Abstract
CD4+CD25+ regulatory T cells (Tregs) have been shown to inhibit graft-versus-host disease (GVHD) in murine models, and this suppression was mediated by Tregs expressing the lymphoid homing molecule l-selectin. Here, we demonstrate that Tregs lacking expression of the chemokine receptor CCR5 were far less effective in preventing lethality from GVHD. Survival of irradiated recipient animals given transplants supplemented with CCR5-/- Tregs was significantly decreased, and GVHD scores were enhanced compared with animals receiving wild-type (WT) Tregs. CCR5-/- Tregs were functional in suppressing T-cell proliferation in vitro and ex vivo. However, although the accumulation of Tregs within lymphoid tissues during the first week after transplantation was not dependent on CCR5, the lack of function of CCR5-/- Tregs correlated with impaired accumulation of these cells in the liver, lung, spleen, and mesenteric lymph node, more than one week after transplantation. These data are the first to definitively demonstrate a requirement for CCR5 in Treg function, and indicate that in addition to their previously defined role in inhibiting effector T-cell expansion in lymphoid tissues during GVHD, later recruitment of Tregs to both lymphoid tissues and GVHD target organs is important in their ability to prolong survival after allogeneic bone marrow transplantation.
Introduction
Acute graft-versus-host disease (GVHD) is a severe and potentially fatal complication of allogeneic bone marrow transplantation (allo-BMT). Acute GVHD is caused by mature donor T cells that recognize alloantigens presented initially by host antigen presenting cells (APCs).1,2 Our group has demonstrated that the accumulation of donor T cells during the peritransplantation period takes place primarily in lymphoid tissues, followed by recruitment to parenchymal tissues such as the gastrointestinal (GI) tract, liver, lung, and skin.3 We and others have recently demonstrated that eliminating expression of the chemokine receptor CCR5 from donor T cells in an experimental GVHD model resulted in exacerbated GVHD and increased T-cell infiltration of the liver and lung in lethally irradiated recipient animals.4,5 These data suggested that the primary role for CCR5 during GVHD in conditioned transplant recipients is not to direct effector-cell recruitment as originally hypothesized, but to down-modulate target organ inflammation.
Chemokines are predominantly 8-kDa to 12-kDa chemotactic proteins, which bind a family of 7-transmembrane–spanning G protein–coupled receptors, and function primarily in leukocyte migration (reviewed in Moser et al6 ). The chemokine receptor CCR5 is expressed on activated T helper-1/T cytotoxic-1 (TH1/TC1) T cells, natural killer (NK) cells, macrophages, and dendritic cells.7 The ligands for this receptor, CCL3, CCL4, and CCL5, are expressed at sites of inflammation during acute GVHD.4,8-11 CCR5 may play a role in directing effector cells to sites of inflammation.12-15 Interestingly however, multiple studies in CCR5-deficient mice, in addition to our GVHD studies, demonstrate enhanced cell-mediated immune responses during pathogen infection,16-18 delayed-type hypersensitivity,19 and tumor vaccination,20 suggesting an immunoregulatory role for CCR5.
Within the past decade, CD4+CD25+ regulatory T cells (Tregs) have been recognized as important mediators of peripheral tolerance, and deficiency of this population is associated with autoimmune inflammation, including type I diabetes,21-23 gastritis,24 inflammatory bowel disease,25 and thyroiditis24 in several murine models. Tregs inhibit T-cell activation, proliferation, and effector function (reviewed in Piccirillo and Shevach26 ). Tregs are also capable of inhibiting allogeneic T-cell responses, such as skin and solid organ allograft rejection.27-30 Treg-mediated inhibition of acute GVHD has been demonstrated by our group and others.31-37 The gene FoxP3, encoding the forkhead family transcription factor Scurfin, is constitutively expressed by Tregs, and is crucial in the development of their suppressive phenotype.38-44
Our group and others have demonstrated the suppression of T-cell expansion within lymphoid tissues during GVHD by Tregs.45,46 The presence of Tregs has been demonstrated within parenchymal organs such as the pancreas21,47 and lung,41 at sites of infection,48-51 and within allografts30 and tumors,52-54 and their presence at these sites has been shown to impact local inflammation. Although a number of studies have demonstrated chemokine receptor expression and responsiveness of Tregs to chemokines,22,53,55-59 no study has definitively demonstrated a role for proinflammatory chemokine receptors in Treg function. Accumulation of FoxP3-expressing cells within cardiac allografts was recently shown to be dependent on CCR4 expression by recipient mice.30 However, a direct requirement for CCR4 expression by Tregs was not demonstrated.
Here we show that the expression of CCR5 by donor Tregs is critical for their ability to suppress lethality due to acute GVHD. The inability of CCR5-/- Tregs to suppress GVHD lethality correlates with reduced accumulation of CCR5-/- as compared with wild-type (WT) Tregs in GVHD target organs more than one week after transplantation.
Materials and methods
Mice
Mice used for transplantation were described previously.4,60 Within each experiment, all recipient mice were the same sex and age, which ranged from 6 to 10 weeks. Donor mice were gender-matched to recipients, and ranged from 6 to 13 weeks of age. Scurfy mice (Sf-/-), which have been described,61 were a kind gift from Dr Virginia Godfrey. All animal experiments were performed in accordance with protocols approved by the University of North Carolina Institutional Animal Care and Use Committee.
Cell isolations
T-cell–depleted bone marrow (TCD-BM) and whole splenic T cells were prepared from WT donors as described.4 CD25- splenic T cells were prepared from WT or CCR5-/- donors as follows: splenic T cells were isolated using Mouse T-cell Immunocolumns (Cedarlane Laboratories, Hornby, ON, Canada), according to the manufacturer's protocol, and stained with anti-CD25-biotin (clone 7D4; BD Biosciences Pharmingen, San Diego, CA), followed by streptavidin–phycoerythrin (PE). Cells were labeled with anti-PE microbeads (Miltenyi Biotech, Gladbach, Germany), and CD25+ cells depleted following methods from the manufacturer (Miltenyi Biotech). The depletion of CD25+ T cells was more than 90%. In some experiments, CD4+CD25+ Tregs were purified by isolating CD25+ cells using the approach described above, staining with anti-CD4-Cy5 (clone RM4-5; BD Biosciences Pharmingen), and sorting the CD4+CD25+ cells to 90% to 97% purity using a MoFlo high speed cell sorter (Cytomation, Fort Collins, CO). In most experiments, Tregs were purified from the spleens of donor mice, to 90% to 95% purity, using a previously described column-purification protocol.45
Bone marrow transplantation
Irradiation, transplantation, and housing of mice were performed as described.4 Transplant recipients received 3 × 106 TCD WT bone marrow cells and 5 × 106 CD25-depleted (CD25-) T cells (WT or CCR5-/-). For survival studies, some groups received transplants supplemented with Tregs from WT or CCR5-/- donors. The dose of Tregs administered for survival and GVHD grading studies was 1.5 × 106 in experiments using Tregs sorted by fluorescence-activated cell sorting (FACS), and 2.75 × 106 in experiments using column-purified Tregs. For in vivo migration studies, recipients were given transplants containing WT TCD-BM and either WT whole splenic T cells or WT CD25- T cells, alone or supplemented with 0.5 × 106 to 1.5 × 106 Tregs purified from WT or CCR5-/- donors. In most migration studies, Treg donors were WT/enhanced green fluorescent protein (eGFP) and CCR5-/-/eGFP.
GVHD grading
Mice were observed twice weekly and GVHD evaluated using a published clinical scoring system.62
Isolation of leukocytes from tissues
Isolation of total infiltrating leukocytes from target organs has been described previously.4 To purify donor cell infiltrates, the total organ leukocytes were stained with anti-H2Kd-PE (clone SF1-1.1; BD Biosciences Pharmingen), followed by incubation with anti-PE microbeads (Miltenyi Biotech), and depletion of H2Kd-expressing host cells according to the manufacturer's instructions. The remaining cells were more than 95% donor.
Antibodies and flow cytometry
Monoclonal antibodies used for FACS were obtained from BD Biosciences Pharmingen and included anti-H2Kd-PE (or a biotinylated version) (SF1-1.1–mouse IgG2a-kappa), anti-mCD4-PerCP (RM4-5–rat IgG2a-kappa), anti-CD25-biotin (clone 7D4–rat IgM). Isotype controls were obtained from BD Biosciences Pharmingen. To generate polyclonal antimurine Scurfin antibody, rabbits were immunized with the peptide CLLGTRGSGGPFQGRDLRSGAH from the N-terminus of the murine Scurfin protein (Proteintech Group, Chicago, IL). Polyclonal antibody was affinity-purified using the immunizing peptide via the SulfoLink Kit (Pierce Biotechology, Rockford, IL). Specificity of the purified antibody was confirmed by Western blot and FACS analysis (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). For intracellular staining, BD FACS permeabilizing solution 2 (BD Biosciences Pharmingen) was used according to the manufacturer's protocol. Cells were stained with antimouse Scurfin antibody or normal rabbit IgG (Caltag Laboratories, Burlingame, CA), followed by goat anti–rabbit IgG (H+L)–PE (Caltag). Flow cytometry and analysis were performed as previously described.4
Treg culture and chemotaxis assay
Tregs and CD4+CD25- T cells were isolated from WT and CCR5-/- donors, and 1 × 106 cells were activated and cultured using immobilized anti-CD3ϵ antibody and interleukin 2 (IL-2).33 After 5 days in culture, cells were counted and chemotaxis assays performed to assess migration to 1, 10, 100, and 1000 ng/mL recombinant murine CCL3, CCL4, or CCL5 (Peprotech, Rocky Hill, NJ) as previously described.4
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of CCR5 and FoxP3 expression
Total RNA isolation, synthesis of cDNA, and quantitative real-time RT-PCR (QPCR) were performed using a previously described method.4 Primers and probe for murine CCR5 were as follows (5′ to 3′): CCR5 forward: GACTCTGGCTCTTGCAGGAT; CCR5 reverse: GCCGCAATTTGTTTCACAT; probe: TCAAGGGTCAGTTCCGACCTATAGC.
In vitro suppression assays
Allogeneic stimulators were prepared from spleen cell suspensions of B6D2 mice, depleted of T cells using anti-CD90 magnetic beads (Miltenyi Biotech), and irradiated with 2100 cGy from a 137Cs source. Stimulators (1 × 105) were incubated with responders (WT CD4+CD25- T cells, 1 × 105) and various ratios of Tregs from WT or CCR5-/- donors. Cultures were incubated for 5 days at 37°C and 1 μCi (0.037 MBq) 3H-thymidine was added for the last 16 hours of culture. Incorporation of 3H-thymidine was assessed as described.4 In other experiments, a previously described protocol was used to measure Treg-mediated suppression.63 To measure suppression by Tregs sorted from GVHD target organs, cultures were prepared as above, using allogeneic stimulators (2.5 × 104), and WT CD4+CD25- responders (2.5 × 104). Infiltrating leukocytes were isolated from GVHD target organs and the GFP-expressing CD4+ Tregs or CD4+CD25- T cells were sorted using a MoFlo high-speed cell sorter (Cytomation). Tregs or CD4+CD25- T cells were added to cultures at the indicated ratios. Anti-CD3ϵ antibody (0.5 μg/mL) was added, and 3H-thymidine incorporation was assessed after 72 hours.
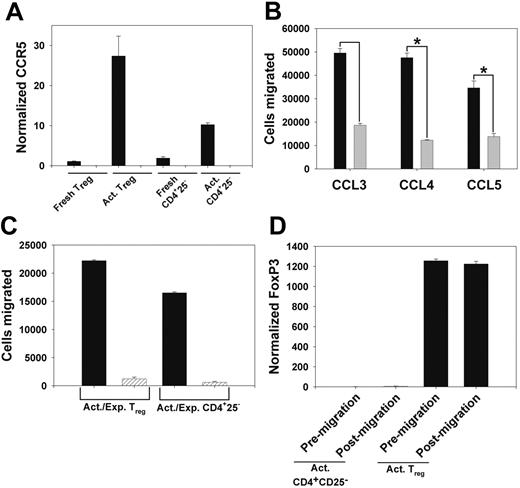
Functional expression of CCR5 on Tregs. (A) QPCR assessment of CCR5 expression in column-purified T CD25- T cells from WT (▪) and CCR5-/- (▨) mice. Shown is CCR5 expression in freshly isolated cells, and cells cultured as in “Materials and methods.” Data are mean ± the standard error of the mean (SEM). (B) WT Tregs (▪) and CD4+CD25- T cells (▦) were cultured as in panel A, and migration in response to 10 ng/mL CCL3, CCL4, and CCL5 was determined. Data are mean ± SEM. *P < .05. (C) Chemotaxis of WT (▪) and CCR5-/- (▨) Tregs and CD4+CD25- T cells, cultured as above, in response to 100 ng/mL CCL4. Data are mean ± SEM. (D) QPCR analysis of FoxP3 mRNA expression in cultured WT Tregs and CD4+regs and CD4+ CD25- T cells prior to chemotaxis and in cells that migrated in response to 100 ng/mL CCL4. Data are mean ± SEM.
Functional expression of CCR5 on Tregs. (A) QPCR assessment of CCR5 expression in column-purified T CD25- T cells from WT (▪) and CCR5-/- (▨) mice. Shown is CCR5 expression in freshly isolated cells, and cells cultured as in “Materials and methods.” Data are mean ± the standard error of the mean (SEM). (B) WT Tregs (▪) and CD4+CD25- T cells (▦) were cultured as in panel A, and migration in response to 10 ng/mL CCL3, CCL4, and CCL5 was determined. Data are mean ± SEM. *P < .05. (C) Chemotaxis of WT (▪) and CCR5-/- (▨) Tregs and CD4+CD25- T cells, cultured as above, in response to 100 ng/mL CCL4. Data are mean ± SEM. (D) QPCR analysis of FoxP3 mRNA expression in cultured WT Tregs and CD4+regs and CD4+ CD25- T cells prior to chemotaxis and in cells that migrated in response to 100 ng/mL CCL4. Data are mean ± SEM.
Apoptosis assay
Tregs were column-purified from WT or CCR5-/- donors, activated, and expanded in culture. Aliquots of cells were taken on days 3 and 5 of culture, and assessed for apoptosis using an Annexin V Apoptosis Detection kit (BD Biosciences Pharmingen), according to the manufacturer's instructions.
Statistical analysis
Estimates of the probability of survival for all groups were determined using the method of Kaplan and Meier.64 Mean survival times were compared using the rank sum test. Cells migrating to chemokines were log transformed and compared by Student t test. Clinical scores, cell infiltration, and FoxP3 expression were compared by Student t test. For all tests, P values less than or equal to .05 were considered significant.
CCR5 deficiency impairs the ability of Tregs to suppress GVHD morbidity and mortality. (A) Survival of lethally irradiated B6D2 recipients of allogeneic BMT consisting of WT TCD-BM and CD25-depleted (CD25-) T cells from WT or CCR5-/- donors. In some groups, mice received WT TCD-BM and WT CD25- T cells, plus Tregs from WT or CCR5-/- donors. Figure represents data pooled from 2 separate experiments yielding similar results. For WT CD25-,n = 10; CCR5-/- CD25-, n = 10; WT CD25- plus WT Tregs, n = 8; WT CD25- plus CCR5-/- Tregs, n = 8. (B) Clinical GVHD scores of recipients above were evaluated at the time points shown. Data represent mean score ± SEM at each time point.
CCR5 deficiency impairs the ability of Tregs to suppress GVHD morbidity and mortality. (A) Survival of lethally irradiated B6D2 recipients of allogeneic BMT consisting of WT TCD-BM and CD25-depleted (CD25-) T cells from WT or CCR5-/- donors. In some groups, mice received WT TCD-BM and WT CD25- T cells, plus Tregs from WT or CCR5-/- donors. Figure represents data pooled from 2 separate experiments yielding similar results. For WT CD25-,n = 10; CCR5-/- CD25-, n = 10; WT CD25- plus WT Tregs, n = 8; WT CD25- plus CCR5-/- Tregs, n = 8. (B) Clinical GVHD scores of recipients above were evaluated at the time points shown. Data represent mean score ± SEM at each time point.
Results
Functional expression of CCR5 on CD4+CD25+ Tregs after activation through the T-cell receptor
Initially, we assessed the expression of CCR5 on Tregs, and the ability of those cells to migrate in response to CCR5-binding chemokines in vitro. We found that CD4+CD25+ T cells isolated from the spleens of unmanipulated C57BL/6 (WT) mice did not express significant levels of CCR5 mRNA. However, after polyclonal activation by culturing on immobilized anti-CD3ϵ monoclonal antibody in the presence of IL-2, expression of CCR5 mRNA was strongly upregulated on WT Tregs (Figure 1A). We observed significant migration of WT Tregs in response to the CCR5-binding chemokines CCL3, CCL4, and CCL5. Responsiveness of activated Tregs to a physiologic concentration (10 ng/mL) of CCL4 and CCL5 was significantly greater than that of similarly activated CD4+CD25- T cells, with a strong trend for increased responsiveness to CCL3 (Figure 1B). Similar trends were observed at chemokine doses of 1 ng/mL and 100 ng/mL (data not shown). Tregs isolated from CCR5-/- mice did not migrate in response to the CCR5-specific chemokine CCL4 (Figure 1C), confirming that the responsiveness of WT Tregs to this chemokine was CCR5 specific. Activated WT Tregs that migrated in response to CCL4 expressed similar levels of FoxP3 mRNA, compared with Tregs evaluated prior to the migration assay (Figure 1D), demonstrating that CCL4 induces the migration of FoxP3-expressing Tregs.
CCR5 expression on Tregs is critical for GVHD suppression
To address the role of CCR5 expression in the function of donor Tregs in vivo during acute GVHD, we used a parent-to-F1 (first filial generation) murine GVHD model similar to that used in our previous study.4 Lethally irradiated B6D2 recipients were given transplants consisting of 3 × 106 WT TCD-BM cells plus 5 × 106 CD25-depleted (CD25-) T cells from WT or CCR5-/- mice. All of these recipients suffered GVHD-related mortality. However, unlike our previous findings using whole (Treg-replete) splenic T cells,4 we did not observe earlier mortality in recipients of CCR5-/- CD25- T cells (Figure 2A). Median survival of recipients of WT or CCR5-/- CD25- T cells was 17 and 19 days, respectively (P = .23), suggesting that the enhanced GVHD found previously using CCR5-/- T cells may be due to impaired in vivo function of CCR5-/- Tregs.
To more specifically assess differences in the ability of WT and CCR5-/- Tregs to inhibit GVHD, we added back WT or CCR5-/- Tregs to transplants containing WT CD25- T cells, and compared their ability to prevent GVHD lethality and reduce signs of GVHD-related morbidity (Figure 2A-B). Adding WT Tregs to transplants was highly effective in preventing GVHD lethality, as median survival increased from 17 days in mice receiving WT CD25- T cells alone, to 59 days (P = .002), with 50% of those mice surviving until termination of the experiment on day 75. This correlated with a significant decrease in clinical GVHD score compared with mice receiving WT CD25- T cells alone, starting one week after transplantation and continuing throughout the course of the experiment. In contrast, adding CCR5-/- Tregs did not prevent lethality, as only 12.5% of recipients survived through day 75. Furthermore, adding CCR5-/- Tregs was significantly less effective in prolonging median survival time compared with adding WT Tregs (P = .02). Adding CCR5-/- Tregs decreased the clinical score of GVHD in the first 4 weeks after transplantation, compared with control mice receiving WT CD25- T cells alone. However, by day 30 and for the remainder of the experiment, clinical scores in mice receiving CCR5-/- Tregs were similar to the peak scores found in the control group. These data demonstrated that CCR5-/- Tregs were significantly impaired in their ability to inhibit GVHD in vivo.
CCR5 expression is not required for FoxP3 expression, development of suppressive function, or expansion and survival of activated Tregs
We next sought to address the mechanism for the impaired in vivo function of CCR5-/- Tregs during GVHD. We compared the suppressive abilities of Tregs isolated from the spleens of unmanipulated WT and CCR5-/- mice using previously published in vitro assays.34,63 CD4+CD25+ T cells were present in comparable proportions in the spleens and peripheral lymph nodes of unmanipulated CCR5-/- and WT mice (Figure 3A). T regs from CCR5-/- mice were comparable to those from WT mice in their ability to suppress the proliferation of WT CD4+CD25- responder cells in response to irradiated B6D2 allogeneic stimulators at all Treg-to-responder ratios (Figure 3B). FoxP3 mRNA expression in CCR5-/- Tregs was comparable to that in WT Tregs (Figure 3C). We also assessed the ability of WT and CCR5-/- Tregs to proliferate and undergo apoptosis when activated and expanded in culture. A similar net expansion of CCR5-/- and WT Tregs was found over 5 days in vitro, and similar proportions of WT and CCR5-/- Tregs underwent apoptosis at days 3 and 5 in culture (data not shown). Thus, CCR5 does not play a critical role in regulating the development, suppressive function, or FoxP3 gene expression of Tregs, and the absence of CCR5 does not impair the proliferation or survival of Tregs in vitro.
CCR5 expression on Tregs is required for infiltration of target organs during GVHD
Previous studies have demonstrated that Treg function during GVHD was dependent on the expression of l-selectin (CD62L) by Tregs.45,46 l-selectin expression on the surface of WT and CCR5-/- Tregs and conventional CD4 T cells was similar both in freshly isolated cells and cells isolated during the first week after transplantation (data not shown).
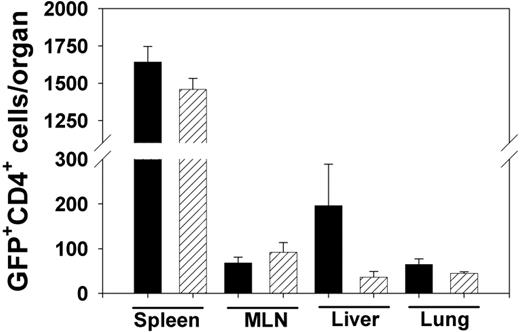
We assessed the accumulation of WT and CCR5-/- Tregs in lymphoid tissues after transplantation by purifying CD4+CD25+ Tregs from WT/eGFP or CCR5-/-/eGFP transgenic mice and adding eGFP-expressing Tregs to transplants containing WT TCD-BM and WT whole splenic T cells. On day 5 after transplantation, no significant differences were observed in the numbers of GFP-positive CD4 T cells in spleen or mesenteric lymph node (MLN) of mice receiving WT or CCR5-/-/eGFP Tregs (Figure 4). Additionally, no significant differences were observed in the numbers of WT/eGFP and CCR5-/-/eGFP Tregs infiltrating the liver and lung. Similar results were found when assessing the accumulation of WT or CCR5-/- Tregs in the spleen, MLN, liver, and lung on day 7 after transplantation (data not shown). Thus, the absence of CCR5 on Tregs did not affect the accumulation of these cells in either lymphoid tissues or GVHD target organs in the first week after transplantation.
CCR5 deficiency does not affect the proportions of Tregs in the periphery, the suppressive function of Tregs, FoxP3 expression, proliferation, or survival. (A) CD4+CD25+ T cells in spleens, and inguinal (ILNs) and mesenteric lymph nodes (MLNs) from male WT and CCR5-/- mice. Shown are cells within the live lymphocyte gate. Data are representative of 2 organs per group. (B) Suppression of WT responder cell proliferation in response to B6D2 alloantigen by WT (▪) and CCR5-/- (▨) Tregs was assessed as described in “Materials and methods.” Data represent mean ± SEM. *P ≤ .05. (C) FoxP3 expression in WT (▪) and CCR5-/- (▨) Tregs and CD4+CD25-, cultured as described in “Materials and methods,” was assessed by QPCR. Data represent mean ± SEM.
CCR5 deficiency does not affect the proportions of Tregs in the periphery, the suppressive function of Tregs, FoxP3 expression, proliferation, or survival. (A) CD4+CD25+ T cells in spleens, and inguinal (ILNs) and mesenteric lymph nodes (MLNs) from male WT and CCR5-/- mice. Shown are cells within the live lymphocyte gate. Data are representative of 2 organs per group. (B) Suppression of WT responder cell proliferation in response to B6D2 alloantigen by WT (▪) and CCR5-/- (▨) Tregs was assessed as described in “Materials and methods.” Data represent mean ± SEM. *P ≤ .05. (C) FoxP3 expression in WT (▪) and CCR5-/- (▨) Tregs and CD4+CD25-, cultured as described in “Materials and methods,” was assessed by QPCR. Data represent mean ± SEM.
The accumulation of Tregs in GVHD target organs in the second week after transplantation was dependent on CCR5. For these experiments, recipients underwent transplantation as described above, except that CD25-depleted T cells as opposed to whole splenic T cells were given. CCR5-/-/eGFP Treg numbers were lower than WT/eGFP Treg numbers in both the liver and spleen on day 10 after transplantation (Figure 5A). No significant differences in CCR5-/- versus WT GFP+CD4 T-cell accumulation were observed in the MLN at this time (data not shown).
Infiltration of WT and CCR5-/- Tregs, measured by quantitation of eGFP+CD4+ cell infiltrates in lymphoid tissues and target organs on day 5 after transplantation. Lethally conditioned B6D2 mice received transplants containing WT TCD-BM and WT whole splenic T cells, with FACS-sorted WT/eGFP (▪) or CCR5-/-/eGFP (▨) Tregs added. The numbers of eGFP+CD4+ cells infiltrating the organs shown were determined by flow cytometry on day 5 after transplantation. n = 4 mice/group. Data represent mean ± SEM.
Infiltration of WT and CCR5-/- Tregs, measured by quantitation of eGFP+CD4+ cell infiltrates in lymphoid tissues and target organs on day 5 after transplantation. Lethally conditioned B6D2 mice received transplants containing WT TCD-BM and WT whole splenic T cells, with FACS-sorted WT/eGFP (▪) or CCR5-/-/eGFP (▨) Tregs added. The numbers of eGFP+CD4+ cells infiltrating the organs shown were determined by flow cytometry on day 5 after transplantation. n = 4 mice/group. Data represent mean ± SEM.
To address the concern that the GFP+ cells evaluated could be contaminated with rapidly proliferating non-Tregs,41,43 we quantified the expression of FoxP3 in donor cell infiltrates from specific tissues isolated from mice at day 10. We found significant levels of FoxP3 mRNA expression in donor cells isolated from target organs only when Tregs were added to the transplants, confirming the utility of this approach to assess Treg accumulation. FoxP3 mRNA expression in donor cell infiltrates isolated from the livers of mice receiving CCR5-/-/eGFP Tregs was lower than that in mice receiving WT Tregs. Decreased FoxP3 expression correlated directly with the reduction in CCR5-/- GFP+CD4 T-cell numbers in livers, demonstrating that the accumulation of CCR5-/-/eGFP Tregs at this site was significantly less than that of WT/eGFP Tregs (Figure 5B). FoxP3 mRNA levels were also reduced in donor cell infiltrates from the lungs of mice receiving CCR5-/- Tregs as compared with WT Tregs (Figure 5C). Interestingly, although we had observed a significant reduction in GFP+CD4 T-cell numbers in the spleens of mice receiving CCR5-/-/eGFP Tregs as compared with WT/eGFP Tregs, FoxP3 mRNA levels were not significantly different between these 2 groups (Figure 5B).
Quantitation of FoxP3 expression in donor cells infiltrating lymphoid tissues and target organs on days 10, 13, and 16 after transplantation. (A) Transplants consisted of WT TCD-BM and WT CD25- T cells, with column-purified WT/eGFP (▪) or CCR5-/-/eGFP (▨) Tregs. The numbers of eGFP+CD4+ cells infiltrating the spleen and liver were determined by flow cytometry on day 10. These data are representative of 2 similar experiments. *P < .05 (B) FoxP3 expression in donor cell infiltrates purified on day 10 from spleens and livers of transplant recipients receiving TCD-BM and WT CD25- T cells, either alone (▦) or with column-purified Tregs from WT/eGFP (▪) or CCR5-/-/eGFP (▨) donors. Donor cell infiltrates were pooled from 3 mice per group. Data shown are representative of 2 separate experiments yielding similar results. *P < .05. (C) FoxP3 expression in donor cell infiltrates purified on day 10 from lungs of mice transplanted as above. Donor cell infiltrates were pooled from 3 mice per group. (D) FoxP3 expression in donor cell infiltrates purified on days 13 and 16 from spleens, livers, and MLNs of recipient mice transplanted as in panel B. Donor cell infiltrates were pooled from 4 mice per group on day 13 and from 3 mice receiving WT/eGFP Tregs and 2 mice receiving CCR5-/-/eGFP Tregs on day 16. (E) Mice underwent transplantation as in panel A, and intracellular Scurfin analysis was performed on leukocytes pooled from the spleen or liver of 3 mice per group on day 10. Total GFP+CD4+Scurfin+ cells per organ were calculated by multiplying the total number of GFP+CD4+ cells in each organ, by the percent Scurfin+. Data shown are mean ± SEM. *P < .05.
Quantitation of FoxP3 expression in donor cells infiltrating lymphoid tissues and target organs on days 10, 13, and 16 after transplantation. (A) Transplants consisted of WT TCD-BM and WT CD25- T cells, with column-purified WT/eGFP (▪) or CCR5-/-/eGFP (▨) Tregs. The numbers of eGFP+CD4+ cells infiltrating the spleen and liver were determined by flow cytometry on day 10. These data are representative of 2 similar experiments. *P < .05 (B) FoxP3 expression in donor cell infiltrates purified on day 10 from spleens and livers of transplant recipients receiving TCD-BM and WT CD25- T cells, either alone (▦) or with column-purified Tregs from WT/eGFP (▪) or CCR5-/-/eGFP (▨) donors. Donor cell infiltrates were pooled from 3 mice per group. Data shown are representative of 2 separate experiments yielding similar results. *P < .05. (C) FoxP3 expression in donor cell infiltrates purified on day 10 from lungs of mice transplanted as above. Donor cell infiltrates were pooled from 3 mice per group. (D) FoxP3 expression in donor cell infiltrates purified on days 13 and 16 from spleens, livers, and MLNs of recipient mice transplanted as in panel B. Donor cell infiltrates were pooled from 4 mice per group on day 13 and from 3 mice receiving WT/eGFP Tregs and 2 mice receiving CCR5-/-/eGFP Tregs on day 16. (E) Mice underwent transplantation as in panel A, and intracellular Scurfin analysis was performed on leukocytes pooled from the spleen or liver of 3 mice per group on day 10. Total GFP+CD4+Scurfin+ cells per organ were calculated by multiplying the total number of GFP+CD4+ cells in each organ, by the percent Scurfin+. Data shown are mean ± SEM. *P < .05.
We assessed FoxP3 expression in donor cells infiltrating the liver, spleen, and MLN at days 13 and 16 after transplantation (Figure 5D). At days 13 and 16, FoxP3 expression in donor cell infiltrates isolated from liver, as well as spleen and MLN, was lower when mice received CCR5-/-/eGFP Tregs, as compared with WT/eGFP Tregs. Importantly, this did not correlate with an overall reduction in GFP-expressing cells in GVHD target tissues over time, as the number of both WT and CCR5-/- GFP+CD4 T cells infiltrating liver (CCR5-/- 167% increase; WT 235%) and spleen (CCR5-/- 135%; WT 91%) increased between days 10 and 13. Thus, although CCR5 expression by Tregs was not required for their accumulation in lymphoid tissues during the first week after transplantation, accumulation of CCR5-/- Tregs in lymphoid tissues was lower than WT Tregs after day 10. The discrepancy between GFP+CD4+ T-cell numbers and FoxP3 mRNA expression in donor cells isolated from spleens at day 10 suggested that a proportion of the GFP+CD4+ T cells infiltrating the spleens were non-Treg.
To resolve the discrepancy between GFP+ Treg numbers and FoxP3 expression data, we used a novel anti-Scurfin polyclonal antibody generated by our group (Figure S1) to determine the total number of GFP+CD4+Scurfin+ cells infiltrating the spleen and liver 10 days after transplantation (Figure 5E). We found no significant difference in GFP+CD4+Scurfin+ cell numbers in the spleens of mice receiving WT/eGFP or CCR5-/-/eGFP Tregs, but a significant 3-fold reduction, which was similar to the difference in FoxP3 mRNA expression, in the number of these cells in the livers of mice receiving CCR5-/-/eGFP compared with WT/eGFP Tregs. Thus, measurement of FoxP3 mRNA expression in donor-cell infiltrates isolated from target organs, or flow cytometric determination of the frequency of FoxP3+ cells, are more rigorous methods of quantifying Treg accumulation during GVHD than use of labeled CD4+CD25+ T cells. This observation is consistent with recent data demonstrating that approximately 25% of CD4+CD25+ cells from normal mice do not express FoxP3 protein, and in fact proliferate when stimulated in vitro.41
CCR5-/- and WT Tregs sorted from target organ–infiltrating cells are suppressive in vitro. Lethally conditioned recipients were given transplants containing WT TCD-BM and WT CD25- T cells, with column-purified WT/eGFP (black bars) or CCR5-/-/eGFP (hatched bars) Tregs or with WT/eGFP CD4+CD25- T cells (dark gray bars). GFP+CD4+ cells were isolated from spleen and liver infiltrates by flow cytometric sorting on day 14, from 3 mice per group. These were used in the indicated Treg/responder cell ratios in an in vitro suppression assay, as described in “Materials and methods.” (Note: From previous work [Figure 5], a significant proportion of GFP+CD4 T cells sorted from target tissues are FoxP3-negative cells. The indicated ratios therefore overestimate Treg numbers and should not be compared to suppression by freshly isolated cells in Figure 3.)
CCR5-/- and WT Tregs sorted from target organ–infiltrating cells are suppressive in vitro. Lethally conditioned recipients were given transplants containing WT TCD-BM and WT CD25- T cells, with column-purified WT/eGFP (black bars) or CCR5-/-/eGFP (hatched bars) Tregs or with WT/eGFP CD4+CD25- T cells (dark gray bars). GFP+CD4+ cells were isolated from spleen and liver infiltrates by flow cytometric sorting on day 14, from 3 mice per group. These were used in the indicated Treg/responder cell ratios in an in vitro suppression assay, as described in “Materials and methods.” (Note: From previous work [Figure 5], a significant proportion of GFP+CD4 T cells sorted from target tissues are FoxP3-negative cells. The indicated ratios therefore overestimate Treg numbers and should not be compared to suppression by freshly isolated cells in Figure 3.)
WT and CCR5-/- Tregspresent in target tissues during GVHD maintain their suppressive phenotype
We sought to determine whether the FoxP3-expressing Tregs residing in target tissues during GVHD maintain their suppressive phenotype. To accomplish this, we isolated GFP-expressing Tregs from the spleens and livers of mice having received transplants containing WT TCD-BM, WT CD25- T cells, and either WT/eGFP or CCR5-/-/eGFP Tregs, 14 days earlier. As a control, a group of recipient mice received transplants containing CD4+CD25- T cells from WT/eGFP donors. Both WT/eGFP and CCR5-/-/eGFP Tregs effectively suppressed responder cell proliferation (Figure 6), when sorted from either the spleen or the liver. GFP+ CD4+CD25- T cells isolated from the spleens of control mice did not suppress responder cell proliferation. Thus, donor Tregs infiltrating both lymphoid and extra-lymphoid GVHD target organs maintained their suppressive phenotype through the first 2 weeks after transplantation. Furthermore, these data indicate that the lack of function of CCR5-/- Tregs in suppressing GVHD lethality was not due to loss of the suppressive phenotype during GVHD in vivo.
Discussion
The function of naturally occurring CD4+CD25+ Tregs in modulating GVHD has been well documented.31-36,46 Our group and others have shown previously that the ability of l-selectinHi Tregs to inhibit effector T-cell expansion within secondary lymphoid tissues early after allo-BMT correlated with their ability to suppress GVHD.45,46 Interestingly, here we demonstrate that recruitment of Tregs to sites of inflammation and lymphoid tissues later after transplantation via the chemokine receptor CCR5 is also crucial for the function of these cells. This study provides the first definitive demonstration of a requirement for a chemokine receptor in the function of these cells in vivo.
Previous studies have demonstrated that CCR5 expression by Tregs is specific to a subset of Tregs, which preferentially infiltrate extralymphoid sites and sites of inflammation.21,41 This Treg subset has been shown to express a more activated phenotype. Our data support the notion that CCR5 expression by Tregs requires an activation stimulus. It is interesting to note that a number of studies have demonstrated enhanced cell-mediated immune responses in CCR5-/- mice, including increased GVHD severity,4,5 tumor immunity,20 responses to pathogens,16-18 and delayed-type hypersensitivity.19 Additionally, humans with the CCR5Δ32 mutation, which reduces CCR5 expression on leukocytes,65 have a propensity for autoimmune diseases of the liver and lung.66-68 Thus, CCR5 expression by Tregs may be important in suppression of inflammation in a specific subset of organs.
Our data suggest that CCR5 is not required for accumulation of Tregs in lymphoid tissues during the first 10 days after transplantation. Although we have demonstrated CCR5-ligand expression within the spleen beginning early after transplantation in this model,4 the similarity in WT and CCR5-/- Treg numbers in spleen and MLN during the first 10 days after transplantation demonstrates that the presence of Tregs in lymphoid tissues does not initially depend on their expression of CCR5. Based on previous studies, l-selectin, perhaps acting in concert with ligands that bind to CCR7, directs the early homing of Tregs to lymphoid tissues after transplantation.22,45,46
Previously, we demonstrated expression of the CCR5-ligands CCL3 and CCL4 in the liver and lung during GVHD, which reached peak levels between days 7 and 14.4 Here, although not affected during the first week, by 10 days after transplantation, CCR5-/- Treg infiltrates were significantly lower than WT Tregs. Lower CCR5-/- Treg infiltration of the liver was also evident at later time points. Thus, the time points at which target organ infiltration by Tregs was affected by CCR5-deficiency correlated with the kinetics of CCR5-ligand expression at these sites. Decreased infiltration of CCR5-/- Tregs in the liver and lung correlated with increased total CCR5-/- CD4+ and CD8+ T cells, and increased tissue pathology found in our previous study at these sites.4 Interestingly, on days 13 and 16, lower CCR5-/- Treg infiltration was observed in spleen and MLN as well. Thus, although CCR5 does not direct the initial entry of Tregs into lymphoid tissues after transplantation, at later time points activated Tregs may recirculate to lymphoid tissues via CCL3, CCL4, or CCL5 produced at these sites.
Reduced infiltration of CCR5-/- Tregs, as compared with WT Tregs, most likely reflects impairment in their migration in response to ligands that bind to CCR5. Conceivably, defects in proliferation or survival could be responsible for these findings, although we were unable to demonstrate these in vitro. Comparable suppressive function of CCR5-/- and WT Tregs sorted from target organ infiltrates on day 14 after transplantation is not consistent with a greater fraction of CCR5-/- Tregs undergoing apoptosis in target tissues. Additionally, we found that the number of eGFP-expressing cells in target organs increased over time in mice receiving either WT/eGFP or CCR5-/-/eGFP Tregs, strongly suggesting that differential expansion or survival of CCR5-/-/eGFP Tregs is not responsible for the differences observed.
We propose a bimodal model for the function of Tregs in suppressing lethal acute GVHD. Early after transplantation, Tregs limit the expansion and differentiation of alloantigen-specific T cells within lymphoid tissues. Activation of Tregs within lymphoid tissues induces expression of CCR5. The presence of ligands for CCR5 in target tissues, which are produced by infiltrating donor T cells,69 would induce Treg migration to those sites and further limit the local expansion and/or cytolytic effector function of alloreactive T cells. Later, the expression of CCR5 may be crucial in the migration of activated Tregs back to inflamed lymphoid tissues.
In conclusion, we have found that the expression of CCR5 on donor Tregs is critical for their infiltration of GVHD target organs later than one week after transplantation and eliminating expression of CCR5 from Treg-exacerbated GVHD in lethally conditioned, MHC-mismatched recipients. Our findings suggest that the CCR5Δ32 allele may be a predisposing factor for the occurrence of lethal GVHD after allogeneic human stem cell transplantation in recipients of myeloablative conditioning. Additionally, inhibition of Treg migration may be clinically important in tumor immunotherapy, as migration of Tregs to the tumor may represent a barrier to therapeutic efficacy.53 We are currently investigating the importance of CCR5 in Treg-mediated tolerance in tumor models.
Prepublished online as Blood First Edition Paper, July 7, 2005; DOI 10.1182/blood-2005-04-1632.
Supported by grants from the National Institutes of Health: CA 58 233, CA 102 052, and AI 064 363 (J.S.S.); AI 34 495, HL 56 067, HL 55 209, HL 63 452, and HL 66 308 (B.R.B.); and AI53 804 and HL72 240 (L.S.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Christin Raimondo, Ivana Ferrer, Nicholas Siefers, V. McNeil Coffield, Shannon Pop, and William Reed for technical assistance, and Larry Arnold and the University of North Carolina Flow Cytometry Facility for FACS-based cell sorting.




![Figure 6. CCR5-/- and WT Tregs sorted from target organ–infiltrating cells are suppressive in vitro. Lethally conditioned recipients were given transplants containing WT TCD-BM and WT CD25- T cells, with column-purified WT/eGFP (black bars) or CCR5-/-/eGFP (hatched bars) Tregs or with WT/eGFP CD4+CD25- T cells (dark gray bars). GFP+CD4+ cells were isolated from spleen and liver infiltrates by flow cytometric sorting on day 14, from 3 mice per group. These were used in the indicated Treg/responder cell ratios in an in vitro suppression assay, as described in “Materials and methods.” (Note: From previous work [Figure 5], a significant proportion of GFP+CD4 T cells sorted from target tissues are FoxP3-negative cells. The indicated ratios therefore overestimate Treg numbers and should not be compared to suppression by freshly isolated cells in Figure 3.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2005-04-1632/6/m_zh80210585980006.jpeg?Expires=1769086135&Signature=Zt1c1GVGEKcx0juNKHr1WRqzZW6oKcDNbjD1cR2mlnxfx7qUBxPc1iXFpFJyLfSGPqLp58Yq6ZWXW3CwEFQGkjaKUn3HVuvuc-ZLmaeSZv2375PqlhxRmFCtZEeqKbW0H1WedPMrJd4hAoeYrPcoa7ZFRLzIFqdAYjRDBVY0fa3Eoy~g6LwGHldTmztJShE9tE7TNW3h6-a3aUuoznVxpXt2lvK3hkcLJFEZDcZ2W2Dqkd969YX7daE7t9WjexDKMG5SbJhaLFQC~mqujO8pB0oFtYlUw1bxqJtNWDWCdPRPKAooj3IM8CROkiYEFADQJqrCkWLlGkEgztMIfTTvvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal