulates to the contact zones with T cells within the first hour of coculture. Specific silencing of CYTIP results in stronger adhesion of DCs to T cells and to fibronectin. When a need for deattachment is created in a T-cell priming assay by only partially loading DCs with antigen, CYTIP silencing causes reduced priming capacity. Thus, CYTIP allows DCs to actively control DC-T-cell interactions.
Introduction
A key event in the successful induction of an immune response is the antigen-specific activation of T cells by antigen-presenting cells. The most prominent among antigen-presenting cells is the dendritic cell (DC). Immature DCs in the periphery very effectively process antigens, and with additional inflammatory signals from the innate immune system they undergo a maturation process. In the secondary lymphoid organs DCs present the antigen to T cells as peptides bound to MHC class I and class II molecules. Specific T cells are primed by mature DCs carrying the relevant antigen. An early event in this priming activity is the scanning of antigen-MHC complexes on DCs by T cells.1 This initial contact of T cells with DCs takes place in the outer paracortex of the lymph nodes and is antigen independent.2 If antigen recognition occurs, the interaction is productive, leading to a strengthening of the conjugate and synapse formation. Otherwise, the interaction between T cell and DC is loose and transient.3 In both cases T cells need to detach from the conjugates either to scan more DCs for a relevant antigen or to clonally expand and leave the secondary lymphoid organs to home to their effector sites. The mechanisms leading to conjugate formation have been shown to depend on T-cell receptors (TCRs) and coreceptors interacting with antigen-loaded MHC, integrins, cadherins, and costimulatory molecules.4 The equally important mechanisms leading to the deattachment of the cells from conjugates are unknown and have not been considered to be an active process.
During maturation, DCs induce the expression of genes coding for molecules critically involved in the function of mature DCs. One of the induced molecules is CYTIP (cytohesin-interacting protein). We showed that overexpression of CYTIP in Jurkat cells mediates the loss of adhesiveness of LFA-1 to ICAM-1 by translocating the β-2 integrin-binding protein cytohesin-1 from the membrane to the cytosol, thereby abolishing its binding-enhancing activity.5 These observations prompted us to investigate whether human DCs might use such a CYTIP-dependent mechanism to regulate the loosening of their binding to other cells, for example, to T cells in the course of T-cell priming.
Materials and methods
Generation of CD14+ monocyte-derived dendritic cells
Human DCs were prepared from peripheral blood monocytes essentially as described.6,7 Anonymous human blood components were obtained from the local blood bank according to the guidelines of the institutional review board of the Innsbruck Medical University and the tenets of the Helsinki Protocol. Monocytes were obtained by isolating CD14+ cells by magnetic sorting (MACS; Miltenyi Biotec, Bergisch-Gladbach, Germany). Briefly, 1.5 × 106 cells/well were plated in 6-well tissue culture plates in 3 mL complete culture medium (RPMI-1640 [PAN]; Biotech GmbH, Aidenbach, Germany), supplemented with 50 μg/mL gentamicin, 2 mM l-glutamine (Sigma Chemicals, St Louis, MO), 2% autologous human serum, 800 U/mL GM-CSF (specific activity 1.1 × 106 U/mg; Leukomax; Novartis, Basel, Switzerland) and 20 U/mL IL-4 (Strathmann, Hamburg, Germany). Culture medium was renewed every other day by removing 1 mL medium and substituting with 1.5 mL fresh medium containing 1600 U/mL GM-CSF and 20 U/mL IL-4.6 On day 6, cells were cultured for 2 additional days with or without a defined cytokine cocktail8 consisting of TNFα (10 ng/mL; kindly provided by Dr G. R. Adolf, Bender, Vienna, Austria), IL-1β (2 ng/mL; PeproTech EC LTD, London, England), IL-6 (1000 U/mL; PeproTech) and PGE2 (1 μg/mL, Prostaglandin E2; Pharmacia & Upjohn SA, Buurs, Belgium) as maturation stimulus.
Generation of CD34+-derived dendritic cells
DCs were generated from CD34+ progenitor cells enriched from mononuclear fractions of umbilical cord blood samples using immunomagnetic beads (Miltenyi Biotech) according to the manufacturer's protocol. The isolated cells were cryopreserved in FCS containing 10% DMSO (Merck, Darmstadt, Germany). After thawing, 4 × 104 cells were seeded in culture flasks (Greiner, Frickenhausen, Germany) in complete culture medium (RPMI 1640, supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine; Sigma Chemicals), 100 U/mL penicillin, 100 μg/mL streptomycin (Irvine Scientific, Santa Ana, CA), 10 mM HEPES (Biochrom KG, Berlin, Germany), rhGM-CSF (100 ng/mL, 200 U/mL; Leukomax; Novartis), rhTNF-α (2.5 ng/mL, 50 U/mL, specific activity 1 × 108 U/mg; kindly provided by Dr G. R. Adolf, Bender), and rhSCF (25 ng/mL, specific activity 5 × 105 U/mg; PeproTech) as described previously.9,10 Medium was changed at day 4 and day 10. For experiments, cells were harvested on days 5 (DC precursors) and 13 (spontaneously matured DCs).
Whole-skin explants
To obtain Langerhans cells and dermal dendritic cells we used an established method.11-13 Briefly, standardized pieces of normal skin were prepared by means of an 8-mm punch from split-thickness skin (0.3 mm). These explants were floated on 1.5 mL culture medium (RPMI 1640, supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine; Sigma Chemicals), 100 U/mL penicillin, 100 μg/mL streptomycin (Irvine Scientific) in 24-well plates (one explant per well) for 2 to 5 days at 37°C. Cells that had emigrated into the culture medium during this time were harvested, counted, and further evaluated by flow cytometry.
Preparation of T cells
Bulk T cells were isolated from the rosettes that had formed with neuraminidase-treated sheep red blood cells during the monocyte isolation procedure by lysing the sheep red blood cells with ammonium chloride as described.13 CD8+ T cells were isolated from bulk T cells using CD8 MicroBeads (Milteny Biotech) according to the manufacturer's protocol.
Preparation of human dermal microvascular endothelial cells
Human dermal microvascular endothelial cells were isolated from surgically removed normal foreskins obtained from newborns and children up to 7 years old essentially as described previously.14 Briefly, foreskins were washed in 70% ethanol and then rinsed in Hank-buffered salt solution (HBSS; Gibco, Paisley, Scotland). Thereafter, skin fragments were cut into small pieces and incubated overnight in 1.2 U Dispase II (Boehringer-Mannheim, Mannheim, Germany) per mL PUCK solution (Sigma Chemicals) at 4°C to separate the epidermis from the dermis. Cells obtained from the dermal layer were plated in tissue culture flasks (Greiner), coated with 1% gelatin (Sigma Chemicals), and cultured in EC basal medium (EBM; Clonetics, Walkersville, MD), supplemented with 10% normal human serum, 5 ng/mL epidermal growth factor (Clonetics), 2 mM L-glutamine, 1 μg/mL hydrocortisone acetate, 5 × 10-5 M dibutyril cyclic adenosine monophosphate (Sigma Chemicals), 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 μg/mL amphotericin B (all purchased from Irvine Scientific).
Quantitation of CYTIP transcripts
Total RNA was extracted using TRIZOL (Gibco BRl, Life Technology). Quantitative polymerase chain reaction (PCR) analysis was performed using real-time PCR (ABI PRISM 7700 sequence detector; Applied Biosystems, Vienna, Austria). Sequences for probes and primers (synthesized by Microsynth, Balgach, CH) specific for CYTIP mRNA molecules were selected using the Primer Express software (Applied Biosystems): 5′-GGAGGACAGCCCAGCTCAC, 5′-GCTCACCATTGATATTTGCAAG, 5′-FAM-TGCTGGCCTGCAAGCTGGTGA-Tamra-3′. For PCR the Brilliant Quantitative PCR Core Reagent Kit from Stratagene (Heidelberg, Germany) was used. Random primed cDNA was prepared (Superscript II RNase H-reverse transcriptase; Life Technologies, Vienna, Austria) from total RNA.
Immunocytochemical analysis
Mature DCs were allowed to adhere to coated glass slides or previously immobilized allogeneic T cells on poly-L-lysine. Coating of glass slides was with fibronectin (Sigma Chemicals) (20 μg/mL, overnight at 4°C) or poly L-lysine (Sigma Chemicals), (100 μg/mL for 1 hour at 37°C) or goat anti-mouse Ig (Jackson Laboratories, West Grove, PA) (10 μg/mL, 1 hour at room temperature) followed by mouse anti-human CD18, -CD11a, -CD11c, -CD49d (VLA-4), -CD54 (ICAM-1), or -CD106 (VCAM-1), (10-50 μg/mL for 1 hour at room temperature) (Becton Dickinson/Pharmingen, San Diego, CA). Cells were fixed in acetone for 10 minutes. Immunostaining was performed with the CYTIP-specific antibody 2F9. Antibody binding was visualized using biotinylated species-specific goat anti-rat Ig (Amersham International, Amersham, United Kingdom) followed by a streptavidin-Alexa 488 conjugate (Molecular Probes, Eugene, OR) and in some cases followed by PKH26 RED Fluorescence Cell Linker (Sigma Chemicals). Immunolabeled specimens were mounted in DAPI containing Vectashield (Vector Laboratories, Burlingame, CA) and viewed on a Zeiss Axiovert 100 M microscope (Carl Zeiss, Jena, Germany) equipped with a LSM 510 scanning head and a Zeiss Plan-Apochromat 63×/1.4 oil-immersion objective lens. Laser lines at 488 nm and 364 nm and 543 nm were used for excitation. Acquisition was performed with Zeiss LSM imaging software version 2.81 (Carl Zeiss).
Silencing of CYTIP
DCs were cultured as described. On day 6 immature DCs were harvested and counted. 2.5 × 106 cells/well were seeded in 24-well plates in 500 μL culture medium without antibiotics (RPMI 1640 supplemented with 2% autologous serum, 2 mM l-glutamine, and 800 U/mL GM-CSF [specific activity 1.1 × 106 U/mg, Leukomax; Novartis]) and 20 U/mL IL-4 (Strathmann). Cells were transfected with 100 pM siRNA (ready to use duplexes purchased from Dharmacon, Lafayette, CO) using Lipofectamine 2000 (Invitrogen, Life Technologies) according to the manufacturer's protocol. Four hours after transfection DCs were stimulated for maturation; 48 hours later, DCs were harvested and used for further experiments. Transfection efficiencies were determined by intracellular fluorescence-activated cell sorting (FACS) analysis or by Western blotting.
Western blotting
Cells were lysed in buffer containing 50 mM Tris HCl pH 7.5, 150 mM NaCl, and 1% (wt/vol) Nonidet P40, 1 μM phenylmethylsulfonyl fluoride (PMSF), 1 μM Di-thiotreithol (DTT) supplemented with 2 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 μg/mL pepstatin A, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). CYTIP was detected by standard Western blot using the CYTIP-specific antibody 2F9 followed by a secondary biotinylated anti-rat antibody and peroxidase conjugated streptavidin. Western blots were developed using the Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Santa Cruz, CA).
Intracellular FACS staining for CYTIP
Cells were permeabilized and stained using the FIX&PERM cell permeabilization kit (GAS-002; An der Grub, Kaumberg, Austria). For CYTIP staining the specific antibody 2F9 was used. Antibody binding was visualized using biotinylated species-specific goat anti-rat Ig (Amersham) followed by a streptavidin-Alexa 488 conjugate (Molecular Probes). The percentage of positive cells was evaluated on a FACSCalibur instrument using CellQuest software (BD Immunocytometry Systems, San Jose, CA).
FACS analysis of surface molecules
Directly labeled primary antibodies specific for CD11a, CD18, CD40, CD54, CD80, CD86, MHCI, and MHCII (Becton Dickinson/Pharmingen), CD11c (DAKO, Glostrup, Denmark), CD49d, CD49e, CD106 (Ancell, Bayport, MN), and CD83 (Immunotech/Coulter, Fullerton, CA) were used. Specimens were analyzed on a FACScalibur using CellQuest software (BD Immunocytometry Systems).
Adhesion assay of calcein-AM-labeled DCs
To measure the influence of CYTIP silencing on adherence either to fibronectin, T cells, or endothelial cells, we used a fluorometric adhesion assay (Vybrant cell adhesion assay kit; Molecular Probes) as previously described.15 Briefly, before labeling with the fluorogenic dye, calcein AM, DCs were washed twice with PBS, resuspended in RPMI 1640 without phenol red, supplemented with 10% FCS, and adjusted to 1 × 106 cells/mL. DCs were then labeled at a final concentration of 50 μM for 30 minutes at 37°C. Labeling was stopped by adding prewarmed (37°C) RPMI without phenol red and serum. DCs were washed twice with RPMI without phenol red and serum, added to coated microtiter plates, and incubated for 30 to 45 minutes at 37°C and 5% CO2. The plates were coated with fibronectin (20 μg/mL, overnight at 4°C), followed by blocking with 0.01% gelatin for 2 hours at room temperature or with poly L-lysine (100 μg/mL for 1 hour) to immobilize 106 T cells to create a confluent layer or with a confluent layer of human dermal microvascular endothelial cells. Unbound cells were removed by gentle washing with PBS twice. Each well was scanned by a fluorescence microtiter plate reader (Fluoroskan Ascent; Labsystems, Helsinki, Finland) to measure the fluorescent signal emitted by the bound DCs using excitation and emission wavelengths of 485 nm and 538 nm, respectively.
Specific cytotoxic T lymphocyte (CTL) priming assay
Expansion of EBV A2.1 peptide-specific CD8+ T cells was induced by stimulation of purified CD8+ T cells from HLA A2.1+ donors with autologous DCs that were either pulsed or unpulsed with 10 μM HLA A2.1-restricted EBV peptide (GLCTLVAML; purchased from ProImmune, Oxford, United Kingdom) for 3 hours at 37°C at 1 × 106 DC/mL complete medium. For stimulation, 106 CD8+ T cells were incubated with DCs at a ratio of 1:10 for 7 days. Expansion of antigen-specific CTLs was quantified by a pentamer staining according to the manufacturer (ProImmune Limited).
Statistical analysis
All experiments were performed at least 3 times with similar results. Error bars are from standard deviations, and P values are from 2-tailed Student t tests.
Results
Expression of CYTIP mRNA in DC subsets
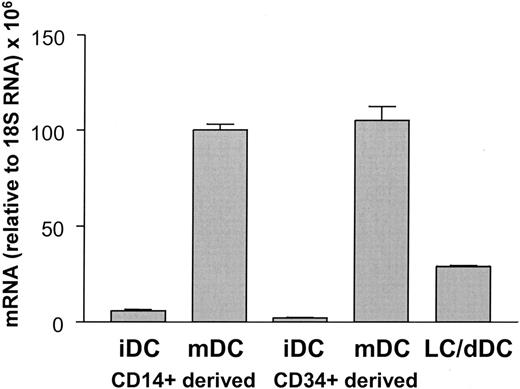
We showed previously that CYTIP is induced during maturation of CD14+ monocyte-derived DCs. To investigate whether other DC populations also express and regulate the expression of this molecule, we performed real-time PCR of reverse transcribed mRNA from CD34+ precursor-derived DCs, skin explant crawl-out cells, and epidermal Langerhans cells (Figure 1). CYTIP mRNA can be detected in all of them, although to varying extents. Thus, CYTIP mRNA is expressed in a variety of DCs and induced on maturation.
CYTIP accumulates at the cell periphery on adherence to fibronectin and on engagement of several adhesion molecules
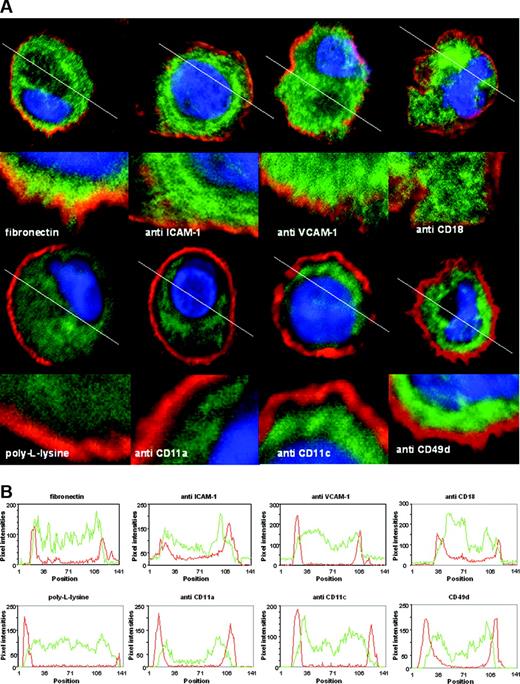
Next, we investigated whether endogenous CYTIP in DCs changes its localization within the cell on integrin engagement, similarly to the overexpressed protein in Jurkat cells.5 On binding to fibronectin, a binding partner of a number of adhesion molecules,16 but not to poly-L-lysine which mediates charge-dependent adhesion, CYTIP localizes to the periphery of the cell in DCs. In addition, peripheral localization of CYTIP also occurs on antibody-mediated binding of the adhesion molecules CD18, CD54 (ICAM-1), and CD106 (VCAM-1) (Figure 2A, upper row), as can be seen by the overlapping of green and red fluorescence (resulting in orange/yellow), representing CYTIP and the plasma membrane, respectively, but not on engagement of other adhesion molecules like CD11a, CD11c, and CD49d (VLA-4) (Figure 2A, lower row), where green and red fluorescence remain separated. The pixel intensity for the red and green fluorescence was measured along the white lines drawn in Figure 2A and shown as line scans in Figure 2B. Overlapping of green and red fluorescence on the cell margins is more pronounced in the upper row. These data show that localization to the proximity of the plasma membrane of CYTIP is specifically induced by a restricted number of adhesion molecules.
Quantitation of CYTIP mRNA expression in DC populations. RNA from CD14+ monocyte-derived immature DCs (iDCs) and mature DCs (mDCs), CD34+ precursor-derived immature and mature DCs, and emigrated Langerhans cells (LCs) and dermal cells from skin explant cultures (dDCs) was collected and subjected to quantitative reverse transcriptase (RT)-PCR using CYTIP-specific primers and probes. Values are relative to 18S RNA. CYTIP mRNA is expressed in a variety of DCs and induced on maturation.
Quantitation of CYTIP mRNA expression in DC populations. RNA from CD14+ monocyte-derived immature DCs (iDCs) and mature DCs (mDCs), CD34+ precursor-derived immature and mature DCs, and emigrated Langerhans cells (LCs) and dermal cells from skin explant cultures (dDCs) was collected and subjected to quantitative reverse transcriptase (RT)-PCR using CYTIP-specific primers and probes. Values are relative to 18S RNA. CYTIP mRNA is expressed in a variety of DCs and induced on maturation.
Silencing of CYTIP expression is specific and does not interfere with the expression of other molecules
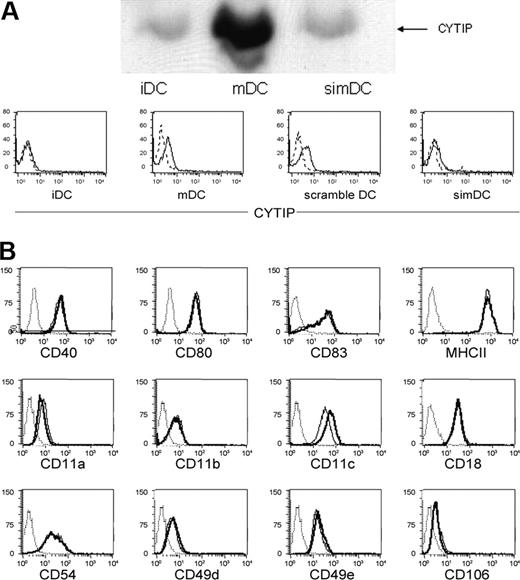
As a tool to investigate the function of CYTIP we silenced its expression during the maturation period using small interfering RNA. CYTIP expression could be kept to the level of immature DCs as controlled by Western blotting and was achieved in 60% to 90% of the cells as shown by intracellular FACS staining for CYTIP (Figure 3A). Expression of molecules known to be induced or up-regulated during maturation of DCs or several adhesion molecules is not influenced by the silencing procedure, as shown by FACS analysis of CD40, CD80, CD83, MHC II, CD11a, CD11b, CD11c, CD18, CD49d, CD49e, CD54, and CD106 (Figure 3B).
Loss of adherence of mature DCs to fibronectin depends on CYTIP expression
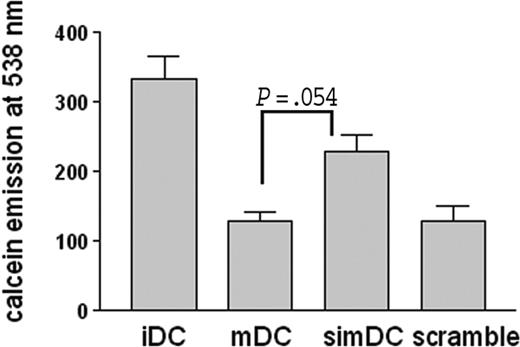
To ensure a functional relevance of the peripheral localization of CYTIP, we measured the adherence of DCs to fibronectin and the function of CYTIP in these adhesion events. Immature DCs, mature DCs, CYTIP-silenced mature DCs, and DCs transfected with an unrelated siRNA were labeled with calcein and allowed to bind to fibronectin-coated 96-well plates. After washing, adherent cells were quantified by measuring calcein in a spectrophotometer. Figure 4 shows adhesion of immature, mature, CYTIP-silenced mature DCs and mature DCs transfected with an unrelated siRNA. Mature DCs adhere less efficiently to fibronectin than immature DCs. Silencing of CYTIP partially restores adherence in mature DCs, whereas the introduction of an unrelated siRNA has no effect on the adherence capacity of mature DCs to fibronectin. Thus, CYTIP mediates deattachment of integrin-induced adhesion of mature DCs.
Cellular CYTIP distribution in response to stimulation of surface molecules by antibodies. (A) Mature monocyte-derived DCs were allowed to bind to antibodies immobilized on glass slides for 20 to 30 minutes. After fixation in acetone, CYTIP was visualized by a specific monoclonal anti-CYTIP antibody detected by Alexa 488 (green fluorescence). DAPI was used to visualize the nuclei (blue fluorescence) and PKH26 to mark the plasma membrane (red fluorescence). Images were obtained by confocal microscopy. White lanes were used to perform line scans shown in panel B. (B) Red lines show pixel intensities for PKH26; green lines show pixel intensities for CYTIP staining. On binding to fibronectin, and antibody-mediated binding of CD18, ICAM-1 (CD54), and VCAM-1 (CD106), but not to poly-L-lysine and anti-CD11a, -CD11c, and -VLA-4 (CD49d), CYTIP moves toward the membrane in DCs.
Cellular CYTIP distribution in response to stimulation of surface molecules by antibodies. (A) Mature monocyte-derived DCs were allowed to bind to antibodies immobilized on glass slides for 20 to 30 minutes. After fixation in acetone, CYTIP was visualized by a specific monoclonal anti-CYTIP antibody detected by Alexa 488 (green fluorescence). DAPI was used to visualize the nuclei (blue fluorescence) and PKH26 to mark the plasma membrane (red fluorescence). Images were obtained by confocal microscopy. White lanes were used to perform line scans shown in panel B. (B) Red lines show pixel intensities for PKH26; green lines show pixel intensities for CYTIP staining. On binding to fibronectin, and antibody-mediated binding of CD18, ICAM-1 (CD54), and VCAM-1 (CD106), but not to poly-L-lysine and anti-CD11a, -CD11c, and -VLA-4 (CD49d), CYTIP moves toward the membrane in DCs.
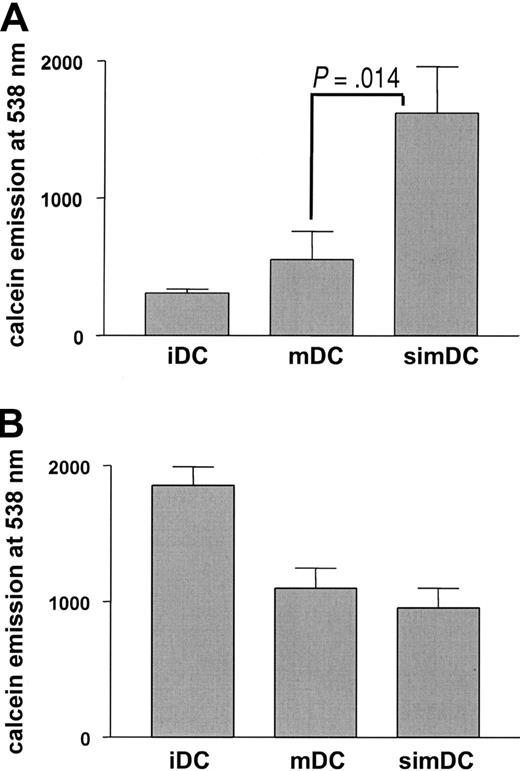
Adherence of mature DCs to T cells but not to endothelial cells is enhanced by CYTIP silencing
In vivo, 2 situations are conceivable where a rapid release of integrin-mediated adhesion may be advantageous for mature DCs. (1) Migration from the periphery to the secondary lymphoid organs requires loosening of integrin-mediated adhesion to allow DCs to leave their surroundings. (2) Screening of T-cell antigen receptors in the secondary lymphoid organs is more efficient when release from integrin-mediated, antigen-nonspecific conjugate formation of DCs and T cells enhances the probability of antigen-specific TCR engagement. To detect an eventual effect of CYTIP in these events we monitored the behavior of CYTIP-silenced mature DCs that were labeled with calcein and allowed to bind for 30 minutes to endothelial cells grown in 96-well plates for the first situation, or to immobilized T cells for the second situation. After washing, adherent cells were quantified by measuring calcein in a spectrophotometer. CYTIP silencing in mature DCs enhanced binding to T cells (Figure 5A) but did not alter binding activities of DCs to endothelial cells (Figure 5B). These data indicate that CYTIP is required for the deattachment of DC-T-cell contacts, but not for DC-endothelial cell interactions.
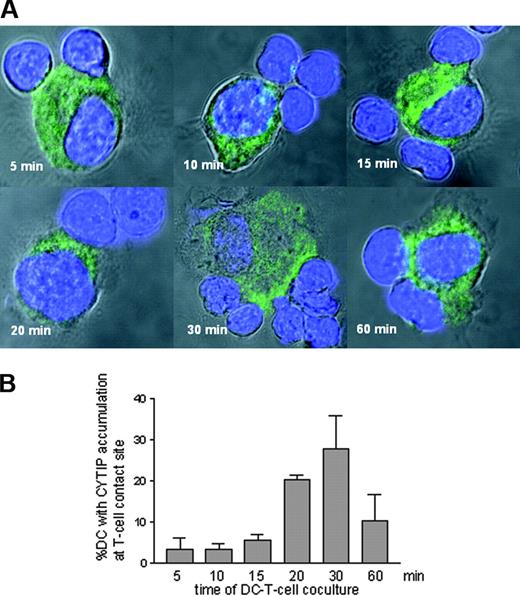
Endogenous CYTIP in DCs accumulates at the contact zones to T cells
To verify a role of CYTIP in early DC-T-cell contact events, we monitored whether CYTIP in DCs relocalizes on contact with T cells, as seen with fibronectin and several integrins. For this we allowed 3 × 104 mature DCs to bind to 105 bulk T cells immobilized on glass slides via poly-L-lysine. After 5, 10, 15, 20, 30, and 60 minutes, cells were fixed in acetone and processed for confocal microscopy. In Figure 6A, cells were stained for CYTIP (Alexa 488, green fluorescence), and nuclei were visualized by DAPI (blue fluorescence). Images were taken with a Zeiss Axiovert 100 M microscope. Accumulation of CYTIP in the contact zone of DCs and T cells can be observed starting at 15 minutes, for a maximum of 30 minutes. For quantification of DCs showing translocation of CYTIP to the contact zone with T cells we scanned 3 times an area of 0.546 mm2 for each time point and counted all DCs having contact with a T cell. Using pseudo-color analysis we then counted all DCs with T-cell contact, where accumulation occurred at the contact site. Accumulation corresponds to change of color (1.5 × gain of intensity).
Silencing of CYTIP is specific and does not interfere with the expression or induction of other molecules. (A) CYTIP expression was monitored 48 hours after silencing by Western blotting and intracellular FACS analysis. The expression level can be kept to the level of immature DCs and was achieved in 60% to 90%. iDC indicates immature DC; mDC, mature DC; simDC, mature DC silenced for CYTIP; and scramble, unrelated control siRNA used in silencing procedure. (B) Expression of other molecules known to be induced or up-regulated during maturation of DCs as well as adhesion structures (CD40, CD80, CD83, MHCII, CD11a, CD11b, CD11b, CD11c, CD18, CD54, CD49d, CD49e, and CD106) are not influenced by the silencing procedure, as shown by FACS analysis. Broken line indicates isotype control; solid line, immature DC; bold line, mDC silenced for CYTIP; and dotted line, mature DC.
Silencing of CYTIP is specific and does not interfere with the expression or induction of other molecules. (A) CYTIP expression was monitored 48 hours after silencing by Western blotting and intracellular FACS analysis. The expression level can be kept to the level of immature DCs and was achieved in 60% to 90%. iDC indicates immature DC; mDC, mature DC; simDC, mature DC silenced for CYTIP; and scramble, unrelated control siRNA used in silencing procedure. (B) Expression of other molecules known to be induced or up-regulated during maturation of DCs as well as adhesion structures (CD40, CD80, CD83, MHCII, CD11a, CD11b, CD11b, CD11c, CD18, CD54, CD49d, CD49e, and CD106) are not influenced by the silencing procedure, as shown by FACS analysis. Broken line indicates isotype control; solid line, immature DC; bold line, mDC silenced for CYTIP; and dotted line, mature DC.
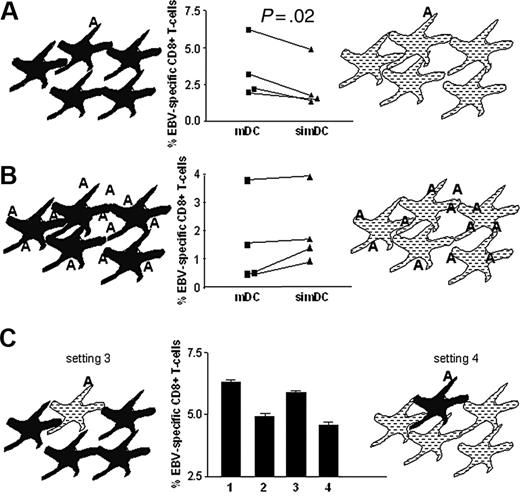
CYTIP silencing impairs T-cell priming capacity of DCs
To evaluate whether the deattaching function of CYTIP has an effect on T-cell priming activity, we performed an in vitro T-cell priming assay, in which DCs were partially loaded with antigen. If CYTIP is needed for the release of T cells from conjugates formed during the T-cell screening process, a setting where all DCs are loaded with antigen should work independently of CYTIP. Otherwise, in a setting where only part of the DCs is loaded with antigen, T cells need to deattach from conjugates with DCs carrying irrelevant antigens to enhance their chance to encounter a DC carrying the relevant antigen and thus able to activate the T cell. In this case CYTIP-silenced DCs are expected to be less efficient in priming T cells. We loaded DCs with an EBV-specific peptide and used them in a T-cell priming assay either alone or mixed with 9 parts of unloaded DCs. We measured T-cell expansion by pentamer staining of CD8+ T cells. In Figure 7A we show that CYTIP-silenced mature DCs have a clearly reduced capacity to prime T cells as compared with control mature DCs in the assay where only 1/10 of DCs is antigen loaded, whereas CYTIP-silenced mature DCs and control mature DCs exert similar priming activity if all DCs are loaded with antigen (Figure 7B). In Figure 7C, in addition to showing the setting described in Figure 7A (bar 1, mDCs; bar 2, simDCs), we show the priming efficiency of 10% pulsed silenced DCs mixed with 90% unpulsed untreated DCs (setting and bar 3) and of 10% untreated pulsed DCs mixed with 90% silenced unpulsed DCs (setting and bar 4). In setting 3, unpulsed untreated DCs allow T cells to leave. The priming efficiency is intact. However, in setting 4 unpulsed silenced DCs do not allow deattachment from unpulsed DCs, and priming capacity is impaired. This indicates that CYTIP is required for the release of T cells from interactions with DCs, presumably during the screening of antigen-specific TCRs.
DCs adherence to fibronectin is CYTIP dependent. CYTIP was specifically silenced in DCs during maturation. DCs were labeled with calcein and allowed to adhere to fibronectin-treated 96-well plates. Adherence to fibronectin was measured by quantifying calcein emission. Mature DCs (mDCs) adhere less efficiently to fibronectin than immature DCs (iDCs). Silencing of CYTIP partially restores adherence in mature DCs (simDCs). The unrelated siRNA (scramble) does not change the adhesion features of mDCs.
DCs adherence to fibronectin is CYTIP dependent. CYTIP was specifically silenced in DCs during maturation. DCs were labeled with calcein and allowed to adhere to fibronectin-treated 96-well plates. Adherence to fibronectin was measured by quantifying calcein emission. Mature DCs (mDCs) adhere less efficiently to fibronectin than immature DCs (iDCs). Silencing of CYTIP partially restores adherence in mature DCs (simDCs). The unrelated siRNA (scramble) does not change the adhesion features of mDCs.
DC adherence to T cells but not to endothelial cells depends on CYTIP. T cells were applied to poly-L-lysine-coated 96-well plates, and endothelial cells were cultured in 96-well plates. Calcein-labeled DCs were allowed to adhere to T cells or endothelial cells for 45 minutes. Adherent cells were quantified by measuring calcein emission. CYTIP silencing in mature DCs (simDCs) enhanced binding to T cells (A) but did not alter binding activities of DCs to endothelial cells (B). iDC indicates imature DC; mDC, mature DC.
DC adherence to T cells but not to endothelial cells depends on CYTIP. T cells were applied to poly-L-lysine-coated 96-well plates, and endothelial cells were cultured in 96-well plates. Calcein-labeled DCs were allowed to adhere to T cells or endothelial cells for 45 minutes. Adherent cells were quantified by measuring calcein emission. CYTIP silencing in mature DCs (simDCs) enhanced binding to T cells (A) but did not alter binding activities of DCs to endothelial cells (B). iDC indicates imature DC; mDC, mature DC.
Cellular localization of CYTIP in DCs in response to T-cell contact. (A) DCs were allowed to adhere to poly-L-lysine immobilized bulk T cells for the indicated time points. CYTIP was visualized by immunostaining (green fluorescence). Nuclei are stained with DAPI (blue fluorescence). Cells were visualized with a confocal microscope. Accumulation of CYTIP in the contact zone of DCs and T cells can be observed starting at 15 minutes with a maximum at 30 minutes. (B) Percentage of contact sites with polarized CYTIP from all DCs with T-cell contact was calculated in 3 scans each of 0.546 mm2 for each time point.
Cellular localization of CYTIP in DCs in response to T-cell contact. (A) DCs were allowed to adhere to poly-L-lysine immobilized bulk T cells for the indicated time points. CYTIP was visualized by immunostaining (green fluorescence). Nuclei are stained with DAPI (blue fluorescence). Cells were visualized with a confocal microscope. Accumulation of CYTIP in the contact zone of DCs and T cells can be observed starting at 15 minutes with a maximum at 30 minutes. (B) Percentage of contact sites with polarized CYTIP from all DCs with T-cell contact was calculated in 3 scans each of 0.546 mm2 for each time point.
Discussion
In a Jurkat cell transfection model we showed recently that CYTIP action on cell adhesion is mediated through capture of the intracellular, adhesion-enhancing, integrin-binding molecule cytohesin-1. Cytohesin-1 enhances the binding activity of LFA-1 to ICAM-1 by binding to the β-2 chain (CD18) of LFA-1. CYTIP binding of cytohesin-1 results in the relocalization of both CYTIP and cytohesin-1 from the membrane to the cytosol, which is accompanied by reduced adhesion. Peripheral localization of overexpressed CYTIP in Jurkat cells was shown on binding to fibronectin and ICAM-1 (CD54).5 We therefore examined the localization of endogenous CYTIP in DCs after binding to fibronectin and antibody-mediated binding of CD18, the β-2 subunit of ICAM-1, the binding partner LFA-1. CD18 in DCs can associate with 3 different α-chains, CD11a, CD11b, and CD11c; therefore, we also examined the effect of antibodies directed against 2 of these subunits. In addition, we used antibodies directed against the integrins VCAM-1 (CD106) and VLA-4 (CD49), known to be expressed on DCs. Localization of CYTIP at the periphery of the cell was obtained, as expected, on DCs binding to fibronectin, anti-ICAM-1 and anti-CD18. Antibody-mediated binding of the integrin VCAM-1 also induced relocalization, whereas binding of VLA-4 and the α-chains CD11a and CD11c had no effect. It is well established that cytohesin-1 binds only to the β-2 chain of integrins.17,18 Therefore, cytohesin-1 capture is probably only one mechanism mediating this loss of adhesion capacity and an additional, as yet unknown, mechanism might be involved in the function of CYTIP. This is supported by binding studies of CYTIP and cytohesin-1. Binding of CYTIP to cytohesin-1 recruits the coiled-coil regions of both proteins, but also the second protein-protein binding site on CYTIP, a PDZ domain, was shown to be required for membrane localization.5 The putative binding partner of this domain is still to be identified.
Expansion of antigen-specific T cells by DCs is impaired by CYTIP silencing. Mature HLA A2.1+ DCs were cocultured with autologous CD8+ T cells for 7 days. T cells were stained with anti-CD8-FITC and PE-labeled pentamers specific for HLA A2.1 loaded with EBV peptide. Percentage of CD8+/Pentamer+ cells are shown. (A) DCs silenced for CYTIP (simDC) or not (mDC) were loaded with EBV peptide and mixed with unloaded CYTIP-silenced DCs or unloaded untreated DCs, respectively, at a ratio of 1:10. The results of 4 independent experiments show that simDCs have a clearly reduced capacity to prime T cells as compared with mDCs in the assay where only 1/10 of DCs is antigen loaded. The P value from a paired Student t test performed with the results of the 4 experiments is .02 (significant). (B) All DCs were loaded with the EBV peptide. simDCs and mDCs exert similar priming activity if all DCs are loaded with antigen. P = .09 (not significant). (C) The bar graphs show the priming capacity of DCs in the setting described in panel A (bar 1, mDCs; bar 2, simDCs) and, in addition, of DCs silenced for CYTIP and loaded with EBV peptide and mixed with 9 parts of untreated unpulsed DCs (setting 3, bar 3) and of untreated DCs loaded with EBV peptide and mixed with 9 parts unloaded CYTIP silenced DCs (setting 4, bar 4). Although 9 parts of unpulsed untreated DCs do not interfere with the priming capacity of loaded silenced DC (bar 3), CYTIP silencing of 9 parts of unloaded DCs impairs priming (bar 4).
Expansion of antigen-specific T cells by DCs is impaired by CYTIP silencing. Mature HLA A2.1+ DCs were cocultured with autologous CD8+ T cells for 7 days. T cells were stained with anti-CD8-FITC and PE-labeled pentamers specific for HLA A2.1 loaded with EBV peptide. Percentage of CD8+/Pentamer+ cells are shown. (A) DCs silenced for CYTIP (simDC) or not (mDC) were loaded with EBV peptide and mixed with unloaded CYTIP-silenced DCs or unloaded untreated DCs, respectively, at a ratio of 1:10. The results of 4 independent experiments show that simDCs have a clearly reduced capacity to prime T cells as compared with mDCs in the assay where only 1/10 of DCs is antigen loaded. The P value from a paired Student t test performed with the results of the 4 experiments is .02 (significant). (B) All DCs were loaded with the EBV peptide. simDCs and mDCs exert similar priming activity if all DCs are loaded with antigen. P = .09 (not significant). (C) The bar graphs show the priming capacity of DCs in the setting described in panel A (bar 1, mDCs; bar 2, simDCs) and, in addition, of DCs silenced for CYTIP and loaded with EBV peptide and mixed with 9 parts of untreated unpulsed DCs (setting 3, bar 3) and of untreated DCs loaded with EBV peptide and mixed with 9 parts unloaded CYTIP silenced DCs (setting 4, bar 4). Although 9 parts of unpulsed untreated DCs do not interfere with the priming capacity of loaded silenced DC (bar 3), CYTIP silencing of 9 parts of unloaded DCs impairs priming (bar 4).
The peripheral localization of endogenous CYTIP in DCs on binding to integrins has a similar effect to that seen for overexpressed CYTIP in Jurkat cells: It is accompanied by an attenuation of binding capacity to fibronectin and T cells. The higher binding capacity of mature DCs specifically silenced for CYTIP both to fibronectin (Figure 4) and to T cells (Figure 5A) indicates a similar functional consequence of endogenous CYTIP relocalization in DCs as that observed for overexpressed CYTIP in Jurkat cells: attenuation of adhesion on peripheral localization of CYTIP. These data and the timely confined accumulation of CYTIP to the contact zone with T cells shortly after starting DC-T-cell cocultures (Figure 6), in addition to the enhanced T-cell binding of CYTIP-silenced DCs allow the interpretation that CYTIP mediates T-cell deattachment from DCs.
Early during the priming of T cells a scanning process takes place, connecting the antigen-presenting cell with the T cell for a limited time.19,20 The outcome of this scanning process is the activation of antigen-specific T cells, whereas antigen-unspecific T cells should remain inactive.3,21,22 In both cases the release of the connection between the 2 cell types is necessary to continue the process of immune response, either to allow for clonal expansion of the activated T cell or to continue the scanning process. Although many molecules are known to build cell conjugates, the termination of them was not studied and was mainly assumed to be a passive process. Given the importance and the calculated incidence of DC-T-cell contacts during the scanning process, which ranges between 500 and 5000 encounters per hour,23 it is well assumable that this process is actively regulated. We here present evidence by 3 independent experimental designs that CYTIP is used by DCs to enhance the termination of DC-T-cell contacts: (1) the relocalization of cytosolic CYTIP in DCs to the contact zone with T cells, taking place almost immediately after encounter and lasting only for a short period of time, (2) the restoration of the binding capacity of mature DCs to fibronectin, a ligand for a number of adhesion molecules, on silencing of CYTIP, and (3) the higher binding capacity of CYTIP-silenced mature DCs to T cells. In addition, the resolution of the contact seems to be restricted to the DC-T-cell contact, because the binding of DCs to endothelial cells is not affected by silencing of CYTIP. A further evidence for the specificity of CYTIP function can be deduced from the differential response of cellular CYTIP relocalization on activation by a series of surface molecules (Figure 2). A functional consequence of CYTIP silencing in mature DCs is the reduced priming capacity for CD8+ T cells, when only part of the DCs are loaded with the relevant antigen (Figure 7). This setting seems more biologically relevant than priming assays with all DCs loaded with antigen, considering that in locations where T-cell screening occurs only a minority of the DCs will carry the antigen.24-26 These data indicate that in the context of T-cell scanning and priming CYTIP activity is needed to allow DCs to quickly release T cells from conjugates, enhancing their possibility for the interaction with a DC carrying the relevant antigen. Our control experiments always resulted in a slightly enhanced priming capacity of CYTIP-silenced mDCs for CD8+ T cells when all DCs were loaded with the relevant antigen (Figure 7B). A speculative interpretation of this results is a more stable productive DC-T-cell interaction when the disintegrating mechanism is silenced. This mechanism can only be applied by mature DCs because CYTIP is induced by maturation. Immature DCs, which have shorter contact times to T cells than mature DCs, may have a very different binding pattern to T cells than mature DCs, given the differences in the expression levels of adhesion molecules, costimulatory molecules and even the number of extracellular MHC molecules. Immature DCs probably apply different adhesion structures, possibly without a need for active disintegration. Thus, our data on CYTIP function suggest an active mechanism for the disintegration of DC-T-cell contacts controlled by mature DCs through CYTIP.
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2005-01-0425.
Supported by the Austrian Science fund “Fonds zur Förderung der wissenschaftlichen Forschung” project (16021-B01) (C.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal