Abstract
The unfavorable clinical evolution in indolent non-Hodgkin lymphomas suggests defective control of neoplastic growth by the immune system. To address this issue, we evaluated phenotype, function, and maturation profile of CD4+ and CD8+ T cells from peripheral-blood, lymph nodes, or bone marrow of patients with B-cell non-Hodgkin lymphoma (NHL) at diagnosis. T cells from these patients frequently showed an activated but apoptosis-prone phenotype with low frequency of tumor-reactive T cells showing a TH2/Tc2 functional profile in the response to autologous tumor. In peripheral blood or in lymph nodes and bone marrow, and, in comparison to healthy donors, patients' T cells showed a skewed differentiation toward Tnaive and Tcentral memory stages, with low expression of granzyme B and perforin. T-cell culture with autologous tumor in the presence of IL-2, IL-15, and autologous bone marrow–derived cells led to massive T-cell expansion and to differentiation of cytotoxic factor+ CD8+ T cells releasing IFN-γ and killing autologous B-cell tumor in an HLA-class I–restricted fashion. These results suggest impaired T-cell differentiation to effector stage in patients with B-cell NHL, but indicate that T-cell responsiveness to γc cytokines is retained, thus allowing to promote generation of antitumor T cells for immune intervention.
Introduction
Indolent non-Hodgkin lymphomas (NHLs) arise from transformation of relatively well-differentiated, antigen-responsive B-lymphocytes present in germinal centers.1 Although neoplastic transformation at early stages of B-cell development usually results in aggressive diseases, malignant clonal proliferation of mature B cells generally leads to indolent B-cell lymphomas,1 although clinical courses can show a considerable heterogeneity even within the same histologic subtype. This reflects the emerging complexity in the biologic and molecular basis of these diseases.2,3
Although the available therapeutic strategies for indolent B-cell lymphoma, such as standard chemotherapy, high-dose chemotherapy, and bone marrow transplantation, have improved the outcome of these diseases, the majority of patients are not cured.1 For these reasons, other approaches, including immunotherapy,4 are being currently investigated, based on the evidence indicating that these tumors may be responsive to T-cell–mediated immunity in vivo. This has been suggested by the evidence for a prognostic significance of CD4+ T cells at tumor site5,6 and, more recently, by the identification of a molecular signature of tumor-infiltrating lymphocytes that predicts improved survival in patients with follicular lymphoma (FL).7 Moreover, several B-cell NHLs (B-NHLs) have been shown to express an array of T-cell–defined epitopes, such as those encoded by the tumor-specific B-cell receptor,8,9 by CD19 and CD20 differentiation antigens,10 or by overexpressed genes as BCL211 and fibromodulin.12 These determinants represent attractive candidates for vaccination approaches, and initial results of studies targeting the B-cell idiotype have documented clinical responses in some patients.4 In addition, these studies have shown that it is possible to boost/promote T-cell–mediated responses to autologous B-cell tumor in the majority of vaccinated patients.12 These findings point to the existence of a significant pool of tumor-specific T cells even in untreated patients. However, the often unfavorable clinical evolution of these diseases suggests that activation of effective antitumor immunity may be frequently impaired in vivo. To investigate this possibility we evaluated phenotype, function, antitumor activity, and maturation profile of CD4+ and CD8+ T cells from a large panel of patients with B-NHL. The results indicated that T cells from tumor site of these patients frequently show an activated phenotype but a low frequency of tumor-reactive effector T cells with a TH2/Tc2 functional profile. Moreover, a skewed T-cell differentiation profile toward T naive (TN) and T central memory (TCM) stages,13 with low to absent expression of cytolytic factors, was found in peripheral blood or tumor site. In the attempt to reverse such impaired T-cell differentiation and to promote activation of antitumor effectors, we evaluated T-cell proliferation and maturation in response to different combinations of γc cytokines IL-2 and IL-15. This strategy led to functional differentiation of antitumor T cells releasing IFN-γ and able to lyse autologous B-cell tumor in an HLA-class I–restricted fashion. Moreover, by adding autologous irradiated bone marrow–derived feeder cells to the T-cell cultures, we also achieved massive T-cell expansion. These results provide the rationale for alternative immunologic strategies in patients with indolent NHL, such as the adoptive cell transfer (ACT) of T lymphocytes activated ex vivo with cytokines of the γc family.
Patients and methods
Patients, donors, and cells
All specimens were obtained following approval by the scientific review committee of the Istituto Nazionale per lo Studio e la Cura dei Tumori. Written informed consent was obtained from each patient before enrolling in the study. Lymphocytes were isolated as described14 from peripheral blood (PBL) and from surgical specimens of involved lymph nodes (LNs) or bone marrow (BM) of adults with indolent B-cell–derived NHL15 at diagnosis (for clinical characteristics, see Supplemental Table S1, available at the Blood website; click on the Supplemental Materials link at the top of the online article). PBL specimens were also obtained from 47 healthy donors. Lymphocytes were also isolated from lymph nodes of patients with breast cancer; the lymph nodes were evaluated as tumor-free by conventional histochemistry. Pathologic lymph nodes were processed by a mechanical disaggregation device yielding single-cell suspensions (BD Medimachine; BD Biosciences, Franklin Lakes, NJ). CD19+ NHL cells were purified from PBL, BM, or LNs by using a high-gradient immunomagnetic technique according to manufacturer's instructions (CD19 microbeads kit; Miltenyi Biotech, Bergisch Gladbach, Germany). Neoplastic B cells were characterized by flow cytometry by staining with PE–anti-CD19, anti-CD23, and FITC–anti-CD5 (BD Biosciences, San Jose, CA). Dendritic cells were generated from monocytes by culture in the presence of GM-CSF and IL-4 as described.16 Immature dendritic cells (DCs) were cocultured with neoplastic B cells previously induced to undergo apoptosis by UV irradiation as described.17 Further DC maturation was then promoted by culture for 48 hours with TNF-α as described.16
Monoclonal antibodies and flow cytometry analysis
Flow cytometry analysis of T cells was performed as described18 with the following mouse antihuman mAbs: APC– or PerCp–anti-CD8, APC– or PerCP–anti-CD4, PE– or PerCp–anti-CD3 (BD Biosciences). To detect CCR7, cells were stained with IgM anti-CCR7 (BD Biosciences), followed by biotin-conjugated rat anti–mouse IgM and then by Cy-Chrome–conjugated streptavidin (BD Biosciences). T cells were stained with PE–anti-CD3, APC–anti-CD4, and PerCp–anti-CD8 as well as with the following FITC-labeled mAbs: CD25, CD44, CD62L, CD69, HLA-DR, CD95 (BD Biosciences), and CD122 (Coulter, Miami, FL). To detect apoptotic cells, freshly isolated lymphocytes from patients or donors were stained with PE- or FITC-labeled annexin V (BD Biosciences). As a positive control of apoptosis, lymphocytes were cultured for 48 hours on plates coated with immobilized anti-CD3 mAb as described.18 To detect intracellular perforin or granzyme B, patients' T cells, either freshly isolated or after culture with 50 ng/mL IL-2 (Chiron, Emeryville, CA), were permeabilized with Cytofix/Cytoperm (BD Biosciences) and then stained with FITC–anti-Perforin (BD Biosciences), or with PE–anti-Granzyme B (CLB, Amsterdam, The Netherlands) in the presence of Perm/Wash solution (BD Biosciences). Analysis of T cells for expression of 19 different T-cell receptor beta-chain variable region (TCRBV) regions was performed with mAbs to TCRBV regions (Immunotech, Marseille, France; Serotec, Oxford, United Kingdom), coupled to staining with anti-CD3, anti-CD4, and anti-CD8, as described.19 All samples were analyzed by a dual-laser fluorescence-activated cell sorting (FACS) Calibur CytoFluorimeter (BD Biosciences) using CellQuest software (BD Biosciences).
Cytokine production assays
Production of IFN-γ and/or IL-4 by patients' and donors' CD4+ or CD8+ T cells was assessed by an intracellular staining assay as described.20 Briefly, T cells were cultured for 4 hours either alone or with live autologous B-cell tumor, or with autologous DCs previously loaded or not with killed neoplastic B cells or killed autologous EBV-transformed lymphoblastoid cell lines (LCLs). After the first hour, GolgiStop (BD Biosciences) was added. Cell-surface staining was then carried out with FITC–anti-CD3 and PerCP–anti-CD8 or PerCp–anti-CD4 MAbs (BD Biosciences), followed by fixation with paraformaldehyde, permeabilization with Perm/Wash (BD Biosciences), and staining with APC–anti-IFN-γ and PE–anti-IL-4 MAbs (BD Biosciences) in the presence of Perm/Wash solution. Cells were analyzed by 4-color flow cytometry. In some control experiments, T cells from patients or from a healthy donor were cocultured for 2 to 3 weeks with irradiated autologous LCLs, in the presence of IL-2, and then evaluated for intracellular cytokine production in response to live LCLs or to DCs loaded with killed autologous LCLs, as described.20 IFN-γ enzyme-linked immunospot (ELISPOT) was performed as described21 in 96-well Multiscreen HA plates (Millipore, Bedford, MA). To this end, T cells cultured with autologous B-cell tumor and γc cytokines were tested in a 24-hour ELISPOT assay for IFN-γ production in response to autologous or allogeneic B-cell tumor. T-cell response to 300 ng/mL IL-2 was used as positive control in the ELISPOT assay. Spots in each well were evaluated by the AID Elispot reader (Autoimmun Diagnostika, Strasburg, Germany).
T-cell culture with γc cytokines
CD3+ T lymphocytes from peripheral blood or tumor site of patients with NHL were purified by high-gradient immunomagnetic technique, according to the manufacturer's instructions (CD3 microbeads kit; Miltenyi Biotec), and then cultured in 24-well plates at 1 × 106 cells/mL, in RPMI 1640 medium (BioWhittaker, Verviers, Belgium) supplemented with 10% pooled human serum, 2 mM L-glutamine (BioWhittaker), 20 mM HEPES buffer (BioWhittaker), and antibiotics. Medium was supplemented with rhIL-2 (Chiron) at either 3 or 300 ng/mL, or rhIL-15 (PeproTech, Rocky Hill, NJ) at 10 ng/mL, or combination of these 2 cytokines. Autologous neoplastic B cells after irradiation (30 Gy) were added at a lymphocyte-tumor ratio of 2:1. In some experiments, autologous irradiated (30 Gy) bone marrow–derived cells, or autologous DCs or autologous monocytes were used as feeder layer at a concentration of 25 × 104 cells/mL. Cell concentration was monitored daily and kept constant to 1 × 106 cells/mL. Medium with cytokines was partially replaced every 3 days.
Cytotoxicity assay
Lysis of neoplastic B cells by T-cell cultures activated with γc cytokines was evaluated as described18 by a 4-hour 51Cr release assay at the effector-target ratio of 25:1. In some experiments, after 51Cr labeling, neoplastic B cells were preincubated with a mAb (w6/32) to a monomorphic determinant of HLA class I antigens for 45 minutes at 37°C before adding effector cells.
Data analysis and statistical evaluation
The proportion of CD3+CD4+ or CD3+CD8+ T cells from peripheral blood or tumor site of patients or from peripheral blood of healthy donors expressing each of the 4 maturation phenotypes13 (namely TN, TCM, T effector memory [TEM], and T terminally differentiated [TTD]) defined by differential expression of CCR7 versus CD45RA were compared by analysis of variance (ANOVA), followed by Student-Newman-Keuls multiple comparison test. The whole data set containing the CD4+ and CD8+ T-cell maturation profiles of patients and donors was also subjected to correspondence analysis22 and hierarchic clustering as described.18 Both correspondence analysis and hierarchic clustering were conducted using J-Express Pro software (Molmine, www.Molmine.com). Data were hierarchically clustered by tissue (PBL or tumor site) and phenotype using a complete linkage as clustering method and the Pearson correlation as the similarity measure. Statistical analysis of IFN-γ ELISPOT assays was carried out by ANOVA followed by Student-Newman-Keuls (SNK) multiple comparison test.
Results
T cells from patients with B-NHL show an activated phenotype but a low frequency of tumor-reactive effector T cells with a TH2/Tc2 functional profile
T cells isolated at diagnosis from patients with B-cell NHL were evaluated for expression of activation markers, of different TCRBV regions, and for recognition of autologous tumor. The analysis for activation markers, carried out in 11 patients (Table 1), indicated that CD4+ and CD8+ T cells at tumor site (involved lymph nodes) frequently expressed CD69, HLA-DR, CD95, and IL-2R-α and -β chains. In contrast, T cells from healthy donors showed a resting phenotype (Supplemental Table S2). The high level of expression of CD95, on CD4+ or CD8+ T cells, suggested that lymphocytes at tumor site could be poised to undergo apoptosis. To evaluate this possibility, freshly isolated T cells from involved lymph nodes, or from bone marrow, or from peripheral blood of 30 patients were evaluated for binding of annexin V. In such assay, freshly isolated PBL from a healthy donor, cultured or not on immobilized anti-CD3 mAb, were used as positive and negative controls for annexin V binding, respectively (Figure 1Ai-iii). In periphery of patients with B-cell NHL, annexin V binding by T cells was generally low (Figure 1B, left for representative results). However, in several instances of involved lymph nodes or bone marrow, up to 20% to 30% of T cells were positive for annexin V binding (Figure 1B, right). In these patients the proportion of annexin V+ lymphocytes was not significantly different in the CD4+ and CD8+ cells nor in the CCR7+ or CCR7– fraction of such subsets (data not shown).
Activation profile of T cells from tumor site or peripheral blood of patients with B-NHL
. | . | Gated on CD3+CD4+ T cells‡ . | . | . | . | . | . | . | Gated on CD3+CD8+ T cells‡ . | . | . | . | . | . | . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue* . | Tumor† . | CD25 (IL-2R-α) . | CD122 (IL-2R-β) . | CD44 . | CD62L . | CD69 . | HLA-DR . | CD95 . | CD25 (IL-2R-α) . | CD122 (IL-2R-β) . | CD44 . | CD62L . | CD69 . | HLA-DR . | CD95 . | ||||||||||||
| LN | FL | 24 | 54 | 88 | 8 | 78 | 66 | 99 | 10 | 64 | 74 | 10 | 61 | 69 | 94 | ||||||||||||
| LN | CLL | 4 | ND | 92 | 2 | 28 | 24 | 94 | 0 | ND | 89 | 8 | 28 | 44 | 99 | ||||||||||||
| LN | FL | 7 | 15 | 31 | 1 | 27 | 42 | 97 | 1 | 14 | 9 | 1 | 30 | 69 | 99 | ||||||||||||
| LN | CLL | 10 | 16 | 89 | 5 | 45 | 34 | 79 | 3 | 16 | 95 | 4 | 22 | 22 | 47 | ||||||||||||
| LN | FL | 17 | 4 | 3 | 3 | 50 | 76 | 95 | 15 | 6 | 2 | 4 | 46 | 65 | 92 | ||||||||||||
| LN | FL | 15 | 2 | 60 | 16 | 67 | 77 | 98 | 14 | 2 | 65 | 14 | 60 | 72 | 95 | ||||||||||||
| PBL | CLL | 1 | 19 | 91 | 11 | 0 | 10 | 4 | 0 | 24 | 87 | 3 | 2 | 16 | 2 | ||||||||||||
| PBL | CLL | 10 | 5 | 99 | 6 | 0 | 10 | 41 | 0 | 4 | 99 | 4 | 1 | 15 | 17 | ||||||||||||
| PBL | CLL | 2 | 1 | 99 | 23 | 0 | 5 | 27 | 0 | 1 | 99 | 8 | 0 | 9 | 10 | ||||||||||||
| PBL | CLL | 2 | 3 | 99 | 5 | 0 | 27 | 24 | 1 | 4 | 98 | 1 | 1 | 50 | 14 | ||||||||||||
| PBL | CLL | 1 | 3 | 77 | 1 | 1 | 3 | 23 | 1 | 3 | 82 | 1 | 2 | 10 | 13 | ||||||||||||
. | . | Gated on CD3+CD4+ T cells‡ . | . | . | . | . | . | . | Gated on CD3+CD8+ T cells‡ . | . | . | . | . | . | . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue* . | Tumor† . | CD25 (IL-2R-α) . | CD122 (IL-2R-β) . | CD44 . | CD62L . | CD69 . | HLA-DR . | CD95 . | CD25 (IL-2R-α) . | CD122 (IL-2R-β) . | CD44 . | CD62L . | CD69 . | HLA-DR . | CD95 . | ||||||||||||
| LN | FL | 24 | 54 | 88 | 8 | 78 | 66 | 99 | 10 | 64 | 74 | 10 | 61 | 69 | 94 | ||||||||||||
| LN | CLL | 4 | ND | 92 | 2 | 28 | 24 | 94 | 0 | ND | 89 | 8 | 28 | 44 | 99 | ||||||||||||
| LN | FL | 7 | 15 | 31 | 1 | 27 | 42 | 97 | 1 | 14 | 9 | 1 | 30 | 69 | 99 | ||||||||||||
| LN | CLL | 10 | 16 | 89 | 5 | 45 | 34 | 79 | 3 | 16 | 95 | 4 | 22 | 22 | 47 | ||||||||||||
| LN | FL | 17 | 4 | 3 | 3 | 50 | 76 | 95 | 15 | 6 | 2 | 4 | 46 | 65 | 92 | ||||||||||||
| LN | FL | 15 | 2 | 60 | 16 | 67 | 77 | 98 | 14 | 2 | 65 | 14 | 60 | 72 | 95 | ||||||||||||
| PBL | CLL | 1 | 19 | 91 | 11 | 0 | 10 | 4 | 0 | 24 | 87 | 3 | 2 | 16 | 2 | ||||||||||||
| PBL | CLL | 10 | 5 | 99 | 6 | 0 | 10 | 41 | 0 | 4 | 99 | 4 | 1 | 15 | 17 | ||||||||||||
| PBL | CLL | 2 | 1 | 99 | 23 | 0 | 5 | 27 | 0 | 1 | 99 | 8 | 0 | 9 | 10 | ||||||||||||
| PBL | CLL | 2 | 3 | 99 | 5 | 0 | 27 | 24 | 1 | 4 | 98 | 1 | 1 | 50 | 14 | ||||||||||||
| PBL | CLL | 1 | 3 | 77 | 1 | 1 | 3 | 23 | 1 | 3 | 82 | 1 | 2 | 10 | 13 | ||||||||||||
ND indicates not done.
Lymphocytes were isolated from peripheral blood (PBL) or involved lymph nodes (LNs).
Patients had follicular lymphoma (FL) or chronic lymphocytic leukemia (CLL).
Results of flow cytometry analysis were expressed as the percentage of positive cells for each of the indicated markers in either the CD3+CD4+ or CD3+CD8+ subsets.
Binding of annexin V by T cells from patients with B-NHL. (A) Freshly isolated lymphocytes from PBL of a healthy donor were stained for binding of annexin V before (i), or, to promote apoptosis, after culture for 48 hours on immobilized anti-CD3 mAb at 10 (ii) and 100 (iii) ng/mL. (B) PBL, or involved LNs, or BM of 5 patients with FL or CLL were stained for binding of annexin V. Lymphocytes were gated on CD3+ T cells and then analyzed for CD4 or CD8 expression versus annexin V binding. Numbers in each dot plot refer to the percentage of positive cells in each quadrant. PT indicates patient.
Binding of annexin V by T cells from patients with B-NHL. (A) Freshly isolated lymphocytes from PBL of a healthy donor were stained for binding of annexin V before (i), or, to promote apoptosis, after culture for 48 hours on immobilized anti-CD3 mAb at 10 (ii) and 100 (iii) ng/mL. (B) PBL, or involved LNs, or BM of 5 patients with FL or CLL were stained for binding of annexin V. Lymphocytes were gated on CD3+ T cells and then analyzed for CD4 or CD8 expression versus annexin V binding. Numbers in each dot plot refer to the percentage of positive cells in each quadrant. PT indicates patient.
To document possible expansion of T cells sharing similar TCR structures, TCR B chain V region repertoire analysis was then carried out in tumor-containing lymph nodes of several patients. Only rare instances of significant expansions of T cells sharing the same TCRBV region were observed in lymph node biopsies (Supplemental Table S3). To identify tumor-reactive T lymphocytes at tumor site and to evaluate their functional profile (TH1 versus TH2, or Tc1 versus Tc2), intracellular IFN-γ and IL-4 production assays were set up in 4 patients, without prior T-cell activation. The T cells were evaluated for response to autologous tumor (ie, after direct antigen presentation) or in response to autologous DCs loaded with killed autologous B-cell tumor (ie, after tumor antigen cross-presentation). In lymphocytes from a healthy donor, activated against an autologous EBV-transformed B-cell line (B-LCL), CD4+ and CD8+ T cells produced IFN-γ and IL-4 in response to live B-LCL, or to autologous DCs loaded with killed B-LCL (Supplemental Figure S1Ai-iv). In contrast, in patients with B-NHL, CD4+ and CD8+ T cells producing IL-4, but not IFN-γ (thus expressing a TH2 and a Tc2 effector phenotype, respectively), and present at low frequency (2%-4%) were found in lymph nodes in response to autologous DCs loaded with killed tumor cells (Figure 2Aii versus Ai, for representative results), or even in response to live autologous neoplastic B cells (Figure 2Aiv versus Aiii). Patients' T cells preactivated by culture with DCs loaded with autologous killed B-cell tumor maintained a TH2/Tc2 functional profile when reassessed on autologous B-cell tumor (Figure 2Bii versus Bi), although these cells could produce even IFN-γ, and not only IL-4, after activation with PMA plus ionomycin (Figure 2Biv). Control experiments suggested that the TH2/Tc2 functional profile of T cells from involved LNs could be restricted to recognition of autologous B-cell tumor. In fact, preactivation of T cells, from the same involved lymph node of patient A, with irradiated autologous EBV-transformed B cells (instead of autologous B-cell tumor), led to activation of CD4+ and CD8+ T cells releasing even IFN-γ, and not only IL-4. This was seen in response to DCs loaded with killed autologous B-LCL (Supplemental Figure S1Bii), or even in response to live B-LCL (Supplemental Figure S1Biii). Taken together, these results indicate that T cells from patients with B-NHL frequently show an activated but apoptosis-prone phenotype with low frequency of tumor-reactive T cells showing a TH2/Tc2 functional profile in the response to autologous tumor.
Skewed T-cell differentiation profile in T cells from peripheral blood or tumor site from patients with B-cell tumor
The low frequency of tumor-reactive T cells, observed when T lymphocytes were tested without prior activation, was consistent with a reduced T-cell differentiation to effector stage in patients with B-NHL. To assess this possibility, differentiation of CD4+ and CD8+ T cells along the CCR7+CD45RA+ naive (TN) to CCR7+CD45RA– central memory (TCM), CCR7– CD45RA– effector memory (TEM), and CCR7–CD45RA+ terminally differentiated (TTD) stages13 were investigated in lymphocytes isolated at diagnosis from peripheral blood or involved lymph nodes or bone marrow of a large panel of patients with B-NHL. The CD4+ and CD8+ T-cell maturation profile of PBL from a panel of 47 healthy donors was also included in the analysis. As a first step in the evaluation of the whole panel of patients and donors, we used correspondence analysis,22 an exploratory technique yielding a two-dimensional projection of all the CD4+ and CD8+ T-cell maturation profiles. By such analysis we found that the T-cell maturation profiles of the patients defined a loose cluster shifted toward the CD4+ and CD8+ early maturation stages (ie, TN and TCM, indicated as 4N, 8N, 4CM, 8CM in Supplemental Figure S2). This cluster was not overlapped with a more differentiated cluster (shifted toward TEM and TTD stages, indicated as 4EM, 8EM, 4TD, and 8TD in Supplemental Figure S2) containing all donors samples. Hierarchic cluster analysis was then performed, as previously described,18 to obtain a comprehensive classification of CD8+ and CD4+ maturation profiles from patients and donors, as defined by CCR7 versus CD45RA phenotype. On the basis of intracluster similarity, but also of the highest intercluster differences for the maturation profile, 3 major maturation groups were identified in the CD4+ and CD8+ subsets. In the CD8+ subset, almost all PBL samples from donors fell in the first cluster (Figure 3Ai) characterized by sizable fractions of lymphocytes in each of the 4 maturation stages from TN to TTD. In contrast, almost all tissue samples (either from PBL or LNs or bone marrow) from patients with B-NHL fell into 2 maturation clusters (Figure 3Aii-iii) characterized by a predominant fraction of T lymphocytes at the early stages of differentiation (TN or TCM). In the CD4+ subset, patients and donors were not as clearly separated as in the CD8+ subset, although, even in this instance, most lymphocyte samples from the patients showed an increased fraction of cells at the TN and TCM stages, and a reduced fraction of cells at the TEM and TTD stages (Figure 3Bii-iii). In 13 patients, 2 different involved tissues could be evaluated (either PBL and tumor-containing LN, or PBL and involved bone marrow) for the T-cell maturation profile. Interestingly, in 9 of 13 cases the CD4+ and CD8+ T cells from the 2 different tissues fell in the same maturation cluster (data not shown). Comparison of each of the T-cell maturation stages defined by CCR7 versus CD45RA by ANOVA and SNK test (Supplemental Figure S3) confirmed significant differences in the CD4+ and CD8+ T-cell maturation profiles between donors and patients. As a further control, lymphocytes from tumor-free lymph nodes of patients with breast cancer were evaluated. In contrast with LNs from patients with B-cell NHL, no evidence for a skewing toward the TN and TCM stages was found in such samples. In fact, differentiated T cells at the TEM (CCR7–CD45RA–) or at the TTD (CCR7–CD45RA+) stages were present in such samples at frequencies similar to those found in PBL of healthy donors (Supplemental Figure S4). No major clinical parameters (such as stage, bone marrow involvement, and international prognostic index) of the patients with B-NHL were found associated with the CD4+ and CD8+ T-cell maturation clusters defined in Figure 3. The skewed T-cell maturation in patients with B-cell NHL, mostly evident in the CD8+ subset, was confirmed by analysis of the CD27 versus CD28 phenotype23 (data not shown).
Effector T cells with a TH2/Tc2 phenotype at tumor site of patients with B-NHL. (A) Intracellular staining for IL-4 and IFN-γ production in gated CD4+ or CD8+ T cells isolated from involved lymph nodes of two patients with FL (A and B). Lymphocytes from patient A were tested without prior activation against autologous DCs either unloaded (i) or loaded with killed autologous B-cell tumor (ii). Lymphocytes from patient B were tested without prior activation either alone (iii) or against live autologous B-cell tumor (iv). (B) Lymphocytes from patient A, isolated from the same lymph node as in panel A, were cultured for 2 weeks with DCs loaded with killed autologous B-cell tumor and then tested either alone (i) or against live autologous B-cell tumor (ii), allogeneic B-cell tumor (iii), or after activation with PMA + ionomycin (iv). Numbers in the dot plots indicate the fraction of positive cells in each quadrant.
Effector T cells with a TH2/Tc2 phenotype at tumor site of patients with B-NHL. (A) Intracellular staining for IL-4 and IFN-γ production in gated CD4+ or CD8+ T cells isolated from involved lymph nodes of two patients with FL (A and B). Lymphocytes from patient A were tested without prior activation against autologous DCs either unloaded (i) or loaded with killed autologous B-cell tumor (ii). Lymphocytes from patient B were tested without prior activation either alone (iii) or against live autologous B-cell tumor (iv). (B) Lymphocytes from patient A, isolated from the same lymph node as in panel A, were cultured for 2 weeks with DCs loaded with killed autologous B-cell tumor and then tested either alone (i) or against live autologous B-cell tumor (ii), allogeneic B-cell tumor (iii), or after activation with PMA + ionomycin (iv). Numbers in the dot plots indicate the fraction of positive cells in each quadrant.
Hierarchic cluster analysis of T-cell maturation profiles from patient with B-NHL and healthy donors. Hierarchic clustering was performed for the CD3+CD8+ (A) and the CD3+CD4+ (B) subsets. In each panel, rows represent individual lymphocyte samples from PBL from healthy donors; PBL, involved lymph nodes, or bone marrow from patients. Columns represent each of the 4 possible phenotypes obtained by analysis of CCR7 versus CD45RA by 4-color flow cytometry and coded as follows: TN, CCR7+CD45RA+; TCM, CCR7+CD45RA–; TEM, CCR7–CD45RA–; TTD, CCR7–CD45RA+. Lymphocyte samples were coded as follows: ○, PBL from healthy donors; □, PBL or LNs from patients with CLL; ▪, PBL or LNs from patients with FL;  , PBL from patients with mantle-cell lymphoma [MCL]; ▴, bone marrow from patients with FL; ▵, bone marrow from patients with CLL. The percentage of positive cells for each of the 4 phenotype subsets was coded by levels of gray shading, as indicated at the bottom of the figure. The 3 major T-cell maturation clusters found in the CD8+ and CD4+ subsets were ranked from i to iii according to a progressive shift toward the most immature phenotypes.
, PBL from patients with mantle-cell lymphoma [MCL]; ▴, bone marrow from patients with FL; ▵, bone marrow from patients with CLL. The percentage of positive cells for each of the 4 phenotype subsets was coded by levels of gray shading, as indicated at the bottom of the figure. The 3 major T-cell maturation clusters found in the CD8+ and CD4+ subsets were ranked from i to iii according to a progressive shift toward the most immature phenotypes.
Hierarchic cluster analysis of T-cell maturation profiles from patient with B-NHL and healthy donors. Hierarchic clustering was performed for the CD3+CD8+ (A) and the CD3+CD4+ (B) subsets. In each panel, rows represent individual lymphocyte samples from PBL from healthy donors; PBL, involved lymph nodes, or bone marrow from patients. Columns represent each of the 4 possible phenotypes obtained by analysis of CCR7 versus CD45RA by 4-color flow cytometry and coded as follows: TN, CCR7+CD45RA+; TCM, CCR7+CD45RA–; TEM, CCR7–CD45RA–; TTD, CCR7–CD45RA+. Lymphocyte samples were coded as follows: ○, PBL from healthy donors; □, PBL or LNs from patients with CLL; ▪, PBL or LNs from patients with FL;  , PBL from patients with mantle-cell lymphoma [MCL]; ▴, bone marrow from patients with FL; ▵, bone marrow from patients with CLL. The percentage of positive cells for each of the 4 phenotype subsets was coded by levels of gray shading, as indicated at the bottom of the figure. The 3 major T-cell maturation clusters found in the CD8+ and CD4+ subsets were ranked from i to iii according to a progressive shift toward the most immature phenotypes.
, PBL from patients with mantle-cell lymphoma [MCL]; ▴, bone marrow from patients with FL; ▵, bone marrow from patients with CLL. The percentage of positive cells for each of the 4 phenotype subsets was coded by levels of gray shading, as indicated at the bottom of the figure. The 3 major T-cell maturation clusters found in the CD8+ and CD4+ subsets were ranked from i to iii according to a progressive shift toward the most immature phenotypes.
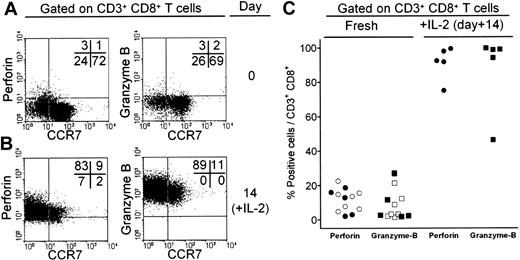
Expression of cytolytic factors in CD8+ T cells from patients with B-NHL. T lymphocytes from a lymph node biopsy of a patient with FL were characterized for expression of CCR7 and perforin or granzyme B before (A) and after (B) culture for 2 weeks with 50 ng/mL IL-2. Numbers in the dot plots indicate the fraction of positive cells in each quadrant. (C) Expression of cytolytic factors perforin (○) and granzyme B (□) in T cells isolated from peripheral blood of patients with CLL, or from lymph node biopsies (•, ▪) of patients with FL. Expression of perforin and granzyme B was also reevaluated after culture with IL-2.
Expression of cytolytic factors in CD8+ T cells from patients with B-NHL. T lymphocytes from a lymph node biopsy of a patient with FL were characterized for expression of CCR7 and perforin or granzyme B before (A) and after (B) culture for 2 weeks with 50 ng/mL IL-2. Numbers in the dot plots indicate the fraction of positive cells in each quadrant. (C) Expression of cytolytic factors perforin (○) and granzyme B (□) in T cells isolated from peripheral blood of patients with CLL, or from lymph node biopsies (•, ▪) of patients with FL. Expression of perforin and granzyme B was also reevaluated after culture with IL-2.
Low expression of cytotoxic factors in CD8+ T cells from patients with B-NHL can be reversed by culture with γc cytokines
In agreement with current models of cytokine-induced T-cell differentiation,13,18 culture of PBL from healthy donors in the presence of IL-2 led to CD8+ T-cell maturation toward the TTD stage, as shown by down-regulation of CCR7, increased expression of CD45RA, and up-regulation of cytolytic factor perforin (Supplemental Figure S5). In patients with B-cell NHL, perforin and granzyme B expression was low in CD8+ T cells isolated from peripheral blood or involved LNs (Figure 4A for representative results and Figure 4C for results on a panel of patients), confirming the skewed maturation of these T cells. In addition, cytotoxic factor expression was mainly associated with the limited fraction of differentiated, CCR7-negative cells (Figure 4A). However, culture for 2 weeks of patients' T cells with cytokines such as IL-2 and IL-15 promoted expression of both perforin and granzyme B, associated with down-modulation of CCR7 (Figure 4B for representative results in one patient and Figure 4C for results on a panel of patients). Such T-cell maturation could be obtained by culture of patients' T cells from peripheral blood or involved LNs of patients with FL and CLL and using either IL-2 or IL-15 (data not shown).
Expansion and functional analysis of T cells from patients with B-NHL after culture with γc cytokines
T cells isolated at diagnosis from 20 patients were purified (CD3+ ≥ 98%) from tumor-containing lymph nodes (FL, n = 10; CLL, n = 6; MCL, n = 4) by immunomagnetic sorting and then were cultured for 3 weeks in the presence of autologous neoplastic B cells in medium supplemented with γc cytokines. Low-dose (3 ng/mL) IL-2 used alone (data not shown), or in the presence of IL-15, induced only an average 2-fold expansion (Figure 5Ai, showing representative results from 10 patients). High-dose IL-2 (300 ng/mL; HD–IL-2) led to an average 16.4 ± 4.6-fold T-cell expansion (Figure 5Aii). No significant T-cell expansion could be achieved in the presence of IL-1β, IL-12, or IL-15 used as single cytokines (data not shown). However, HD–IL-2 plus IL-15 led to an average 43.5- ± 3.7-fold expansion (Figure 5Aiii). Addition, to HD–IL-2 and IL-15, of an irradiated feeder layer of autologous bone marrow–derived cells (Figure 5Aiv), or of autologous monocytes (Figure 5Av), led to a further increase in T-cell growth (101.1 ± 8.1-fold and 69.8 ± 6.5-fold expansion, respectively). In contrast, autologous DCs markedly suppressed T-cell growth in response to HD–IL-2 and IL-15 (Figure 5Avi).
ELISPOT analysis was then used to evaluate the frequency of IFN-γ–producing T cells in response to autologous or allogeneic neoplastic B cells after T-cell culture with γc cytokines. In 10 patients investigated, the frequency of IFN-γ–producing T cells in response to the autologous B-cell tumor was significantly higher than in response to allogeneic, HLA-mismatched B-cell tumor (Figure 5B, for representative results from one patient), and the highest frequency of tumor-reactive IFN-γ–producing T cells was achieved after culture with HD–IL-2, IL-15, and autologous bone marrow–derived feeder cells. By cytotoxicity assays we found that T cells cultured with γc cytokines could lyse the autologous neoplastic B cells and that the lysis could be significantly inhibited in the presence of mAb to a monomorphic determinant of HLA class I antigens (Figure 5C). Lysis of autologous B-cell tumor, by the T-cell cultures activated with γc cytokines, was 30% ± 5% at E/T ratio of 30:1 (average of 10 patients, data not shown).
Growth and functional analysis of T cells from patients with B-cell NHL after culture with γc cytokines. (A) Purified CD3+ T cells from tumor-containing lymph nodes of 10 patients with B-NHL were seeded at the initial concentration of 1 × 106/mL and cultured for 3 weeks with autologous B-tumor cells in the presence of IL-2 at 3 ng/mL (LD–IL-2) or at 300 ng/mL (HD–IL-2). IL-15 was used at 10 ng/mL. Irradiated autologous bone marrow–derived cells (BM), autologous monocytes (MØ), or autologous monocyte-derived DCs (DCs) were also added in some cultures. Total number of viable cells was assessed by trypan blue exclusion and the number of CD3+ cells by immunophenotyping on days 0, 7, 14, and 21 of culture. Viable cells were always greater than 97% CD3+ T cells. (B) T cells isolated from tumor-containing lymph nodes were cultured for 3 weeks with HD–IL-2 alone (1), or HD–IL-2 plus IL-15 (2), or HD–IL-2 plus IL-15 and irradiated autologous bone marrow–derived cells (3). The T-cell cultures were then evaluated in a 24-hour IFN-γ–ELISPOT assay in response to no stimulus (white bars, negative control), autologous (light gray bars), or allogeneic (dark gray bars) B-cell tumor, or IL-2 at 300 ng/mL (black bars, positive control). (C) T cells were cultured with HD–IL-2 plus IL-15 and autologous bone marrow–derived cells and then tested for lysis of autologous B-cell tumor preincubated (empty bars) or not (black bars) with a mAb to a monomorphic determinant of HLA class I antigens. T cells were also tested for lysis of an allogeneic HLA-mismatched B-LCL (gray bars). (B-C) Error bars indicate SD of the mean. Statistical analysis was annotated as follows: *P < .05, **P < .01.
Growth and functional analysis of T cells from patients with B-cell NHL after culture with γc cytokines. (A) Purified CD3+ T cells from tumor-containing lymph nodes of 10 patients with B-NHL were seeded at the initial concentration of 1 × 106/mL and cultured for 3 weeks with autologous B-tumor cells in the presence of IL-2 at 3 ng/mL (LD–IL-2) or at 300 ng/mL (HD–IL-2). IL-15 was used at 10 ng/mL. Irradiated autologous bone marrow–derived cells (BM), autologous monocytes (MØ), or autologous monocyte-derived DCs (DCs) were also added in some cultures. Total number of viable cells was assessed by trypan blue exclusion and the number of CD3+ cells by immunophenotyping on days 0, 7, 14, and 21 of culture. Viable cells were always greater than 97% CD3+ T cells. (B) T cells isolated from tumor-containing lymph nodes were cultured for 3 weeks with HD–IL-2 alone (1), or HD–IL-2 plus IL-15 (2), or HD–IL-2 plus IL-15 and irradiated autologous bone marrow–derived cells (3). The T-cell cultures were then evaluated in a 24-hour IFN-γ–ELISPOT assay in response to no stimulus (white bars, negative control), autologous (light gray bars), or allogeneic (dark gray bars) B-cell tumor, or IL-2 at 300 ng/mL (black bars, positive control). (C) T cells were cultured with HD–IL-2 plus IL-15 and autologous bone marrow–derived cells and then tested for lysis of autologous B-cell tumor preincubated (empty bars) or not (black bars) with a mAb to a monomorphic determinant of HLA class I antigens. T cells were also tested for lysis of an allogeneic HLA-mismatched B-LCL (gray bars). (B-C) Error bars indicate SD of the mean. Statistical analysis was annotated as follows: *P < .05, **P < .01.
Discussion
Analysis for activation markers and for the cytokine profile of CD4+ and CD8+ T cells from patients with B-NHL frequently indicated an activated, apoptosis-prone phenotype with low frequency of tumor-reactive T cells showing a TH2/Tc2 functional profile in the response to autologous tumor. The activated, apoptosis-prone T-cell phenotype is in agreement with previous results24 in NHL and in nonmalignant secondary lymphoid organs, supporting the notion that such phenotype may be a common feature of T lymphocytes from chronic inflammatory lesions. Furthermore, the results on CD95 expression, and on the extent of early apoptosis in patients' T cells, are consistent with a possible tumor escape mechanism that might contribute to eliminate activated antitumor T cells at the tumor site. This possibility is in agreement with the limited evidence for expansion of T cells sharing the same TCRBV region. Previous results have indicated CD95 expression on peripheral-blood T cells from patients with CLL25,26 and that CD19+CD95-L+ B-CLL cells can kill CD95+ cell lines.27 However, the evidence for a direct killing of CD95+ T cells by CD95-L+ neoplastic B cells is still controversial. CD95-L expression has been documented in myeloma cells and Hodgkin lymphomas,28,29 but not confirmed in low-grade B-cell lymphomas such as CLL.30 Moreover, analysis of different B-cell lymphomas has provided morphologic evidence of apoptosis, caspase-3 activation, and caspase-dependent PARP cleavage only in CD20+ neoplastic B cells.31 Thus, the mechanism leading to increased binding of annexin V in T cells from patients with B-NHL remains to be elucidated. One possibility, to be investigated, is that T-cell apoptosis in patients with B-NHL may be promoted by tumor-derived microvesicles, as described in circulating T cells from patients with solid tumors.32
The functional analysis of T cells from the tumor site provided evidence for tumor-reactive T cells with ability to produce IL-4, but not of IFN-γ, in response to autologous B-cell tumor, or to DCs loaded with killed neoplastic B-NHL cells. In the light of the evidence indicating that IL-4 promotes survival of B-NHL cells, such as CLL,33 these results suggest that a TH2-polarized antitumor response may in fact promote neoplastic B-cell survival, rather than contributing to control neoplastic-cell growth. In agreement with this possibility, supernatants of CD8+ T cells from PBL of patients with CLL have been shown to suppress apoptosis of neoplastic B cells by an IL-4–dependent mechanism,34 indicating a paracrine function of T-cell–derived IL-4 in CLL. Additional results have suggested the existence of an altered balance in TH1/TH2 T-cell subsets in patients with B-NHL. Thus, an increased IL-4 production, with a shift toward the TH2 T-cell profile, has been found in peripheral-blood T cells of patients with advanced stage CLL,35 or in patients compared with healthy donors,36 after activation with PMA and ionomycin, or with PHA. Further evidence for a polarization toward the TH2/Tc2 functional profile in T cells from patients with B-NHL was provided in this study, by assessing T-cell production of IFN-γ and IL-4 in response to autologous B-cell tumor, or to DCs loaded with killed tumor cells. Interestingly, such polarization was not found when T cells from the tumor site were tested against autologous EBV-transformed B cells. This suggests that the TH2/TC2 skewing was restricted to the response against autologous B-cell tumor and did not reflect an antigen-independent T-cell polarization known to be generated in different tumors (see Balkwill37 for a review).
T cells from the tumor site or peripheral blood of patients with B-NHL showed a reduced maturation along the naive to effector and memory pathway, with a reduced frequency of differentiated T cells at the TEM and TTD stages. Accordingly, only a low fraction of T cells from patients with B-NHL expressed cytolytic factors such as granzyme B and perforin. In fact, the expression of these cytotoxic factors has been shown to be low to absent in TCM cells and acquired when T cells differentiate to the TEM stage.13,18 The reduced expression of cytolytic factors in T cells from patients with B-NHL suggests that impaired T-cell differentiation may prevent effective elimination of neoplastic cells in these patients. This possibility is in agreement with the proposed role of cytotoxic factors and of cytolytic cells (either natural killer or T) in the protection against lymphomas, as documented in murine models and, more recently, in patients with lymphoma. In fact, in murine models, perforin expression has been shown to be required in the protection against lymphomagenesis, and cytotoxic T lymphocytes have been shown to mediate the elimination of lymphomas arising in perforin-deficient mice.38 The relevant role of the cytolytic factors released by cytotoxic lymphocytes has been recently supported even in patients with lymphoma, as documented by the identification of mutations affecting the perforin gene in patients with lymphoma.39,40
Several mechanisms may account for the skewed T-cell maturation found in patients with B-NHL. In principle, either impaired T-cell differentiation or accelerated elimination of differentiated T cells may yield the observed skewing of T-cell phenotype. On the one hand, impaired T-cell differentiation may be generated as a result of immunosuppressive factors released by neoplastic cells,41 of poor antigen-presenting function of B-NHL,42 or even of defective functional maturation of professional APCs, as described in patients with B-NHL.43 However, alternative explanations are possible, such as the rapid turnover of differentiated T cells, as described for mature (CD28–) CD8+ T cells in peripheral blood of patients with head and neck cancer.44 Interestingly, in such patients, the accelerated turnover of differentiated T cells, compared with naive T lymphocytes, has been correlated to the increased susceptibility to apoptosis of cells at the effector stage.45 On the other hand, we did not find evidence for increased annexin V binding in CCR7– T cells from patients of this study, compared with CCR7+ cells, as it may be expected to support an increased rate of elimination, by apoptosis, of differentiated T cells.
Despite their defective maturation, T cells from patients with B-NHL retained responsiveness to γc cytokines such as IL-2 and IL-15, which promoted activation, proliferation, and functional differentiation of CD8+ T cells to cytotoxic factor+, IFN-γ-secreting effectors. These results suggest that patients with B-NHL possess a pool of antitumor T cells that can be differentiated by γc cytokines toward the TC1 functional profile, despite the initial TC2 skewing found at tumor site. However, the differentiation of T cells to cytolytic effectors by γc cytokines, although necessary, in itself would not be sufficient for the development of effective immune intervention strategies, such as the adoptive cell-transfer therapies, unless coupled to an efficient procedure for achieving massive T-cell expansion. In fact, the results obtained in other solid tumors such as melanoma46 or EBV+ Hodgkin lymphomas47 have shown that clinically significant antitumor responses after ACT, require multiple injections of differentiated T cells at numbers at or above the 108 to 109 range. The results of this study suggest that such level of T-cell expansion might be feasible, in principle, even in patents with B-cell NHL. In fact, even when starting from as little as 1 × 106 T cells, the addition to γc cytokines of an adequate feeder layer of irradiated BM-derived cells or of monocytes allowed to achieve, respectively, an average approximately 100- or approximately 70-fold increase in T-cell number in a 3-week culture. Moreover, ACT with ex vivo γc cytokine–activated T cells may be considered as an alternative to immunotherapy with cytokines such as IL-2. In fact, the latter approach, often adopted in combination with rituximab, has recently been shown to be well tolerated, but to achieve limited clinical responses in patients with B-cell NHL.48,49
Taken together, these results provide evidence that γc cytokine–mediated reversal of impaired T-cell differentiation could be considered as an effective tool for development of immune intervention strategies in B-NHL.
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2005-06-2234.
Supported in part by grants from Istituto Superiore di Sanità, Rome, within the framework of Italy-USA Program on therapy of tumors and from Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy.
A.A., R.M., A.M.G., C.C., and M.D.N. contributed to the conception, analysis of data, and writing of the manuscript. A.C. contributed to the conception of the work. L.R. and P.B. contributed to the execution of the research. All authors checked the final version of the manuscript.
A.A. and R.M. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients for their generous participation in this study. We thank Ms Stefania Cresta for her excellent technical help. We also thank Dr Liliana Devizzi and Dr Paola Matteucci, of “Cristina Gandini” Bone Marrow Transplantation Unit of our Institute, for their contribution to the clinical management of patients.



![Figure 3. Hierarchic cluster analysis of T-cell maturation profiles from patient with B-NHL and healthy donors. Hierarchic clustering was performed for the CD3+CD8+ (A) and the CD3+CD4+ (B) subsets. In each panel, rows represent individual lymphocyte samples from PBL from healthy donors; PBL, involved lymph nodes, or bone marrow from patients. Columns represent each of the 4 possible phenotypes obtained by analysis of CCR7 versus CD45RA by 4-color flow cytometry and coded as follows: TN, CCR7+CD45RA+; TCM, CCR7+CD45RA–; TEM, CCR7–CD45RA–; TTD, CCR7–CD45RA+. Lymphocyte samples were coded as follows: ○, PBL from healthy donors; □, PBL or LNs from patients with CLL; ▪, PBL or LNs from patients with FL; , PBL from patients with mantle-cell lymphoma [MCL]; ▴, bone marrow from patients with FL; ▵, bone marrow from patients with CLL. The percentage of positive cells for each of the 4 phenotype subsets was coded by levels of gray shading, as indicated at the bottom of the figure. The 3 major T-cell maturation clusters found in the CD8+ and CD4+ subsets were ranked from i to iii according to a progressive shift toward the most immature phenotypes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-06-2234/4/m_zh80020689290003.jpeg?Expires=1766011584&Signature=e285mWqdkaFzPZBz0qDueD3Xcc8Cgwy59FWh-x0H7UPY6GmA7UpTT7VbHYrdbJIePBWhod0iALRgoL0OEAOCQA7e3yJKVdOLkfQP22kxZMrLkaq6zNeXHav3eB6e8KvMV14BHZoj7ElLAtsQmXPv8QEoko8SWJzS~~0FD2bDAOwBzpXHRbNPRM-omzR4ZZehWc3cCs7JS6NS80zMTcjR25uNh3aMJAu7yCkmTKX1nf8EAQaDutyNFv4kEm-b-6uHlihiTzb-LRRXyq37ZwvCpXqim~EmZa~VW6wtpVEKoseUPwjaaJ2qKU8hD8UHbupi11jiIdIGMAdttet6mNS3xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal