Abstract
Phenotypic maturation, cytokine secretion, and migration are distinct functional characteristics of dendritic cells (DCs). These functions are independently regulated by a number of extracellular variables, such as type, strength, and persistence of an array of soluble and membrane-bound mediators. Since the exact composition of these variables in response to infection may differ between individuals, the intracellular signaling pathways activated by these extracellular networks may more closely correlate with DC function and predict the course of adaptive immunity. We found that activation of p38 kinase (p38K), extracellular signal–related kinase 1/2 (ERK1/2), and phosphatidylcholine-specific phospholipase C (PC-PLC) enhanced cytokine secretion, whereas p38K, cyclic adenosine monophosphate (cAMP), and PC-PLC enhanced migration. In contrast, phosphatidylinositol 3-kinase (PI3K)/Akt-1 and cAMP inhibited cytokine secretion while ERK1/2 inhibited migration. Migration and cytokine secretion further differed in their sensitivity to inhibition over time. However, although DCs could be manipulated to express migration, cytokine secretion, or both, the level of activation or persistence of intracellular pathway signaling was not predictive. Our results suggest a modular organization of function. We hypothesize that the expression of specific DC functions integrates a large variety of activating and inhibitory variables, and is represented by the formation of a functional unit of molecular networks—the signal response module (SRM). The combined activities of these modules define the functional outcome of DC activation.
Introduction
Dendritic cells (DCs) form a sentinel system of antigen-presenting cells resident in peripheral tissues that samples the microenvironment for molecules signaling “danger,” such as those released during cellular stress, tissue damage, and pathogen invasion. Upon exposure to these factors, DCs undergo complex changes in their phenotype and function, which in their sum are termed maturation. These include up-regulated surface expression of costimulatory molecules (CD80, CD86), acquisition of migratory function toward draining lymph nodes, and secretion of cytokines such as interleukin (IL)–6 and IL-12p70. Their ability to capture antigen in peripheral tissues and to transport it into draining lymph nodes for presentation to T cells empowers DCs to regulate adaptive immune responses.1
Acquisition of migratory capacity and cytokine secretion are not default functional outcomes following DC activation. We have previously reported that strength and persistence of the activation stimulus together with a variety of extracellular factors modulate the functional phenotype of DCs.2 Secretion of IL-12p70 requires persistent stimulation, is enhanced by other cytokines such as interferon-γ (IFN-γ), 3 IL-4,4 IL-1β, 5 and IFN-α, 6 and is inhibited by prostaglandin E2 (PGE2) and adenosine triphosphate (ATP). In contrast, migration toward chemokine C-C motif receptor 7 (CCR7) ligands requires a weak and nonpersistent activation signal and is inhibited by persistent CD40 signaling. Exposure to PGE2 or ATP increases the migrating capacity of monocyte-derived DCs (MoDCs).7-9 Interestingly, CD1c+ DCs purified from peripheral blood (PBDCs) represent migratory-type DCs secreting low amounts of cytokines. This functional profile correlated with a nonpersistent signaling induced by CD40 ligation in this DC population.2
Within the local microenvironment of infection, the composition of the extracellular network of soluble and membrane-bound variables influencing distinct DC functions may vary substantially between individuals. This led us to investigate whether intracellular signaling pathways activated by the extracellular networks more closely correlate with expression of DC functions and thus allow one to predict the direction an adaptive immune response may take.
Signaling pathways mediating DC activation include the mitogen-activated protein kinases (MAPKs) p38 kinase (p38K) and extracellular signal–related kinase 1/2 (ERK1/2), as well as the phosphatidylcholine-specific phospholipase C (PC-PLC). We have recently shown that persistent and nonpersistent phosphorylation of ERK1/2 and p38K correlated with the differentiation of distinct DC phenotypes.2 Although p38K activity has already been recognized to be essential for DC activation,10,11 ERK1/2 has been associated with both inhibitory effects as well as with positive effects on lipopolysaccharide (LPS)–induced DC activation.10,11 Moreover, we have recently reported that ERK, while synergizing with p38K on cytokine induction, had inhibitory effects on migration,2 demonstrating the versatility of this pathway.
We and others have shown that PGE2 is an efficient cofactor inducing migration and inhibiting cytokine secretion in the context of other MoDC maturation factors in vitro.7,8 An anti-inflammatory and migration-inducing role for PGE2 has subsequently been demonstrated in vivo. Mice lacking the EP4 receptor for PGE2 express a reduced capacity for Langerhans cell migration toward draining lymph nodes,12 whereas more severe inflammation develops upon exposure to irritants.13 Other cyclic adenosine monophosphate (cAMP)–elevating agents also suppress cytokine secretion by DCs.14-16 The mechanism by which cAMP modifies DC activation is not fully understood. cAMPcan directly17,18 and indirectly19,20 inhibit Raf-1 and ERK phosphorylation. This inhibition is cell-type specific, as B-Raf is activated by cAMP, resulting in increased phosphorylation of ERK1/2 in cells expressing distinct isoforms of B-Raf.21 Although cAMP can activate p38K in some cell types,22 LPS-induced p38K phosphorylation was not altered by cAMP in DCs.23
A potent negative regulator of DC activation and IL-12p70 secretion is phosphoinositide 3-kinase (PI3K; reviewed in Fukao and Koyasu24 ). In murine DCs, PI3K inhibits p38K, while PI3K inhibitors enhance p38K phosphorylation and IL-12 secretion.25 In other cell types, the PI3K substrate Akt-1 inhibits Raf-1 and B-Raf, resulting in reduced ERK phosphorylation.26-28 Whether this is also the case in DCs has not been investigated.
The multitude of interacting extracellular factors and signaling pathways that a DC perceives will require some level of integration necessary for determining whether the DC responds by acquiring migratory capacity toward draining lymph nodes for interaction with adaptive immune effector cells, or whether it remains at the site of infection and secretes cytokines for recruitment and activation of innate immune effectors. This study investigates whether the activities of signaling pathways influencing DC differentiation predict the resulting functional DC phenotype. If so, the information derived from a complex network of extra- and intracellular variables would be integrated at the level of interacting signaling cascades.
It is now recognized that a cell's functional properties are ultimately encoded by a complex intracellular network of molecular interactions.29,30 The building blocks of this organization are likely compartmentalized into spatially or chemically isolated functional modules which carry discrete functions and are distinctly regulated. Our results support the hypothesis that functional characteristics such as migration, T-cell activation, and cytokine secretion are regulated as independent intracellular modules, which we introduce here as signal response modules (SRMs).
We suggest that the formation of these SRMs is an integrative response to information derived from extracellular and intracellular variables resulting in a functional decision (eg, migration or cytokine secretion). The combination of these constitutes the DC's functional outcome.
Materials and methods
Media
DC were cultured in RPMI 1640 (Sigma, Taufkirchen, Germany) supplemented with 60 mg/L penicillin G, 12.6 mg/L streptomycin, 2 mM L-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (FCS; Sigma) in a 5% CO2 incubator.
Monoclonal antibodies, enzyme-linked immunosorbent assay kits, cytokines, and chemokines
Flow cytometric analysis of DCs was performed using the following monoclonal antibodies (mAbs): fluorescein isothiocyanate (FITC)–conjugated immunoglobulin G1 (IgG1) isotype control; phycoerythrin (PE)–conjugated IgG1 isotype control; anti-CD86/B70/B7-2–FITC (PharMingen, San Diego, CA); and anti-CD83–PE (BD, Heidelberg, Germany). Cytokine enzyme-linked immunosorbent assay (ELISA) kits (Opteia brand) for IL-6 and IL-12p70 were purchased from PharMingen/Becton Dickinson (Heidelberg, Germany). The following cytokines were added to DC cultures: recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF, 40 ng/mL; Immunex, Seattle, WA), rh tumor necrosis factor-α (TNF-α; 10 ng/mL), rhIL-4 (500 U/mL); IFN-α2a (2000 IU/mL) and IFN-γ (all from PromoCell, Heidelberg, Germany). The BHK cell line expressing CD40L was a gift from Dr E. Leo (University of Heidelberg, Germany). Expression of CD40 ligand (CD40L) was confirmed by flow cytometry using an anti-CD40L mAb (Becton Dickinson). PGE2 (1 μM final concentration) was purchased from ICN Biomedicals (Aurora, OH). CCL21 (6Ckine) was purchased from PromoCell and used in migration assays at 40 ng/mL. The MAPK kinase (MEK) inhibitor PD98059 and the p38K inhibitor as well as the nuclear factor κB (NFκB) inhibitors MG-132 and Bay11-7985 were purchased from Calbiochem (Merck Biosciences, Darmstadt, Germany). The membrane-permeable synthetic cAMP analog and activator of protein kinase A (PKA; Sp-5,6-DCl-cBIMPS) was purchased from Biolog (Bremen, Germany). The PI3K inhibitor wortmannin was purchased from Sigma-Aldrich (St Louis, MO), and D609 (tricyclodecan-9-yl xanthate potassium) was purchased from Biomol (Hamburg, Germany). The Amplex-Red Assay kits for measuring PC-PLC and phospholipase D (PLD) activity were purchased from Molecular Probes (Eugene, OR).
MoDCs
Peripheral blood mononuclear cells (PBMCs) were obtained from buffycoat preparations from healthy donors from the Red Cross Blood Bank (Heidelberg, Germany) and used to produce monoclonal DCs (MoDCs). CD14+ monocytes were affinity purified using the magnetic-activated cell-sorting (MACS) CD14 isolation kit (Miltenyi Biotech, BergischGladbach, Germany) and cultured at 5 × 105 cells/mL in culture medium supplemented with GM-CSF (40 ng/mL), and IL-4 (500 U/mL) in 24-well plates. By day 7, MoDCs represented more than 95% of cultured cells. On day 7, all wells were washed and cell density readjusted to 1 to 2 × 105 DCs/mL. Maturation-inducing factors were added, and cells and supernatants were harvested on day 9. All cytokines and DC stimuli used in the present study have previously been tested in dose titration analyses, and the concentrations used in the figures represent those found to be optimal.2,7
Cytokine ELISA
Cytokine secretion by stimulated MoDCs was measured by ELISA. IL-6 and IL-12p70 ELISA were performed on supernatants of MoDC cultures according to the manufacturer's instructions using Maxisorp plates (Nunc, Wiesbaden, Germany). The horseradish peroxidase (HRP) substrate was tetramethylbenzidine (TMB) peroxidase (BD Pharmingen, Heidelberg, Germany); the color reaction was terminated by adding 100 μL orthophosphoric acid (1 M). Plates were read in a Sunrise microplate reader (Tecan, Salzburg, Austria).
Migration assays
MoDCs matured with the indicated stimuli for 36 to 48 hours were harvested, washed, and tested for migration toward the chemokine CCL21 (6Ckine, a ligand for CCR7) in a standard transwell assay.2,7 Briefly, lower chambers of transwell plates (3.0 μm pore size; Costar, Corning, NY) were filled with 350 μL culture medium with or without CCL21 (40 ng/mL). 1-2 × 104 DCs were added in 50 μL RPMI/10% FCS into the upper chamber, and cells were incubated at 37°C for 3 to 4 horus. Cells in the lower chambers were harvested, concentrated to 50 μL volumes in Eppendorf tubes, and counted with a hemocytometer. Each condition was performed in duplicate wells.
Western blot analysis
MoDCs activated for 30 minutes to 48 hours with the indicated stimuli were harvested, washed, resuspended at a densitiy of 5 × 106 cells/mL in Western Sample Buffer (100 mM Tris/HCL [pH 6.8], 4% sodium dodecyl sulfate [SDS], 0.2% Bromophenol-Blue, 20% glycerol, and 200 mM DTT) and snap-frozen in –80°C. Prior to analysis, lysates were thawed, heated for 3 minutes to 96°C, homogenized with a sonicator and 10 μL extract (corresponding to 5 × 104 cells) per lane was separated onto 10% SDS–polyacrylamide gel electrophoresis (PAGE) followed by electroblotting. Blocking was performed in phosphate-buffered saline (PBS) + 5% nonfat milk powder for 2 hours. Membranes were incubated with the following primary antibodies in blocking buffer + 0.1% Tween 20 overnight at 4°C: anti–phospho-ERK1/2 (Thr202/Tyr204, 1:1000; Cell Signaling, New England Biolabs, Frankfurt AM Main, Germany), anti–phospho-p38K (1:1000; Cell Signaling), anti-ERK1/2 (1:2000; Santa Cruz Biotechnology, Heidelberg, Germany), anti-p38K (1:2000; Santa Cruz Biotechnology), anti–phospho-Akt-1 and anti-PTEN (phosphatase and tensin homolog deleted on chromosome 10; a major negative regulator of PI3K/Akt1 signaling) and anti–phospho-PTEN (all 1:1000; Cell Signaling). After washing, secondary antibodies were applied in blocking buffer for 2 hours at room temperature (RT): anti–rabbit HRP for anti–phospho-mAb (1:3000; Cell Signaling) and goat anti–rabbit HRP for nonphosphorylated mAb (1:2000; Santa Cruz Biotechnology). Membranes were washed followed by detection of immunoreactive proteins using the enhanced chemiluminescence (ECL) Western blot system (Santa Cruz Biotechnology). Exposure times ranged from 30 seconds to 15 minutes. For quantitative Western blot analysis, the ECL-Signal was quantified using the Lumi-Imager F1 and the LumiAnalyst Version 3.1 for Windows Software (Roche Diagnostics, Mannheim, Germany).
PC-PLC activity
Amplex Red Assays were performed according to the manufacturer's instructions. Cells were harvested 2 to 24 hours following activation, gently washed and resuspended in lysis buffer (pH 6.9) containing 60 mM PIPES, 25 mM HEPES, 10 mM EDTA, and 2 mM MgCl2. Lysates were stored at –80°C. Color reactions were read after 30 minutes at 530/590 nm using a Spectra MAX GeminiXS Plate Reader (Molecular Devices, Sunnyvale, CA).
Results
Independent regulation of migration and cytokine secretion by cAMP (cBIMPS) and PI3K: CD40L-induced DC activation
PI3K and cAMP are 2 important signaling pathways mediating hormonal and neuroendocrine signals. They are implicated in modulating DC responses, and their different activities in individual immune systems of patients might alter the effect of incoming activation stimuli. The effects of modulating PI3K and cAMP signaling on DC function were therefore investigated. Using the BHK-CD40L cell line as stimulator cells (Figure S1, available on the Blood website; see the Supplementary Figures link at the top of the online article), we investigated the effects of adding a membrane permeable synthetic cAMP analog and activator of PKA (Sp-5,6-DCl-cBIMPS)31 and a PI3K inhibitor (wortmannin)32,33 on the MoDC cytokine secretion, phenotypic maturation, and migratory function. As with PGE2, cBIMPS blocked IL-12p70 secretion, did not influence IL-6 secretion, and induced migration (Figure 1A,C). In contrast, wortmannin strongly enhanced secretion of both IL-6 and IL-12p70 (Figure 1A). Although wortmannin increased the migratory potential of DCs in the set of parallel experiments presented in Figure 1C, this effect varied from donor to donor. Neither cBIMPS nor wortmannin increased the levels of CD80/83/86 expression above that seen with BHK-CD40L alone (Figure 1B). Interestingly, the combinations of cBIMPS (or PGE2) with wortmannin strongly induced all 3 DC functions, resulting in a mature DC phenotype capable of both migration and cytokine secretion (Figure 1A-C). These results support the hypothesis that the expression of these functions is not necessarily linked and that intracellular signaling pathways can independently enhance or inhibit their activation.
Independent regulation of migration and cytokine secretion by cAMP (cBIMPS) and PI3K: CD40L-induced DC activation. Immature MoDCs were washed and resuspended in culture medium at a concentration of 1 to 3 × 105 cells/mL. BHK-CD40L cells were irradiated and added at a concentration of 1:20 to MoDCs either alone or together with PGE2 (1 μM), wortmannin (100 ng/mL), and the cAMP analog and activator of protein kinase A (Sp-5,6-DCl-cBIMPS; 50 μM). Supernatants and cells were harvested after 36 to 48 hours. (A) Secretion of IL-6 and IL-12p70 measured by ELISA (means ± SEM of 7 experiments; *P < .05, **P < .01 compared with activation with the BHK-CD40L cell line). (B) Mean fluorescence intensity (MFI) of CD80, CD83, and CD86 expression for MoDCs activated with the BHK-CD40L cell line in the presence of inhibitors relative to MoDCs activated without inhibitors (means ± SD, n = 6-16 separate donors; *P < .05, **P < .01 compared with activation with the BHK-CD40L cell line).(C) Migration toward CCL21 (means ± SEM, n = 5-8 separate donors; *P < .05 compared with activation with the BHK-CD40L cell line).
Independent regulation of migration and cytokine secretion by cAMP (cBIMPS) and PI3K: CD40L-induced DC activation. Immature MoDCs were washed and resuspended in culture medium at a concentration of 1 to 3 × 105 cells/mL. BHK-CD40L cells were irradiated and added at a concentration of 1:20 to MoDCs either alone or together with PGE2 (1 μM), wortmannin (100 ng/mL), and the cAMP analog and activator of protein kinase A (Sp-5,6-DCl-cBIMPS; 50 μM). Supernatants and cells were harvested after 36 to 48 hours. (A) Secretion of IL-6 and IL-12p70 measured by ELISA (means ± SEM of 7 experiments; *P < .05, **P < .01 compared with activation with the BHK-CD40L cell line). (B) Mean fluorescence intensity (MFI) of CD80, CD83, and CD86 expression for MoDCs activated with the BHK-CD40L cell line in the presence of inhibitors relative to MoDCs activated without inhibitors (means ± SD, n = 6-16 separate donors; *P < .05, **P < .01 compared with activation with the BHK-CD40L cell line).(C) Migration toward CCL21 (means ± SEM, n = 5-8 separate donors; *P < .05 compared with activation with the BHK-CD40L cell line).
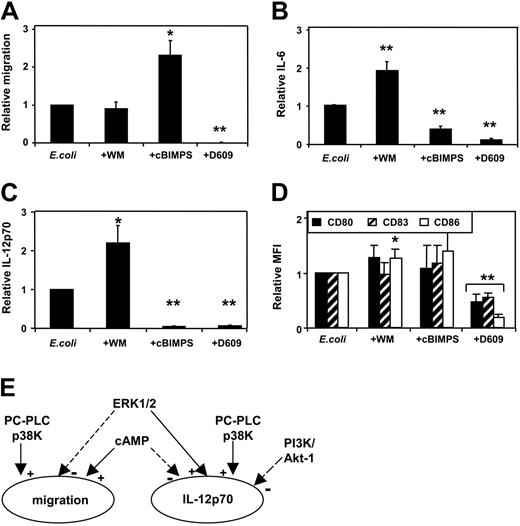
Independent regulation of migration and cytokine secretion by cAMP (cBIMPS), PI3K, and PC-PLC/PLD: E coli –induced DC activation
We next examined the effect of these intracellular modulators on MoDCs activated with intact Escherichia coli bacteria.2 In this setting, we also analyzed the effect of D609 (tricyclodecan-9-yl xanthate potassium), an inhibitor of PC-PLC and PLD activity.34,35 As observed with CD40L activation, the addition of cBIMPS to E coli–stimulated MoDCs enhanced their migration and inhibited IL-12p70 secretion (Figure 2A,C). In this context, cBIMPS also reduced IL-6 secretion (Figure 2B). Interestingly, although wortmannin did not influence MoDC migration, its addition to E coli–stimulated MoDCs enhanced both IL-12p70 and IL-6 secretion. Since phenotypic maturation markers are potently up-regulated on E coli–activated MoDCs, addition of cBIMPS or wortmannin in this context resulted in only a minor further enhancement in CD80/83/86 expression (Figure 2D). Interestingly, nontoxic doses of D609 blocked all 3 DC functions, highlighting the importance of PC-PLC/PLD signaling on MoDC function (Figure 2A-C). These results demonstrate that the effects of cBIMPS and wortmannin are similar in CD40L- and E coli–activated MoDCs, and that PC-PLC strongly enhanced E coli–induced DC activation. Furthermore, our results demonstrate that for both CD40L- and E coli–induced DC activation, migration and secretion of IL-12p70 are independently regulated by distinct intracellular signaling pathways (Figure 2E).
Independent regulation of migration and cytokine secretion by cAMP (cBIMPS), PI3K, and PC-PLC/PLD: E coli –induced DC activation. MoDCs were activated using intact E coli in the presence or absence of inhibitors. Inhibitors were added to MoDCs 20 to 40 minutes prior to E coli exposure. Wortmannin and cAMP were used as in Figure 1. D609 (tricyclodecan-9-yl xanthate potassium) was used at a concentration of 300 μM. Cells and supernatants were harvested 36 to 48 hours later. (A) Migration toward CCL21 (means ± SEM, n = 8-9 separate donors; *P < .05, **P < .01 compared with activation without inhibitor). Secretion of IL-6 (B) and IL-12p70 (C) measured by ELISA (mean values ± SEM, n = 5-12 separate donors for IL-6 and n = 10-13 for IL-12p70; *P < .05, **P < .01 compared with activation without inhibitor). (D) MFI of CD80, CD83, and CD86 expression for MoDCs activated with E coli in the presence of inhibitors relative to MoDCs activated without inhibitors (means ± SD, n = 4-6 separate donors; *P < .05, **P < .01 compared with activation without inhibitors). (E) Schematic of the independent regulation of migration and IL-12p70 secretion of DCs. Both functions are enhanced by p38K and PC-PLC, whereas the PI3K/Akt-1 pathway inhibited only IL-12p70 secretion. In contrast, cAMP enhanced migration and inhibited IL-12p70, whereas ERK1/2 inhibited migration and enhanced IL-12p70.
Independent regulation of migration and cytokine secretion by cAMP (cBIMPS), PI3K, and PC-PLC/PLD: E coli –induced DC activation. MoDCs were activated using intact E coli in the presence or absence of inhibitors. Inhibitors were added to MoDCs 20 to 40 minutes prior to E coli exposure. Wortmannin and cAMP were used as in Figure 1. D609 (tricyclodecan-9-yl xanthate potassium) was used at a concentration of 300 μM. Cells and supernatants were harvested 36 to 48 hours later. (A) Migration toward CCL21 (means ± SEM, n = 8-9 separate donors; *P < .05, **P < .01 compared with activation without inhibitor). Secretion of IL-6 (B) and IL-12p70 (C) measured by ELISA (mean values ± SEM, n = 5-12 separate donors for IL-6 and n = 10-13 for IL-12p70; *P < .05, **P < .01 compared with activation without inhibitor). (D) MFI of CD80, CD83, and CD86 expression for MoDCs activated with E coli in the presence of inhibitors relative to MoDCs activated without inhibitors (means ± SD, n = 4-6 separate donors; *P < .05, **P < .01 compared with activation without inhibitors). (E) Schematic of the independent regulation of migration and IL-12p70 secretion of DCs. Both functions are enhanced by p38K and PC-PLC, whereas the PI3K/Akt-1 pathway inhibited only IL-12p70 secretion. In contrast, cAMP enhanced migration and inhibited IL-12p70, whereas ERK1/2 inhibited migration and enhanced IL-12p70.
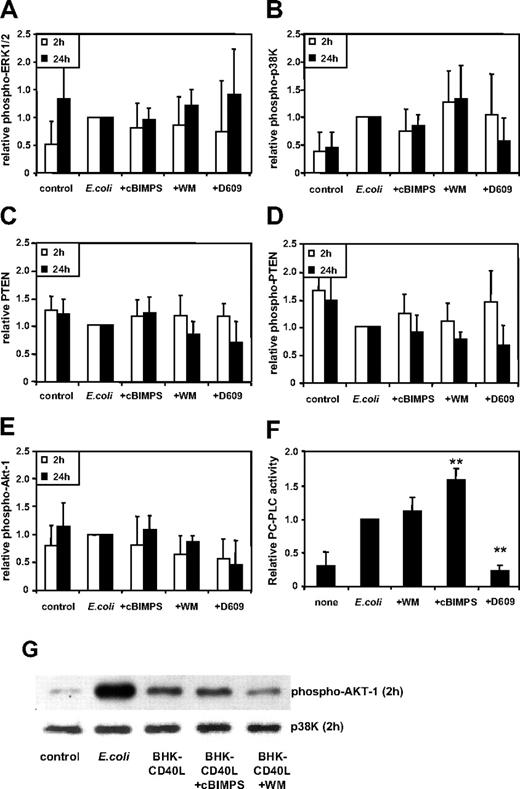
Activity of intracellular signaling pathways does not predict migratory capacity or cytokine secretion by DCs
The potent attenuating effects of MAPK inhibitors upon MoDC activation2 as well as the correlation of MAPK activity with strength and persistence of extracellular activation led us to hypothesize that the effect of intracellular modulators may likewise be explained by a modulation of p38K and ERK1/2 activity. This, however, could not be substantiated using quantitative immunoblotting (Figure 3A-B). Although a trend toward higher levels of phosphorylated p38K in the presence of wortmannin and toward lower levels of MAPK activity in the presence of cBIMPS and D609 was observed, these differences did not reach significance.
We further examined whether strength and persistence of the tumor suppressor phosphatase, PTEN, the protein kinase B, Akt-1, and NFκB-p65 activity might correlate with the type of DC function expressed. Here, although wortmannin and D609 slightly reduced Akt-1 phosphorylation, the functions finally expressed by the variously activated DC types (ie, proinflammatory DC via E coli, or E coli + wortmannin, or migratory DCs via E coli + cBIMPS) could not be predicted simply based on the intracellular levels of PTEN (Figure 3C) or phosphorylated PTEN (inactive form of PTEN; Figure 3D), or phosphorylated Akt-1 (Figure 3E), when measured 2 and 24 hours following activation.
Next, we analyzed the effects of wortmannin, cBIMPS and D609 on the PC-PLC activity of MoDCs. Figure 3F shows that D609 inhibited PC-PLC activity induced by intact E coli. Interestingly, cBIMPS significantly increased PC-PLC activity (Figure 3F), whereas wortmannin did not alter the activity of this enzyme relative to E coli–activated MoDCs. This suggests that PC-PLC activity is a crucial signaling pathway involved in MoDC maturation in response to E coli, and that cAMP signaling further enhances this pathway. However, the inhibitory effects of D609 on both migration and cytokine secretion are not reflected in the levels of intracellular PC-PLC activity.
These signaling pathways (MAPK, Akt-1, PTEN) were also analyzed using MoDCs activated with BHK-CD40L cells with or without cBIMPS and wortmannin. Similarly, no correlation was observed between the strength and persistence of the activities of these pathways and the functional outcome of DC differentiation (n = 4, data not shown). Comparison of the different activation protocols (ie, CD40L-BHK or intact E coli) demonstrated that the highest activation levels for Akt-1 were detected in E coli–activated MoDCs (Figure 3G).
Activity of intracellular signaling pathways does not predict migratory capacity or cytokine secretion by DCs. MoDCs were activated using intact E coli and inhibitors added (as in Figure 2) 20 to 40 minutes prior to E coli exposure, and cells were harvested at 2 and 24 hours later, washed, and resuspended in lysis buffer. Strength of phosphorylation of (A) ERK1/2, (B) p38K, (C) PTEN, (D) unphosphorylated (active) PTEN, and (E) Akt-1 was analyzed. Activation of these pathways was assessed by quantitative immunoblotting. Data shown as means ± SD (ERK-1/2 and p38K at 2 hours from n = 9 and 24 hours from n = 5-6; all other proteins, n = 4 separate donors). *P < .05; **P < .01 compared with activation without inhibitors. (F) PC-PLC activity was measured in cellular lysates harvested 24 hours after activation (means ± SD, n = 6-8 separate donors), **P < .01 compared with activation without inhibitors (E coli). (G) Western blot analysis of MoDCs 2 hours following activation. Phosphorylation of Akt-1 is representative of n = 4 separate donors. p38K analysis acts as protein loading control.
Activity of intracellular signaling pathways does not predict migratory capacity or cytokine secretion by DCs. MoDCs were activated using intact E coli and inhibitors added (as in Figure 2) 20 to 40 minutes prior to E coli exposure, and cells were harvested at 2 and 24 hours later, washed, and resuspended in lysis buffer. Strength of phosphorylation of (A) ERK1/2, (B) p38K, (C) PTEN, (D) unphosphorylated (active) PTEN, and (E) Akt-1 was analyzed. Activation of these pathways was assessed by quantitative immunoblotting. Data shown as means ± SD (ERK-1/2 and p38K at 2 hours from n = 9 and 24 hours from n = 5-6; all other proteins, n = 4 separate donors). *P < .05; **P < .01 compared with activation without inhibitors. (F) PC-PLC activity was measured in cellular lysates harvested 24 hours after activation (means ± SD, n = 6-8 separate donors), **P < .01 compared with activation without inhibitors (E coli). (G) Western blot analysis of MoDCs 2 hours following activation. Phosphorylation of Akt-1 is representative of n = 4 separate donors. p38K analysis acts as protein loading control.
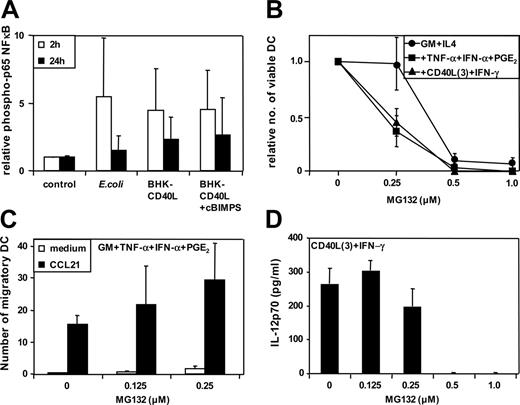
Induction of proapoptosis signals in MoDCs by the global NFκB inhibitor MG132
NFκB are a family of transcription factors mediating immune and inflammatory responses. NFκB activity correlates with strength and persistence of extracellular activation of MoDCs.2 Evaluation of the phosphorylation levels of p65 NFκB in the variously activated MoDCs indicated that there were no discernable differences in p65 NFκB phosphorylation levels between proinflammatory and migratory-type MoDCs when examined at 2 and 24 hours following activation (Figure 4A). Furthermore, the use of the pan-NFκB inhibitors MG132 (or Bay 11-7985; data not shown) were proapoptotic at doses higher than 0.5 μM (Figure 4B), and when used at concentrations lower than 0.5 μM, did not significantly affect MoDC migration (Figure 4C) or IL-12p70 secretion (Figure 4D). It is therefore unclear from these data how distinct NFκB family members influence, at the molecular level, the expression of specific DC functional profiles.
Discussion
Maturation of MoDCs is a finely tuned adaptive process resulting in functional heterogeneity of DC functional profiles.2,7 The present study demonstrates that DCs not only process extracellular information such as the strength and persistence of specific classes of activation stimuli, but detect and adaptively respond to this information in the context of complex networks of inhibitory and activating extracellular factors. In addition, a network of interacting intracellular signaling pathways can also modulate DC activation. The activity of these pathways is presumably not only influenced by the DCs activating stimulus themselves, but also by a plethora of hormones and constitutively produced soluble factors (such as those produced by the autonomous nerve system),36 suggesting that homeostasis is based on balancing the activity of these pathways within the immune system.
Functional DC profiles are not predicted by the strength and persistence of NFκB signaling pathways. MoDCs were activated using intact E coli and inhibitors added as in Figure 3. (A) Strength of phosphorylation of p65 NFκB. Activation was assessed by quantitative immunoblotting. Data represent the means ± SD of n = 4 separate donors. (B) Relative number of viable MoDCs 36 to 48 hours following activation (no inhibitor = 1). Immature MoDCs were washed and resuspended in culture medium at a concentration of 1 to 3 × 105 cells/mL. Cells were either continued in GM-CSF + IL-4 (immature DCs) or stimulated with GM-CSF + TNF-α + IFN-α + PGE2 or with CD40L trimers + IFN-γ as previously described.2 Supernatants and cells were harvested after 36 to 48 hours, and viability was assessed by trypan blue cell count (means ± SEM of 3 separate donors). (C) Migration toward CCL21 by MoDCs activated with GM-CSF + TNF-α + IFN-α + PGE2 (means ± SEM of 3-6 separate donors). (D) Secretion of IL-12p70 by MoDCs activated with CD40L trimers + IFN-γ (means ± SEM of 3-6 separate donors).
Functional DC profiles are not predicted by the strength and persistence of NFκB signaling pathways. MoDCs were activated using intact E coli and inhibitors added as in Figure 3. (A) Strength of phosphorylation of p65 NFκB. Activation was assessed by quantitative immunoblotting. Data represent the means ± SD of n = 4 separate donors. (B) Relative number of viable MoDCs 36 to 48 hours following activation (no inhibitor = 1). Immature MoDCs were washed and resuspended in culture medium at a concentration of 1 to 3 × 105 cells/mL. Cells were either continued in GM-CSF + IL-4 (immature DCs) or stimulated with GM-CSF + TNF-α + IFN-α + PGE2 or with CD40L trimers + IFN-γ as previously described.2 Supernatants and cells were harvested after 36 to 48 hours, and viability was assessed by trypan blue cell count (means ± SEM of 3 separate donors). (C) Migration toward CCL21 by MoDCs activated with GM-CSF + TNF-α + IFN-α + PGE2 (means ± SEM of 3-6 separate donors). (D) Secretion of IL-12p70 by MoDCs activated with CD40L trimers + IFN-γ (means ± SEM of 3-6 separate donors).
The initial conditions present in the microenvironment during the induction phase of an adaptive immune response will likely vary from individual to individual, making identification of correlates of function difficult. We therefore investigated whether the activity of intracellular signaling pathways, which exert strong influences on DC differentiation, may represent a more accurate correlate of the functional DC profile. These pathways could then be used as surrogate markers in order to characterize or predict the type and quality of adaptive immune responses in vivo (eg, in human tissue sections or by microarray or proteomic analyses).
In this study, we investigated a number of extracellular factors influencing DC maturation as well as membrane-permeable modulators of intracellular signaling pathways. We demonstrate that specific functions (such as migration or secretion of cytokines) can be independently manipulated and differ in their sensitivity to abrogation of intracellular signaling pathways. In addition to the previously reported dichotomy of migratory- and proinflammatorytype DCs induced by CD40L activation, we identified that a functional DC phenotype can be produced that secreted high levels of cytokines and migrated toward CCL21 if MoDCs were exposed to CD40L in the presence of wortmannin and cAMP analogs (cBIMPS). This mixed functional phenotype was previously shown for MoDCs activated with E coli,2,7 while a similar functional type of MoDCs has been reported when MoDCs are stimulated with a complex array of toll-like receptor (TLR) agonists and proinflammatory cytokines.37
Our results indicate that differences in the intracellular signaling activities of p38K, ERK1/2, p65 NFκB, PTEN, PC-PLC, and Akt-1 could not sufficiently explain the different functional DC profiles induced by CD40L or E coli activation. Although p38K and ERK1/2 activity correlated closely with the strength and persistence of extracellular activation signals and the subsequent DC functional profile expressed,2 this observation cannot be generalized to apply to all situations where the effect of one extracellular activation stimulus is further modulated by different activities of intracellular pathways (eg, PI3K or cAMP activity). The strong activation of the inhibitory PI3K/Akt-1 pathway in E coli–activated MoDCs (Figure 3G) suggests that both enhancing and inhibitory pathways are simultaneously activated following DC activation. This highlights the complexities of translating microenvironmental signals into DC functional responses.
Surprisingly, no linear relationships were observed between the strength and persistence of extracellular receptor–ligand interactions, or the activity of specific intracellular signaling pathways, and the resulting DC functional profile. Rather, these parameters appear to represent variables (stimulatory and inhibitory) that can influence the net outcome of an adaptive DC functional response. In this regard, the final DC functional profile expressed cannot be predicted by identifying which intracellular pathways are activated (weakly or strongly), or which of them persist. Finally, distinct DC functional profiles (eg, migration, IL-12p70, or IL-6 secretion) were not linked but independently regulated or influenced by these variables. Therefore, these variables appear to be inhibitory or enhancing only with regards to any single specific function, but may vary in their effects for other functions, as seen for PGE2 enhancing migration yet shutting down IL-12p70 secretion.
The clearly independent regulation of migration and cytokine secretion suggests a modular intracellular organization of function. In this regard, the findings of the present study indicate that signal transduction pathways influence rather than direct the functional profile that DCs express. The concept of biological system networks proposes that any cell function is the net outcome of an interactive network of extracellular signals and intracellular signaling pathways.29,30 The concept developed in the present study classifies all intracellular players that are part of the molecular network required for the expression of a specific function as a functional unit or “module.” Since these modules or networks of function are not constitutively expressed but rather recruited into action in response to extracellular activation stimuli, we propose the term signal response module (SRM). In contrast, the molecular entities that influence the establishment of an SRM, or its function, are termed “variables.” In this way, the formation of an SRM represents an integration of all enhancing and inhibitory variables into a functional decision (eg, migration or cytokine secretion). Our hypothesis (Figure S3) suggests that the complexity of responses to extracellular stimuli is integrated at the level of the intracellular SRM mediating a given specific function (eg, migration).
These SRMs have to be characterized at a molecular level in order to find surrogate markers for migratory or proinflammatory function. Model systems like the one presented here are particularly suited to address this question. As minor alterations in the initial activation protocols (BHK-40L or BHK-40L + PGE2) change the expression of SRMs in the recipient DC population, the molecular composition of distinct modules can now be analyzed in DCs derived from the same donor. Examining very similar DC populations that differ in the expression of only one SRM may help to solve one of the problems of microarray data analyses: finding functional relevance for the overwhelming number of differentially expressed genes.38,39
A detailed in vitro analysis of intracellular networks mediating adaptive cellular differentiation may help to design clinical strategies aimed at influencing human immune responses at multiple levels. For instance, small-molecule antagonists interfering with a variety of signaling pathways such as ERK1/2, p38K, AKT-1, and PKA are currently under clinical investigation.40-44 Such compounds add to the increasing apparatus of antihypertensive and antiasthmatic drugs, which interfere with cAMP signaling, calcium signaling, and phospholipase activity, and influence immune responses in vitro and in vivo (reviewed in Elenkov et al36 ). Although there is still only preliminary clinical data, a common observation when using these drugs is the wide gap of in vitro versus in vivo efficacy of the single compounds. In contrast with in vitro and murine in vivo experiments where all but a few variables are held constant, clinical trials test the efficiency of modifiers against the network of all variables influencing a functional response. Our working hypothesis may help to develop rational approaches on how to handle complexity in clinical settings where the composition of this network of extra- and intracellular players cannot be known in detail.
Prepublished online as Blood First Edition Paper, March 9, 2006; DOI 10.1182/blood-2005-04-1501.
Supported by grants from the Deutsche Forschungsgemeinschaft (LU830/1-3), and the Tumorzentrum Heidelberg-Mannheim. M.S. is supported by a Max-Eder-Scolarship of the Deutsche Krebshilfe. E.M. is an employee of CSL Ltd and an Honorary Senior Research Fellow of the Ludwig Institute for Cancer Research and an Associate Professor of the University of Melbourne.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr U. Klingmüller for supporting us with the use of the LumiImager and LumiAnalyst Software.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal