Abstract
Adaptive immunity is triggered at the immune synapse, where peptide-major histocompatibility complexes and costimulatory molecules expressed by dendritic cells (DCs) are physically presented to T cells. Here we describe transmission of the inflammatory monoamine serotonin (5-hydroxytryptamine [5-HT]) between these cells. DCs take up 5-HT from the microenvironment and from activated T cells (that synthesize 5-HT) and this uptake is inhibited by the antidepressant, fluoxetine. Expression of 5-HT transporters (SERTs) is regulated by DC maturation, exposure to microbial stimuli, and physical interactions with T cells. Significantly, 5-HT sequestered by DCs is stored within LAMP-1+ vesicles and subsequently released via Ca2+-dependent exocytosis, which was confirmed by amperometric recordings. In turn, extracellular 5-HT can reduce T-cell levels of cAMP, a modulator of T-cell activation. Thus, through the uptake of 5-HT at sites of inflammation, and from activated T cells, DCs may shuttle 5-HT to naive T cells and thereby modulate T-cell proliferation and differentiation. These data constitute the first direct measurement of triggered exocytosis by DCs and reveal a new and rapid type of signaling that may be optimized by the intimate synaptic environment between DCs and T cells. Moreover, these results highlight an important role for 5-HT signaling in immune function and the potential consequences of commonly used drugs that target 5-HT uptake and release.
Introduction
Dendritic cells (DCs) are highly specialized to integrate diverse stimuli for the generation of antigen-specific immunity and life-long immunologic memory. As immature cells, they are ubiquitously distributed in peripheral tissues where they serve as immune sensors, sampling foreign and self antigens.1,2 Microbial antigens recognized by pattern recognition receptors,3 and self molecules released by dead and dying cells,4 trigger the migration of DCs. While en route to secondary lymphoid tissues, DCs up-regulate their expression of cell surface major histocompatibility complex (MHC) and costimulatory molecules and the secretion of cytokines and chemokines necessary for the initiation of adaptive immune responses.1,2
The intimate and dynamic interactions that occur between DCs and cognate lymphocytes in secondary lymphoid tissues are equally specialized. It is now clear that T-cell activation requires a zone of adhesive, direct contact with antigen-presenting cells (APCs), popularly known as the immune synapse.5 From the DC vantage, the formation of immune synapses involves cytoskeletal reorganization and the focal redistribution of antigen-MHCs and accessory molecules.6-9 The immune synapse appears to maximize the physical interactions between DCs and lymphocytes and forms a stable platform for the recruitment and concentration of signaling molecules.10,11
The immune synapse exhibits many similarities with classical neuronal synapses, including a requirement for cell-to-cell adhesion and close membrane apposition. In addition, triggering of cell surface receptors leads to intracellular signal transduction in both structures.10,11 The tight synaptic cleft between neurons (20-40 nm) and the neuromuscular junction (∼100 nm) facilitates the rapid diffusion of neurotransmitters.11 Although a relatively transient structure, the immune synapse also features a synaptic cleft (15-40 nm).9,10,12 We therefore considered the possibility that the immune synapse may also support the regulated secretion of labile molecules that could contribute to signaling between DCs and lymphocytes.
Langerhans cells, the archetypal DCs, are known to accumulate neurotransmitters.13 Serotonin (5-hydroxytryptamine [5-HT]) is a classical neurotransmitter and vasoactive amine involved in signaling a variety of behavioral and physiologic states including appetite, sleep, mood, and pain.14 Moreover, 5-HT has significant effects on inflammation and immunity. Mast cells and platelets both express the 5-HT–specific transporter (SERT), which enables them to sequester 5-HT from the microenvironment. In turn, this 5-HT is released in response to injury or inflammatory signals such as IgE complexes, complement components, and platelet-activating factor.15,16 Exocytosed 5-HT regulates platelet aggregation and modulates leukocyte motility and airway hyperresponsiveness.16,17
5-HT is also reported to modulate T-cell activation and differentiation.16,18,19 The mechanisms by which T cells may encounter 5-HT, however, are unclear, particularly within secondary lymphoid tissues. We hypothesized that DCs may sequester 5-HT released by platelets and mast cells at sites of injury and inflammation or from sympathetic nerves that innervate lymphoid tissues14 and secrete this 5-HT on interaction with T cells. In support of this hypothesis, we demonstrate that DCs possess the machinery for the selective uptake and regulated release of 5-HT. Of importance, we identify the expression of type 1 tryptophan hydroxylase (TPH-1) by activated T cells that indicates that they are capable of producing 5-HT. We propose that DCs may sequester 5-HT released from their microenvironment, or directly from activated T cells, and transmit this 5-HT to naive T cells. Thus, 5-HT transmission represents a novel form of rapid communication between DCs and T cells that may influence early T-cell signaling.
Materials and methods
DC culture
Animals were used in accordance with guidelines set forth by the Georgetown University Animal Care and Use Committee and the Canadian Council on Animal Care. Bone marrow (BM) cells were isolated from the femurs and tibias of normal C57Bl/6 mice (7-12 weeks old) and cultured for 5 days at 3 × 105 cells/mL in the presence of GM-CSF and IL-4 as previously described.20,21 For some experiments, BMDCs were activated using LPS (50 ng/mL; L2654 Sigma, St Louis, MO) during the last 18 hours of culture. Spleen DCs were expanded in vivo using Flt3 ligand (15 μg/d, intraperitoneally, for 10 days) and matured by overnight culture.22,23 Unless stated otherwise, DCs were enriched from spleen or BM cultures to approximately 85% to 90% by centrifugation over histodenz (140 mg/mL, 20 minutes, 500g; Sigma). In some experiments, DCs were labeled with anti-CD11c FITC or biotinylated anti-CD11c plus streptavidin (SA)–PE Cy7 (HL3) and purified to more than 95% using a fluorescence-activated cell sorting (FACS) Vantage cell sorter (BD Biosciences, San Jose, CA). Labeling with anti-CD86 PE (GL1; BD Biosciences) was used to separate immature (CD11c+ CD86-) and mature (CD11c+ CD86+) DCs.
T-cell isolation and culture
T cells were purified from C57Bl/6 spleen cells using SpinSep T-cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada) and cultured at 1 × 106 cells/mL in RPMI 1640 (Biosource, Rockville, MD) supplemented with 10% FCS (Sigma or Hyclone, Logan, UT), nonessential amino acids, l-glutamine, sodium pyruvate, penicillin-streptomycin, and 2-ME (Sigma, or Invitrogen, Carlsbad, CA) with 2.5 to 5 μg/mL concanavalin A (Con A; Sigma or Biomeda, Foster City, CA) for 2 days. Con A-activated T cells (1 × 106 cells/mL) were rested for 4 days in complete media supplemented with IL-2 (10 IU/mL; PeproTech, Rocky Hill, NJ).
RT-PCR
RNA was extracted using TRIzol (Invitrogen), first-strand cDNA synthesis was performed using Advantage reverse transcription for polymerase chain reaction (RT-PCR; BD Biosciences), and cDNA was amplified using Taq DNA polymerase (Eppendorf, Hamburg, Germany). Comparable quantities of cDNA were ensured by amplification of GAPDH (forward 5′-GCC GCC TGG AGA AAC CTG CCA AGT-3′ and reverse 5′-TAT TCA AGA GAG TAG GGA GGG CTC-3′). All other primers were as follows: SERT, forward 5′-ACA ACA TCA CCT GGA CAC TCC ATT C-3′ and reverse 5′-CCG CAT ATG TGA TGA AAA GGA GGC T-3′; TPH-1, forward 5′-GAA CAA AGA CCA TTC CTC CGA AAG AG-3′ and reverse 5′-GTG AGC TGA TCG GGC GAG TCC A-3′; TPH-2, forward 5′-CGA TCT GGC TTC ACA GTG AG-3′ and reverse 5′-GAT TTC ACA CAC GCC TTG TC-3′; monoamine oxidase A (MAO-A), forward 5′-GCT CTG CAG CCC GTC CAT TAT GAA-3′ and reverse 5′-ATC ATG CAG CCA CAA TAG TC-3′; and MAO-B forward 5′-GCT CTG CAG CCC GTC CAT TAT GAA-3′ and reverse 5′-GGG TCG TGC AGG GAC ATC GAA AGA TTC TTG TTC T-3′. Transcripts were amplified by 32 to 38 cycles of the following: cDNA denaturation (20 seconds at 95°C), followed by 30 seconds of primer annealing, and 30 seconds of extension at 72°C. Annealing temperatures were as follows: GAPDH (57°C), SERT (58°C), TPH-1 (54°C), TPH-2 (53°C), MAO-A (53°C), and MAO-B (57°C). PCR products were resolved as single bands by agarose gel electrophoresis and visualized with SYBR Safe (Molecular Probes, Eugene, OR) fluorescence.
SERT and 5-HT immunolabeling
BMDCs were adhered to poly-l-lysine–coated slides (30 minutes, 37°C) and fixed with 95% ethanol. BMDCs were incubated with anti-SERT antibody (1 hour, room temperature, 4A2.2; Advanced Targeting Systems, San Jose, CA) conjugated with Xenon Alexa Fluor 546 (Molecular Probes) and biotinylated anti-CD11c (2 hours, room temperature; BD Biosciences) plus SA-Alexa Fluor 488 (1 hour, room temperature; Molecular Probes). Irrelevant hamster IgG1 and mouse IgG2b were used as negative controls for CD11c and SERT labeling, respectively (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). The isotype of the anti-SERT monoclonal antibody (mAb) was determined using the ImmunoPure mAb isotyping kit II (AP/PNPP; Pierce Biotechnology, Rockford, IL).
Sorted CD11c+ BMDCs preloaded with 100 μM 5-HT (1 hour, 37°C) were cytocentrifuged onto slides and fixed with 4% paraformaldehyde. 5-HT was labeled with 5-HT antisera (72 hours, room temperature, S-5545; Sigma) followed by biotinylated anti–rabbit immunoglobulin (24 hours, 4°C) and SA-Alexa Fluor 546 (1 hour, room temperature; Molecular Probes). LAMP-1 mAb (1D4B, 24 hours, 4°C; BD Biosciences) was detected with anti–rat Alexa Fluor 488 (1 hour, room temperature; Molecular Probes). Irrelevant rat IgG2a was used as the negative control for LAMP-1 staining. BMDCs incubated without 5-HT, and 5-HT antisera absorbed with 100 μM 5-HT (18 hours, 4°C), were used as negative controls for 5-HT labeling (Figure S2). Slides were examined using a Zeiss LSM 510 META microscope (Oberkochen, Germany) using a Plan-Apochromat 63×/1.4 numeric aperture oil immersion objective. Pinhole settings were 1 airy unit. Images were acquired and analyzed with LSM510 software (Zeiss). Figures were compiled using Adobe Photoshop (Adobe Systems, San Jose, CA).
SERT radioligand binding
BMDCs were resuspended in 5 mM Tris-HCl buffer (pH 7.4) containing 5 mM EDTA and protease inhibitors (buffer A). BMDCs were homogenized using a Polytron (Brinkmann Instruments, Westbury, NY) and debris was sedimented by gentle centrifugation (1000g, 10 minutes). Membranes were enriched by ultracentrifugation of the supernatant (30 000g, 30 minutes). Quantitation of SERT was performed using the specific SERT ligand, [3H]-citalopram (Perkin Elmer, Wellesley, MA). Membrane pellets were resuspended in 500 μL 50 mM Tris-HCl (pH 7.4), 120 mM NaCl, and 5 mM KCl (buffer B). Samples were incubated with [3H]-citalopram (2 nM, 1 hour, room temperature) in the presence or absence of paroxetine (2 μM; Sigma), to define nonspecific binding. Samples were collected onto polyethyleneimine-treated Whatman GF/C glass-fiber filters using a cell harvester (Brandel, Gaithersburg, MD) and washed with 10 mL ice-cold buffer B to separate bound and unbound ligand. Binding of [3H]-citalopram was determined by scintillation counting. Mouse brain membrane fractions were used as the positive control and were prepared as described for BMDCs.
Serotonin uptake and release
BMDCs and whole brain synaptosomes were incubated with [3H]5-HT (3-300 nM, Perkin Elmer) in Hanks balanced salt solution (HBSS) for 4 minutes at 0°C or 37°C. Samples were washed extensively in ice-cold HBSS (5 times) to remove excess [3H]5-HT, or collected onto glass-fiber filters using a cell harvester (see “SERT radioligand binding”). [3H]5-HT uptake was measured by liquid scintillation counting. Specific uptake was calculated by subtracting uptake at 0°C from uptake at 37°C. 5-HT release was measured using BMDCs preloaded with 100 nM [3H]5-HT, 100 μM l-ascorbic acid, and 30 μM tranylcypromine in supplemented RPMI 1640 (1 hour, 37°C). BMDCs were washed 4 to 5 times with HBSS and allowed to equilibrate in complete RPMI 1640 (12-18 hours, 37°C). BMDCs were washed 3 more times with HBSS prior to stimulation with 10 μM ionomycin or 500 μM ATP in HBSS (room temperature).
Amperometry
BMDCs were preloaded with 0.5 mM serotonin and 0.1 mM l-ascorbic acid (3 hours, 37°C), washed thoroughly, and used within 6 hours. No change in cell viability was observed. Amperometry recording used 5-μm carbon fibers (ALA Scientific Instruments, Westbury, NY) and an EPC8 amplifier (HEKA, Southboro, MA) at a polarization of 750 mV. Data were filtered at 3 kHz and sampled at 10 kHz. ATP was applied from a pressure-injection pipette (1-2 μm in diameter) positioned at about 100 μm from the cell of interest.
Antibody labeling and ELISA
BMDCs were incubated for 2 days with LPS (50 ng/mL), cross-linked anti-CD40 (5 μg/mL; HM40-3; BD Biosciences) or CD152/Fc (40 μg/mL; Sigma). BMDCs were labeled with anti-CD11c FITC and anti-CD86 PE or anti-MHC class II PE (25-9-17; BD Biosciences) and analyzed using a FACSCalibur flow cytometer and Cell Quest software (BD Biosciences). Culture supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) for IL-6 and IL-12 (BioLegend, San Diego, CA). cAMP from T-cell lysates was quantified by Correlate-EIA Direct Cyclic AMP Enzyme Immunoassay Kit (Assay Designs, Ann Arbor, MI). The basal level of cAMP in naive T cells was about 0.55 pmol/mL/106 cells.
Statistical analysis
Errors are the SEM unless otherwise indicated. Statistical comparisons are based on the Student t test or ANOVA.
Results
SERT expression is developmentally regulated
To explore the mechanism of monoamine uptake by DCs, we tested for the presence of selective transporters for 5-HT (SERT), dopamine (DAT), and norepinephrine (NET). Using RT-PCR, SERT mRNA was identified in DCs cultured from BM (BMDCs) and in DCs directly isolated from bulk spleen cells (Figure 1A). Thus, SERT is expressed in both in vitro–generated and naturally occurring DCs. Of interest, SERT expression increased as DCs matured and expressed cell surface CD86. SERT expression was further enhanced following activation with a potent and well-characterized DC activation stimulus, lipopolysaccharide (LPS), a TLR-4 ligand. SERT protein was confirmed in BMDCs by immunostaining. Day 5 BMDCs, which are heterogeneous in their expression of CD86, were labeled with anti-CD11c and anti-SERT antibodies (Figure 1B). Although all CD11c+ BMDCs were SERT+, SERT- lymphocytes were clearly visible (Figure 1B arrowhead). Furthermore, using a specific and potent ligand ([3H]-citalopram), we characterized SERT expression by radioligand-binding assays. Specific binding for SERT in brain and day 5 BMDCs was 335 ± 6 and 50 ± 8 fmol/mg, respectively. Thus, SERT levels in unstimulated BMDCs are approximately 15% of brain.
In contrast to SERT, the expression of DAT and NET mRNA was very low to undetectable in BMDCs or spleen DCs (data not shown). Although DCs express SERT, they apparently do not synthesize 5-HT because expression of the rate-limiting synthetic enzymes for 5-HT, TPH-1 or TPH-2, were not detected (Figure 1A). They do express MAO-A and MAO-B, however, which are key catabolic enzymes. Of interest, expression of MAO-A and MAO-B by BMDCs was inversely regulated compared with SERT (Figure 1A). Spleen DCs exhibited a similar profile for expression of MAO-A; however, significant expression of MAO-B was not detected.
Expression and developmental regulation of the 5-HT transporter and MAO in mouse DCs. (A) CD11c+ CD86- (immature DCs) or CD11c+ CD86+ (mature DCs) were purified by cell sorting from BMDC cultures or spleen cells. BMDCs were activated by overnight exposure to LPS (50 ng/mL) prior to sorting CD11c+ CD86+ (activated DCs). Gene expression for SERT, TPH-1 and TPH-2, MAO-A, and MAO-B was examined by RT-PCR. (B) Density gradient-enriched day 5 BMDCs were immunolabeled and analyzed by confocal microscopy. CD11c (mouse DC marker) and SERT were visualized with antibodies labeled with Alexa Fluor 488 (green; i) and Alexa Fluor 546 (red; ii), respectively. Nuclei were counterstained with TO-PRO-3 (blue). In the red, green, and blue overlay (iii), the yellow signal indicates regions of red/green overlap. The bright-field image (iv) includes a CD11c and SERT double-negative cell with lymphocytic morphology (arrowhead). Scale bar represents 10 μm. Data are representative of 2 independent experiments.
Expression and developmental regulation of the 5-HT transporter and MAO in mouse DCs. (A) CD11c+ CD86- (immature DCs) or CD11c+ CD86+ (mature DCs) were purified by cell sorting from BMDC cultures or spleen cells. BMDCs were activated by overnight exposure to LPS (50 ng/mL) prior to sorting CD11c+ CD86+ (activated DCs). Gene expression for SERT, TPH-1 and TPH-2, MAO-A, and MAO-B was examined by RT-PCR. (B) Density gradient-enriched day 5 BMDCs were immunolabeled and analyzed by confocal microscopy. CD11c (mouse DC marker) and SERT were visualized with antibodies labeled with Alexa Fluor 488 (green; i) and Alexa Fluor 546 (red; ii), respectively. Nuclei were counterstained with TO-PRO-3 (blue). In the red, green, and blue overlay (iii), the yellow signal indicates regions of red/green overlap. The bright-field image (iv) includes a CD11c and SERT double-negative cell with lymphocytic morphology (arrowhead). Scale bar represents 10 μm. Data are representative of 2 independent experiments.
Serotonin uptake by DCs
To confirm that SERT was functional in BMDCs we measured the specific uptake of [3H]5-HT. Figure 2A shows a representative example of 5-HT uptake at 37°C versus nonspecific uptake at 0°C. The difference between these curves (specific uptake) exhibits a saturable relationship. The mean values for Vmax and Km from 3 separate experiments were 16 ± 0.3 fmol/106 cells/min and 85 ± 15 nM, respectively. This Km value is similar to the value for uptake by whole brain synaptosomes of 87 ± 23 nM (n = 3, Figure 2B; note that the total protein concentration in brain was ∼5 times greater than BMDCs). Moreover, the specific SERT inhibitor, fluoxetine, inhibited uptake of [3H]5-HT in BMDCs and brain (Figure 2C). Together, these data indicate that SERT in BMDCs has functionally similar properties to SERT present in neuronal tissue.
We also demonstrated 5-HT uptake by the direct visualization of 5-HT within DCs. 5-HT is a labile molecule requiring rapid fixation for successful immunodetection. Because the mouse DC marker CD11c is not fixation stable, day 5 CD11c+ BMDCs were first sorted and then loaded with 5-HT (100 μM, 1 hour, 37°C). Granules of 5-HT labeled with 5-HT antisera are readily visualized in the cytosol (Figure 2D-E). Preabsorption of the 5-HT antisera with 100 μM 5-HT inhibited binding to 5-HT–loaded BMDCs (Figure S2). Because the bright-field micrographs indicated that 5-HT labeling was coincident with vesicular compartments, we colabeled for the lysosomal-associated membrane protein, LAMP-1. Apparent costaining of 5-HT and LAMP-1 in BMDCs is evident (yellow; Figure 2D), suggesting that 5-HT is trafficked into lysosomal compartments following uptake. Thus, these data indicate that similar to platelets, DCs can accumulate 5-HT through a regulated uptake mechanism.
Serotonin uptake by BMDCs. (A-B) [3H]5-HT uptake by BMDCs and whole mouse brain synaptosomes at 4°C (▪) and 37°C (○) and specific uptake (▵) versus 5-HT concentration. Data are from a single experiment performed in duplicate and are representative of 3 independent experiments. Note that brain protein concentration was about 5 times greater than BMDCs. The smooth curves are fits to a hyperbolic function used to derive Km and Vmax (see “Serotonin uptake by DCs”). The stated Km values are the means (± SE) from 3 experiments with BMDCs and brain synaptosomes. (C) Mean inhibition of specific [3H]5-HT (100 nM) uptake by fluoxetine (200 nM) in BMDCs and brain. Data are means (± SD) from 2 experiments (*P < .05, paired t test). (D) Day 5 BMDCs were purified by cell sorting for CD11c-PE-Cy7+ cells and then loaded with 5-HT (100 μM). LAMP-1 and 5-HT were visualized by confocal microscopy with antibodies labeled with Alexa Fluor 488 (green; i) and Alexa Fluor 546 (red; ii), respectively. Nuclei were counterstained with TO-PRO-3 (blue). In the red, green, and blue overlay (iii), the yellow signal indicates regions of red/green overlap. Scale bar represents 20 μm. (E) Magnification of bright field (i) and red/green/blue overlay (ii) from a single DC. Scale bar represents 10 μm.
Serotonin uptake by BMDCs. (A-B) [3H]5-HT uptake by BMDCs and whole mouse brain synaptosomes at 4°C (▪) and 37°C (○) and specific uptake (▵) versus 5-HT concentration. Data are from a single experiment performed in duplicate and are representative of 3 independent experiments. Note that brain protein concentration was about 5 times greater than BMDCs. The smooth curves are fits to a hyperbolic function used to derive Km and Vmax (see “Serotonin uptake by DCs”). The stated Km values are the means (± SE) from 3 experiments with BMDCs and brain synaptosomes. (C) Mean inhibition of specific [3H]5-HT (100 nM) uptake by fluoxetine (200 nM) in BMDCs and brain. Data are means (± SD) from 2 experiments (*P < .05, paired t test). (D) Day 5 BMDCs were purified by cell sorting for CD11c-PE-Cy7+ cells and then loaded with 5-HT (100 μM). LAMP-1 and 5-HT were visualized by confocal microscopy with antibodies labeled with Alexa Fluor 488 (green; i) and Alexa Fluor 546 (red; ii), respectively. Nuclei were counterstained with TO-PRO-3 (blue). In the red, green, and blue overlay (iii), the yellow signal indicates regions of red/green overlap. Scale bar represents 20 μm. (E) Magnification of bright field (i) and red/green/blue overlay (ii) from a single DC. Scale bar represents 10 μm.
Triggered release of serotonin
It is striking that the up-regulation of SERT mRNA expression during DC maturation and activation is inversely correlated with expression of the major catabolic enzymes for 5-HT, MAO-A, and MAO-B (Figure 1A). This suggests that 5-HT sequestered by DCs in LAMP-1+ vesicles is not destined for degradation. Of interest, synaptic polarization and Ca2+-regulated release of preassembled vesicular cytokine stores has been reported for DCs.24-27 Ca2+ transients are also evident in DCs during interactions with T cells.28 We therefore tested whether DCs could release 5-HT through regulated secretion. We found that extracellular ATP, a physiologic DC stimulus that mobilizes intracellular Ca2+, and the Ca2+ ionophore, ionomycin, evoked significant release of [3H]5-HT from DCs (up to 3-fold above baseline; Figure 3A).
Regulated cytokine secretion by DCs has been inferred by detection of elevated extracellular cytokine concentrations in response to stimuli that elicit intracellular Ca2+ rises. To confirm that 5-HT release from BMDCs occurred via exocytosis we performed amperometry. This technique enables the direct detection of oxidizable transmitters as they are released following vesicle docking/fusion with the plasma membrane (Figure 3B). Application of extracellular ATP induced well-resolved, amperometric spikes reflecting the exocytosis of individual quanta of oxidizable 5-HT (Figure 3C-D). These spikes occurred at varying latencies following ATP application and exhibited a wide range of amplitudes from about 2 or 3 to 100 pA (Figure 3D-E). Analysis of the spike area indicated the release of about 30 000 to 130 000 molecules of 5-HT/fusion event and are comparable to those reported for exocytosis in neuronal PC12 cells.29 Significantly, these data constitute the first direct measurement of membrane exocytosis in DCs.
Exogenous 5-HT modulates naive T-cell levels of cAMP
We next asked whether the regulated exocytosis of 5-HT from DCs may affect DC–T-cell encounters. Early events in the T-cell intracellular signaling pathway include a marked and transient increase in cAMP.30-32 Persistent high levels of cAMP, however, inhibit T-cell responses.33-35 Thus, the generation of a productive T-cell response requires TCR recognition of cognate ligands to be coordinated with cAMP degradation, and the inhibition of further cAMP synthesis. 5-HT is reported to enhance T-cell proliferation by signaling at type 1 5-HT receptors and reducing intracellular levels of cAMP.18 We therefore tested the effects of 5-HT on freshly isolated, naive splenic T cells (Figure 4A). 5-HT alone (1-10 μM) did not alter basal cAMP levels. Next we raised cAMP (∼200%-300%) by a short exposure to the PDE4 inhibitor, rolipram. Cotreatment with 5-HT significantly reduced the rolipram-evoked increase in cAMP by about 65% to 75%. This 5-HT–mediated reduction in cAMP was prevented by pretreatment with a type 1 5-HT receptor antagonist (S-Way 100135, 1 μM; Figure 4A). The concentration of 5-HT that produced half-maximal inhibition (IC50) was about 500 nM (Figure 4B) and the effect saturated at about 10 μM. Thus, 5-HT signaling via type-1 5-HT receptors may limit the magnitude and duration of the increase in cAMP levels following TCR signaling and, thereby, promote T-cell activation.
Ca2+-triggered exocytosis of serotonin in BMDCs. (A) [3H]5-HT release (% of control) from BMDCs in response to extracellular ATP (100 μM) and ionomycin (10 μM). Data are means ± SE (n = 3; *P < .05; **P < .01). (B) BMDCs with apposed 5-μm carbon-fiber electrode; scale bar represents 20 μm. (C) Amperometric spikes evoked by application of extracellular ATP (100 μM) in a cell preloaded with 5-HT. (D) Representative spikes are shown on an expanded time scale. (E) Distribution of spike amplitudes (2 pA bins) from a total of 336 single events taken from 37 cells.
Ca2+-triggered exocytosis of serotonin in BMDCs. (A) [3H]5-HT release (% of control) from BMDCs in response to extracellular ATP (100 μM) and ionomycin (10 μM). Data are means ± SE (n = 3; *P < .05; **P < .01). (B) BMDCs with apposed 5-μm carbon-fiber electrode; scale bar represents 20 μm. (C) Amperometric spikes evoked by application of extracellular ATP (100 μM) in a cell preloaded with 5-HT. (D) Representative spikes are shown on an expanded time scale. (E) Distribution of spike amplitudes (2 pA bins) from a total of 336 single events taken from 37 cells.
T cells express TPH-1, the rate-limiting enzyme for 5-HT synthesis
5-HT is reported to enhance the proliferation of T cells, both in vitro and in vivo. The source of this 5-HT is unclear and may derive from enterochromaffin cells, sympathetic nerves,14 or from endogenous T-cell production. We tested for the latter possibility using RT-PCR. Figure 5A shows that T cells produce mRNA for TPH-1 but not TPH-2. Of importance, TPH-1 gene expression is barely detectable in naive T cells but is dramatically up-regulated following mitogen activation. Thus, in contrast to DCs, T cells have the capacity to synthesize 5-HT, and this potential is enhanced during their activation.
Because DCs participate in spatially intimate interactions with T cells, we investigated whether DCs could functionally sequester 5-HT emanating from T cells. As previously described,36 T cells were loaded with [3H]5-HT, washed, and incubated at 37°C for 1 hour, during which time they passively released 5-HT into the culture supernatant. DCs exposed to this culture supernatant were able to take up [3H]5-HT at room temperature, but not at 0°C, and this uptake was almost completely inhibited by fluoxetine (Figure 5B). Thus, DCs can efficiently sequester 5-HT that is released in small quantities from activated T cells.
Serotonin inhibits naive T-cell production of cAMP. (A) Change in levels of cAMP in naive T cells in response to single or combined treatment as indicated with 5-HT (10 μM), the PDE-4 inhibitor, rolipram (30 μM), and the type 1 5-HT receptor inhibitor, S-Way 100135 (1 μM). Each data point is the mean (± SE) of 4 to 5 experiments (*P < .01 compared with control; †P < .05 compared with other rolipram groups by ANOVA). (B) Dose-response relationship for inhibition of cAMP by 5-HT in T cells pretreated with rolipram. The smooth line is the fit to a Hill equation.
Serotonin inhibits naive T-cell production of cAMP. (A) Change in levels of cAMP in naive T cells in response to single or combined treatment as indicated with 5-HT (10 μM), the PDE-4 inhibitor, rolipram (30 μM), and the type 1 5-HT receptor inhibitor, S-Way 100135 (1 μM). Each data point is the mean (± SE) of 4 to 5 experiments (*P < .01 compared with control; †P < .05 compared with other rolipram groups by ANOVA). (B) Dose-response relationship for inhibition of cAMP by 5-HT in T cells pretreated with rolipram. The smooth line is the fit to a Hill equation.
T cells express the enzyme for 5-HT synthesis and T-cell 5-HT is sequestered by BMDCs. (A) Expression of TPH-1 and TPH-2 was determined by RT-PCR in naive T cells, Con A- (5 μg/mL) activated T cells, and Con A-activated T cells rested for 4 days in IL-2 (10 μg/mL). Comparable quantities of cDNA were ensured by amplification of GAPDH. Data are representative of 2 experiments. (B) BMDC uptake of [3H]5-HT from T-cell supernatants at 0°C, room temperature, and room temperature with fluoxetine (100 nM). Freshly purified, naive T cells were loaded with 1 μM [3H]5-HT (1 hour, 37°C). Cells were washed and resuspended in HBSS (1 hour, 37°C) to passively release [3H]5-HT. BMDCs were resuspended in T-cell supernatant (2 hours, room temperature). Data are means ± SD (n = 2; *P < .001,t test).
T cells express the enzyme for 5-HT synthesis and T-cell 5-HT is sequestered by BMDCs. (A) Expression of TPH-1 and TPH-2 was determined by RT-PCR in naive T cells, Con A- (5 μg/mL) activated T cells, and Con A-activated T cells rested for 4 days in IL-2 (10 μg/mL). Comparable quantities of cDNA were ensured by amplification of GAPDH. Data are representative of 2 experiments. (B) BMDC uptake of [3H]5-HT from T-cell supernatants at 0°C, room temperature, and room temperature with fluoxetine (100 nM). Freshly purified, naive T cells were loaded with 1 μM [3H]5-HT (1 hour, 37°C). Cells were washed and resuspended in HBSS (1 hour, 37°C) to passively release [3H]5-HT. BMDCs were resuspended in T-cell supernatant (2 hours, room temperature). Data are means ± SD (n = 2; *P < .001,t test).
T cell-derived signals regulate SERT expression
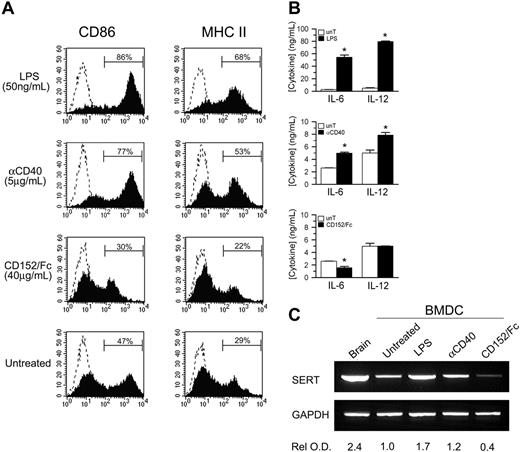
Having established that T cells may be one source of 5-HT for DCs, we investigated if cross-talk between DCs and T cells could have an impact on 5-HT transport. As shown in Figure 1A, SERT cDNA expression increases with DC maturation; therefore, we asked whether T-cell ligands known to modulate DC maturation could also regulate SERT expression. BMDCs were cultured with anti-CD40 mAb or CD152/Fc, which simulate ligation by T-cell molecules that, respectively, deliver activating and inhibitory signals to DCs,37,38 or with LPS. Relative to untreated BMDCs, LPS dramatically enhanced the expression of CD86 and the cell surface expression of MHC class II molecules (Figure 6A). In addition, functional maturation was demonstrated by significant increases in the secretion of IL-6 and IL-12 into the culture supernatant (Figure 6B). Similar, but less pronounced, effects were observed in response to receptor cross-linking with anti-CD40 mAb. In contrast, phenotypic maturation and cytokine secretion by BMDCs were suppressed by CD152/Fc. Of importance, SERT cDNA expression mirrored the induction and inhibition of DC maturation (Figure 6C). Signals that enhance DC maturation (anti-CD40 or LPS), and in turn drive T-cell proliferation, also up-regulated SERT cDNA expression. As indicated by the relative optical density of SERT RT-PCR products, LPS was a more potent stimulus for enhanced SERT cDNA expression, compared with anti-CD40 mAb. In contrast, signaling via the inhibitory T-cell ligand CD152 resulted in substantial inhibition of SERT cDNA expression. Thus, these ligands affect SERT cDNA expression similar to their regulation of DC maturation and cytokine production. Based on these observations, we suggest that SERT expression appears to be regulated, at least in part, by the dynamic and reciprocal interactions that occur between DCs and T cells.
Discussion
5-HT is a simple monoamine neurotransmitter abundant in the gastrointestinal tract and crucial in the regulation of vascular smooth muscle tone, mood, appetite, and pain.14 5-HT is also recognized as an inflammatory mediator; platelets sequester 5-HT, which they release in response to inflammatory signals including IgE complexes, complement, and platelet-activating factor.15,16 We describe a novel role of 5-HT as a signaling molecule that may be sequestered by DCs at sites of inflammation/injury, or nearby nerve endings, and transmitted between DCs and T cells via an exocytotic pathway (Figure 7). Significant evidence indicates that 5-HT can regulate T-cell function,16,18,19 but the origins of this 5-HT is unclear. The focal release of 5-HT by DCs at synaptic junctions with T cells may suppress cAMP production and thereby facilitate the activation and differentiation of naive T cells.
SERT expression is coupled to DC functional maturation. (A) BMDCs were incubated for 2 days with LPS (50 ng/mL), cross-linked anti-CD40 mAb (5 μg/mL), or CD152/Fc (40 μg/mL), or left untreated. Solid histograms show the expression of CD86 and MHC II molecules by gated, CD11c+ BMDCs relative to isotype controls (dashed histograms). (B) Immature (CD11c+ CD86-) BMDCs were sorted and were cultured for 2 days as described. IL-6 and IL-12 concentration in the culture supernatants was determined by ELISA for treated BMDCs (▪) relative to untreated controls (unT; □). Data are means ± SD from triplicate assays (n = 3; *P < .05, t test). (C) The expression of SERT mRNA relative to GAPDH controls was examined by RT-PCR for each treatment group prepared as described in panel B. The optical density of each band relative to untreated BMDCs was determined and normalized for GAPDH signal. Data are representative of 2 independent experiments.
SERT expression is coupled to DC functional maturation. (A) BMDCs were incubated for 2 days with LPS (50 ng/mL), cross-linked anti-CD40 mAb (5 μg/mL), or CD152/Fc (40 μg/mL), or left untreated. Solid histograms show the expression of CD86 and MHC II molecules by gated, CD11c+ BMDCs relative to isotype controls (dashed histograms). (B) Immature (CD11c+ CD86-) BMDCs were sorted and were cultured for 2 days as described. IL-6 and IL-12 concentration in the culture supernatants was determined by ELISA for treated BMDCs (▪) relative to untreated controls (unT; □). Data are means ± SD from triplicate assays (n = 3; *P < .05, t test). (C) The expression of SERT mRNA relative to GAPDH controls was examined by RT-PCR for each treatment group prepared as described in panel B. The optical density of each band relative to untreated BMDCs was determined and normalized for GAPDH signal. Data are representative of 2 independent experiments.
This study reveals several novel aspects of 5-HT signaling in leukocytes. First, we show that DCs express a functional SERT that is recognized by the antidepressants citalopram and fluoxetine, specific inhibitors of 5-HT uptake. Thus, DCs resident in peripheral tissues, including the skin and mucosal tissues, may take up 5-HT that is released by activated platelets, degranulating mast cells, or sympathetic nerves. We show that expression of SERT in DCs, and thereby their capacity to sequester 5-HT, is enhanced by agents that promote DC maturation, including the microbial stimulus LPS, and CD40 ligation. It is conceivable that activated platelets serve not only as 5-HT reservoirs but also mediate DC maturation through their expression of CD40 ligand.39 Significantly, the IgE-mediated degranulation of mast cells is reported to trigger the increased migration of epidermal DCs to draining lymph nodes.40 This appears to be dependent, in part, on histamine, which is released along with 5-HT. In this manner, 5-HT bioavailability and uptake are linked to the differentiation of functionally mature DCs.
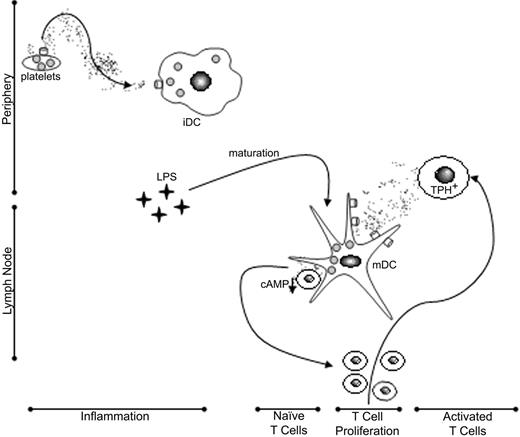
A conceptual model for 5-HT uptake and release by DCs and the immunologic consequences. Immature DCs (iDC) may sequester 5-HT released by platelets or mast cells at sites of injury and inflammation, which they in turn secrete via a Ca2+-dependent exocytotic pathway on encountering naive, cognate T cells. High local concentrations of 5-HT within DC–T-cell synaptic junctions suppress further production of cAMP and facilitate T-cell proliferation. CD40-CD40 ligand signaling up-regulates DC SERT expression and thereby promotes uptake of 5-HT passively released from activated T cells. Mature DCs (mDC) may shuttle 5-HT between activated and naive T cells, thereby amplifying adaptive immune responses.
A conceptual model for 5-HT uptake and release by DCs and the immunologic consequences. Immature DCs (iDC) may sequester 5-HT released by platelets or mast cells at sites of injury and inflammation, which they in turn secrete via a Ca2+-dependent exocytotic pathway on encountering naive, cognate T cells. High local concentrations of 5-HT within DC–T-cell synaptic junctions suppress further production of cAMP and facilitate T-cell proliferation. CD40-CD40 ligand signaling up-regulates DC SERT expression and thereby promotes uptake of 5-HT passively released from activated T cells. Mature DCs (mDC) may shuttle 5-HT between activated and naive T cells, thereby amplifying adaptive immune responses.
Second, our data show that DCs store sequestered 5-HT in intracellular vesicles, and this is supported by the punctuate pattern of immunostaining with 5-HT antisera that colocalize with the lysosomal marker LAMP-1. Furthermore, we show that DCs can release this stored 5-HT via Ca2+-triggered exocytosis. Amperometric experiments reveal quantal secretion of 5-HT consistent with the fusion of 5-HT–laden vesicular compartments with the plasma membrane. These real-time measurements of vesicle release constitute the first direct evidence for Ca2+-triggered exocytosis in DCs. The reduced expression of MAO-A and MAO-B, the major catabolic enzymes of 5-HT, concomitant with DC maturation further supports a 5-HT secretory function. This decrease in 5-HT degradative capacity, accompanied by increased levels of SERT, appears coordinated to maximize stores of 5-HT in mature DCs that are specialized for antigen presentation.
Released 5-HT can function to alter cAMP levels in T cells by acting at type 1 5-HT receptors. Significantly, this effect requires near micromolar levels of 5-HT. Although 5-HT is labile and physiologic levels in sera seldom exceed nanomolar concentrations,14 the release of 5-HT into the synaptic space between DCs and T cells would produce a localized, high concentration of 5-HT able to act over short distances and signal 5-HT receptors. In this regard, it is significant that lysosomes and intracellular stores of IL-12 within DCs have been demonstrated to polarize toward alloreactive T cells26 and natural killer (NK) cells.25 Thus, the accumulation of 5-HT in polarized LAMP-1+ vesicles would allow for the focal release of 5-HT. It should be noted that secretion of cytokines from DCs can powerfully influence T-cell fate and NK cell activation. Unlike monoamine exocytosis, however, cytokine secretion is constitutive in the vast majority of cases and regulated at the level of transcription. Moreover, cytokines can act at considerable distance from their site of release and therefore their signaling is not spatially restricted. Exocytosed 5-HT may terminate the initial rise in cAMP that follows T-cell receptor engagement by APCs.31,32 Thus, triggered secretion of 5-HT may represent a novel signaling pathway by which DCs can modulate T-cell proliferation, differentiation, or both.
Third, we show that T cells express TPH-1, a requisite and rate-limiting enzymatic component for 5-HT synthesis in peripheral tissues,41 and that this expression is increased by mitogen activation. This finding suggests that 5-HT may normally play an important role in T-cell activation. The predominant expression of TPH-1 in activated rather than naive T cells also emphasizes the potential function of DCs in shuttling 5-HT to naive cells that cannot yet synthesize 5-HT. The developmental regulation of TPH-1 in T cells exactly parallels that of SERT in DCs. DCs are known to participate in sequential interactions with many T cells and, thus, they could effectively sequester 5-HT from an initial contact with an activated T cell (indeed our data demonstrate that DCs can take up [3H]5-HT from T cells [Figure 5]) and subsequently release 5-HT to naive T cells. In this way DCs could provide a “5-HT bridging” mechanism that directly couples the activation of naive T cells to activated T cells. Further support for such a mechanism is provided by the regulation of SERT expression in DCs following T-cell interactions. CD152, a negative regulator of T cells, both inhibits DC maturation38 and SERT mRNA expression. In contrast, T cell-derived signals that promote DC survival and maturation (eg, CD40 ligation)37 enhance SERT expression. Thus, the capacity for DCs to take up 5-HT (and subsequently release it) is profoundly regulated by these dynamic T-cell encounters.
Of interest, the immediate precursor of 5-HT, tryptophan, has also been implicated in the regulation of T-cell responses. Catabolism of tryptophan by certain DC subsets that express indoleamine 2,3-dioxygenase (IDO) has been shown to suppress/inhibit T-cell proliferation.42-44 Of importance, DC IDO and SERT are reciprocally regulated by T-cell–derived signals. Inhibitory signals delivered by CD152/Fc activate IDO45 and suppress SERT expression, whereas ligation of CD40 produces the opposite effect46 (Figure 6). Thus, it appears that tryptophan metabolism, and 5-HT production and sequestration, may also constitute an important regulatory point in T-cell activation pathways. Further investigation of DC subtypes, specifically human DC subtypes, with regard to their relative roles in 5-HT transport versus IDO mediated-tryptophan catabolism is therefore warranted.
Our data highlight potential clinical consequences of drugs that interact with SERT. Commonly prescribed antidepressants including tricyclics and selective serotonin reuptake inhibitors (SSRIs) are known to acutely inhibit SERT and alter long-term SERT expression.16 Our data show that the SSRI, fluoxetine, is an effective antagonist of 5-HT uptake in DCs. Significantly, in 2004, there were about 147 million prescriptions for SSRIs in the United States alone (Intercontinental Marketing Services, Fairfield, CT). Moreover, several drugs of abuse including methamphetamines, ecstasy, and cocaine, interact at SERT causing release of 5-HT.16 Therefore, the impact of altered or dysregulated 5-HT uptake and release produced by these drugs with regard to immune competence needs to be carefully established.16 In addition, the specificity of these pharmacologic agents for monoamine transporters raises the intriguing possibility that they may be used therapeutically to modify or augment immune responses.
Prepublished online as Blood First Edition Paper, October 13, 2005; DOI 10.1182/blood-2005-07-2903.
Supported by an operating grant, NIAID AI054450, from the National Institutes of Health (G.P.A. and P.J.O.). M.L.P. was supported by the Premiers' Research Excellence Award (PREA; P.J.O.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ken Kellar for advice regarding 5-HT uptake and radioligand binding experiments, Dr Michael Bader (Berlin-Buch, Germany) for advice regarding RT-PCR for tryptophan hydroxylase, Dägna Solveig Sheerar for cell sorting, and Lianne Dale for assistance with confocal microscopy. We appreciate the assistance of Mia Merrill and Rosa Miyares with the preparation of graphics and the critical review of this manuscript by Dr Michael Rieder. We thank Amgen for the gift of recombinant human Flt3 ligand.


![Figure 2. Serotonin uptake by BMDCs. (A-B) [3H]5-HT uptake by BMDCs and whole mouse brain synaptosomes at 4°C (▪) and 37°C (○) and specific uptake (▵) versus 5-HT concentration. Data are from a single experiment performed in duplicate and are representative of 3 independent experiments. Note that brain protein concentration was about 5 times greater than BMDCs. The smooth curves are fits to a hyperbolic function used to derive Km and Vmax (see “Serotonin uptake by DCs”). The stated Km values are the means (± SE) from 3 experiments with BMDCs and brain synaptosomes. (C) Mean inhibition of specific [3H]5-HT (100 nM) uptake by fluoxetine (200 nM) in BMDCs and brain. Data are means (± SD) from 2 experiments (*P < .05, paired t test). (D) Day 5 BMDCs were purified by cell sorting for CD11c-PE-Cy7+ cells and then loaded with 5-HT (100 μM). LAMP-1 and 5-HT were visualized by confocal microscopy with antibodies labeled with Alexa Fluor 488 (green; i) and Alexa Fluor 546 (red; ii), respectively. Nuclei were counterstained with TO-PRO-3 (blue). In the red, green, and blue overlay (iii), the yellow signal indicates regions of red/green overlap. Scale bar represents 20 μm. (E) Magnification of bright field (i) and red/green/blue overlay (ii) from a single DC. Scale bar represents 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-07-2903/4/m_zh80030690010002.jpeg?Expires=1769105062&Signature=gzgvnGp-~gS3dKLVwujSTngymSRoIdhw49S0mXu9IGYk5WCqmobPqXPO4UKNCZXJMKD8TLn9MvDjKc~u1CV-cK4guuupWE3-GmWejDRbTaeWYWm2OAB9LGy-oIg6RBfDU7mV1Dpmf7AR2pVIo3ZD7eszgT0ZbNCRBM6tzYA9z13IIQylwAJgWDfK0T6JO6r1L~ltAuUhnc4JdCTIuiG4sLnJ0YlIDpUQdxiLgKqA-BVFANlm~9MDphLTHt16HicanjN~y2TYD386-z3TINUXhNNGW43KX0POkA8n8vrEZcYBlCs7wdAIUrrwFtL6lreELWa4M8Ab6MhA432HqoP6Bg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Ca2+-triggered exocytosis of serotonin in BMDCs. (A) [3H]5-HT release (% of control) from BMDCs in response to extracellular ATP (100 μM) and ionomycin (10 μM). Data are means ± SE (n = 3; *P < .05; **P < .01). (B) BMDCs with apposed 5-μm carbon-fiber electrode; scale bar represents 20 μm. (C) Amperometric spikes evoked by application of extracellular ATP (100 μM) in a cell preloaded with 5-HT. (D) Representative spikes are shown on an expanded time scale. (E) Distribution of spike amplitudes (2 pA bins) from a total of 336 single events taken from 37 cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-07-2903/4/m_zh80030690010003.jpeg?Expires=1769105062&Signature=4j9IEPBK6tMTEBNI9XqCWB8n9GaAvI6dUqo-l1XWa6nxsDKqThxN2fcSbC8iwNBBWws9ZPDAJFZpfaG0cpU-jVXRkkuQcRBp6bKt3JVLI7D9GXormQ06JmaycMVutwxT0r9M5aN1GXM0TrE1ZmEV9WLLo6y2B5X7~J6lX6-Yo4w9y8PsOaSQmgmGEUnjkdFNtbRtZfzhQt~eaihUtjACnxk6oUyCodtZqGAQHi9y3BNXmop1aQ0clMyjUgslB9xTH42gIVIf7hn15s-h6qdNNRqz3SXOX2Fb5MnbxjsLJ8VjoND8OvKQ1Ek4WDY3H30IuCIVymmoEEF654ayhUWwtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. T cells express the enzyme for 5-HT synthesis and T-cell 5-HT is sequestered by BMDCs. (A) Expression of TPH-1 and TPH-2 was determined by RT-PCR in naive T cells, Con A- (5 μg/mL) activated T cells, and Con A-activated T cells rested for 4 days in IL-2 (10 μg/mL). Comparable quantities of cDNA were ensured by amplification of GAPDH. Data are representative of 2 experiments. (B) BMDC uptake of [3H]5-HT from T-cell supernatants at 0°C, room temperature, and room temperature with fluoxetine (100 nM). Freshly purified, naive T cells were loaded with 1 μM [3H]5-HT (1 hour, 37°C). Cells were washed and resuspended in HBSS (1 hour, 37°C) to passively release [3H]5-HT. BMDCs were resuspended in T-cell supernatant (2 hours, room temperature). Data are means ± SD (n = 2; *P < .001,t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-07-2903/4/m_zh80030690010005.jpeg?Expires=1769105062&Signature=jdFr3QeOAq4Mmd512vezt2eJz6VrHUn9adv~5sle4XW3neRM6MI8j1ln~PvyWQ2psZgwn43qN7oLca6i8vY~6A4s~5Z9RcTcGAHjSzFZu33rYtgoVp5DAY7LIaTfMk45Pbd1xr9ig4xlelqtU3LUfds60HNHlfQIeTTrsv8pLbSisyjxx9VmfIKb2627kR8vBjn2q2a1xikTNbVkAj~8eAYuSppNTeeKjrBd5pJt96xNneyqf~Oeu5DMo3mOxUA~Tm4Nl0IKpFolZDvgg-JIMTVQJRXy0yZZMPg4Js81RkVEmWI570zbgSqpWfc7l8~Ko3YpgDMxVZ-WRGtjNHpmRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal