We recently reported that specific genetic polymorphisms, particularly polymorphisms in thymidylate synthase (TYMS) and glutathione S-transferase M1 (GSTM1) predicted the risk of relapse among children with acute lymphoblastic leukemia (ALL).1 An accompanying commentary noted surprise that the thiopurine methyltransferase (TPMT) genotype was not predictive of relapse risk.2 The answer may lie in the fact that the starting dose of mercaptopurine (75 mg/m2 per day) in our study was higher than some groups use, and that therapy was individualized based on TPMT status.
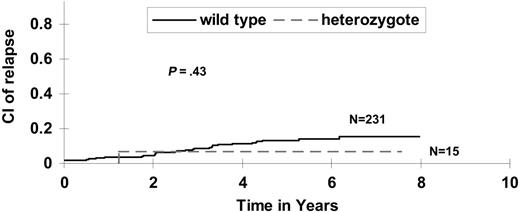
TPMT methylates and inactivates mercaptopurine. The approximately 10% of populations that inherit intermediate or absent TPMT activity is at higher risk of myelosuppression and its attendant toxicity if prescribed “normal” doses of thiopurines3 ; in addition, such patients appear at higher risk of secondary cancers.4,5 TPMT activity is inherited as a monogenic autosomal codominant trait, with the vast majority of inactivating alleles accounted for by single nucleotide polymorphisms at amino acids 238, 460, and 719.6 TPMT activity can also be measured directly in erythrocytes.7 Thus, patients can be clinically screened for TPMT status and then prescribed doses of mercaptopurine that are tailored to their TPMT genotype or phenotype.6,8 At St Jude Children's Research Hospital, since the early 1990s, we have used a combination of measurement of thiopurine metabolites, TPMT status, and clinical tolerance to continuation therapy to selectively decrease the dose of mercaptopurine (without decreasing the nonthiopurine therapy) in patients with low or intermediate TPMT activity, to counsel patients on compliance if thiopurine metabolites are low, and to increase doses of chemotherapy in patients demonstrating persistently high white blood cell counts. Because we have previously found that constant administration (ie, avoiding interruption) in thiopurine therapy resulted in fewer relapses,9 our goal has been to maintain the highest dose of daily mercaptopurine that is tolerable. Using this approach, TPMT genotype was not predictive of hematologic relapse risk in our study Total XIIIB (Figure 1), with 5-year cumulative incidences of 13.2% ± 2.3 versus 6.7% ± 6.7% among patients with the wild-type versus low-activity genotypes, respectively (P = .46).
As Zwann indicated, after 2 weeks of including a somewhat lower dose of mercaptopurine than we used (60 mg/m2 per day), patients with deficient or heterozygous TPMT genotype in a front-line BFM trial in ALL had a lower level of minimal residual disease than those with wild-type TPMT.10 Whether a similar relationship between TPMT genotype and ultimate relapse risk will be observed over the longer term, in the context of multiagent chemotherapy that involves higher doses of mercaptopurine as well as other agents, remains to be seen, and will likely be influenced by the strategies used for dosage adjustment during continuation therapy.
SJCRH protocol total XIIIB: cumulative incidence of relapse. Cumulative incidence of relapse is not higher among TPMT heterozygotes than wild-type patients, adjusting dose of 6MP down among heterozygotes with prospective TPMT assessment.
SJCRH protocol total XIIIB: cumulative incidence of relapse. Cumulative incidence of relapse is not higher among TPMT heterozygotes than wild-type patients, adjusting dose of 6MP down among heterozygotes with prospective TPMT assessment.
Our finding that long-term outcome was not related to TPMT status, in a setting in which dosages were individualized based partly on each patient's TPMT status, is evidence that pharmacogenetic dosage individualization strategies can be used to mitigate toxicity without compromising efficacy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal