Abstract
Scleromyxedema, the most severe manifestation of the spectrum of lichen myxedematosus, is characterized by cutaneous mucinosis, extracutaneous manifestations, and a monoclonal gammopathy. Seven of 8 patients evaluated at our center were treated with high-dose melphalan (180 mg/m2 intravenously) and autologous peripheral blood stem cell transplantation, with marked improvement of gastrointestinal, central nervous system, pulmonary manifestations, and Karnofsky performance status. Five patients obtained a cutaneous complete remission and 2 patients had partial remissions. Three patients with slight progression in the skin at 12, 8, and 4 months after treatment received a second cycle of high-dose melphalan and had further symptomatic improvement. The lichen myxedematosus–scleromyxedema spectrum appears to be a continuum that requires the presence of a serum paraprotein and differs in severity of skin lesions, extracutaneous manifestations, and performance status. High-dose melphalan followed by autologous transplantation appears effective for improving the symptoms and systemic manifestations of scleromyxedema.
Introduction
Scleromyxedema, the most generalized and rare variant of lichen myxedematosus (LM) recently classified by Rongioletti and Rebora1 (Table 1), is characterized by a paraprotein and widespread cutaneous mucinosis. Patients present with papular mucinosis (waxy papules on the face, neck, upper trunk, forearms, hands, and thighs) that becomes increasingly confluent, leading to a scleroderma-like appearance (Figure 1). Common extracutaneous manifestations include upper gastrointestinal dysmotility, muscle weakness, joint contractures, and neurologic symptoms such as seizures, encephalopathy, coma, and obstructive or restrictive pulmonary disease.
Classification of lichen myxedematosus (LM)
|
|
Data are from Rongioletti and Rebora.1
Previously used treatments include prednisone,2 immunoglobulins,3 photopheresis, and low-dose melphalan.2-6 High-dose melphalan has been used successfully to treat polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome, which is also characterized by a monoclonal gammopathy causing polyneuropathy.7 Amyloidosis (AL), another plasma cell dyscrasia associated with severe clinical manifestations, has also been treated with high-dose melphalan with reported survival benefit.8 Approximately 80% of scleromyxedema cases are associated with a monoclonal gammopathy, usually IgG-lambda, supporting the rationale for using high-dose melphalan therapy in this disease.
Patients, materials, and methods
Patients
Between April 2000 and November 2003, 8 patients with scleromyxedema were evaluated for possible high-dose chemotherapy and autologous peripheral blood stem cell transplantation (PBSCT). Seven patients, including one previously reported,9 received the treatment and 1 died prior to treatment. Informed consent was obtained for therapy under the University of Texas M. D. Anderson Cancer Center institutional Compassionate Investigational New Drug protocol, in accordance with the Declaration of Helsinki. Patient demographics and disease status are summarized in Table 2.
Patient demographics and disease status
Patient no. . | Age, y . | Sex . | Paraprotein . | Paraprotein level before PBSCT . | Skin involvement . | Extracutaneous manifestations . | Karnofsky performance status before PBSCT, % . |
|---|---|---|---|---|---|---|---|
| 1 | 47 | M | IgG-λ* | 0.1 g/dL | Generalized | GI, CNS, PLM, JC | 30-40 |
| 2 | 55 | F | IgG-λ | 0.1 g/dL | Generalized | None | 80 |
| 3 | 55 | F | IgG-λ | Intermittent positivity never greater than 0.1 g/dL | Acral papules; limited | GI, CT, PN | 70 |
| 4 | 46 | M | IgG-λ | 0.7 g/dL | Generalized | GI | 30-40 |
| 5 | 74 | F | IgG-κ | 0.5 g/dL | Limited | GI | 70 |
| 6 | 58 | M | IgG-λ | 0.3 g/dL | Generalized | GI | 60 |
| 7 | 47 | F | IgG-λ | 0.9 g/dL† | Generalized | PLM, RN | 30 |
| 8 | 43 | F | IgG-λ | 0.5 g/dL | Generalized | CNS, GI, PLM, JC | 50 |
Patient no. . | Age, y . | Sex . | Paraprotein . | Paraprotein level before PBSCT . | Skin involvement . | Extracutaneous manifestations . | Karnofsky performance status before PBSCT, % . |
|---|---|---|---|---|---|---|---|
| 1 | 47 | M | IgG-λ* | 0.1 g/dL | Generalized | GI, CNS, PLM, JC | 30-40 |
| 2 | 55 | F | IgG-λ | 0.1 g/dL | Generalized | None | 80 |
| 3 | 55 | F | IgG-λ | Intermittent positivity never greater than 0.1 g/dL | Acral papules; limited | GI, CT, PN | 70 |
| 4 | 46 | M | IgG-λ | 0.7 g/dL | Generalized | GI | 30-40 |
| 5 | 74 | F | IgG-κ | 0.5 g/dL | Limited | GI | 70 |
| 6 | 58 | M | IgG-λ | 0.3 g/dL | Generalized | GI | 60 |
| 7 | 47 | F | IgG-λ | 0.9 g/dL† | Generalized | PLM, RN | 30 |
| 8 | 43 | F | IgG-λ | 0.5 g/dL | Generalized | CNS, GI, PLM, JC | 50 |
GI indicates gastrointestinal dysmotility; CNS, central nervous system presenting as seizures; PLM, pulmonary dysfunction; JC, joint contractures; CT, carpal tunnel syndrome; PN, peripheral neuropathy; and RN, renal dysfunction.
With bone marrow involvement.
Level at initial evaluation; did not receive PBSCT high-dose treatment.
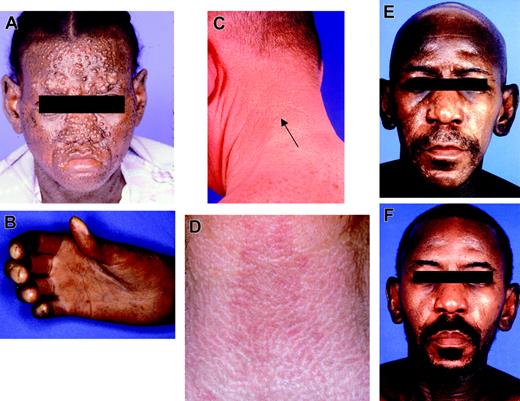
The most severe patient, who died prior to treatment, had multiple large papules over the central face as well as severe pulmonary hypertension and sclerodactyly (Figure 1A-B). The less severe patients had small waxy papules on the face, neck, posterior ears, and dorsal hands (Figure 1C-D). Six of the 7 treated patients had received prior systemic therapy: prednisone/dexamethasone (3 patients), plaquenil (2 patients), interferon-alpha (2 patients), azathioprine (1 patient), chlorambucil (1 patient), psoralen and long-wave ultraviolet radiation (PUVA)/extracorporeal photopheresis (2 patients), cyclophosphamide (1 patient), and penicillamine (1 patient). Of 2 patients pretreated with pulse dexamethasone, one patient had a partial remission in the skin with slight progression and the other had a transient response followed by rapid progression and escalation of disease upon steroid tapering. The other 5 were progressing prior to transplantation and had an impaired performance status. Two of these patients had a Karnofsky performance status10 of 30% to 40%, requiring a gastrostomy feeding tube and repeated hospitalizations.
Treatment methods
Peripheral blood stem cell collection. Patients received filgrastim (5 μg/kg) rounded to the nearest vial size subcutaneously twice daily until completion of apheresis. Apheresis was performed starting on day 4 of filgrastim administration and continued until at least 5 × 106 CD34+ cells/kg were collected. The CD34+ cell content was evaluated immediately after apheresis and the cells were subsequently cryopreserved using programmed freezing.
High-dose chemotherapy. All but one patient was hospitalized for the high-dose chemotherapy administration. Melphalan (90 mg/m2 per day) was given intravenously over 30 minutes on days –3 and –2. To minimize oral mucositis, patients were instructed to keep ice chips in their mouths starting 5 minutes before and for 30 minutes after the melphalan infusion. Peripheral blood stem cells were infused on day 0.
Supportive care. Filgrastim (5 μg/kg rounded to the nearest vial size) was given subcutaneously daily from day 0 until the absolute neutrophil count reached at least 1.5 × 109/L. Infection prophylaxis included levofloxacin (500 mg orally daily), fluconazole (200 mg orally daily), and valacyclovir (500 mg orally daily). Transfusions were given to maintain a hemoglobin level higher than 80 g/L (8.0 g/dL) and platelet levels higher than 15 × 109/L.
Results
Patients
Seven patients were treated with a total of 10 cycles of high-dose melphalan. Three patients received 2 cycles of high-dose melphalan. The second cycle was administered due to return of skin lesions and systemic symptoms. All patients were assessed for toxicity and response. One patient (no. 7) receiving pulse dexamethasone died of pneumonia and severe pulmonary hypertension prior to stem cell mobilization and treatment (Figure 1A). The median time from the initial symptoms until transplantation was 3.5 years (range, 1-6 years). All progressed from initial papular skin rash as the first manifestation of the disease to generalized mucinosis and/or development of extracutaneous symptoms.
Lesional skin biopsies were taken from all patients to confirm the diagnosis and were stained with hematoxylin, eosin, and colloidal iron to detect dermal mucin. The epidermis was not involved (Figure 2A). A proliferation of predominantly spindle and epitheloid cells, and a fibromyxoid stroma containing thick bundles of collagen and increased mucin were present (Figure 2B). The degree of cellularity ranged from scattered cells in the dermis to markedly hypercellular infiltrates involving the entire reticular dermis. Immunohistochemical studies were performed to detect expression of factor XIIIa (a marker of dermal dendrocytes) and procollagen 1 (a precursor of collagen 1). There was expression of factor XIIIa in up to 50% of cells in the dermis, consistent with a proliferation of dermal dendrocytes. Procollagen 1 was expressed in the basement membrane area.
Toxicity
There was no transplant-related mortality and no grade 3 or 4 toxicity (Common Terminology Criteria for Adverse Events version 3.0, National Cancer Institute, National Institutes of Health, Bethesda, MD). The most common toxicity was neutropenic fever observed in 9 of the 10 patients. Three patients with upper GI dysmotility had radiologic evidence of pneumonia, suspected to be secondary to aspiration. One patient had grade 2 mucositis and transient rise in creatinine. One patient had transient indirect hyperbilirubinemia, and 1 patient had grade 2 diarrhea. The median time to neutrophil recovery higher than 0.5 × 109/L was 9 days. The median time for platelet recovery higher than 20 × 109/L and 100 × 109/L was 10 and 16 days, respectively.
Response
Resolution of the cutaneous lesions started during the pancytopenic phase and continued up to 6 months after transplantation. Five of the 7 treated patients had a complete resolution of the skin lesions. One patient had a near complete remission with a few residual papules in the neck and posterior auricular areas (Figure 1E-F). One patient had a partial remission in the skin with continued improvement 4 months after transplantation.
We observed a complete remission of all GI, CNS, and pulmonary manifestations. GI dysmotility, present in 6 patients, completely resolved, and gastrostomy tubes were removed. Two patients with pulmonary symptoms (nos. 1 and 6) noted improvement in pulmonary symptoms. Pulmonary function tests showed significant improvement in both (47% and 24% improved forced expiatory vital capacity [FEVC] and 27% improvement in diffusion capacity [DLCO] for both). One patient with scleromyxedema-associated seizures had successful withdrawal of anticonvulsant therapy. Contractures of the hands and peripheral neuropathy only partially improved. As shown in Table 2, 6 patients had a decrease in their paraprotein levels and one patient had no change (stable paraprotein level of 0.1 g/dL). Significant improvement in Karnofsky performance status (PS) was universal, and reached 80% or higher for all patients (100% for 4 patients, 90% for 2 patients with residual contractures, and 80% for 1 patient with residual peripheral neuropathy).
Clinical manifestations of scleromyxedema. (A-B) Patient 8: most severe facial papules (A) and sclerodactyly with joint contractures (B). (C) Patient 6: small waxy papules on the neck. (D) Patient 5: white ridges on neck. (E-F) Patient 4: before transplantation, with facial papules, glabellar thickening, and alopecia (E), and after transplantation (F), with resolution.
Clinical manifestations of scleromyxedema. (A-B) Patient 8: most severe facial papules (A) and sclerodactyly with joint contractures (B). (C) Patient 6: small waxy papules on the neck. (D) Patient 5: white ridges on neck. (E-F) Patient 4: before transplantation, with facial papules, glabellar thickening, and alopecia (E), and after transplantation (F), with resolution.
With a median follow-up of 823 days (range, 72-1133 days), 3 patients with the most extensive disease at the time of transplantation (2 patients requiring gastric-tube [G-tube] feeding) had evidence of limited mild disease progression in the skin at 12, 8, and 4 months. All 3 patients received a second PBSCT with a complete response in the skin; although, in 1 case the response was transient (3 months). This patient presently has stable and limited cutaneous disease with a performance status of 90% at 19 months after second PBSCT and is not requiring any therapy.
Histology of lesional skin biopsy. (A) Normal epidermis with a proliferation of fibroblasts (magnification, × 10). (B) Increased mucin deposition in the dermis (magnification, × 40). Images were obtained using an Olympus BX-40 microscope (Olympus, Melville, NY) and 10×/0.25 numeric aperture (NA) and 40×/0.65 NA objectives. Images were acquired using an Olympus DP10 camera and were processed using Adobe Photoshop 8.0 (Adobe Systems, San Jose, CA).
Histology of lesional skin biopsy. (A) Normal epidermis with a proliferation of fibroblasts (magnification, × 10). (B) Increased mucin deposition in the dermis (magnification, × 40). Images were obtained using an Olympus BX-40 microscope (Olympus, Melville, NY) and 10×/0.25 numeric aperture (NA) and 40×/0.65 NA objectives. Images were acquired using an Olympus DP10 camera and were processed using Adobe Photoshop 8.0 (Adobe Systems, San Jose, CA).
Discussion
Scleromyxedema and the family of lichen myxedematosus were first classified by Montgomery and Underwood in 1953.11 The disease was revised to include 3 categories by Rongioletti and Rebora in 2001 (Table 1).1 Since our patients presented with a paraprotein and skin manifestations of papular mucinosis and/or lichen myxedematosus and advanced to generalized scleromyxedema with systemic involvement, we propose that these mucin deposition entities form a disease spectrum of severity, related to the time in the course of their evolution. The monoclonal gammopathy seen is predominantly an IgG-lambda, usually less than 3 g/dL. IgG-kappa is more rarely seen. Possibly due to the low level of paraprotein, the latter may be detected intermittently and immunofixation should be performed in negative cases.12 The paraprotein has been shown to stimulate fibroblast production of mucin, suggesting a causal role and a rationale for lowering it.13,14
No established standard therapy exists for the systemic treatment of scleromyxedema. Steroids1 and low-dose melphalan are commonly used with mixed results and concerns about the risk of infection and secondary malignancy.4 In our patients, whose symptoms interfered with function and quality of life, high-dose melphalan followed by autologous stem cell transplantation led to complete resolution of most cutaneous and systemic GI and pulmonary symptoms with improvement in performance status. The impairment in performance was secondary to physical limitations due to skin involvement and/or extracutaneous disease. The progressive skin tightness from the papular mucinosis limited the ability of patients to open their mouths and caused joint contractures, sclerodactily, and carpal tunnel syndrome. Organ involvement leads to loss of swallowing due to upper GI dysmotility or severe pulmonary limitations. The extent of the cutaneous and extracutaneous organ involvement may be best evaluated by the patients' overall Karnofsky performance status.10 Four patients had slight progression of systemic symptoms and increased skin lesions, for which a second course of therapy was administered with subsequent sustained remissions at 46, 34, 16, and 9 months after treatment.
Thus, high-dose melphalan appears to be a safe and effective treatment, and we recommend this therapy for patients with stage II or III scleromyxedema (Table 3).
Proposed criteria for diagnosis and staging of scleromyxedema
Diagnosis |
| Histologic presence of dermal mucin deposit with increased collagen deposition and fibroblast proliferation and presence of a serum monoclonal gammopathy |
| Staging |
| Clinical stage I: limited cutaneous papular mucinosis |
| Clinical stage II: generalized cutaneous mucinosis and/or extracutaneous manifestation(s) |
| Clinical stage III: generalized cutaneous mucinosis and/or extracutaneous manifestation(s) and disease-related Karnofsky PS < 50% |
Diagnosis |
| Histologic presence of dermal mucin deposit with increased collagen deposition and fibroblast proliferation and presence of a serum monoclonal gammopathy |
| Staging |
| Clinical stage I: limited cutaneous papular mucinosis |
| Clinical stage II: generalized cutaneous mucinosis and/or extracutaneous manifestation(s) |
| Clinical stage III: generalized cutaneous mucinosis and/or extracutaneous manifestation(s) and disease-related Karnofsky PS < 50% |
Although there was no strict interpatient correlation between the extent of the disease and the paraprotein level, most patients had a modest decrease in the paraprotein levels following treatment. Pretransplantation pulse dexamethasone induction may benefit patients who are too debilitated to undergo transplantation, although the possibility of rapid disease progression upon steroid taper is a concern. The toxicity of high-dose single-agent melphalan with autologous stem cell transplantation has been well described, mostly in the context of multiple myeloma.15 The long-term risk of secondary myelodysplastic syndrome or leukemia in patients with multiple myeloma receiving low-dose melphalan has been reported to be 9.8%, with a median cumulative dose administered of 1440 mg.16 The risk of secondary leukemia following the administration of high-dose melphalan correlates mostly with the pretransplantation chemotherapy exposure.17 Given the rarity of scleromyxedema, a multicenter clinical trial to further assess this therapy should be initiated.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2004-12-4870.
Supported in part by K24-CA86815 (M.D.) and CA16672.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal