Abstract
The complement system is an essential element in our innate defense against infections with Neisseria meningitidis. We describe 2 cases of meningococcal septic shock, 1 of them fatal, in 2 children of a Turkish family. In the surviving patient, alternative pathway activation was absent and factor D plasma concentrations were undetectable. Concentrations of mannose-binding lectin (MBL), C1q, C4 and C3, factor B, properdin, factor H, and factor I were normal. Mutation analysis of the factor D gene revealed a T638 > G (Val213 > Gly) and a T640 > C (Cys214 > Arg) mutation in the genomic DNA from the patient, both in homozygous form. The consanguineous parents and an unaffected sister had these mutations in heterozygous form. In vitro incubation of factor-D–deficient plasma of the boy with serogroup B N meningitidis showed normal MBL-mediated complement activation but no formation of the alternative pathway C3-convertase C3bBbP, and severely decreased C3bc formation and terminal complement activation. The defect was restored after supplementation with factor D. In conclusion, this is the second report of a factor D gene mutation leading to factor D deficiency in a family with meningococcal disease. This deficiency abolishes alternative-pathway dependent complement activation by N meningitidis, and leads to an increased susceptibility to invasive meningococcal disease.
Introduction
As stated in 1969 by Goldschneider et al1,2 and confirmed by large scale vaccination campaigns at the start of this century,3,4 specific antibodies offer complete protection against invasive meningococcal disease. Without antibodies, the primary humoral defense against Neisseria meningitidis invasion relies solely on the complement system.
Complement activation contributes to the clearance of meningococci by C3b and the membrane attack complex (MAC). C3b(i), fixed to meningococci, opsonizes the bacterium. In addition, C3b-coated bacterial particles are shuttled to and cleared by the reticuloendothelial system in liver and spleen by binding to complement receptor type 1 (CR1; CD35) on erythrocytes.5,6 MAC induces bacteriolysis through insertion in the meningococcal outermembrane of a macromolecular complex composed of the terminal complement components C5b, C6, C7, C8, and 1 or more C9 molecules, which become activated in a relay race fashion after adherence of C3b. Deficiency of 1 of the terminal complement factors leads to inadequate MAC formation and a 1000-fold increased risk for meningococcal infections.7,8 Interestingly, most of these infections are caused by relatively rare serogroups and run a mild course.9-11 In cases of insufficient proximal complement activation, not only MAC formation but also C3b-mediated clearance is missing. In these cases, the defense against meningococci is more seriously affected and a more severe course may be expected. However, extensive complement activation in meningococcal infections also has deleterious effects to the host by the generation of excess amounts of anaphylatoxin C3a and C5a,12 which are known to induce the generation of reactive oxygen species by phagocytes,13 up-regulate production of proinflammatory cytokines,14 increase disseminated intravascular coagulation,15 and aggravate cardiodepression.16
Initial complement activation, aimed to activate C3 and to bind C3b(i) to a microbial or cellular surface, can be started by the classical pathway, the mannose-binding lectin (MBL) pathway, or the alternative pathway. Engagement of the classical pathway requires specific antibodies or C-reactive protein (CRP) attached to the bacterium for binding and activation of C1qrs. In the very early stage of meningococcal disease, specific antibodies and CRP are still absent.17,18 Therefore, only the lectin and the alternative pathway will play a role at this stage. The relative contribution of these pathways is still a subject of study.19,20 As we recently showed in vitro, maximal complement activation by meningococci requires involvement of both pathways,13 with an initiating role for the lectin pathway and an amplifying role for the alternative pathway.21
Clinically, MBL deficiency is associated with a higher risk for invasive meningococcal disease although it runs a less severe course in MBL-deficient patients.22-24 Deficiency of factor B, the primary alternative pathway initiator, has never been found. However, deficiency of properdin (P), the protein that stabilizes the alternative pathway C3-convertase C3bBb, is associated with a high risk for severe disease.25-27 In 2001, we reported a factor D gene mutation leading to complete deficiency of factor D, in a Dutch family with 2 cases of meningococcal disease.28 Combined with the earlier observation of Hiemstra et al,29 this finding suggests that factor D deficiency also predisposes to invasive meningococcal disease.
In the present study, we describe the clinical course of meningococcal disease in 2 children of a Turkish family with a novel factor D gene mutation leading to undetectable factor D plasma concentrations. The family had a normal genetic makeup for MBL. Therefore, we were able to explore, by additional in vitro experiments, the relative importance of the lectin and the alternative pathway in complement activation by N meningitidis.
Patients, materials, and methods
Patient A
A 9-month-old girl was admitted in 1996 to a local hospital because of purpura for 2 hours and 45 minutes. She had a history of high fever for 6 days, coughing, diarrhea, and vomiting. At admission the temperature was 40°C, she was tachypneic, and had signs of a poor peripheral circulation and moderate nuchal rigidity. The pulse rate was 220 beats per minute and blood pressure was 141/80 mm Hg. Laboratory examination at admission showed: CRP level, 53 mg/L; leukocyte count, 3.9 × 109/L; platelet count, 234 × 109/L; creatinine, 78 μM; arterial pH, 7.19; PCO2, 4.2 mM; bicarbonate, 11 mM; and base excess, –15.2 mM. Blood cultures were negative. Cerebrospinal fluid (CSF) examination revealed only mild signs of inflammation: leukocytes, 20 × 106/L; glucose, 3.8 mM; and protein, 280 mg/L. CSF cultures grew N meningitidis serogroup B:P1:15. The chest x-ray showed an infiltrate in the right upper quadrant. Shortly after lumbar puncture, generalized seizures occurred and treatment was started with phenobarbital, ampicillin, dexamethasone, bicarbonate, and paracetamol. Unfortunately, the severity of disease was not sufficiently recognized and the patient was admitted to a regular pediatric ward.
After 1.5 hours, blood pressure fell to 79/52 mm Hg. In spite of 200 mL/kg fluid resuscitation during the first 24 hours, blood pressure remained low and complete renal failure and thrombopenia (38 × 109/L) developed. No inotropics, ventilatory support, or fresh plasma were given. Thirty-six hours after admission, blood pressure decreased suddenly to 49/29 mm Hg followed by respiratory arrest. Cardiopulmonary resuscitation was needed and transfer to our Pediatric Intensive Care Unit (PICU) was requested. At arrival, 36 hours after hospital admission, the patient had poorly perfused extremities and was persistently hypotensive in spite of high dosages of inotropic and vasopressive medication. The electroencephalogram was compatible with postresuscitation cerebral damage. The girl died 11 hours later because of refractory shock. Autopsy was not allowed.
Patient B
Four years later, the 13-month-old brother of patient A was admitted to the same local hospital because of sudden onset of fever and a petechial rash. At the age of 4 months he had been hospitalized for a respiratory syncytial virus infection complicated by bacterial superinfection. Later, he suffered several times from recurrent upper or lower respiratory tract infections that required frequent antibiotic treatment. Now, at admission, he was covered with small purpuric lesions, had decreased capillary refill, and no signs of meningismus. His body temperature was 39.7°C, pulse rate 180 beats per minute and a respiration rate of 36 breaths per minute. Laboratory results showed: CRP level, 119 mg/L; leukocyte count, 10.9 × 109/L; platelet count, 324 × 109/L; creatinine, 52 μM, activated partial thromboplastin time (APTT), 46 seconds (normal, 25-35 seconds); and plasma D-dimers, 1.35 mg/L (normal, < 0.5 mg/L). The chest X-ray was normal. Blood cultures grew N meningitidis B.1.P1-4.
Directly after admission, treatment was started with ceftriaxone, dexamethasone, and fluid challenges (2 × 23 mL/kg 0.9% NaCl). After consultation with our PICU staff by telephone, 4 hours later, dobutamine (5 μg/kg/min) and 15 mL/kg fresh-frozen plasma (FFP) was given. In the first 18 hours, the situation remained stable. Laboratory analysis after 18 hours showed creatinine at 45 μM, a leukocyte count of 40.6 × 109/L, a normal platelet count, and signs of compensated disseminated intravascular coagulation (APTT, 41 seconds; fibrinogen, 7.9 g/L; and D-dimers, 4.47 mg/L). During the following day he received another 2 × 15 mL FFP. Recovery was uneventful. Two weeks after admission he was discharged in good condition.
Because the boy was the second patient with severe meningococcal disease in the same family and his parents appeared to be consanguineous, further immunologic analysis was performed. The concentrations of immunoglobulin classes and subclasses were normal, as were the antibodies against polysaccharide capsule of Streptococcus pneumoniae serogroup 3, 4, and 9 and Haemophilus influenza type B. Analysis of the complement pathway showed normal concentrations of MBL, C1q, C4, and C3, but a complete absence of alternative pathway activation in the alternative pathway hemolytic activity 50 (AP50) test (AP50 < 5%; normal, 75%-125%). Factor B level was 84 IU/mL (normal, 49-129 IU/L). Factor H level was 0.31 g/L (normal, 0.2-0.6 mg/L). Properdin as well as factor I, determined qualitatively, were present. However, factor D was undetectable at less than 0.03 mg/L (normal, 1.0-2.0 mg/L). Because of this deficiency the boy was put on antibiotic prophylaxis (1 × 100 mg clarythromycin daily) and was vaccinated with the conjugated N meningitidis serogroup C vaccine and the unconjugated serogroup A plus C vaccine. With informed consent of his parents, the patient's factor D gene defect was analyzed and additional in vitro complement activation tests were performed.
Informed consent statement
Studies were conducted in accordance with the local guidelines for human experiments (Commissie Mensgebonden Onderzoek regio Arnhem-Nijmegen, Radboud University Nijmegen Medical Centre). Informed consent (according to the Declaration of Helsinki) was obtained from the patient, his mother, and the healthy controls prior to performing the experiments.
Factor D protein determination and factor D gene sequencing
The activity and the protein concentration of factor D were measured as described previously.28 The factor D gene polymerase chain reaction (PCR) was performed with primers annealing to intron sequences close to each exon (Table 1). The PCR conditions and the sequence reactions were as described by Hiemstra et al.29
Sequence of genomic PCR primers used in this study
. | Primer . |
|---|---|
| Complement factor D | |
| CFD-ex1-fw | 5′-GAGTCTGGCAGGAGGTAACCCAGTC-3′ |
| CFD-ex1-rev | 5′-GCGTTCAGAGCCTTCCATTAGTGAG-3′ |
| CFD-ex2-fw | 5′-GAGAGCTGGGATCCCGTCAGGCAGC-3′ |
| CFD-ex2-rev | 5′-GAGTCCGCGGTCGGTGCCAGCCGACTC-3′ |
| CFD-ex3-fw | 5′-GAGTCGGCTGGCACCGACCGCGGACTC-3′ |
| CFD-ex3-rev | 5′-CATGCAGCAGGAGAGGTCGAGGCTGG-3′ |
| CFD-ex4-fw | 5′-CTCCCCGAGCCTAGCGGCATTCTCC-3′ |
| CFD-ex4-rev | 5′-TCATGCTCCGCCCATCTTCCAGTTC-3′ |
| CFD-ex5-fw | 5′-ACTAGTGAAGACCAAATTAACACGG-3′ |
| CFD-ex5-rev | 5′-GCAGGAGTGGATGACTTCATTGCTCG-3′ |
| MBL | |
| MBL-L-fw | 5′-TTAGCACTCTGCCAGGGCCAACG-3′ |
| MBL-L-rev | 5′-CCCATCTTTGTATCTGGGCAGCTGA-3′ |
| MBL-X-fw | 5′-TCTTTGGATCACCAAAAGCTTTCAGCTC-3′ |
| MBL-X-rev | 5′-GAGGGGTTCATCTGTGCCTAGAC-3′ |
| MBL-ABCD-fw | 5′-AGTTTTCTCACACCAAGGTG-3′ |
| MBL-ABCD-rev | 5′-ATCCCCAGGCAGTTTCCTCTGGAAG-3′ |
. | Primer . |
|---|---|
| Complement factor D | |
| CFD-ex1-fw | 5′-GAGTCTGGCAGGAGGTAACCCAGTC-3′ |
| CFD-ex1-rev | 5′-GCGTTCAGAGCCTTCCATTAGTGAG-3′ |
| CFD-ex2-fw | 5′-GAGAGCTGGGATCCCGTCAGGCAGC-3′ |
| CFD-ex2-rev | 5′-GAGTCCGCGGTCGGTGCCAGCCGACTC-3′ |
| CFD-ex3-fw | 5′-GAGTCGGCTGGCACCGACCGCGGACTC-3′ |
| CFD-ex3-rev | 5′-CATGCAGCAGGAGAGGTCGAGGCTGG-3′ |
| CFD-ex4-fw | 5′-CTCCCCGAGCCTAGCGGCATTCTCC-3′ |
| CFD-ex4-rev | 5′-TCATGCTCCGCCCATCTTCCAGTTC-3′ |
| CFD-ex5-fw | 5′-ACTAGTGAAGACCAAATTAACACGG-3′ |
| CFD-ex5-rev | 5′-GCAGGAGTGGATGACTTCATTGCTCG-3′ |
| MBL | |
| MBL-L-fw | 5′-TTAGCACTCTGCCAGGGCCAACG-3′ |
| MBL-L-rev | 5′-CCCATCTTTGTATCTGGGCAGCTGA-3′ |
| MBL-X-fw | 5′-TCTTTGGATCACCAAAAGCTTTCAGCTC-3′ |
| MBL-X-rev | 5′-GAGGGGTTCATCTGTGCCTAGAC-3′ |
| MBL-ABCD-fw | 5′-AGTTTTCTCACACCAAGGTG-3′ |
| MBL-ABCD-rev | 5′-ATCCCCAGGCAGTTTCCTCTGGAAG-3′ |
MBL protein determination and gene analysis
MBL protein was determined in a solid-phase enzyme-linked immunosorbent assay (ELISA) with mannan coated to a 96-well plate and detection with monoclonal antibody (mAb) αMBL-1 (biotinylated mouse anti–human MBL, immunoglobulin G1 [IgG1], 10 μg/mL; Sanquin, Amsterdam, the Netherlands). Briefly, microtiter plates were coated with 100 μg/mL mannan in 0.1 M NaHCO3 (pH 9.6) overnight at room temperature. The microtiter plates were washed 5 times with H2O. Plasma or serum samples and MBL standards (standard serum, 1.5 μg/mL MBL) were diluted in TTG/Ca2+ (20 mM Tris [pH 7.4]/150 mM NaCl/0.02% Tween-20/0.2% gelatin/10 mM CaCl2), with 10 U/mL heparin, added to the plates, and incubated by shaking at room temperature for 1 hour. After washing, the plates were incubated for 1 hour with biotinylated αMBL-1 in TTG/Ca2+, washed with H2O, and incubated by shaking at room temperature for 30 minutes with streptavidin poly–horseradish peroxidase (HRP) at a ratio of 1:10 000 in TBS/Ca2+/2% milk (20 mM Tris [pH 7.4]/150 mM NaCl/10 mM CaCl2/2% milk). After washing, the color was developed with tetramethyl-3,3′,5,5′-benzidine (TMB)/0.01% H2O2 in 0.1 M Na acetate (pH 5.5), stopped with 2 M H2SO4, and measured spectrophotometrically at 405 nm (BioAssay Reader Sunrise; Tecan, Salzburg, Austria).
MBL gene analysis for mutations in exon 1 and polymorphisms in the promoter region was performed by sequencing of PCR products obtained with primers described in Table 1. The PCR and sequencing conditions were as for factor D.
Complement activation experiments
Lepirudin (Refludan; Pharmion, Tiel, the Netherlands)–anticoagulated (50 μg/mL final concentration) blood from patient B, his mother, and 4 healthy volunteers was drawn in polyproplylene tubes on ice (2-4 years after presentation with meningococcal sepsis) and centrifuged immediately. The plasma was stored at –80°C until further experiments. Complement activation was tested by adding heat-killed serogroup B N meningitidis H44/76. This strain is an isolate of a patient with invasive disease and an international reference strain, serologically classified as B:15:P1.7,16, immunotype L3,7,9.30
For the complement activation experiments, 300 μL plasma was incubated in polypropylene 96-well plates (NUNC, Roskilde, Denmark) with 75 μL phosphate-buffered saline (PBS) or N meningitidis (final concentration, 1 × 108/mL) suspended in PBS (75 μL) and purified human factor D at 75 μL to a final concentration of 2 mg/L (QUIDEL, San Diego, CA) or murine mAb against human factor D (clone 166-32) at 75 μL in a final concentration of 25 μg/mL (kindly provided by M. Fung, Tanox, Houston, TX).31 Sample preparation was always on ice, and incubation was for 1 hour at 37°C. To stop complement activation after 1 hour, the plates were put on ice and 10 mM EDTA was added. Thereafter, samples were stored at –80°C until complement analysis. Complement factor 1 rs (C1rs)–C1-inhibitor (C1rs-C1inh) complexes, C4bc, C3bBbP, C3bc, and soluble terminal complement complex (TCC) was assayed by ELISA as described previously in detail.13,32
For comparison, plasma of 2 healthy adult controls was incubated in all experiments in the same run.
Results
Factor D and MBL values in the patient, his parents, and his sister
Patient B had an undetectable level of factor D protein in his blood; his mother, father, and unaffected sister had strongly decreased levels of factor D (Table 2). The circulating MBL protein levels of these individuals were also measured to exclude increased susceptibility for Neisseria infection due to MBL deficiency. All members of this family had normal MBL levels, although the father of the patient was heterozygous for an Arg52 > Cys mutation (allele D) (Table 2).
Results of family investigations
. | Factor D protein, mg/L* . | Factor D gene mutation . | MBL protein, mg/L† . | MBL gene‡ . |
|---|---|---|---|---|
| Patient B | < 0.03 | Homozygous | 2.6 | HYPA/HYPA |
| Mother | 0.44 | Heterozygous | 1.3 | HYPA/HYPA |
| Father | 0.14 | Heterozygous | 1.4 | HYPD/HYPA |
| Sister | 0.34 | Heterozygous | 2.6 | HYPA/HYPA |
. | Factor D protein, mg/L* . | Factor D gene mutation . | MBL protein, mg/L† . | MBL gene‡ . |
|---|---|---|---|---|
| Patient B | < 0.03 | Homozygous | 2.6 | HYPA/HYPA |
| Mother | 0.44 | Heterozygous | 1.3 | HYPA/HYPA |
| Father | 0.14 | Heterozygous | 1.4 | HYPD/HYPA |
| Sister | 0.34 | Heterozygous | 2.6 | HYPA/HYPA |
Normal range, 1.0-2.0 mg/L.
Normal range, > 0.8 mg/L.
Normal, HYPA/HYPA.
Genetic analysis of the factor D gene in the patient and his family
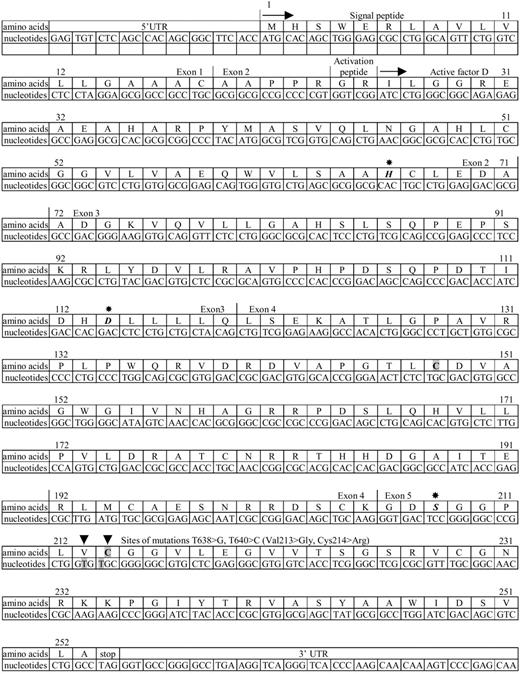
Mutation analysis of the factor D gene revealed a T638 > G (Val213 > Gly) and a T640 > C (Cys214 > Arg) mutation in the genomic DNA from the patient, both in apparently homozygous form. Both parents and the unaffected sister had these mutations in a heterozygous form. These mutations are in conserved residues, close to Ser208 in the catalytic center of the enzyme (Figure 1). Moreover, the Cys214 > Arg mutation destroys an internal disulfide bond between 2 conserved cysteines.34
N meningitidis–induced complement activation
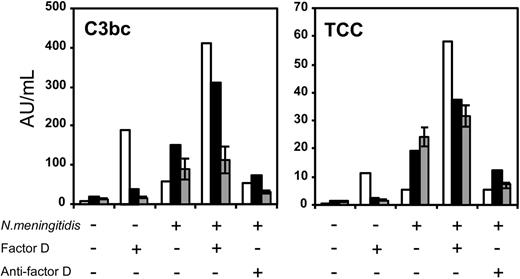
Upon stimulation with 108/mL N meningitidis, factor D–deficient plasma of the patient produced clearly less C3bc and TCC compared with healthy individuals (see Figure 2). Plasma of the heterozygous mother had a C3bc and TCC production approximately similar to that of the healthy controls. Addition of a monoclonal antibody against factor D inhibited complement activation in the mother and the controls, but had no effect on complement activation in the patient. Thus, factor D is important for the activation of C3 as well as the activation of the terminal complement cascade.
Addition of purified human factor D alone in the absence of other stimuli had no effect on complement activation in plasma of the controls and in plasma of the mother. However, some C3bc and TCC were induced in the factor D–deficient plasma of the patient. Factor D supplementation to plasma stimulated with meningococci restored C3bc and TCC activation in the patient's plasma and that of his mother, for the patient even to values higher than that seen in controls.
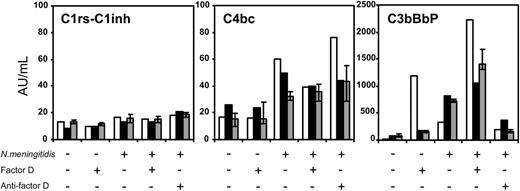
Next, we assayed complement intermediates indicative for initial pathway complement activation. As can be seen in Figure 3, meningococci did not induce C1rs-C1inh complexes in amounts exceeding that of the baseline. This shows that no classical pathway activation occurred.
However, C4bc, reflecting lectin pathway activation (because classical pathway activation was absent), was induced after stimulation with meningococci in the patient and his mother, possibly to a higher extent than in the controls. As expected, addition of purified human factor D or anti–factor D had no effect on C4bc formation.
Finally, after stimulation with meningococci, C3bBbP reflecting alternative pathway activation was increased in the mother and the controls but not in the factor D–deficient patient. Addition of purified factor D to the patient's plasma, not stimulated or stimulated with meningococci, increased the production of C3bBbP in the patient to much higher values than in the mother or the controls. Anti–factor D with meningococcal stimulation abolished C3bBbP in the mother and the controls. Taken together, these results indicate that meningococci can induce complement activation via the lectin and alternative pathways, and that complement activation by meningococci is reduced in factor D deficiency, owing to a defect in the alternative pathway.
Amino-acid and cDNA nucleotide sequence of human complement factor D. The sequence data are from White et al33 and Biesma et al.28 5′UTR indicates 5′untranslated region; first arrow (→), start of protein synthesis; second arrow, start of circulating protein sequence; asterisks, positions of catalytic triad Asp114, His66, and Ser208; arrowheads, positions of mutations; and 3′UTR, part of 3′untranslated region. A disulfide bond exists in the wild-type factor D between Cys148 and Cys214, which is indicated in bold on a gray background (gray boxes).
Amino-acid and cDNA nucleotide sequence of human complement factor D. The sequence data are from White et al33 and Biesma et al.28 5′UTR indicates 5′untranslated region; first arrow (→), start of protein synthesis; second arrow, start of circulating protein sequence; asterisks, positions of catalytic triad Asp114, His66, and Ser208; arrowheads, positions of mutations; and 3′UTR, part of 3′untranslated region. A disulfide bond exists in the wild-type factor D between Cys148 and Cys214, which is indicated in bold on a gray background (gray boxes).
Proximal and terminal complement pathway activation byN meningitidisin plasma from the patient and his mother. The formation of C3bc and sTCC, reflecting proximal complement activation and terminal pathway activation, respectively, in plasma of the homozygous factor D-deficient patient B (□), his heterozygous mother (▪), and the median ± interquartile range (IQR) of 4 healthy controls (▦). The plasma was incubated for 1 hour at 37°C without stimulus or with 108/mL meningococci in the absence or presence of recombinant factor D or anti–factor D, as indicated. The experiment was repeated once, yielding virtually identical results (not shown).
Proximal and terminal complement pathway activation byN meningitidisin plasma from the patient and his mother. The formation of C3bc and sTCC, reflecting proximal complement activation and terminal pathway activation, respectively, in plasma of the homozygous factor D-deficient patient B (□), his heterozygous mother (▪), and the median ± interquartile range (IQR) of 4 healthy controls (▦). The plasma was incubated for 1 hour at 37°C without stimulus or with 108/mL meningococci in the absence or presence of recombinant factor D or anti–factor D, as indicated. The experiment was repeated once, yielding virtually identical results (not shown).
Discussion
In the present study, we report 2 patients, one of them with a fatal course, with invasive meningococcal serogroup B disease in a family of consanguineous parents. The surviving child, a boy of 13 months, had no alternative pathway complement activation as measured by the AP50 assay. This was explained by 2 novel homozygous mutations in the factor D gene, leading to undetectable factor D protein plasma concentrations. Mother, father, and unaffected sister were heterozygous for the mutated gene and had a partial factor D protein deficiency. The genetics of the deceased sister are not known. In vitro incubation of the factor D–deficient plasma of the boy with meningococci showed normal MBL-mediated complement activation but no formation of the alternative pathway C3-convertase C3bBbP, and severely decreased C3bc formation and terminal complement activation. The defect was restored in an overcompensated fashion after supplementation with factor D at a physiologic concentration.
Classical, lectin, and alternative complement pathway activation byN meningitidisin plasma from the patient and his mother. The formation of C1rs-C1inh complexes (marker of classical pathway activation), C4bc (marker of classical or lectin pathway), and C3bBbP (marker of the alternative pathway) in plasma of the homozygous factor D–deficient patient (□), his heterozygous mother (▪), and the median (± IQR) of 4 healthy controls (▦). The plasma was incubated for 1 hour at 37°C without stimulus or with 108/mL meningococci, in the absence or presence of recombinant factor D or anti–factor D, as indicated. The experiment was repeated once, yielding virtually identical results (not shown).
Classical, lectin, and alternative complement pathway activation byN meningitidisin plasma from the patient and his mother. The formation of C1rs-C1inh complexes (marker of classical pathway activation), C4bc (marker of classical or lectin pathway), and C3bBbP (marker of the alternative pathway) in plasma of the homozygous factor D–deficient patient (□), his heterozygous mother (▪), and the median (± IQR) of 4 healthy controls (▦). The plasma was incubated for 1 hour at 37°C without stimulus or with 108/mL meningococci, in the absence or presence of recombinant factor D or anti–factor D, as indicated. The experiment was repeated once, yielding virtually identical results (not shown).
Factor D is a serine protease that,35 in contrast to most other complement proteins, does not require enzymatic cleavage for the expression of its proteolytic activity.36 Instead, factor D activity is attained by reversible conformational changes occurring after contact with its exclusive substrate; that is, factor B attached to C3b.34,37 Factor D catalyzes the cleavage of factor B to Bb. Subsequently, the complex C3bBb is stabilized by properdin (P) forming the alternative pathway C3-convertase: C3bBbP. Factor D activity is the rate-limiting step in the formation of C3bBbP. The gene for factor D is located on chromosome 19p13.3.34,38 Factor D is produced as a preproprotein with a signaling polypeptide of 23 amino acids. The proprotein is formed by amino acids 24-253. The mature protein consists of amino acids 26-253. The 2 mutations found in the patient lead to 2 amino-acid substitutions: Val213 > Gly and Cys214 > Arg. Heterozygosity for these missense mutations leads to protein concentrations in plasma—assayed by a polyclonal ELISA—less than 50% of the normal concentration. Homozygosity leads to complete absence of the protein. Because Cys214 in the wild-type protein forms an S-S bond with Cys148, we presume that the absence of detectable protein is caused by an abnormal folding of the peptide with impeded cellular secretion and/or increased instability.
Pivotal in the cascade of complement activation is cleavage, by the classical (C4b2b) or the alternative pathway C3-convertase (C3bBbP), of the α-chain of C3, which generates C3b. This cleavage results in exposure of an internal thioester that enables C3b to bind covalently to hydroxyl or amino groups present on (meningococcal) surfaces. Because classical pathway activation on meningococci does not occur during meningococcal septic shock, as there is no antibody or CRP yet,17,18 the C3-convertase required for C3b generation will be delivered by the lectin pathway or by the alternative pathway. As reported, MBL binds to outer-membrane proteins of serogroup B meningococci.39 The MBL-associated serine protease MASP2 subsequently cleaves C4 and C2, which will bind to the surface as C4b and C2a and form the classical/lectin pathway C3-convertase C4b2a.40 Factor D induces the formation of the alternative pathway C3-convertase C3bBbP. Alternative route activation is under tight control by various membrane-bound regulatory proteins on human cells and by fluid-phase regulatory proteins such as factor I, factor H, and C4 binding protein (C4BP). Factor I degrades C3b in the presence of the cofactor molecules C4BP or factor H.41,42 Factor H also accelerates the dissociation of Bb from active C3bBb. Both the classical/lectin- and the alternative pathway C3-convertase function, in the presence of an additional C3b molecule, as C5-convertase. Cleaving of C5 is the initiating step of MAC formation.
We recently described a whole blood in vitro system for the evaluation of complement activation by meningococci.13,32 In the present study, this model was slightly modified by the use of plasma instead of whole blood. Using this model, we found that the factor D–deficient patient had severely reduced C3 activation (measured as C3bc) and terminal complement activation (measured as TCC). Analysis of the intermediates showed no classical route activity (no C1rs-C1inh), confirming previous results.13,32,43 Thus, the C4 activation (measured as C4bc) that occurred in the patient's plasma was mediated solely by the lectin pathway. Notably, as can be seen in the factor-D–deficient serum of the patient and in the experiments with anti–factor D, MBL-induced C4 activation induces only minimal amounts of C3bc and TCC when the alternative pathway activation is switched off. Thus, the alternative route functions as an amplification mechanism for MBL-initiated complement activation by meningococci.
Interestingly, supplementation of factor D to the factor D–deficient plasma of the patient, in the absence of another stimulus, induced some spontaneous alternative route activation (C3bBbP) with C3b formation (C3bc) and terminal complement activation (TCC). Furthermore, in the presence of meningococci, supplementation with factor D gave more complement activation in comparison with the mother and the controls. This may be explained by a greater intrinsic activity of the purified factor D. However, it may also reflect a lower concentration of factor H, factor I, or another regulatory protein in the plasma of the factor D–deficient patient. In our patient, factor H was 0.31 g/L, a normal value, and factor I was present, rendering it unlikely that a lower amount or deficiency of these proteins explains these findings.
Clinically, invasive meningococcal disease presents with varying severity. One side of the spectrum is formed by benign meningococcemia and meningitis, both characterized by a moderate outgrowth of meningococci in the bloodstream. On the opposite side fulminant meningococcal septic shock (MSS) is found, characterized by the rapidly overwhelming intravascular outgrowth of meningococci, high concentrations of endotoxin and massive induction of proinflammatory mediators.44 In case of complement deficiency, the outgrowth of meningococci in the bloodstream is not inhibited and high concentrations of bacteria may develop with massive release of proinflammatory mediators, until start of antibiotic treatment. However, as soon as meningococcal proliferation is stopped by the timely start of antibiotics, the generation of lower amounts of anaphylatoxins may be beneficial. Therefore, the ultimate outcome of meningococcal disease in a complement-deficient individual is determined by the interplay between virulence of the bacterium, the initial host response, and early recognition with timely start of adequate therapy. Reportedly, deficiency of terminal complement factors or MBL is found in patients with mild disease, while properdin deficiency leads to severe disease. This underscores the indispensability of an intact alternative pathway. We have not been able to analyze the complement status in patient A but suspect that she also was factor D deficient. Her fatal outcome may be explained by this defect and by the failure to recognize the severity of disease in time with installation of appropriate antishock therapy. Treatment in patient B was guided by an intensive care physician experienced in the treatment of MSS. Interestingly, this patient received as a part of the initial treatment, before factor D deficiency was known, FFP that contained factor D as well as the alternative pathway regulatory proteins in balanced amounts. The question whether the infusion of FFP has contributed to the favorable outcome—next to early antibiotic treatment and adequate resuscitation—requires further investigation.45
In conclusion, we describe in a Turkish patient of consanguineous parents, 2 novel homozygous mutations of the factor D gene leading to the complete absence of detectable factor D protein in plasma. This deficiency leads to reduced alternative-pathway–dependent complement activation by meningococci, and explains the increased susceptibility to invasive meningococcal disease.
Prepublished online as Blood First Edition Paper, March 9, 2006; DOI 10.1182/blood-2005-07-2820.
Supported by the Norwegian Foundation for Health and Rehabilitation, the Norsk Revmatikerforbund, The Family Blix Foundation (T.E.M.), and the Dutch Organisation for Scientific Research (NWO) grant number 920-03-176 (T.S.).
T.S. wrote the paper and performed the in vitro complement activation experiments; D.R. identified the factor D deficiency and gene defect; C.W. identified the complement deficiency in the surviving patient; C.N. and C.L.M.G. treated the patients during the meningococcal infection; T.E.M. performed the ELISA determinations of the complement activation products; and M.v.D. wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank André Hannema (Sanquin Research at CLB, Amsterdam, the Netherlands) for the factor D assay, Martin de Boer (Sanquin Research at CLB, Amsterdam, the Netherlands) for the mutation analyses, and Grethe Bergseth (Nordlandssykehuset, Bodo, Norway) for the assay of the complement intermediates. M. Fung (Tanox, Houston, TX) is thanked for providing the anti–factor D monoclonal antibody.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal