Abstract
Inducible costimulator (ICOS) is expressed on activated and memory T cells and is involved in the regulation of cytokine production. We studied the role of ICOS on alloreactive T cells in graft versus host disease (GVHD) and determined that ICOS expression was up-regulated on alloreactive T cells in recipients of an allogeneic hematopoietic stem cell transplantation (allo-HSCT) with GVHD. We compared ICOS-/- T cells with wild-type (WT) T cells in 2 GVHD models. In both models, recipients of ICOS-/- T cells demonstrated significantly less GVHD morbidity and mortality, which was associated with less intestinal and hepatic GVHD but increased cutaneous GVHD. In addition, recipients of ICOS-/- donor T cells displayed a slight decrease in graft versus leukemia (GVL) activity. Further analysis of alloreactive ICOS-/- T cells showed no defect in activation, proliferation, cytotoxicity, and target organ infiltration. Recipients of ICOS-/- T cells had decreased serum levels of interferon-γ (IFN-γ), while interleukin-4 (IL-4) and IL-10 levels were increased, suggesting that alloreactive ICOS-/- T cells are skewed toward T helper-2 (Th2) differentiation. These data suggest a novel role for ICOS in the regulation of Th1/Th2 development of activated T cells. In conclusion, alloreactive ICOS-/- donor T cells induce less GVHD due to a Th2 immune deviation while GVL activity is slightly diminished.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for hematologic malignancies and certain benign hematopoietic disorders. Apart from the antitumor effect of the conditioning regimen, graft versus leukemia (GVL) activity by alloreactive donor T cells plays an important role in the prevention of posttransplantation tumor relapse. However, alloreactive donor T cells are also responsible for graft versus host disease (GVHD), a major cause of posttransplantation morbidity and mortality.1 Thus, a major challenge for allo-HSCT is to inhibit GVHD while maintaining the beneficial effects of T-cell–mediated GVL activity.
T cells require 2 signals for an effective immune response. The first signal is generated by the interaction of the T-cell receptor (TCR) with a cognate peptide: major histocompatibility complex (MHC) on the surface of an antigen-presenting cell (APC).2 T cells that are stimulated via the TCR alone fail to produce the appropriate cytokines, are unable to sustain proliferation, and will often undergo apoptosis.3 The second signal is a costimulatory signal required to prevent apoptosis and induce full activation and differentiation into effector and memory T cells.4 Because full T-cell activation requires 2 signals, blocking T-cell costimulatory molecules is a strategy utilized to induce tolerance in solid organ transplantation5 and may be useful in allo-HSCT to inhibit GVHD. Costimulatory blockade has been studied in allo-HSCT models to induce immune deviation or suppression after transplantation.6 Inducible costimulator (ICOS) is a relatively new member of B7 family involved in the costimulation of T cells.7-10 This costimulatory family also includes CD28, cytotoxic T-lymphocyte antigen-4 (CTLA-4), and programmed death-1 (PD-1).3 The most studied receptor is CD28, which is constitutively expressed on naive T cells and regulates the threshold for T-cell activation and interleukin-2 (IL-2) production.11 ICOS is expressed upon T-cell activation and contributes to the production of effector cytokines including interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-4, IL-5, and IL-10 but may have little impact on IL-2 production.12-14 ICOS shares 28% amino acid identity and 39% similarity with CD287 and contains several highly conserved motifs that are found in CD28.15 ICOS expression is restricted to activated T cells present in lymphoid organs such as spleen, lymph nodes, and Peyer patches.7,10,16 Mice genetically deficient for ICOS have normal thymi and normal peripheral T-cell development.12-14 However, because ICOS signaling induces CD40L expression on T cells, ICOS-/- mice are deficient in germinal center formation and immunoglobulin (Ig) class switching. This phenotype can be rescued by in vivo administration of an antibody that stimulates CD40 signaling.13
The ICOS ligand (ICOSL) is constitutively expressed on unstimulated B cells, splenic and peritoneal macrophages, and blood-derived dendritic cells.17,18 Thus, ICOSL expression may be induced in peripheral tissues during inflammatory processes.
Recently, a number of studies have found that ICOS is involved in allograft rejection and that blockade of ICOS can promote allograft survival.19-24 Hancock and colleagues21 performed studies using a vascularized cardiac allograft model and demonstrated that ICOS expression was up-regulated during allograft rejection. In addition, administration of ICOS-Fc (fusion to Ig Fc region) could prevent graft rejection and suppress the up-regulation of IFN-γ and IL-10. Similarly, Guo et al24 showed that treatment with a blocking anti–mouse ICOS antibody increased the acceptance of liver transplantation in a rat model.
Thus far only one study has analyzed the effect of ICOS blockade in allo-HSCT, suggesting that the role of ICOS blockade could inhibit or treat GVHD as well as promote bone marrow engraftment.25 In an attempt to better understand the effects of ICOS blockade in allo-HSCT recipients, we used ICOS-/- T cells in allo-HSCT models for GVHD and GVL activity. In this study we show that recipients of alloreactive ICOS-/- T cells display T helper-2 (Th2) immune deviation and have significantly less GVHD, while GVL activity is less affected.
Materials and methods
Cell lines and tissue culture
A20 and P815 were obtained from the American Type Culture Collection (Manassas, VA). 32Dp210 was kindly provided by J. Griffin (Dana Farber Cancer Institute, Boston, MA).26 A20-TGL was generated by transducing A20 with a retroviral vector containing a fusion reporter gene coding for HSV1-TK, enhanced green fluorescent protein (eGFP), and firefly luciferase27 (a kind gift from Dr Vladimir Ponomarev, Memorial Sloan-Kettering Cancer Center). After transduction individual clones with high eGFP expression were sorted into 96-well plates using a FACS Vantage cell sorter (Becton Dickinson, San Jose, CA). Tissue culture medium consisted of RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine.
Mice and allo-HSCT
Female C57BL/6 (B6) (H-2b), C3FeB6F1 (H-2b/k), BALB/c (H-2d), C57BL/6-Ly5.1+ (B6-Ly5.1) (H-2b), and B6D2F1 (H-2b/d) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6.ICOS-KO (ICOS-/-) mice were kindly provided by Dr Richard Flavell (Yale University School of Medicine, New Haven, CT).12 Mice used in allo-HSCT experiments were between 8 and 12 weeks of age. Bone marrow transplantation (BMT) protocols were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. Bone marrow cells (BM) were removed aseptically from femurs and tibias of donor mice and were T-cell depleted (TCD) by incubation with anti–Thy-1.2 for 40 minutes at 4°C followed by incubation with Low-TOX-M rabbit complement (Cedarlane Laboratories, Hornby, ON, Canada) for 40 minutes at 37°C. Splenic T cells were obtained by purification over a nylon wool column. Cells (5 × 106 BM with or without splenic T cells) were resuspended in Dulbecco modified Eagle medium (Life Technologies, Grand Island, NY) and transplanted by tail vein infusion (0.25 mL total volume) into lethally irradiated recipients on day 0. Before transplantation, on day 0, recipients received 900 cGy (BALB/c) or 1300 cGy (C3FeB6F1 and B6D2F1) total body irradiation (137Cs source) as a split dose, with 3 hours between doses (to reduce gastrointestinal toxicity). Mice were housed in sterilized microisolator cages and received normal chow and autoclaved hyperchlorinated drinking water (pH 3.0).
Assessment of GVHD
Mice were randomly assigned to treatment groups and were followed for changes in weight and GVHD status over a period of 12 weeks. Survival was monitored daily, and mice were individually weighed and scored weekly for GVHD. The clinical GVHD score was generated as previously described by Cooke et al.28 The area under the curve (AUC) was used to obtain a P value (see “Statistical analysis”).
Lymphocyte isolation from liver and gut
Mice were killed, and small intestine was dissected from the gastric-duodenal junction to the ileocecal junction. Intestines were washed and flushed with 10% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 10% fetal bovine serum (FBS), and cut into pieces 1 cm long with the luminal side exposed. Pieces were incubated for 1 hour at 37°C while continuously shaking. Subsequently, intestinal pieces were vortexed, and the supernatant was strained and centrifuged at 300 g for 5 minutes. Cells were resuspended in 70% Percoll (Sigma Aldrich, Poole, United Kingdom), overlaid with 40% Percoll, and centrifuged at 1200 g for 30 minutes. Lymphocytes were recovered from the interface. Livers were removed, homogenized, and passed through a cell strainer. Cells were resuspended in 70% Percoll (Sigma Aldrich) overlaid with 40% Percoll and were centrifuged at 1200 g for 30 minutes.
Tumor induction and determination of tumor death
Animals received either 2 × 106 A20, 0.5 × 106 A20-TGL cells, or 1 × 103 P815 cells intravenously in a separate injection on day 0 of allo-HSCT, and survival was monitored daily. The cause of death was determined by necropsy and histopathology. Briefly, all animals with macroscopic liver/spleen metastasis at autopsy were recorded as tumor deaths. Spleens and livers from animals that did not exhibit any gross evidence of tumor were analyzed by histopathology for evidence of microscopic tumor by a veterinary pathologist (Dr Krista LePerle, Cornell University Medical College, New York, NY), and cause of death was subsequently determined. If no evidence of tumor was detected and the animals displayed signs of GVHD, the cause of death was established as GVHD. If tissues were lysed at autopsy the cause of death was labeled as not analyzed.
Bioluminescent imaging
Animals that received A20-TGL were given 150 mg/kg (intraperitonally) of D-Luciferin (Xenogen, Alameda, CA). Fifteen minutes after injection, mice were anesthetized with isoflurane and placed into the Xenogen IVIS bioluminescence (BLI) imaging system (Xenogen) in a supine position and recorded for 5 minutes. Pseudo color images showing the whole body distribution of bioluminescent signal were superimposed on the conventional gray-scale photographs.
GVHD histopathologic analysis
GVHD target organ pathology for small and large bowel and liver was assessed in a blinded fashion. Formalin-preserved organs were paraffin embedded, sectioned, hematoxylin and eosin (H&E) stained, and scored with a semiquantitative scoring system. Bowel and liver were scored for 18 to 22 different parameters associated with GVHD as previously described,29 and skin was evaluated for the number of apoptotic cells per millimeter of epidermis as previously described.30
Flow cytometric analysis
Cells were washed in fluorescence-activated cell sorter (FACS) buffer (PBS with 0.5% bovine serum albumin [BSA] and 0.1% sodium azide), and 106 cells per milliliter were incubated for 20 minutes at 4°C with CD16/CD32 Fc receptor (FcR) block. Subsequently, cells were incubated for 30 minutes at 4°C with antibodies and washed twice with FACS buffer. The stained cells were resuspended in FACS buffer and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) with Cell Quest software or with Flowjo software (Treestar, San Carlos, CA). All of the following fluorochrome-labeled antibodies against murine antigens were obtained from BD PharMingen (San Diego, CA): rat IgG2a (LOU), Ly9.1 (30C7), rat IgG2a (LOU), rat IgG2b (A95-1), CD3 (145-2C11), CD44 (IM7), CD62L (MEL-14), ICOS (7E.17G9), TCRβ (H57-597), rat IgG2a (LOU), CD4 (RM4-5), CD8 (53-6.7), rat IgG2a (R35-95), CD44 (IM7), CD8 (53-6.7), and H-2Kb (AF6-88.5).
Intracellular cytokine staining
In vivo priming. Splenic donor T cells were harvested from recipients of an allo-HSCT with GVHD (as previously described) on day 11 after transplantation.
In vitro priming. Splenocytes were initially incubated in a mixed lymphocyte reaction (MLR) with irradiated (2000 cGy) C3FeB6F1 (allogeneic), C57BL/6 (syngeneic), BALB/c (third-party control) stimulators at a 1:3 effector-stimulator ratio and cocultured for 7 days. Subsequently, cells were harvested and restimulated for 14 hours with either allogeneic or syngeneic irradiated stimulator cells (TCD to reduce contamination with cytokine-expressing stimulator cells) at a 1:4 effector-stimulator ratio in the presence of brefeldin A (10 μg/mL; Sigma, St Louis, MO). Cells were washed and stained with primary (surface) fluorochrome-conjugated antibodies. Next, they were fixed and permeabilized with the Cytofix/Cytoperm Kit (BD PharMingen) and subsequently stained with a secondary antibody. FACS analysis was conducted by gating for the designated populations.
CFSE labeling
Cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) as previously described.31 Briefly, T cells were incubated with CFSE at a final concentration of 2.5 μM (Molecular Probes, Eugene, OR) in Hanks balanced salt solution (HBSS) at 37°C for 15 minutes. Cells were washed 3 times with HBSS before intravenous injection.
51Chromium release assay
Target cells (allogeneic P815 and syngeneic EL4) were labeled with 3.7 MBq chromium 51 (51Cr) at 3 × 106/mL for 1 hour at 37°C and 5% CO2 Labeled targets were plated at 5 × 103 cells per well in U-bottom plates (Costar, Cambridge, MA). Effector cells were prepared (see below) and added at various effector-target ratios and were incubated for 4 hours at 37°C and 5% CO2 Subsequently, 35 μL of supernatant was removed from each well and counted in a gamma counter to determine chromium release (TopCount-Packard, Meriden, CT). Spontaneous release was obtained from wells receiving target cells and media only, and total release was obtained from wells receiving 10% Triton X-100. Percentage lysis was calculated by the following formula: % cytotoxicity = 100 × (experimental release - spontaneous release)/(total release - spontaneous release).
In vitro priming. Wild-type (WT) or ICOS-/- splenocytes were incubated in an MLR with irradiated allogeneic C3FeB6F1 stimulators for 5 days at a 1:2 effector-stimulator ratio.
In vivo priming. Splenic donor T cells were harvested from recipients of an allo-HSCT on day 14 after transplantation.
Enzyme-linked immunosorbent assay (ELISA)
In parallel experiments, blood from animals with GVHD and control animals was harvested and tested by ELISA, which was performed according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical analysis
Statistical analysis of thymic and splenic cellularity and proliferation assays were performed with the nonparametric unpaired Mann-Whitney U test, whereas the Mantel-Cox log-rank test was used for survival data. The statistical analysis performed to test whether a differential change occurred between treatment groups was the pairwise difference in the AUC between groups, using all possible pairwise contrasts. Not all the mice were followed for the full length of the study (12 weeks). To account for informative dropouts, the AUCs were calculated up to the minimum follow-up time for each pairwise difference.32 A P value of .05 or less was considered statistically significant.
Microscopy
Histopathological images were acquired with a Nikon Eclipse 80i (Nikon, Melville, NY) with the following objectives: 1× /0.04 NA Plan UW, WD 3.2; 4× /0.2 NA Plan Apo, WD 15.7; 10× /0.45 Plan Apo DIC L, WD 4.0; 20× /0.75 Plan Apo, DIC M/N2, WD 1.0; 40× /0.95 Plan Apo DIC M/N2, WD 0.14; and 100× /0.04 Plan UW, WD 3.2 (El Segundo, CA). Images were recorded with an Insight SPOT Firewire digital camera with SPOT software version 4.5.9.1 (Diagnostic Instruments, Sterling Heights, MI).
Results
ICOS is expressed on alloreactive T cells during GVHD
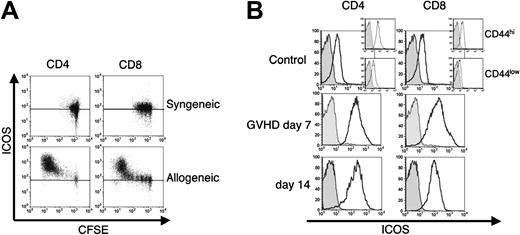
To determine whether expression of ICOS was induced upon alloactivation of T cells in an MHC class I and II disparate model, CFSE-labeled C57BL/6 (B6) splenic T cells were infused into sublethally irradiated syngeneic B6-Ly5.1 and allogeneic C3FeB6F1 hosts (Figure 1A). Proliferation kinetics were evaluated using flow cytometry 72 hours after the adoptive transfer. We have previously shown that CFSE-labeled T cells in allogeneic recipients can be separated into 3 different populations based on their rate of proliferation and the expression of cell-surface activation markers.33 These populations consist of nonproliferative cells (high CFSE content and low or absent expression of activation markers), slow proliferative cells undergoing homeostatic expansion (intermediate CFSE content and lack of activation markers), and fast proliferative, alloactivated T cells (low CFSE content and high expression of activation markers). We found that ICOS expression was present at low levels on donor T cells (both CD4+ and CD8+) infused into syngeneic hosts (Figure 1A, top 2 panels). Donor T cells infused into an allogeneic host showed low levels of ICOS expression in the nondividing or homeostatically expanding sub-population, while ICOS expression was highly up-regulated in the fast proliferative, alloreactive CD4+ and CD8+ T cells (Figure 1A, bottom panels). ICOS up-regulation on these alloreactive T cells was consistent with an activated phenotype because CD44 was also up-regulated (data not shown).
We next examined ICOS expression during the development of GVHD across a full MHC disparity (B6→BALB/c) (Figure 1B). TCD allo-HSCs and allogeneic B6 T cells were transplanted into lethally irradiated recipients, and ICOS expression was determined on splenic donor T cells at days 7 and 14 after transplantation. Low levels of ICOS expression were detected on CD4+ and CD8+ T cells from control mice while ICOS expression was up-regulated on allogeneic donor T cells. Donor T cells with high ICOS expression also demonstrated an activated phenotype (CD25+, CD44hi, and CD62L-; data not shown). We conclude that alloreactive donor T cells express high levels of ICOS during GVHD.
ICOS-/- donor T cells induce less GVHD morbidity and mortality after allo-HCST
To assess the role of ICOS in the development of GVHD after allo-HSCT, we used 2 well-described MHC disparate murine allo-HSCT models: B6→C3FeB6F1 and B6→BALB/c. In both models, lethally irradiated recipients received TCD allo-HSCs from WT B6 mice along with either WT or ICOS-/- T cells. We found in both models that recipients of ICOS-/- T cells developed significantly less GVHD morbidity and mortality (Figure 2).
ICOS expression is up-regulated upon alloactivation and during GVHD. (A) In vivo–alloactivated T cells up-regulate ICOS expression. B6 splenocytes were labeled with CFSE and transferred into a sublethally irradiated (750 cGy) syngeneic B6 or allogeneic C3FeB6F1 recipients. Transferred cells were analyzed after 72 hours for ICOS expression. A horizontal line represents the fluorescence intensity of negative controls for both isotype-stained and unstained cells. T cells were stained for donor T-cell origin and were analyzed by gating on Ly9.1-, CD3+, and CD4+, or CD8+. Data from 1 mouse is representative of 2 independent experiments (n = 3 per experiment). (B) ICOS is highly expressed on alloreactive T cells during GVHD. C3FeB6F1 mice were lethally irradiated (1300 cGy split) and received transplants with TCD allo-HSCs and 1 × 106 T cells from B6 mice. Donor T cells were harvested from recipients' spleens 7 and 14 days after transplantation (GVHD day 7, day 14) and analyzed for ICOS cell surface expression in CD44hi cells. As controls, splenocytes from B6 mice not receiving transplants were used (control), and insets show CD44 subpopulations. Gray histograms represent the isotype control, and the black histograms represent staining with anti-ICOS monoclonal antibody. Shown are representative data of 1 mouse each from 1 independent experiment (normal mouse and day 7, n = 5) and 2 independent experiments (day 14, n = 5 to 8).
ICOS expression is up-regulated upon alloactivation and during GVHD. (A) In vivo–alloactivated T cells up-regulate ICOS expression. B6 splenocytes were labeled with CFSE and transferred into a sublethally irradiated (750 cGy) syngeneic B6 or allogeneic C3FeB6F1 recipients. Transferred cells were analyzed after 72 hours for ICOS expression. A horizontal line represents the fluorescence intensity of negative controls for both isotype-stained and unstained cells. T cells were stained for donor T-cell origin and were analyzed by gating on Ly9.1-, CD3+, and CD4+, or CD8+. Data from 1 mouse is representative of 2 independent experiments (n = 3 per experiment). (B) ICOS is highly expressed on alloreactive T cells during GVHD. C3FeB6F1 mice were lethally irradiated (1300 cGy split) and received transplants with TCD allo-HSCs and 1 × 106 T cells from B6 mice. Donor T cells were harvested from recipients' spleens 7 and 14 days after transplantation (GVHD day 7, day 14) and analyzed for ICOS cell surface expression in CD44hi cells. As controls, splenocytes from B6 mice not receiving transplants were used (control), and insets show CD44 subpopulations. Gray histograms represent the isotype control, and the black histograms represent staining with anti-ICOS monoclonal antibody. Shown are representative data of 1 mouse each from 1 independent experiment (normal mouse and day 7, n = 5) and 2 independent experiments (day 14, n = 5 to 8).
Trafficking of ICOS-/- T cells to GVHD target organs is intact while tissue damage is decreased
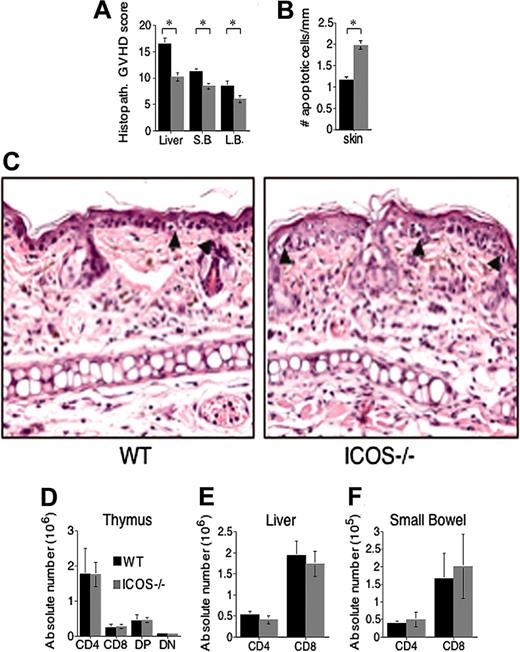
To further assess the role of ICOS in GVHD, we performed histopathologic analysis of specific target organs (Figure 3A). Tissues were harvested on day 14 (Figure 3A) or day 21 (Figure 3B-C) from lethally irradiated C3FeB6F1 mice that received TCD allo-HSCT and either WT or ICOS-/- T cells. Using a semiquantitative scoring system, recipients of ICOS-/- T cells showed significantly less histopathologic damage in liver, small bowel, and large bowel (Figure 3A), while skin pathology demonstrated increased damage in the ICOS-/- T-cell recipients at day 21 (Figure 3B-C) and day 14 (data not shown). Contrary to GVHD in gut, liver, and skin, there was no significant difference in thymic GVHD, which is associated with a decrease in overall thymic cellularity as well as a decrease in the percentage of CD4+CD8+ thymocytes (Figure 3D). These results indicate that alloreactive ICOS-/- T cells generate less GVHD-associated liver and intestinal damage but more GVHD-associated skin damage. Lack of intestinal GVHD with severe skin GVHD has been described in Th2-mediated GVHD where signal transducer and activator of transcription-4-/- (STAT-4-/-) alloreactive T cells were infused in the allograft.34
We then studied if protection from liver and intestinal damage in recipients of ICOS-/- T cells was due to a defect in their capacity to infiltrate target organs. We determined the absolute numbers of donor T cells during GVHD in liver and small intestine (Figure 3E-F) and detected no differences between recipients of ICOS-/- T cells versus WT T cells. These results indicate that alloreactive ICOS-/- T cells generate less GVHD-associated liver and intestinal damage but are able to infiltrate these target organs to the same extent as WT T cells.
ICOS-/- donor T cells cause significantly less GVHD mortality and morbidity in allo-HSCT recipients. (A-C) Parent→F1 model. C3FeB6F1 mice were lethally irradiated (1300 cGy split) and received transplants with TCD allo-HSCs from WT B6 mice (5 × 106) and 1 × 106 WT or ICOS-/- B6 T cells. (D-F) MHC class I and II disparate model. BALB/c recipients were lethally irradiated (900 cGy split) and received transplants with TCD allo-HSCs from WT B6 mouse bone marrow (5 × 106) and splenic WT or ICOS-/- B6 T cells (0.5 × 106). Panels A and D represent survival curves; B and E, weight loss curves; and C and F, clinical GVHD scores. BM-only control groups (n = 0 to 4) and T-cell recipient groups (n = 16 to 20) are derived from 2 combined experiments. Allo-HSC control group (
ICOS-/- donor T cells cause significantly less GVHD mortality and morbidity in allo-HSCT recipients. (A-C) Parent→F1 model. C3FeB6F1 mice were lethally irradiated (1300 cGy split) and received transplants with TCD allo-HSCs from WT B6 mice (5 × 106) and 1 × 106 WT or ICOS-/- B6 T cells. (D-F) MHC class I and II disparate model. BALB/c recipients were lethally irradiated (900 cGy split) and received transplants with TCD allo-HSCs from WT B6 mouse bone marrow (5 × 106) and splenic WT or ICOS-/- B6 T cells (0.5 × 106). Panels A and D represent survival curves; B and E, weight loss curves; and C and F, clinical GVHD scores. BM-only control groups (n = 0 to 4) and T-cell recipient groups (n = 16 to 20) are derived from 2 combined experiments. Allo-HSC control group (
ICOS deficiency protects from histopathologic GVHD damage without affecting homing to target organs. Lethally irradiated (1300 cGy split) C3FeB6F1 recipients received transplants with WT B6 TCD allo-HSCs (5 × 106) and splenic T cells (1 × 106) from WT or ICOS-/- B6 donors. Each group contained 6 to 8 animals. (A-C) Histopathologic analysis of GVHD target organ damage. Recipients were killed on day 14 (liver, small and large bowel), and day 21 (skin) target organs were collected. (A-B) H&E-stained slides of liver, intestines, and skin were analyzed and scored for GVHD histopathologic damage. The graphics represent the average scores ± SEM. ⋆Statistical analyses: liver, P < .01; small bowel (S.B.), P < .01; large bowel (L.B.), P < .05; (B) skin, P < .008. (C) Skin histopathology in recipients of WT (left panel) and ICOS-/- T cells (right panel). Arrowheads denote apoptotic epidermal cells associated with infiltration by effector lymphocytes. Both qualitatively and quantitatively, epidermal injury was more severe in recipients of ICOS-/- T cells (× 400). (D) Thymic cellularity. Recipients were killed at day 21, thymi were harvested, and cells analyzed by flow cytometry. Data are shown as the average of absolute T-cell numbers ± SEM. Statistical analysis: no difference. (E) Donor T-cell content in liver. Recipients were killed at day 21, livers were harvested, and T cells were isolated and analyzed by flow cytometry. Data are shown as the average of absolute T-cell numbers ± SEM. Statistical analysis: no difference. (F) Donor T-cell content in gut. Recipients were killed at day 21, small intestines were harvested, and T cells were isolated and analyzed by flow cytometry. Data are shown as the average of absolute T-cell numbers ± SEM. Statistical analysis: no difference. Day 14 harvest data are representative of 1 experiment of 2 independent experiments (n = 6 to 8 animals). Day 21 harvest data are from 1 independent experiment (n = 6 to 8 animals).
ICOS deficiency protects from histopathologic GVHD damage without affecting homing to target organs. Lethally irradiated (1300 cGy split) C3FeB6F1 recipients received transplants with WT B6 TCD allo-HSCs (5 × 106) and splenic T cells (1 × 106) from WT or ICOS-/- B6 donors. Each group contained 6 to 8 animals. (A-C) Histopathologic analysis of GVHD target organ damage. Recipients were killed on day 14 (liver, small and large bowel), and day 21 (skin) target organs were collected. (A-B) H&E-stained slides of liver, intestines, and skin were analyzed and scored for GVHD histopathologic damage. The graphics represent the average scores ± SEM. ⋆Statistical analyses: liver, P < .01; small bowel (S.B.), P < .01; large bowel (L.B.), P < .05; (B) skin, P < .008. (C) Skin histopathology in recipients of WT (left panel) and ICOS-/- T cells (right panel). Arrowheads denote apoptotic epidermal cells associated with infiltration by effector lymphocytes. Both qualitatively and quantitatively, epidermal injury was more severe in recipients of ICOS-/- T cells (× 400). (D) Thymic cellularity. Recipients were killed at day 21, thymi were harvested, and cells analyzed by flow cytometry. Data are shown as the average of absolute T-cell numbers ± SEM. Statistical analysis: no difference. (E) Donor T-cell content in liver. Recipients were killed at day 21, livers were harvested, and T cells were isolated and analyzed by flow cytometry. Data are shown as the average of absolute T-cell numbers ± SEM. Statistical analysis: no difference. (F) Donor T-cell content in gut. Recipients were killed at day 21, small intestines were harvested, and T cells were isolated and analyzed by flow cytometry. Data are shown as the average of absolute T-cell numbers ± SEM. Statistical analysis: no difference. Day 14 harvest data are representative of 1 experiment of 2 independent experiments (n = 6 to 8 animals). Day 21 harvest data are from 1 independent experiment (n = 6 to 8 animals).
Alloreactive ICOS-/- T cells have intact activation, proliferation, and cytotoxicity
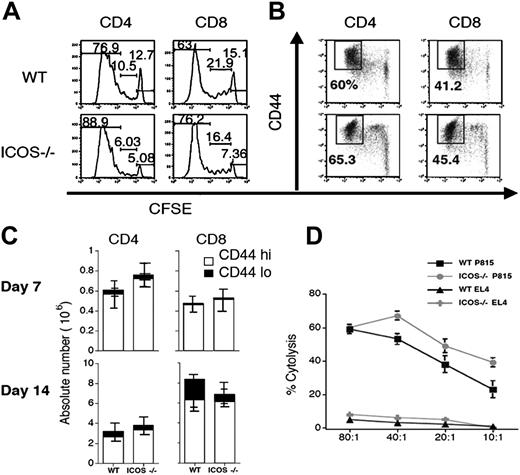
The diminished GVHD activity of alloreactive ICOS-/- T cells could be due to an intrinsic defect in activation, proliferation, or cytotoxicity. To determine the capacity of ICOS-/- T cells to undergo alloreactive proliferation in vivo, CFSE-labeled T cells were transferred into an irradiated allogeneic host (B6→C3FeB6F1), and proliferation kinetics were compared with those of CFSE-labeled WT T cells. There was no significant difference in proliferation kinetics of ICOS-/- versus WT T cells. In some experiments both CD4+ and CD8+ ICOS-/- T cells showed a mild increase in the percentage of fast proliferative, alloreactive cells (Figure 4A). Also, activation of ICOS-/- T cells, determined by CD44 up-regulation, was comparable to WT T-cell activation (Figure 4B), indicating that alloreactive ICOS-/- T cells have intact activation and proliferation kinetics. We confirmed these data by examining the number of activated (CD44hi) donor T cells in spleens of allo-HSCT recipients with GVHD (Figure 4C). Again, no significant differences were detected in the numbers of activated donor T cells present in the spleen at days 7 and 14 after transplantation.
ICOS-/- T cells have intact activation, proliferation, and cytolytic activity. (A) In vivo proliferation and (B) up-regulation of activation markers. WT and ICOS-/- B6 T cells were labeled with CFSE and transferred into sublethally irradiated (750 cGy) allogeneic C3FeB6F1 recipients. Transferred T cells were analyzed by flow cytometry 72 hours after infusion. (C) Donor T-cell expansion during GVHD. Lethally irradiated (1300 cGy split) C3FeB6F1 recipients received transplants with B6 TCD allo-HSCs (5 × 106) and splenic T cells (1 × 106) from WT or ICOS-/- B6 donors. Each group contained 6 animals. Recipients were killed on days 7 and 14 after allo-HSCT. Splenocytes were isolated and analyzed by flow cytometry. Absolute numbers of cells were calculated from total cell counts and percentage of donor origin determined by flow cytometry. Data are shown as the average of absolute T-cell numbers ± SEM. Statistical analysis: no difference. Data shown are representative of 2 independent experiments (n = 5 to 10 animals). (D) Cytolytic activity of WT and ICOS-/- T cells. The murine cell lines P815 (H-2d) and EL4 (H-2b) were labeled with 51Cr and incubated with in vitro–primed effector T cells from WT or ICOS -/- B6 mice at the ratios indicated. Cells were cocultured for 4 hours before specific lysis was determined.
ICOS-/- T cells have intact activation, proliferation, and cytolytic activity. (A) In vivo proliferation and (B) up-regulation of activation markers. WT and ICOS-/- B6 T cells were labeled with CFSE and transferred into sublethally irradiated (750 cGy) allogeneic C3FeB6F1 recipients. Transferred T cells were analyzed by flow cytometry 72 hours after infusion. (C) Donor T-cell expansion during GVHD. Lethally irradiated (1300 cGy split) C3FeB6F1 recipients received transplants with B6 TCD allo-HSCs (5 × 106) and splenic T cells (1 × 106) from WT or ICOS-/- B6 donors. Each group contained 6 animals. Recipients were killed on days 7 and 14 after allo-HSCT. Splenocytes were isolated and analyzed by flow cytometry. Absolute numbers of cells were calculated from total cell counts and percentage of donor origin determined by flow cytometry. Data are shown as the average of absolute T-cell numbers ± SEM. Statistical analysis: no difference. Data shown are representative of 2 independent experiments (n = 5 to 10 animals). (D) Cytolytic activity of WT and ICOS-/- T cells. The murine cell lines P815 (H-2d) and EL4 (H-2b) were labeled with 51Cr and incubated with in vitro–primed effector T cells from WT or ICOS -/- B6 mice at the ratios indicated. Cells were cocultured for 4 hours before specific lysis was determined.
We then assessed the cytolytic capacity of ICOS-/- T cells, after in vitro stimulation with host antigens, against host tumor targets (Figure 4D). We observed that ICOS-/- T cells had slightly more cytolytic activity than alloreactive WT T cells. Similar results were observed with in vivo–stimulated alloreactive T cells derived from recipients of an allo-HSCT (B6→C3FeB6F1). Again we observed a slight increase in cytotoxicity against host target cells (32Dp210 leukemia) (data not shown). These results indicate that ICOS deficiency does not impair the activation, proliferation, and cytotoxic activity of alloreactive T cells.
Th2 immune deviation in recipients of ICOS-/- T cells
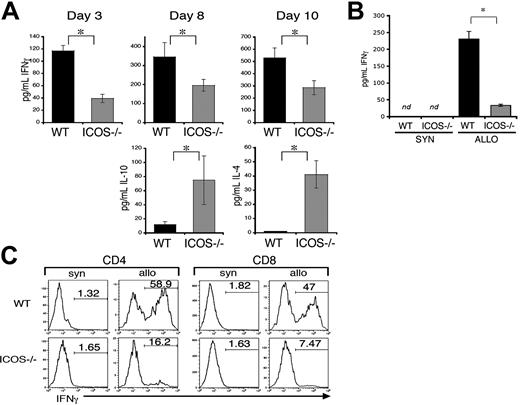
Previous studies have demonstrated that ICOS plays a role in the regulation of effector cytokine production by activated and memory T cells. Thus, protection from GVHD damage in recipients of ICOS-/- T cells could be due to differences in Th1/Th2 cytokine profiles. To address this question, cytokine levels in sera from allo-HSCT recipients were tested by enzyme-linked immunosorbent assay (ELISA) (Figure 5). Sera were harvested at different days after transplantation in 6 independent experiments. At days 3, 8, and 10, there was a significant decrease of IFN-γ in sera of ICOS-/- T-cell recipients (Figure 5A). In contrast, there were increased serum levels of IL-10 (Figure 5A, left bottom panel) and IL-4 (Figure 5A, right bottom panel) compared with WT T-cell recipients. To further assess differences in IFN-γ production by donor WT versus ICOS-/- donor T cells, splenocytes from GVHD hosts were purified and stimulated in vitro with host antigen (Figure 5B). ELISA analysis from supernatants collected after 5 days of coculture showed no detectable IFN-γ when donor B6 WT or ICOS-/- T cells were cultured with B6 splenocytes (syn). When donor T cells were stimulated with C3FeB6F1 (allo) host splenocytes, again we found a significant decrease in IFN-γ production by ICOS-/- T cells. Supernatants of T cells stimulated with allogeneic C3FeB6F1 splenocytes showed increased levels of IL-10 when comparing ICOS-/- T cells with WT T cells (WT = 14.68 ± 6.85 pg/mL; ICOS-/- = 42.34 ± 13.99 pg/mL; P = .04; ± represents SEM).
We also found that intracellular IFN-γ production was more than 3-fold decreased in CD4+ and 6-fold in CD8+ ICOS-/- donor T cells compared with WT T cells (Figure 5C). These data suggest that ICOS deficiency in alloreactive T cells can result in a Th2 immune deviation with decreased IFN-γ production and increased IL-4 and IL-10 production.
ICOS-/- donor T cells display intact GVL activity
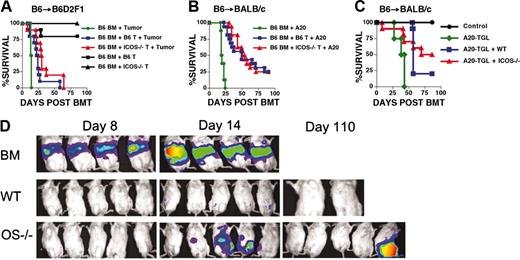
To assess the effects of ICOS deficiency on the GVL activity of alloreactive T cells, we performed experiments in 2 well-characterized GVHD/GVL models: B6→B6D2F1 with P815 mastocytoma and B6→BALB/c with A20 lymphoma (Figure 6; Table 1). In the B6→B6D2F1 experiments (Figure 6A) a low dose of donor T cells (0.5 × 106) was used to decrease GVHD mortality and allow for a better measurement of GVL activity. Indeed, we observed no or minimal mortality in recipients of WT or ICOS-/- T cells without tumor challenge. Mortality in recipients that received TCD alloHSCs with T cells and P815 was significantly delayed compared with recipients of TCD alloHSCs and P815, but no differences were observed between recipients of WT versus ICOS-/- T cells. These results suggested that ICOS-/- T cells had intact GVL activity compared with WT T cells. Similar results were obtained in a second GVHD/GVL model (B6→BALB/c) with A20. In the first set of experiments (Figure 6B) no differences were noted in the rate of mortality comparing recipients of WT versus ICOS-/- T cells when challenged with A20 cells. In a second experiment, mice were challenged with A20-TGL to measure tumor burden by BLI (Figure 6C-D). Mice that did not receive T cells died early (Figure 6C), and A20-TGL cells could be detected in all recipients by BLI. Mice that received WT T cells succumbed to GVHD because there was no BLI signal for A20-TGL throughout the experiment. Mice that received ICOS-/- T cells demonstrated delayed mortality and delayed tumor progression. These results suggest that ICOS-/- T cells may not be as efficient in rejecting A20-TGL as WT T cells and that GVL activity may vary depending on the tumor used for challenge. From these experiments we conclude that ICOS-deficient alloreactive T cells in some cases display slightly decreased GVL activity.
Cause of death in allo-HSCT recipients challenged with tumor cells determined by bioluminescence and/or histopathologic analysis of liver and spleen
. | Tumor . | GVHD . | N/A . |
|---|---|---|---|
| Experiment A | |||
| P815 | 4/4 | 0/4 | 0/4 |
| P815 + WT | 7/10 | 0/10 | 3/10 |
| P815 + ICOS-/- | 6/10 | 0/10 | 4/10 |
| Experiment B | |||
| A20 | 8/8 | 0/8 | 0/8 |
| A20 + WT | 1/16 | 3/16 | 8/16 |
| A20 + ICOS-/- | 4/16 | 1/16 | 9/16 |
| Experiment C | |||
| A20-TGL | 4/4 | 0/4 | 0/4 |
| A20-TGL + WT | 0/10 | 3/10 | 5/10 |
| A20-TGL + ICOS-/- | 4/10 | 1/10 | 0/10 |
. | Tumor . | GVHD . | N/A . |
|---|---|---|---|
| Experiment A | |||
| P815 | 4/4 | 0/4 | 0/4 |
| P815 + WT | 7/10 | 0/10 | 3/10 |
| P815 + ICOS-/- | 6/10 | 0/10 | 4/10 |
| Experiment B | |||
| A20 | 8/8 | 0/8 | 0/8 |
| A20 + WT | 1/16 | 3/16 | 8/16 |
| A20 + ICOS-/- | 4/16 | 1/16 | 9/16 |
| Experiment C | |||
| A20-TGL | 4/4 | 0/4 | 0/4 |
| A20-TGL + WT | 0/10 | 3/10 | 5/10 |
| A20-TGL + ICOS-/- | 4/10 | 1/10 | 0/10 |
N/A indicates not analyzed due to tissue necrosis.
Alloreactive ICOS-/- T cells display Th2 immune deviation. Lethally irradiated (1300 cGy split) C3FeB6F1 recipients received transplants with B6 TCD allo-HSCs (5 × 106) and splenic T cells (1 × 106) from WT or ICOS-/- B6 donors. Mice from 6 independent transplantations (n = 5 to 10) were killed at different days after transplantation, and cytokine production was analyzed. (A) Th2 cytokine profile allo-HSCT recipients of ICOS-/- T cells. IFN-γ, IL-10, and IL-4 levels were determined by ELISA in serum of allo-HSCT recipients of WT or ICOS-/- T cells. ⋆Statistical analyses for IFN-γ: day 3, P = .01; day 8, P = .05; day 10, P = .04. Statistical analyses for IL-10: P = .004. Statistical analyses for IL-4: P = .006. These results are representative of 2 independent experiments at each time point. (B-C) Impaired IFN-γ production in ICOS-/- T cells. Donor T cells were isolated from mice with GVHD at day 10 after allo-HSCT and stimulated with syngeneic (B6) or allogeneic (C3FeB6F1) splenocytes for 5 days. IFN-γ levels were determined by ELISA(B) or by intracellular cytokine staining of T cells from individual mice (C) (ELISA n = 5, intracellular stain n = 2). ⋆Statistical analysis: P < .02; nd indicates not detected.
Alloreactive ICOS-/- T cells display Th2 immune deviation. Lethally irradiated (1300 cGy split) C3FeB6F1 recipients received transplants with B6 TCD allo-HSCs (5 × 106) and splenic T cells (1 × 106) from WT or ICOS-/- B6 donors. Mice from 6 independent transplantations (n = 5 to 10) were killed at different days after transplantation, and cytokine production was analyzed. (A) Th2 cytokine profile allo-HSCT recipients of ICOS-/- T cells. IFN-γ, IL-10, and IL-4 levels were determined by ELISA in serum of allo-HSCT recipients of WT or ICOS-/- T cells. ⋆Statistical analyses for IFN-γ: day 3, P = .01; day 8, P = .05; day 10, P = .04. Statistical analyses for IL-10: P = .004. Statistical analyses for IL-4: P = .006. These results are representative of 2 independent experiments at each time point. (B-C) Impaired IFN-γ production in ICOS-/- T cells. Donor T cells were isolated from mice with GVHD at day 10 after allo-HSCT and stimulated with syngeneic (B6) or allogeneic (C3FeB6F1) splenocytes for 5 days. IFN-γ levels were determined by ELISA(B) or by intracellular cytokine staining of T cells from individual mice (C) (ELISA n = 5, intracellular stain n = 2). ⋆Statistical analysis: P < .02; nd indicates not detected.
GVL activity is not significantly impaired in ICOS-/- T cells. (A) B6 into B6D2F1 and challenge with P815. B6D2F1 mice were lethally irradiated (1300 cGy) and received transplants with TCD B6 allo-HSCs (5 × 106). The tumor challenge control group was not infused with T cells and received 1 × 103 P815 cells (• P815) (n = 4). Control groups for GVHD received a dose of 1 × 106 WT or ICOS-/- B6 T cells (▪ WT and ▴ ICOS-/-)(n = 10) and no tumor challenge. GVL groups received 0.5 × 106 WT (▪) or ICOS-/- (▴) B6 T cells and were challenged with a separate intravenous injection containing 1 × 103 P815 cells (n = 10). Statistical analyses: • versus ▪, P = .02; and • versus ▴, P = .003. The cause of death (GVHD versus tumor) for the GVL groups is shown in Table 1. Shown are combined data from 2 independent experiments. (B) B6 into BALB/c and challenge with A20. BALB/c recipients were lethally irradiated (900 cGy split) and received transplants with TCD B6 allo-HSCs (5 × 106). The tumor challenge control group was not infused with T cells and received 2 × 106 A20 cells (•; n = 8). GVL groups received 0.5 × 106 WT (▪) or ICOS-/- (▴; n = 16) B6 T cells and were challenged in a separate intravenous injection with 2 × 106 A20 cells (▪,n = 18; ▴,n = 10). Shown are data combined from 2 independent experiments. Statistical analyses: • versus ▪ and • versus ▴, P < .001. The cause of death (GVHD versus tumor) for the GVL groups is shown in Table 1. (C) B6→BALB/c and challenge with A20-TGL. BALB/c recipients were lethally irradiated (900 cGy split) and received transplants with TCD B6 allo-HSCs (•)(5 × 106). The tumor challenge control group was not infused with T cells and received 0.5 × 106 A20-TGL cells (•;n = 4). GVL groups received 0.5 × 106 WT (▪) or ICOS-/- (▴) B6 T cells and were challenged with a separate intravenous injection containing 0.5 × 106 A20-TGL cells. (D) Mice were tracked for in vivo luminescence using firefly luciferin injected intraperitoneally, and bioluminescence was determined on days 8, 14, and 110. Mice shown are representative of the entire group. The cause of death (GVHD versus tumor) for the GVL groups is shown in Table 1.
GVL activity is not significantly impaired in ICOS-/- T cells. (A) B6 into B6D2F1 and challenge with P815. B6D2F1 mice were lethally irradiated (1300 cGy) and received transplants with TCD B6 allo-HSCs (5 × 106). The tumor challenge control group was not infused with T cells and received 1 × 103 P815 cells (• P815) (n = 4). Control groups for GVHD received a dose of 1 × 106 WT or ICOS-/- B6 T cells (▪ WT and ▴ ICOS-/-)(n = 10) and no tumor challenge. GVL groups received 0.5 × 106 WT (▪) or ICOS-/- (▴) B6 T cells and were challenged with a separate intravenous injection containing 1 × 103 P815 cells (n = 10). Statistical analyses: • versus ▪, P = .02; and • versus ▴, P = .003. The cause of death (GVHD versus tumor) for the GVL groups is shown in Table 1. Shown are combined data from 2 independent experiments. (B) B6 into BALB/c and challenge with A20. BALB/c recipients were lethally irradiated (900 cGy split) and received transplants with TCD B6 allo-HSCs (5 × 106). The tumor challenge control group was not infused with T cells and received 2 × 106 A20 cells (•; n = 8). GVL groups received 0.5 × 106 WT (▪) or ICOS-/- (▴; n = 16) B6 T cells and were challenged in a separate intravenous injection with 2 × 106 A20 cells (▪,n = 18; ▴,n = 10). Shown are data combined from 2 independent experiments. Statistical analyses: • versus ▪ and • versus ▴, P < .001. The cause of death (GVHD versus tumor) for the GVL groups is shown in Table 1. (C) B6→BALB/c and challenge with A20-TGL. BALB/c recipients were lethally irradiated (900 cGy split) and received transplants with TCD B6 allo-HSCs (•)(5 × 106). The tumor challenge control group was not infused with T cells and received 0.5 × 106 A20-TGL cells (•;n = 4). GVL groups received 0.5 × 106 WT (▪) or ICOS-/- (▴) B6 T cells and were challenged with a separate intravenous injection containing 0.5 × 106 A20-TGL cells. (D) Mice were tracked for in vivo luminescence using firefly luciferin injected intraperitoneally, and bioluminescence was determined on days 8, 14, and 110. Mice shown are representative of the entire group. The cause of death (GVHD versus tumor) for the GVL groups is shown in Table 1.
Discussion
Our study provides evidence, in a Th1-mediated disease, that ICOS blockade can result in immune deviation from Th1 to Th2 responses, which has not been previously described. This is in contrast to studies in allograft rejection, also a Th1-mediated disease, where ICOS inhibition ameliorated disease but did not induce Th2 deviation, because no Th2 cytokines were detected.21 Here we show that ICOS expression is induced during alloactivation of both CD4+ and CD8+ T cells and that alloreactive donor T cells in mice that have received an allo-HSCT and are undergoing GVHD also increase their ICOS expression. Recipients of an allo-HSCT containing ICOS-/- donor T cells had significantly less GVHD morbidity and mortality compared with mice that received WT donor T cells. ICOS deficiency in T cells had no effect on their in vivo activation, proliferation, and infiltration of target organs, but cytokine production was skewed toward a Th2 phenotype as demonstrated by a decrease in serum levels of IFN-γ and increased levels of IL-4 and IL-10.
The role of ICOS in allo-HSCT was first studied in a graft versus host reaction (GVHR) models, where alloreactive T cells are adoptively transferred into an immunocompetent host.35 In this study, blockade of ICOS signaling accelerated acute GVHR.36 However, GVHR models are not comparable to true GVHD models for a variety of reasons. For example, differences between both models may be due to the lack of host myeloablative conditioning in the GVHR model. The conditioning regimen is critical for the earliest phase of acute GVHD and starts before donor cells are infused.1 Tissue damage of intestinal mucosa, liver, and other epithelial and endothelial surfaces activates host cells to secrete inflammatory cytokines. This cytokine cascade induces up-regulation of adhesion molecules and MHC antigens and enhances donor T-cell responses. The enhanced risk of GVHD after clinical HSCT is associated with extensive tissue damage, and this relationship between conditioning intensity, cytokine production, and GVHD severity has been supported in several animal models.29 Apart from the lack of myeloablative conditioning, GVHR models do not include the transfer of donor HSCs, host versus graft reactivity, posttransplantation hematopoietic reconstitution, and classic target organ GVHD. Morbidity and mortality in most GVHR models are due to donor T-cell reactivity against host hematopoietic cells resulting in pancytopenia. Therefore, the pathophysiology of GVHR is significantly different from GVHD, and it is difficult to extrapolate findings in GVHR models to GVHD models and clinical GVHD. Moreover, the use of an inhibitory reagent in the GVHR models instead of ICOS-deficient donor T cells does not discriminate whether ICOS blockade impacts on host or donor-derived T cells.
Because Th1 inflammatory cytokines (like IFN-γ) play an important role in the cytokine cascade during the pathogenesis of GVHD as demonstrated by Ferrara and colleagues,37-39 a Th1 to Th2 shift in the initial response of donor T cells alleviates GVHD38,40 and preserves GVL activity.41 However, some studies show that while Th2 skewing increases survival, Th2 cells are required for the induction of hepatic and severe skin GVHD.34 Therefore, a bias toward Th2 cytokine production by ICOS-/- T cells may explain why in our model intestinal GVHD is decreased, resulting in an overall decrease in GVHD mortality, while skin GVHD is increased.
ICOS stimulation was initially thought to induce Th2 differentiation because blockade of ICOS signaling inhibited in vitro and in vivo Th2 differentiation.42-44 However, other in vivo models for Th1-mediated disorders, including allograft rejection,21,22,24 experimental autoimmune encephalomyelitis (EAE),45,46 and the Th1/Th2 model of collagen-induced arthritis,47 have demonstrated that blockade of ICOS/ICOSL interaction ameliorated disease. In most models of solid organ transplantation, blockade of ICOS/ICOSL signaling prevented or delayed graft rejection.24,48,49 Conflicting results arise when ICOS/ICOSL blocking reagents are administered at different phases of the immune response. Experiments in the EAE model show that blockade of ICOS/ICOSL during the priming phase exacerbates clinical symptoms; however, if blocking reagents are administered during the effector phase of the immune response, the clinical symptoms are abrogated.46 Similarly, in GVHR, blockade of ICOS signaling increased acute GVHR, while the same treatment abrogated chronic GVHR.36 Still, in most models of solid organ transplantation, blockade of ICOS/ICOSL signaling prevents or delays graft rejection.24,48,49
Experiments testing heart allograft survival in ICOS-/- mice showed similar results as using blocking reagents and demonstrated that lack of ICOS signaling increases heart allograft survival and reduced chronic rejection.21 In these experiments, lack of ICOS signaling resulted in a decrease in IFN-γ expression, similar to our results and to previous studies.15,45,47 Still, the role of ICOS in IFN-γ production is controversial. While most studies demonstrate that ICOS stimulation increases IFN-γ production,16,18,44,50 blocking ICOS/ICOSL interaction does not always impair IFN-γ expression.44,46 This discrepancy extends to the capacity of ICOS-/- T cells to produce cytokines. For example, ICOS-/- mice show weak Th2 in vivo responses, but cytokine production and Th2-mediated Ig class switching is rescued by the use of a strong adjuvants in vivo.12-14 Thus, the inflammatory milieu in which ICOS-/- T-cell activation occurs will determine the extent and type of cytokines produced. Importantly, in vitro differentiation of ICOS-/- T cells into Th1 and Th2 subpopulations shows that once ICOS-/- T cells have differentiated, Th1 cells generate less IFN-γ while IL-10 production is intact. Differentiated ICOS-/- Th2 T cells generate equal or slightly higher amounts of IL-4 and IL-10 than differentiated WT T cells.12 Our data in allo-HSCT are consistent with this finding, where alloreactive ICOS-/- T cells, once differentiated into Th2 type T cells, produce lower levels of IFN-γ and higher levels of IL-4 and IL-10 than WT Th2 T cells. However, we cannot exclude that cytokine production of alloreactive ICOS-/- T cells may vary depending on differences in the conditioning regimen, cytokine milieu, strain combinations, and other factors in different GVHD models.
A recent study by Taylor et al25 used GVHD models and found that ICOS blockade by administration of anti-ICOS antibodies or using ICOS-/- donor T cells resulted in a significant delay and decrease in GVHD morbidity and mortality. Interestingly, a greater survival benefit by ICOS blockade was observed in the setting of an intact STAT6 (Th2) pathway, which is in agreement with our observation that ICOS blockade might operate by skewing toward Th2.25
Recent experiments have indicated that ICOS stimulation preferentially induces transendothelial migration and polarization in Th1 cells and blocking of ICOS signaling inhibits Th1 migration but has no impact on Th2 differentiated T cells.51 Because our data in allo-HSCT suggest ICOS-/- T cells become Th2 effectors, it is to be expected that equal amounts of lymphocytes will infiltrate target organs. Indeed, our results show no difference in the infiltration of WT versus ICOS-/- T into GVHD target organs.
Importantly, we found that the in vitro cytolytic activity of ICOS-/- T cells was not impaired but showed slightly less in vivo GVL activity. Our studies and those of others have demonstrated that perforin-mediated cytolytic activity is required for optimal GVL in this particular GVHD/GVL model with P815 mastocytoma, even under conditions of Th2 polarization.41,52,53 However, because IFN-γ is required for optimal GVL activity in some GVHD/GVL models,54 we cannot exclude the possibility that inhibition of ICOS in these cases would interfere with GVL activity.
Current strategies to block ICOS/ICOSL interactions are being generated and have been effective in the treatment of murine models for EAE,46 solid organ transplantation,21,22,24 lung inflammatory diseases,43,44 and collagen-induced arthritis.47 In conclusion, our results indicate that blocking ICOS/ICOSL signaling could be an effective therapeutic target for the abrogation of GVHD through Th2 deviation, whereas GVL activity is less affected.
Prepublished online as Blood First Edition Paper, June 23, 2005; DOI 10.1182/blood-2005-01-0410.
Supported by grants HL69929, HL72412, and CA107096 from the National Institutes of Health and awards from the Emerald Foundation and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by William H. Goodwin and Alice Goodwin and the Commonwealth Foundation for Cancer Research, and an award from Golfers Against Cancer (M.R.M.v.d.B.). T.H.T. is the recipient of a Deutscher Akademischer Austausch Dienst (DAAD) research award.
V.M.H., J.M.E., and T.R.-M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the staff of the Research Animal Resource Center at Memorial Sloan-Kettering Cancer Center (MSKCC) for excellent animal care. We also thank Marsinay Smith, Sydney Lu, Thomas van Huystee, and Susanne McGoldrick for their assistance.

![Figure 2. ICOS-/- donor T cells cause significantly less GVHD mortality and morbidity in allo-HSCT recipients. (A-C) Parent→F1 model. C3FeB6F1 mice were lethally irradiated (1300 cGy split) and received transplants with TCD allo-HSCs from WT B6 mice (5 × 106) and 1 × 106 WT or ICOS-/- B6 T cells. (D-F) MHC class I and II disparate model. BALB/c recipients were lethally irradiated (900 cGy split) and received transplants with TCD allo-HSCs from WT B6 mouse bone marrow (5 × 106) and splenic WT or ICOS-/- B6 T cells (0.5 × 106). Panels A and D represent survival curves; B and E, weight loss curves; and C and F, clinical GVHD scores. BM-only control groups (n = 0 to 4) and T-cell recipient groups (n = 16 to 20) are derived from 2 combined experiments. Allo-HSC control group (\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \({\odot}\) \end{document}), WT allo-HSCs + WT T-cell recipients (▪), and WT allo-HSCs + ICOS-/- T-cell recipients (▵). Statistical analyses shown in all graphics represent recipients of WT versus ICOS-/- T-cell recipients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2005-01-0410/6/m_zh80210586430002.jpeg?Expires=1765921555&Signature=oGA2EbIs4Gu-OTK2jBxC4WsnErxioOz4TFqDDDgDWu~Hgs38uJyjlaF1CuaWIm-cC33DGyEHFl5FHiXeqNX4ESkH7X30Y3kejhILD9ZzfjV3AtsT~ZtbMvQcQx6kltjIwEiZaaxvmgwpySgO9B7d1bzbi9S18m6SbJrP8Kkbc3SVEWjiJzqwlArhLj1Cx4A3pecMzLD7BJpPSMgY6mZk5V4d1Rd2GbRHL7TiPf2Qi7uNs8sqBOww5kEjNCO~e7hI4BAURzjsZrgh0v-Zou56S35kTvKJisAXBA6hSIUMd2WC3nx72YUj0qruK2fb2uamySg9viL4E4DdV8oKtFw2wg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal