Abstract
Current prognostic factors for acute myeloblastic leukemia (AML) are not sufficient to accurately predict the group of patients in the intermediate-risk category who will successfully respond to treatment. Distinct patterns of inherited functional genomic polymorphisms might explain part of these heterogeneous prognoses. We used the allelic discrimination method to identify polymorphisms in GSTT1, SULT1C2, CDA, SXR (drug metabolic pathways), XPD, XPA, XPG, ERCC1, TOP2A (DNA repair), VEGF (angiogenesis), and MDR1 (multidrug resistance) genes in 110 adult patients with intermediate-risk AML, enrolled in the CETLAM-99 prospective trial. A multivariate prognostic model adjusted for age, white blood cell (WBC) count, French-American-British group, cytogenetics, MLL rearrangement, internal tandem duplication of FLT3 (FLT3-ITD), induction courses to achieve complete remission, and germline polymorphisms, was used to detect independent risk factors associated with clinical outcome. This analysis showed an increased risk of refractoriness to chemotherapy in the group of patients with XPA variant alleles (RR = 14; P = .02). In the same model, increased relapse risk was associated with SULT1C2 heterozygosity (RR = 4.1; P = .004), FLT3-ITD (RR 3.3; P = .003), and MDR1 variant alleles (RR = 2.4; P = .02). Adverse prognostic variables for overall survival were XPA (RR = 3.4; P = .02) and MDR1 (RR = 2.1; P = .02) variant alleles, and WBC count (RR = 2.1; P = .02). These findings might be useful in selecting risk-adapted treatment strategies in intermediate-risk AML.
Introduction
Acute myeloblastic leukemia (AML) is characterized by an increased number of immature myeloid cells in bone marrow, which results in hematopoietic insufficiency. Despite improvements in chemotherapy and supportive care measures, the death rate among patients younger than 65 years is still 60%.1 Patients with AML present diverse clinical features, genetic abnormalities, variable response to treatment, and heterogeneous prognosis.2 A number of clinical and biologic features are used to predict clinical outcome. Cytogenetics of blast cells is the most important biologic characteristic used to identify AML patients with a different response to treatment. There is a general agreement to subclassify AML into 3 broad prognostic subgroups based on cytogenetic findings: favorable, intermediate, and poor.1,2 However, it should be noted that there is some controversy regarding the prognostic significance of some cytogenetic abnormalities.3-6 Treatment choice is relatively well defined for cases in the favorable and poor-risk categories. However, for patients in the intermediate-risk category, accounting for over half the cases of this disease, the most appropriate treatment is controversial.2,7-10 As a reflection of this, treatment varies among centers with some advocating chemotherapy and others recommending autologous or even allogeneic stem-cell transplantation (allo-SCT).2,7-10 These facts make necessary a better prognostic stratification of patients deemed to have intermediate-risk AML by conventional criteria.
Molecular analyses for mutations in genes such as the fms-like tyrosine kinase 3 (FLT3), CCAAT/enhance binding protein alpha (CEBPA), mixed-lineage leukemia (MLL), brain and acute leukemia cytoplasmic (BAALC), and nucleophosmin (NPM) can distinguish groups of patients with intermediate-risk AML and different relapse risk.11,12 Whether these genetic markers define a clinically relevant subgroup in intermediate-risk AML remains uncertain.7 Recently, gene-expression profiling has been shown to be a promising tool for providing prognostic information in AML beyond that obtained with conventional methods,13,14 although this approach has financial and technical limitations. Moreover, gene-expression profiling detects the quantity of mRNA, but not qualitative differences of mRNA that may lead to a different functional role of the protein.15,16
Constitutive genetic characteristics of the patient may play an important role in the prognosis, yet this has scarcely been investigated in AML. In this regard, it is well recognized that most drugs exhibit wide interpatient variability in their efficacy and toxicity.17,18 For many drugs, such differences are due in part to polymorphisms in genes encoding drug-metabolizing enzymes (eg, CYPs, CDA, GSTT1, and SULT1C2), drug transport proteins (eg, MDR1, MRP2, and MRP3), and genes that recognize and repair DNA lesions14 (eg, XPA, XPG, and XPD). These polymorphisms represent common variations in a DNA sequence that may lead to either reduced or increased activity of the encoded gene.18 The most frequent type of polymorphisms is the so-called single nucleotide polymorphism (SNP), which accounts for over 90% of genetic variation in the human genome.19 In solid tumors and in acute lymphoblastic leukemia of children, it has been demonstrated that germline SNP has an impact on the effects of anticancer drugs.20-26 These aspects have not been fully investigated in adults with AML.27,28 The goal of this study was to identify among 110 adults with intermediate-risk AML SNPs in key genes influencing response to chemotherapy, to better stratify this poorly defined prognostic AML category. Candidate genes (summarized in Table 1) exhibited polymorphisms and encoded proteins that were involved in the pharmacokinetics or pharmacodynamics of antileukemic agents. Priority was given to polymorphisms associated with phenotypes in both clinical and preclinical studies and to those located in exons or in region UTR5′ or UTR3′, to those with a frequency higher than 10% in the general population, and to those validated. Most of the drugs used for treatment of patients with AML (eg, idarubicin, cytarabine, mitoxantrone, and etoposide) are substrates for cytochrome P450, which is activated by SXR, and are extruded by p-glycoprotein (MDR1). The parent drugs or metabolites of idarubicin and etoposide are substrates for glutathione-S-transferases (GSTT1 and SULT1C2). The mechanism of action of most of these drugs is by damaging DNA of leukemic cells or by inhibiting topoisomerase IIa (TOPIIa). DNA lesions are repaired by enzymes of nucleotide excision repair family (XPA, XPD, XPG, and ERCC1). Moreover, cytarabine, a key drug in the treatment of AML, is metabolized by cytidine deaminase (CDA).
Genes participating in drug metabolic pathways, multidrug resistance, and DNA repair included in the study
Gene . | Function . | Reference . |
|---|---|---|
| Drug metabolic pathway | ||
| CDA | The enzyme cytidine deaminase catalyzes the hydrolytic deamination of cytidine or deoxycytidine to uridine or deoxyuridine, respectively. | Salinas & Wong29 |
| SXR | The human nuclear pregnane X receptor (PXR) activates cytochrome P450-3A expression in response to a wide variety of xenobiotics and plays a critical role in mediating drug-drug interactions. | Chao et al30 |
| GSTT1 | Glutathione S-transferase (GST) θ 1 (GSTT1) is a member of a superfamily of proteins that catalyze the conjugation of reduced glutathione to a variety of electrophilic and hydrophobic compounds. | Davies et al31 |
| SULT1C2 | Sulfotransferase enzymes catalyze the sulfate conjugation of many hormones, neurotransmitters, drugs, and xenobiotic compounds. | Aplenc & Lange,24 Evans et al,25 Mcleod et al26 |
| Multidrug resistance | ||
| MDR1 | This protein is a member of the MDR/TAP subfamily. Members of the MDR/TAP subfamily are involved in multidrug resistance. It is responsible for decreased drug accumulation in multidrug-resistant cells and often mediates the development of resistance to anticancer drugs. | Illmer et al,28 Kim et al32 |
| Angiogenesis | ||
| VEGF | Vascular endothelial growth factor (VEGF), a major mediator of vascular permeability and angiogenesis, may play a pivotal role in mediating the development and progression of cancer. | Ribatti33 |
| DNA repair | ||
| XPD | The nucleotide excision repair pathway is a mechanism to repair damage to DNA. The protein encoded by this gene is involved in transcription-coupled nucleotide excision repair and is an integral member of the basal transcription factor BTF2/TFIIH complex. The gene product has ATP-dependent DNA helicase activity and belongs to the RAD3/XPD subfamily of helicases. | Efferth & Volm,19 Gurubhagavatula et al23 |
| XPA | It is involved in damage recognition after XPC/hHR23B. | Efferth & Volm,19 Bosken et al,34 Gu et al,35 Honecker et al36 |
| ERCC1 and ERCC5 | ERCC1 and ERCC5 are two structure specific endonucleases, incise on the 5′ and 3′ sites of the DNA adduct, respectively, to remove the 24-32 base oligonucleotides that contain the lesion. | Efferth & Volm,19 Bosken et al,34 Gu et al,35 Honecker et al36 |
| TOP2A | This nuclear enzyme is involved in processes such as chromosome condensation, chromatid separation, and the relief of torsional stress that occurs during DNA transcription and replication. | Lang et al37 |
Gene . | Function . | Reference . |
|---|---|---|
| Drug metabolic pathway | ||
| CDA | The enzyme cytidine deaminase catalyzes the hydrolytic deamination of cytidine or deoxycytidine to uridine or deoxyuridine, respectively. | Salinas & Wong29 |
| SXR | The human nuclear pregnane X receptor (PXR) activates cytochrome P450-3A expression in response to a wide variety of xenobiotics and plays a critical role in mediating drug-drug interactions. | Chao et al30 |
| GSTT1 | Glutathione S-transferase (GST) θ 1 (GSTT1) is a member of a superfamily of proteins that catalyze the conjugation of reduced glutathione to a variety of electrophilic and hydrophobic compounds. | Davies et al31 |
| SULT1C2 | Sulfotransferase enzymes catalyze the sulfate conjugation of many hormones, neurotransmitters, drugs, and xenobiotic compounds. | Aplenc & Lange,24 Evans et al,25 Mcleod et al26 |
| Multidrug resistance | ||
| MDR1 | This protein is a member of the MDR/TAP subfamily. Members of the MDR/TAP subfamily are involved in multidrug resistance. It is responsible for decreased drug accumulation in multidrug-resistant cells and often mediates the development of resistance to anticancer drugs. | Illmer et al,28 Kim et al32 |
| Angiogenesis | ||
| VEGF | Vascular endothelial growth factor (VEGF), a major mediator of vascular permeability and angiogenesis, may play a pivotal role in mediating the development and progression of cancer. | Ribatti33 |
| DNA repair | ||
| XPD | The nucleotide excision repair pathway is a mechanism to repair damage to DNA. The protein encoded by this gene is involved in transcription-coupled nucleotide excision repair and is an integral member of the basal transcription factor BTF2/TFIIH complex. The gene product has ATP-dependent DNA helicase activity and belongs to the RAD3/XPD subfamily of helicases. | Efferth & Volm,19 Gurubhagavatula et al23 |
| XPA | It is involved in damage recognition after XPC/hHR23B. | Efferth & Volm,19 Bosken et al,34 Gu et al,35 Honecker et al36 |
| ERCC1 and ERCC5 | ERCC1 and ERCC5 are two structure specific endonucleases, incise on the 5′ and 3′ sites of the DNA adduct, respectively, to remove the 24-32 base oligonucleotides that contain the lesion. | Efferth & Volm,19 Bosken et al,34 Gu et al,35 Honecker et al36 |
| TOP2A | This nuclear enzyme is involved in processes such as chromosome condensation, chromatid separation, and the relief of torsional stress that occurs during DNA transcription and replication. | Lang et al37 |
Patients, materials, and methods
Patient selection
Subjects included in this analysis were selected from a group of adult patients with primary AML who were enrolled and treated in 12 Spanish institutions on the prospective trial of the Grupo Cooperativo para el Estudio y Tratamiento de las Leucemias Agudas y Mielodisplasias (CET-LAM-99). This study was opened for accrual in November 1998 and was closed in July 2003. The corresponding institutional review boards approved the study and all patients signed an informed consent prior to entering the study. Inclusion criteria were as follows: (1) age between 18 and 60 years, (2) diagnosis of AML according to the French-American-British (FAB) classification, (3) no history of myelodysplasia or previous cytotoxic drugs or radiation, and (4) absence of severe concomitant disease. Patients with acute promyelocytic leukemia were excluded and treated in a separate trial. A total of 326 consecutive patients were registered. In short, induction chemotherapy consisted of idarubicin 12 mg/m2 intravenously (days 1, 3, 5), cytarabine 500 mg/m2/12 h intravenously (days 1, 3, 5, 7), and etoposide (VP-16) 100 mg/m2 intravenously (days 1, 2, 3); intensification included cytarabine 500 mg/m2/12 h intravenously (days 1-6) and mitoxantrone 12 mg/m2 intravenously (days 4-6). Subsequent treatment was as follows: patients showing the cytogenetic abnormality inversion 16, t(16;16), or t(8;21) received cytarabine 3 g/m2/12 h intravenously (days 1, 3, 5); those with a normal karyotype were allocated for autologous peripheral-blood transplantation. Finally, those patients with normal cytogenetics and requiring only one cycle of induction chemotherapy to achieve complete remission were assigned to receive an autologous peripheral-blood transplantation as postremission strategy. The remaining patients (ie, patients with cytogenetic abnormalities different from t(8;21) or inv(16), those without valuable mitoses, or those with normal karyotype AML requiring 2 cycles to achieve complete remission) were allocated to allo-SCT or autologous transplantation according to HLA-identical sibling donor availability. Of the 326 patients included in the study, 182 were in the intermediate-risk group, as defined in the Medical Research Council (MRC) AML 10 trial.5 Of these, 110 patients had, besides complete clinical, cytogenetic, and molecular (MLL, FLT3-ITD) data, DNA available for genotyping. The main characteristics of these 110 patients and of the 72 intermediate-risk patients not included in the analysis are listed in Table 2.
Characteristics of the patients and comparison with those included in the CETLAM-99 protocol and not evaluable for the study
. | Included in this study . | Not included . | P . |
|---|---|---|---|
| No. patients | 110 | 72 | |
| Age, median (range), y | 44 (16-60) | 45 (18-60) | .7 |
| Older than 50 y (%) | 38 (34) | 30 (42) | |
| Sex, no. (%) | |||
| Male | 66 (60) | 40 (56) | .6 |
| Female | 44 (40) | 32 (44) | |
| Leukocyte count, × 109/L, median (range) | 30 (0.8-410) | 14 (0.8-410) | .1 |
| FAB classification, no. (%) | 0.8 | ||
| M0 | 8 (7) | 2 (3) | |
| M1 | 25 (23) | 17 (23) | |
| M2 | 22 (20) | 15 (21) | |
| M4 | 15 (13) | 15 (21) | |
| M5 | 31 (28) | 16 (22) | |
| M6 | 2 (2) | 2 (3) | |
| AML and dysplasia | 4 (4) | 3 (4) | |
| Not classifiable | 3 (3) | 2 (3) | |
| Cytogenetics, no. (%) | .5 | ||
| Normal, at least 20 metaphases | 70 (64) | 46 (64) | |
| Karyotype complex, fewer than 5 abnormalities | 1 (1) | 1 (1) | |
| +8 | 5 (4) | 3 (4) | |
| 11q23 | 10 (9) | 2 (3) | |
| Other abnormalities | 24 (22) | 20 (28) | |
| FLT3, no. performed (%) | 106 | 34 | .8 |
| Germinal | 71 (67) | 24 (71) | |
| Internal tandem duplication | 35 (33) | 10 (29) | |
| FLT3-D835, no. performed (%) | 107 | 36 | .999 |
| Germinal | 99 (92) | 34 (94) | |
| Mutated | 8 (8) | 2 (6) | |
| MLL, no. (%) | 95/110 | 37/72 | .999 |
| Germinal | 79 (72) | 29 (85) | |
| Rearranged | 16 (28) | 5 (15) | |
| Induction response, no. (%) | .6 | ||
| Complete remission | 86 (78) | 53 (76) | |
| Refractoriness | 11 (10) | 10 (14) | |
| Death | 13 (12) | 7 (10) | |
| Courses induction to CR, no. (%) | .8 | ||
| 1 course | 71 (83) | 43 (81) | |
| 2 courses | 15 (17) | 10 (19) | |
| Stem-cell transplantation | .2 | ||
| Autologous | 43 (68) | 29 (64) | |
| Allo-TPH Allogeneic | 20 (32) | 16 (36) | |
| Overall survival at 2 y, % | 47 | 42 | .8 |
| Probability of relapse at 2 y, % | 53 | 57 | .2 |
. | Included in this study . | Not included . | P . |
|---|---|---|---|
| No. patients | 110 | 72 | |
| Age, median (range), y | 44 (16-60) | 45 (18-60) | .7 |
| Older than 50 y (%) | 38 (34) | 30 (42) | |
| Sex, no. (%) | |||
| Male | 66 (60) | 40 (56) | .6 |
| Female | 44 (40) | 32 (44) | |
| Leukocyte count, × 109/L, median (range) | 30 (0.8-410) | 14 (0.8-410) | .1 |
| FAB classification, no. (%) | 0.8 | ||
| M0 | 8 (7) | 2 (3) | |
| M1 | 25 (23) | 17 (23) | |
| M2 | 22 (20) | 15 (21) | |
| M4 | 15 (13) | 15 (21) | |
| M5 | 31 (28) | 16 (22) | |
| M6 | 2 (2) | 2 (3) | |
| AML and dysplasia | 4 (4) | 3 (4) | |
| Not classifiable | 3 (3) | 2 (3) | |
| Cytogenetics, no. (%) | .5 | ||
| Normal, at least 20 metaphases | 70 (64) | 46 (64) | |
| Karyotype complex, fewer than 5 abnormalities | 1 (1) | 1 (1) | |
| +8 | 5 (4) | 3 (4) | |
| 11q23 | 10 (9) | 2 (3) | |
| Other abnormalities | 24 (22) | 20 (28) | |
| FLT3, no. performed (%) | 106 | 34 | .8 |
| Germinal | 71 (67) | 24 (71) | |
| Internal tandem duplication | 35 (33) | 10 (29) | |
| FLT3-D835, no. performed (%) | 107 | 36 | .999 |
| Germinal | 99 (92) | 34 (94) | |
| Mutated | 8 (8) | 2 (6) | |
| MLL, no. (%) | 95/110 | 37/72 | .999 |
| Germinal | 79 (72) | 29 (85) | |
| Rearranged | 16 (28) | 5 (15) | |
| Induction response, no. (%) | .6 | ||
| Complete remission | 86 (78) | 53 (76) | |
| Refractoriness | 11 (10) | 10 (14) | |
| Death | 13 (12) | 7 (10) | |
| Courses induction to CR, no. (%) | .8 | ||
| 1 course | 71 (83) | 43 (81) | |
| 2 courses | 15 (17) | 10 (19) | |
| Stem-cell transplantation | .2 | ||
| Autologous | 43 (68) | 29 (64) | |
| Allo-TPH Allogeneic | 20 (32) | 16 (36) | |
| Overall survival at 2 y, % | 47 | 42 | .8 |
| Probability of relapse at 2 y, % | 53 | 57 | .2 |
CR indicates complete remission.
Response criteria
Complete remission was defined as the presence of less than 5% of blast cells in a bone marrow smear after the first or second course of induction therapy. Patients with less than a 50% reduction in marrow leukemia cells after the first course, and those who did not achieve a complete remission after 2 courses, were considered as refractory and withdrawn from the study. Disease-free survival was defined as the time from complete remission to relapse or death. Overall survival was calculated from diagnosis to death from any cause and risk of relapse was measured from complete remission to relapse, censoring deaths in complete remission.
Genotyping
DNA was extracted from patients' peripheral blood; primers and probes were obtained from Applied Biosystems (Foster City, CA) assays on demand-SNP genotyping product (www.appliedbiosystems.com). The characteristics of the probes used to detect the SNP, according to the supplier's information, are shown in Table 3. Probes were synthesized with reporter dye FAM or VIC covalently linked at the 5′ end and a quencher dye MGB linked to the 3′ end of the probe.
Genes and polymorphisms
Gene . | Allele* . | Characteristic* . | Context sequence . |
|---|---|---|---|
| Drug metabolic pathways | |||
| CDA | Lys27Gln | Missense change | CAGCTGCTGGTTTGCTCCCAGGAGGCCAAG [A/C] AGTCAGCCTACTGCCCCTACAGTCACT |
| SXR (1) | -1567T>C | UTR 5′ | AATCCAGTATTTCACTTACTCTTTT [C/T] CTTTCCAATATCCTCATGACATTCA |
| SXR (2) | -35C>T | UTR 5′ | AATAAGCTAATACTCCTGTCCTGAA [A/C] AAGGCAGCGGCTCCTTGGTAAAGCT |
| GSTT1 | Ex5-182C>T | UTR 3′ | TGCTCTGGGACTTGGGCAAGTCTTA [G/A] GCAAGCCATTCCTGCTTTCTGGGCC |
| SULT1C2 | Asp5Glu | Missense change | TTCTTACACTAATGGCCTTACACGA [C/G] ATGGAGGATTTTACATTTGATGGAA |
| Multidrug resistance | |||
| MDR1 (1) | Ex29-193C>T | UTR3′ | AAAATTTATAATGCAGTTTAAACTA [C/T] GATTTCTCTCCACTTGATGATGTCT |
| MDR1 (2) | Ilet 144Met | Missense change | TGTTGGCCTCCTTTGCTGCCCTCAC [A/G] ATCTCTTCCTGTGACACCACCCGGC |
| Angiogenesis | |||
| VEGF (1) | Ex7-913A>G | UTR 3′ | CACCTGCTTCTGAGTTGCCCAGGAG [A/G] CCACTGGCAGATGTCCCGGCGAAGA |
| VEGF (2) | -634G>C | UTR 5′ | CGCGCGGGCGTGCGAGCAGCGAAAG [C/G] GACAGGGGCAAAGTGAGTGACCTGC |
| DNA repair | |||
| XPD | Lys751Gln | Nonsense change | GCAGCTAGAATCAGAGGAGACGCTG [C/A] AGAGGATAGAGCAGATTGCTCAGCA |
| XPA | -4A>G | UTR 5′ | GGGAGCTAGGTCCTCGGAGTGGGCC [G/A] GAGATGGCGGCGGCCGACGGGGCTT |
| ERCC1 | Lys259Thr | Missense change | AAGAAGAGGAAGAAGCCCAAAGGGA [A/C] AGAAACCTTCGAGCCAGAAGACAAG |
| ERCC5 | His46His | Silent change | CACTTAAAGGAGTCCGGGATCGCCA [C/T] GGGAACTCAATAGAAAATCCTCATC |
| TOP2A | Ex39-531 G>A | Intron/UTR 3′ | TAGACACAGCCAAAGTGTTTTTCTT [C/T] GGCCTCTGATGATTTGAGAAGATGA |
Gene . | Allele* . | Characteristic* . | Context sequence . |
|---|---|---|---|
| Drug metabolic pathways | |||
| CDA | Lys27Gln | Missense change | CAGCTGCTGGTTTGCTCCCAGGAGGCCAAG [A/C] AGTCAGCCTACTGCCCCTACAGTCACT |
| SXR (1) | -1567T>C | UTR 5′ | AATCCAGTATTTCACTTACTCTTTT [C/T] CTTTCCAATATCCTCATGACATTCA |
| SXR (2) | -35C>T | UTR 5′ | AATAAGCTAATACTCCTGTCCTGAA [A/C] AAGGCAGCGGCTCCTTGGTAAAGCT |
| GSTT1 | Ex5-182C>T | UTR 3′ | TGCTCTGGGACTTGGGCAAGTCTTA [G/A] GCAAGCCATTCCTGCTTTCTGGGCC |
| SULT1C2 | Asp5Glu | Missense change | TTCTTACACTAATGGCCTTACACGA [C/G] ATGGAGGATTTTACATTTGATGGAA |
| Multidrug resistance | |||
| MDR1 (1) | Ex29-193C>T | UTR3′ | AAAATTTATAATGCAGTTTAAACTA [C/T] GATTTCTCTCCACTTGATGATGTCT |
| MDR1 (2) | Ilet 144Met | Missense change | TGTTGGCCTCCTTTGCTGCCCTCAC [A/G] ATCTCTTCCTGTGACACCACCCGGC |
| Angiogenesis | |||
| VEGF (1) | Ex7-913A>G | UTR 3′ | CACCTGCTTCTGAGTTGCCCAGGAG [A/G] CCACTGGCAGATGTCCCGGCGAAGA |
| VEGF (2) | -634G>C | UTR 5′ | CGCGCGGGCGTGCGAGCAGCGAAAG [C/G] GACAGGGGCAAAGTGAGTGACCTGC |
| DNA repair | |||
| XPD | Lys751Gln | Nonsense change | GCAGCTAGAATCAGAGGAGACGCTG [C/A] AGAGGATAGAGCAGATTGCTCAGCA |
| XPA | -4A>G | UTR 5′ | GGGAGCTAGGTCCTCGGAGTGGGCC [G/A] GAGATGGCGGCGGCCGACGGGGCTT |
| ERCC1 | Lys259Thr | Missense change | AAGAAGAGGAAGAAGCCCAAAGGGA [A/C] AGAAACCTTCGAGCCAGAAGACAAG |
| ERCC5 | His46His | Silent change | CACTTAAAGGAGTCCGGGATCGCCA [C/T] GGGAACTCAATAGAAAATCCTCATC |
| TOP2A | Ex39-531 G>A | Intron/UTR 3′ | TAGACACAGCCAAAGTGTTTTTCTT [C/T] GGCCTCTGATGATTTGAGAAGATGA |
NCIB/Celera.
PCR allelic discrimination
SNP analysis was performed using a real-time polymerase chain reaction (PCR) allelic discrimination TaqMan assay (Applied Biosystems) with minor modifications. All PCRs were run in duplicate and contained 100 ng patient DNA, 12.5 μL TaqMan Universal Master Mix (Applied Biosystems), 1.2 μL primers and probes, and water for a final volume of 25 μL. Appropriate negative controls were also run. Real-time PCR was performed on an ABI Prism 7500 Sequence Detection System (Applied Biosystems) using the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of amplification (95°C for 15 seconds and 60°C for 1 minute). For each cycle, the software determined the fluorescent signal from the VIC- or FAM-labeled probe. Serial dilution of a specific SNP-DNA into water revealed a detection limit between 0.1% and 0.01%.
RNA extraction and cDNA synthesis
Blood samples for RNA expression analysis were collected in a K3/EDTA-containing tube. After standard erythrocyte lyses, RNA was isolated using RNeasy Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. The concentration, purity, and amount of total RNA were determined by UV spectrophotometry, and the integrity and quality of RNA were assessed by electrophoresis on 1% agarose gel. RNA from samples was quantified in triplicate after 1:50 dilution in water using a GeneQuant spectrophotometer (Pharmacia, Uppsala, Sweden). cDNA was synthesized using TaqMan Reverse Transcription Reagents Kit (Applied Biosystems). Reverse transcription (RT) was performed using 300 ng total RNA in 10 μL TaqMan RT buffer, 22 μL of 25 mM magnesium chloride, 20 μL dNTPs, 5 μL random hexamers, 2 μL RNase inhibitor, 2.5 μL MultiScribe RT, and RNA sample plus RNase-free water, for a final volume of 100 μL, in the following thermal cycler conditions: 10 minutes 25°C, 48 minutes 30°C, and 5 minutes 95°C.
Primers and probes for mRNA expression
Applied Biosystems supplied primers and probes, with the assistance of the assay-on-demand gene expression system (www.appliedbiosystems.com/myscience). Probes were labeled at the 5′ end with the reporter dye molecule FAM. According to the supplier, the primers and probes were designed for the following regions: XPA: CTGCGGCTACTGGAGGCATGGCTAA; MDR1(ABCD1): GCAGGTACCATACAGAAACTCTTTG; VEGF: CACCATGCCAAGTGGTCCCAGGCTG; SULT1C2: TACCTATCCTAAAGCAGGAACAACA.
GUSb gene probe, labeled at the 5′ end with the reporter dye molecule FAM (Applied Biosystems), was used as housekeeping gene.
Gene expression and quantification with RT-QPCR
RT-quantitative PCR (RT-QPCR) was performed in a total volume of 20 μL in the ABI Prism 7700 Sequence Detection System (Applied Biosystems). All samples for each gene were run in duplicate for 40 cycles using the following master mix: 10 μL TaqMan universal PCR master mix, 1 μL of the primers and probes, 3 μL cDNA, and 6 μL RNase-free water. Thermal cycler conditions were: 2 minutes 50°C, 10 minutes 95°C, 15 seconds 95°C, and 1 minute 60°C. gDNA was used as negative control in each run. Fluorescent emission data were captured, and mRNA concentrations were quantified by using the critical threshold value and the curve standard method and quantitative normalization of cDNA in each sample was performed using the expression of total RNA concentration as an internal control.38
Statistical analysis
Descriptive statistical analysis of the main characteristics of the patients was performed. Differences between groups were measured by Fisher exact test or the Kruskal-Wallis test. Survival probabilities were estimated using the Kaplan-Meier method. Survival curves were compared by the log-rank test, and the Cox proportional hazards model was used to estimate the risk ratio of events after controlling for prognostic variables. We considered the following characteristics: age (< 50 years versus > 50), white blood cell (WBC) count (< 20 × 109/L versus > 20 × 109/L), FAB group, MLL rearrangement, FLT3-ITD, number of induction courses to achieve complete remission, cytogenetics (normal versus others), and germline polymorphisms. All prognostic variables in the univariate analysis with a P value at or below .2 were included in the multivariate analysis, to eliminate the redundancy among highly correlated characteristics, each of which may be individually significant. For resistant disease the variables were: age, WBC count, and polymorphisms of XPA and ERCC1; for relapse: age, WBC count, cytogenetics, FLT3-ITD, number of induction courses to achieve complete remission, and polymorphisms of XPD, MDR1, TOP2A, SULT1C2, and VEGF; for overall survival: age, WBC count, cytogenetics, FLT3-ITD, number of induction courses to achieve complete remission, and polymorphisms of XPD, XPA, and MDR1. This multivariate analysis was performed using the stepwise proportional hazard Cox regression model. The proportional hazard assumption of the Cox model was analyzed separately for each covariate, before performing the regression analysis, by a graphical and analytic method. The graphs of loge [–loge S (t)] versus loge (t) for each dichotomous covariate were obtained to test whether the curves were roughly parallel. Additionally, for each covariate a time-dependent covariate [covariate*log (t)] was obtained and checked to determine whether the coefficient of the latter significantly differed from 0. Multivariate analysis was performed by means of SPSS 9.0.1 (1999, SPSS, Chicago, IL) statistical software.
Results
Patients, overall response to treatment, and follow-up characteristics
A total of 110 patients, consisting of 66 men (60%) and 44 women (40%) with a median age of 44 years (range, 16-60 years), were evaluable for response. Of these, 86 (78%) achieved a complete remission, 11 (10%) were chemoresistant, and 13 (12%) died during induction. Actuarial probability of relapse was 58% (95% CI, 44%-72%). After a median follow-up of the whole series of 12 months (range, 3-64 months), overall survival was 47% (95% CI, 37%-57%) at 2 years. Characteristics of this group of patients did not differ from those patients (n = 72) with intermediate-risk AML included in the CETLAM-99 study and not included in this analysis (Table 2).
Allele frequencies
Genotype frequencies in the group of 110 patients were comparable to those found in a healthy white population (Table 4). No associations were found between genotype and age, sex, or other prognostic variables (data not shown).
Allele frequencies in the group of 110 patients and in a healthy white population
. | Patients . | . | . | White population* . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genes . | A, % . | B, % . | C, % . | A, % . | B, % . | C, % . | ||||
| MDR1 (1) | 73 | 26 | 1 | 74 | 24.04 | 1.96 | ||||
| MDR1 (2) | 31 | 50 | 19 | 41.4 | 45.9 | 12.6 | ||||
| TOP2A | 62 | 32 | 6 | 52.5 | 39.9 | 7.5 | ||||
| SXR (1) | 43 | 44 | 13 | 36 | 48 | 16 | ||||
| SXR (2) | 42 | 44 | 14 | 47.8 | 42.7 | 9.5 | ||||
| GSTT1 | 87 | 7 | 6 | 53.6 | 39.2 | 7.2 | ||||
| SULT1C2 | 50 | 39 | 11 | 65.3 | 31 | 3.7 | ||||
| VEGF (1) | 19 | 61 | 20 | 38.8 | 47 | 14.2 | ||||
| VEGF (2) | 46 | 40 | 14 | 49 | 42 | 9 | ||||
| XPD | 44 | 46 | 10 | 34.8 | 48.4 | 16.8 | ||||
| XPA | 48.3 | 40.5 | 11.1 | 38.8 | 47 | 14.2 | ||||
| ERCC1 | 72 | 25 | 3 | 59.4 | 35.4 | 5.2 | ||||
| ERCC5 | 32 | 45 | 23 | 29.2 | 49.7 | 21.2 | ||||
| CDA | 49.5 | 39.4 | 11.1 | — | — | — | ||||
. | Patients . | . | . | White population* . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genes . | A, % . | B, % . | C, % . | A, % . | B, % . | C, % . | ||||
| MDR1 (1) | 73 | 26 | 1 | 74 | 24.04 | 1.96 | ||||
| MDR1 (2) | 31 | 50 | 19 | 41.4 | 45.9 | 12.6 | ||||
| TOP2A | 62 | 32 | 6 | 52.5 | 39.9 | 7.5 | ||||
| SXR (1) | 43 | 44 | 13 | 36 | 48 | 16 | ||||
| SXR (2) | 42 | 44 | 14 | 47.8 | 42.7 | 9.5 | ||||
| GSTT1 | 87 | 7 | 6 | 53.6 | 39.2 | 7.2 | ||||
| SULT1C2 | 50 | 39 | 11 | 65.3 | 31 | 3.7 | ||||
| VEGF (1) | 19 | 61 | 20 | 38.8 | 47 | 14.2 | ||||
| VEGF (2) | 46 | 40 | 14 | 49 | 42 | 9 | ||||
| XPD | 44 | 46 | 10 | 34.8 | 48.4 | 16.8 | ||||
| XPA | 48.3 | 40.5 | 11.1 | 38.8 | 47 | 14.2 | ||||
| ERCC1 | 72 | 25 | 3 | 59.4 | 35.4 | 5.2 | ||||
| ERCC5 | 32 | 45 | 23 | 29.2 | 49.7 | 21.2 | ||||
| CDA | 49.5 | 39.4 | 11.1 | — | — | — | ||||
A indicates homozygous wild type; B, heterozygous; C, homozygous for SNP; and —, not available.
NCBI/Celera.
Association between genetic polymorphisms and induction failure
The variant UTR 5′ genotype –4A>G of the DNA repair gene XPA was associated with chemoresistant disease. Individuals with the wild-type (WT) genotype (AA) of XPA had a probability of resistant disease of 6.5%; those with heterozygous genotype (AG) of 11%, and patients with the homozygous variant genotype (GG) had a probability of refractoriness to chemotherapy of 30%. Fisher exact test (2-tailed) of GG cases versus the rest of the patients was marginally significant (P = .07). In the multivariate model, XPA GG genotype was the only independent factor increasing the risk of resistant disease (RR = 14; P = .02; Tables 5, 6). Fifty percent of patients with XPA GG genotype were either chemoresistant or died during the induction, as compared to 22% for the rest of the patients (P = .053). No single factor was found to be associated with induction mortality.
Univariate analysis of association of polymorphisms with outcome
. | . | Chemoresistance, Fisher . | . | . | . | Overall survival, log-rank . | . | . | . | Relapse rate, log-rank . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | No. . | % . | P1 . | P2 . | P3 . | % . | P1 . | P2 . | P3 . | % . | P1 . | P2 . | P3 . | |||||||||
| XPD | .5 | .4 | .3 | .2 | .07 | .6 | .2 | .08 | .8 | |||||||||||||
| WT (Lys/Lys) | 44 | 7 | 28 | 51 | ||||||||||||||||||
| Heterozygous (Lys/Gln) | 51 | 12 | 28 | 66 | ||||||||||||||||||
| Homozygous SNP (Gln/Gln) | 13 | 15 | 36 | 49 | ||||||||||||||||||
| ERCC1 | .03 | — | — | .9 | — | — | .6 | — | — | |||||||||||||
| WT (Lys/Lys) | 78 | 14 | 26 | 63 | ||||||||||||||||||
| 1 or 2 variant alleles (Lys/Thr-Ths/Thr) | 31 | 0 | 31 | 51 | ||||||||||||||||||
| XPA | .09 | .7 | .06 | .1 | .4 | .1 | .7 | .6 | .6 | |||||||||||||
| WT (AA) | 46 | 6.5 | 49 | 45 | ||||||||||||||||||
| Heterozygous (AG) | 36 | 11 | 29 | 66 | ||||||||||||||||||
| Homozygous SNP (GG) | 10 | 30 | 0 | 34 | ||||||||||||||||||
| MRD1 (1) | .7 | — | — | .1 | — | — | .02 | — | — | |||||||||||||
| WT (TT) | 75 | 10 | 37 | 45 | ||||||||||||||||||
| 1 or 2 variant alleles (CT-TT) | 34 | 12 | 14 | 84 | ||||||||||||||||||
| TOP2a | .7 | .5 | .4 | .6 | .6 | .4 | .2 | .2 | .2 | |||||||||||||
| WT (GG) | 62 | 8 | 28 | 65 | ||||||||||||||||||
| Heterozygous (GA) | 40 | 12 | 25 | 48 | ||||||||||||||||||
| Homozygous SNP (AA) | 7 | 14 | 28 | 0 | ||||||||||||||||||
| SULT1C2 | .7 | .5 | .9 | .8 | .6 | .8 | .2 | .07 | .9 | |||||||||||||
| WT (CC) | 57 | 9 | 16 | 68 | ||||||||||||||||||
| Heterozygous (CG) | 39 | 13 | 47 | 34 | ||||||||||||||||||
| Homozygous SNP (GG) | 1 | 8 | 0 | 74 | ||||||||||||||||||
| VEGF2 | .6 | .7 | .6 | .9 | .9 | .8 | .02 | .2 | .06 | |||||||||||||
| WT (GG) | 49 | 8 | 32 | 62 | ||||||||||||||||||
| Heterozygous (GC) | 47 | 11 | 21 | 41 | ||||||||||||||||||
| Homozygous SNP (CC) | 12 | 17 | 31 | 86 | ||||||||||||||||||
| ERCC5 | .3 | .7 | .4 | .7 | .4 | .6 | .5 | .3 | .3 | |||||||||||||
| WT | 39 | 10 | 14 | 67 | ||||||||||||||||||
| Heterozygous | 47 | 7 | 30 | 56 | ||||||||||||||||||
| Homozygous SNP | 17 | 17 | 50 | 51 | ||||||||||||||||||
| CDA | .4 | .3 | .3 | .6 | .3 | .6 | .8 | .6 | .7 | |||||||||||||
| WT | 50 | 6 | 25 | 59 | ||||||||||||||||||
| Heterozygous | 44 | 14 | 37 | 57 | ||||||||||||||||||
| Homozygous SNP | 13 | 15 | 39 | 54 | ||||||||||||||||||
| SXR1 | .7 | .9 | .6 | .9 | .9 | .8 | .7 | .4 | .7 | |||||||||||||
| WT | 43 | 12 | 29 | 59 | ||||||||||||||||||
| Heterozygous | 46 | 11 | 31 | 52 | ||||||||||||||||||
| Homozygous SNP | 18 | 6 | 58 | 57 | ||||||||||||||||||
| SXR2 | .3 | .9 | .3 | .9 | .9 | .9 | .4 | .8 | .8 | |||||||||||||
| WT | 42 | 12 | 34 | 58 | ||||||||||||||||||
| Heterozygous | 49 | 12 | 29 | 51 | ||||||||||||||||||
| Homozygous SNP | 18 | 0 | 37 | 75 | ||||||||||||||||||
| GSTT1 | .2 | .1 | .4 | .9 | .7 | .8 | .9 | .8 | .8 | |||||||||||||
| WT | 70 | 9 | 29 | 62 | ||||||||||||||||||
| Heterozygous | 7 | 28 | 53 | 60 | ||||||||||||||||||
| Homozygous SNP | 8 | 17 | 41 | 63 | ||||||||||||||||||
. | . | Chemoresistance, Fisher . | . | . | . | Overall survival, log-rank . | . | . | . | Relapse rate, log-rank . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable . | No. . | % . | P1 . | P2 . | P3 . | % . | P1 . | P2 . | P3 . | % . | P1 . | P2 . | P3 . | |||||||||
| XPD | .5 | .4 | .3 | .2 | .07 | .6 | .2 | .08 | .8 | |||||||||||||
| WT (Lys/Lys) | 44 | 7 | 28 | 51 | ||||||||||||||||||
| Heterozygous (Lys/Gln) | 51 | 12 | 28 | 66 | ||||||||||||||||||
| Homozygous SNP (Gln/Gln) | 13 | 15 | 36 | 49 | ||||||||||||||||||
| ERCC1 | .03 | — | — | .9 | — | — | .6 | — | — | |||||||||||||
| WT (Lys/Lys) | 78 | 14 | 26 | 63 | ||||||||||||||||||
| 1 or 2 variant alleles (Lys/Thr-Ths/Thr) | 31 | 0 | 31 | 51 | ||||||||||||||||||
| XPA | .09 | .7 | .06 | .1 | .4 | .1 | .7 | .6 | .6 | |||||||||||||
| WT (AA) | 46 | 6.5 | 49 | 45 | ||||||||||||||||||
| Heterozygous (AG) | 36 | 11 | 29 | 66 | ||||||||||||||||||
| Homozygous SNP (GG) | 10 | 30 | 0 | 34 | ||||||||||||||||||
| MRD1 (1) | .7 | — | — | .1 | — | — | .02 | — | — | |||||||||||||
| WT (TT) | 75 | 10 | 37 | 45 | ||||||||||||||||||
| 1 or 2 variant alleles (CT-TT) | 34 | 12 | 14 | 84 | ||||||||||||||||||
| TOP2a | .7 | .5 | .4 | .6 | .6 | .4 | .2 | .2 | .2 | |||||||||||||
| WT (GG) | 62 | 8 | 28 | 65 | ||||||||||||||||||
| Heterozygous (GA) | 40 | 12 | 25 | 48 | ||||||||||||||||||
| Homozygous SNP (AA) | 7 | 14 | 28 | 0 | ||||||||||||||||||
| SULT1C2 | .7 | .5 | .9 | .8 | .6 | .8 | .2 | .07 | .9 | |||||||||||||
| WT (CC) | 57 | 9 | 16 | 68 | ||||||||||||||||||
| Heterozygous (CG) | 39 | 13 | 47 | 34 | ||||||||||||||||||
| Homozygous SNP (GG) | 1 | 8 | 0 | 74 | ||||||||||||||||||
| VEGF2 | .6 | .7 | .6 | .9 | .9 | .8 | .02 | .2 | .06 | |||||||||||||
| WT (GG) | 49 | 8 | 32 | 62 | ||||||||||||||||||
| Heterozygous (GC) | 47 | 11 | 21 | 41 | ||||||||||||||||||
| Homozygous SNP (CC) | 12 | 17 | 31 | 86 | ||||||||||||||||||
| ERCC5 | .3 | .7 | .4 | .7 | .4 | .6 | .5 | .3 | .3 | |||||||||||||
| WT | 39 | 10 | 14 | 67 | ||||||||||||||||||
| Heterozygous | 47 | 7 | 30 | 56 | ||||||||||||||||||
| Homozygous SNP | 17 | 17 | 50 | 51 | ||||||||||||||||||
| CDA | .4 | .3 | .3 | .6 | .3 | .6 | .8 | .6 | .7 | |||||||||||||
| WT | 50 | 6 | 25 | 59 | ||||||||||||||||||
| Heterozygous | 44 | 14 | 37 | 57 | ||||||||||||||||||
| Homozygous SNP | 13 | 15 | 39 | 54 | ||||||||||||||||||
| SXR1 | .7 | .9 | .6 | .9 | .9 | .8 | .7 | .4 | .7 | |||||||||||||
| WT | 43 | 12 | 29 | 59 | ||||||||||||||||||
| Heterozygous | 46 | 11 | 31 | 52 | ||||||||||||||||||
| Homozygous SNP | 18 | 6 | 58 | 57 | ||||||||||||||||||
| SXR2 | .3 | .9 | .3 | .9 | .9 | .9 | .4 | .8 | .8 | |||||||||||||
| WT | 42 | 12 | 34 | 58 | ||||||||||||||||||
| Heterozygous | 49 | 12 | 29 | 51 | ||||||||||||||||||
| Homozygous SNP | 18 | 0 | 37 | 75 | ||||||||||||||||||
| GSTT1 | .2 | .1 | .4 | .9 | .7 | .8 | .9 | .8 | .8 | |||||||||||||
| WT | 70 | 9 | 29 | 62 | ||||||||||||||||||
| Heterozygous | 7 | 28 | 53 | 60 | ||||||||||||||||||
| Homozygous SNP | 8 | 17 | 41 | 63 | ||||||||||||||||||
P1 is a multigroup comparison.
P2 is a comparison between WT and heterozygous groups.
P3 is a comparison between WT and homozygous groups.
Multivariate analysis of association of clinical and biologic characteristics with outcome
Variable . | Genotype . | P . | RR (95% CI) . |
|---|---|---|---|
| Patients with intermediate-risk AML* | |||
| Chemoresistance | |||
| XPA | GG | .02 | 14 (2-23) |
| Overall survival | |||
| XPA | GG | .02 | 3.4 (2-9.4) |
| WBC count greater than 20,000 | — | .02 | 2.1 (1.1-4.1) |
| MDR1 (1) | TC/CC | .02 | 2.1 (1.1-4.1) |
| Relapse | |||
| SULT1C | CC/GG | 0.004 | 4.1 (1.6-10.7) |
| FLT3-ITD | — | 0.003 | 3.3 (1.5-7.3) |
| MDR1 (1) | TC/CC | 0.02 | 2.4 (1.1-5.4) |
| Patients with normal cytogenetics† | |||
| Chemoresistance | |||
| XPA | GG | .01 | 66 (2.3-193) |
| Overall survival | |||
| XPA | GG | .02 | 4 (1.1-13) |
| WBC count greater than 20,000 | — | < .001 | 2.6 (1.2-5.4) |
Variable . | Genotype . | P . | RR (95% CI) . |
|---|---|---|---|
| Patients with intermediate-risk AML* | |||
| Chemoresistance | |||
| XPA | GG | .02 | 14 (2-23) |
| Overall survival | |||
| XPA | GG | .02 | 3.4 (2-9.4) |
| WBC count greater than 20,000 | — | .02 | 2.1 (1.1-4.1) |
| MDR1 (1) | TC/CC | .02 | 2.1 (1.1-4.1) |
| Relapse | |||
| SULT1C | CC/GG | 0.004 | 4.1 (1.6-10.7) |
| FLT3-ITD | — | 0.003 | 3.3 (1.5-7.3) |
| MDR1 (1) | TC/CC | 0.02 | 2.4 (1.1-5.4) |
| Patients with normal cytogenetics† | |||
| Chemoresistance | |||
| XPA | GG | .01 | 66 (2.3-193) |
| Overall survival | |||
| XPA | GG | .02 | 4 (1.1-13) |
| WBC count greater than 20,000 | — | < .001 | 2.6 (1.2-5.4) |
— indicates not applicable.
n = 110.
n = 70.
Association between genetic polymorphisms and relapse rate
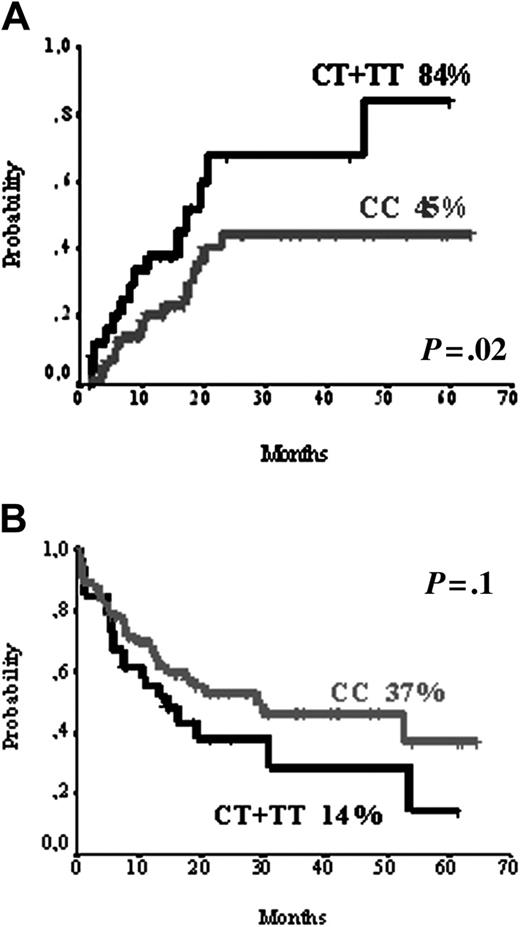
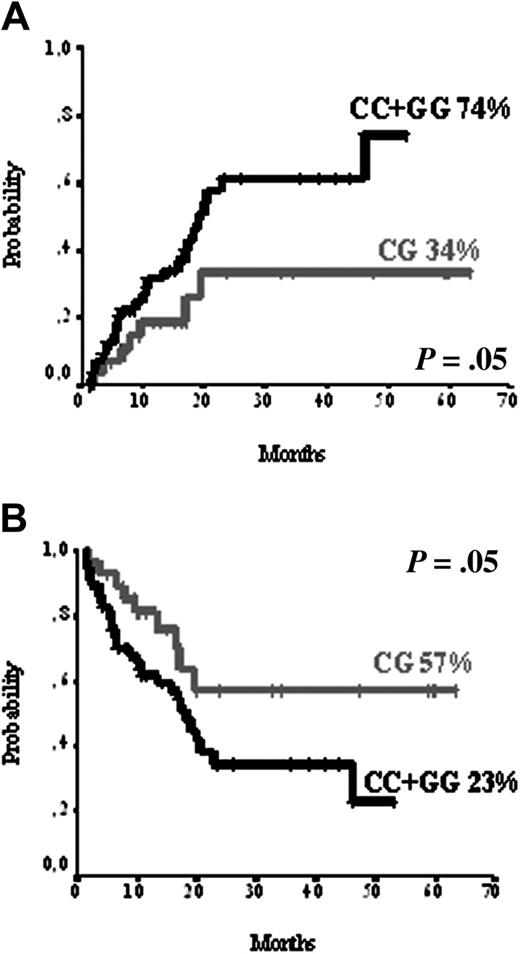
Variant form in UTR 3′ genotype Ex29-193C>T of MDR1 gene was significantly associated with relapse rate. Actuarial probability of relapse was 45% for individuals WT (TT), and 84% for individuals with the variant genotype (CT or CC) by log-rank test (P = .02). In the multivariate analysis only molecular factors were found to be predictive for relapse: SULT1C2 homozygosity (CC or GG; RR 4.1; P = .004), FLT3-ITD (RR 3.3; P = .003), and variant forms of MDR1 (CT or CC; RR 2.4; P = .02) as shown in Figures 1, 2 and Tables 5, 6.
Association between genetic polymorphisms and overall survival
Consistent with the association of XPA –4A>G variant alleles and chemoresistance, overall survival for patients with AA, AG, and GG alleles of XPA gene was 49%, 29%, and 0%, respectively (log-rank test, P = .1). We determined the influence of XPA gene polymorphism on overall survival with a Cox regression model. The most important factor influencing survival was XPA GG genotype (RR 3.4; P = .02). Other factors associated with overall survival in the multivariate analysis were WBC counts and variant alleles of MDR1 gene (Figures 1, 2; Tables 5, 6).
MDR1 polymorphism. (A) MDR1 polymorphism and relapse rate. (B) MDR1 polymorphism and overall survival.
MDR1 polymorphism. (A) MDR1 polymorphism and relapse rate. (B) MDR1 polymorphism and overall survival.
SULT1C2 polymorphism. (A) SULT1C2 polymorphism and relapse rate. (B) SULT1C2 polymorphism and disease-free survival.
SULT1C2 polymorphism. (A) SULT1C2 polymorphism and relapse rate. (B) SULT1C2 polymorphism and disease-free survival.
Association between genetic polymorphisms and clinical outcome in the group of patients with normal cytogenetics
When we restricted the univariate analysis to the group of patients with normal cytogenetics (n = 70), we found identical prognostic factors as those identified in the whole series of 110 patients (Table 5). In the multivariate analysis for overall survival, WBC (RR 2.6; P < .001) and homozygous XPA (RR 4; P = .02) were independent prognostic factors, whereas in the multivariate analysis for chemoresistance only homozygous XPA (RR 66; P = .01) was found to have a statistical significance (Table 6).
Relationship of gene polymorphisms and cDNA expression
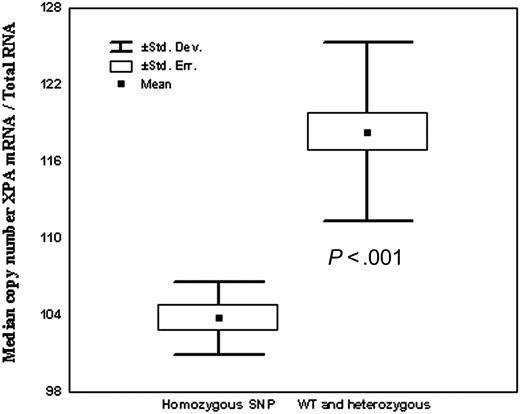
We analyzed the relationship of gene polymorphisms and cDNA expression of the 3 genes (XPA, MDR, and SULT1C2) found to be associated with clinical outcome. In one of these, XPA, polymorphism was located in the promoter area. We hypothesized that the SNP in XPA modified mRNA expression of the gene. For this reason, XPA UTR 5′ –4A>G polymorphism and cDNA expression were assessed in 37 healthy volunteer blood donors from the geographic area of Barcelona. Of them, 15 (35%) had a WT genotype, 14 (50%) were heterozygous, and 6 (15%) were homozygous for the variant genotype XPA (–4A>G). We found a close association between XPA alleles and mRNA XPA expression. Thus, individuals with XPA GG genotype demonstrated much lower XPA expression levels as compared with AA and AG genotypes (P < .001; Figure 3). Polymorphisms of MDR1 and of SULT1C2 are located at 3′UTR and in the exonic area, respectively. As expected, there was no association of SNP in these locations with mRNA expression (data not shown).
Relationship of XPA UTR5′ (–4) polymorphism and XPA cDNA expression.
Discussion
We investigated for an association of polymorphisms of 11 genes with clinical outcome in 110 patients with intermediate-risk AML. Polymorphisms from 4 genes showed no association with outcome (SXR, GSTT1, ERCC5, and CDA), whereas polymorphisms of 7 genes (Table 5) showed in the univariate analysis an association with induction failure (ERCC1 and XPA), relapse (XPD, MRD1, TOP2A, SULT1C2, and VEGF), and overall survival (XPD, XPA, and MRD1). Polymorphisms of 3 genes were independent prognostic factors in the multivariate analysis: XPA for chemoresistance and overall survival, MDR1 for relapse and overall survival, and SULT1C2 for relapse (Table 6).
One of the most striking findings in this work was the association of polymorphisms in the DNA repair gene XPA with the clinical outcome of adult patients with intermediate-risk AML. XPA gene forms part of the nucleotide excision repair (NER) system, which is the only human system that protects against the carcinogenic effects of sunlight by removing UV light–induced DNA adducts. Along with light-induced lesions, the NER system recognizes and excises a broad spectrum of bulky lesions, including those induced by chemotherapy.39-41 Because DNA repair enzymes are correctives for DNA damage induced by anticancer agents, it was hypothesized that polymorphisms in DNA repair genes might influence treatment outcome of neoplasm.23 We have observed, for the first time, an association of XPA genotype and outcome of therapy for AML, in which individuals with the genotype XPA –4A>G GG had an inferior outcome, largely caused by an increase in chemoresistant cases. Moreover, findings in this study suggest an allele dosage effect of XPA A>G with respect to outcome. Thus, the proportion of individuals with resistant disease was 6.5% for XPA AA, 11% for those AG, and 30% for patients with the GG genotype. Consistent with this, actuarial probability of overall survival at 5 years was 50% for XPA AA, 29% for AG, and 0% for GG. In the multivariate analysis, adjusted for the most important clinical and biologic characteristics, XPA GG genotype was the most important factor associated with chemoresistance and with overall survival. This association of XPA GG genotype and clinical outcome might be due to changes in the functional capacity of the protein to repair DNA.40 In this sense, it is of note that XPA –4A>G polymorphism is located in the promoter area, which might affect its translation efficiency. Indeed, we found a strong correlation between XPA –4A>G polymorphism and quantity of mRNA expression, healthy controls with XPA GG genotype having the lowest XPA mRNA expression (Figure 2). This is an intriguing finding because it has been considered that a higher, and not the opposite, efficient DNA repair system might lead to a lower efficacy of chemotherapy.34 However, recent data seem to support that reduced DNA repair capacity might also lead to resistance to chemotherapy, by interrupting cell signals to undergo apoptosis. Thus, chemoresistance in solid tumors has been associated with enhanced expression of XPA in some34 but not in all studies,35,36 and it has been recently reported that reduced mismatch repair efficiency may cause resistance to cytotoxic drugs, by an inability to detect DNA damage, thus preventing cells from undergoing DNA damage–induced apoptosis.42-46 Moreover, in hematologic malignancies, Allan et al have recently reported that a polymorphism in XPD that protects against cell death is associated with poorer prognosis in patients with AML.27 Further support of the notion that DNA repair and apoptosis are functionally linked comes from the experiment in which p53-dependent apoptosis is compromised in fibroblasts from patients with XPD variant alleles, a phenotype that can be restored by expression of XPD wild type.47 Alternatively, the association of XPA variants and poorer outcome might be explained by the fact that inefficient DNA repair leads to additional instability and more aggressive tumors.48-52
In the present study, the relationship between polymorphisms in MDR1, the most studied gene concerning protection of host cells from toxics, and relapse rate and overall survival in intermediate-risk AML is also of note. We have observed that polymorphisms in UTR3′ of the MDR1 gene (Ex29-193C>T) were associated with increased relapse rate (45% for WT and 84% for individuals with the variant genotype) and poorer overall survival (37% for WT individuals and 14% for individuals with the variant genotype). Similar to the results herein presented, Illmer et al reported that MDR1 gene polymorphisms in exons 12, 21, and 26 affected therapy outcome in patients with AML.28 In that study, those polymorphisms associated with poor survival had the lowest median of MDR1 mRNA expression. A significant correlation of polymorphisms in MDR with both increased levels and function of the protein has also been observed.32,53 We did not perform immunophenotypic analysis to exclude a higher MDR protein expression on blast cells, but we found no correlation between alleles and MDR expression at mRNA level. The influence of MDR1 protein in the response to chemotherapy is widely known, because MDR1 mutation is frequently acquired in many tumor types following cytotoxic chemotherapy and confers chemoresistance through gene amplification.24 The results herein presented, and those of Illmer et al,28 support that the role of MDR1 in response to treatment of AML is not exclusively due to acquired mutations, but also to constitutional polymorphisms.
Another constitutive genetic finding that was associated with response to the treatment in our study was the homozygosity of SULT1C2 for the polymorphism Asp5Glu. SULT1C2 is a glutathione sulfur transferase (GST), an enzyme that adds sulfur molecules to a wide range of acceptor molecules29 and that plays an important role in determining the cytotoxicity of chemotherapeutic drugs, including anthracyclines,30 a key drug in the treatment of patients with AML. Thus, recent reports support the idea that reduced GST activity is associated with toxic deaths,31 whereas increased GST activity is associated with a higher incidence of relapse.54 We found no effect of SULT1C2 Asp5Glu genotype on overall survival but did demonstrate a higher relapse rate (Figure 2A) and reduced disease-free survival (Figure 2B) in patients with SULT1C2 homozygous (CC and GG) genotype, suggesting a more efficient activity of this genotype. We tried to find an explanation for the relationship between SULTIC2 heterozygosis and reduced probability of relapse. It has been shown that heterozygosis may lead to a different functional capacity of the encoded protein and to a different mRNA expression.28 The first possibility cannot be ruled out because we did not perform functional studies. With respect to the second, we found no association of SULT1C2 genotype and mRNA expression.
Finally, although the precise functional mechanism of the effect of polymorphisms of XPA, MDR1, and SULT1C2 in AML therapy remains to be determined, the findings presented here strongly support the importance of considering these genomic polymorphisms as prognostic markers in patients with intermediate-risk AML.
Appendix
The centers and investigators contributing to the CETLAM study were as follows: Hospital Clínic, Barcelona (Jordi Esteve, Mireia Camós, María Rozman, Neus Villamor, Dolors Costa); Hospital Del Mar, Barcelona (Carmen Pedro, Lourdes Florensa, Francesc Soler); Hospital de la Santa Creu i Sant Pau, Barcelona (Salut Brunet, Josep Fr. Nomdédeu, Anna Aventín, Granada Perea, Jorge Sierra); Hospital Vall d'Hebrón, Barcelona (Javier Bueno, Carmen sanchez, Teresa Vallespí); Clínica Tecknon, Barcelona (Pilar Vivancos); Institut Català d'Oncologia (ICO)/Hospital Germans Trias i Pujol, Badalona (Josep M Ribera, Isabel Granada, Albert Oriol); ICO/Duran i Reynals, Hospitalet de LLobregat (Joan Berlanga, David Gallardo, Alicia Domingo); Hospital Juan Canalejo, A Coruña (Pio Torres); Hospital Clínico, Malaga (M. Paz Queipo De Llano); Hospital Clínico Universitario, Valencia (Mar Tormo); Hospital Josep Trueta, Girona (Ramon Guárdia); Hospital Son Dureta, Palma (Joan Besalduch, Marta Barnués); Hospital Son Llatzer, Palma (Joan Bargay); Hospital Joan XXIII, Tarragona (Andreu Llorente, Lourdes Escoda); Hospital Arnau de Vilanova, Lleida (Juan M. Sanchez, Montserrat Teixidó); Hospital Verge de la Cinta, Tortosa (Xavier Ortin); Hospital Mutua Terrassa, Terrassa (Josep M. Martí-Tutusaus, Cristina Estany).
Prepublished online as Blood First Edition Paper, February 28, 2006; DOI 10.1182/blood-2005-08-3272.
Acomplete list of the members of the Grupo Cooperativo para el Estudio y Tratamiento de las Leucemias Agudas y Mielodisplasias (CETLAM) appears in the “Appendix.”
Supported by RTICCC03/10 and G03/008 from Instituto de Salud Carlos III (ISCIII); JS02-04 and BM05-219-0 from Fundació La Caixa; Fondo investigaciones sanitarias de la seguridad social (FIS) 02/0509 and FISS 02/350; and Fundació Escola d'Hematologia Farreras Valentí.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal