Abstract
Telomerase catalytic subunit (hTERT) has been shown to play a critical role not only in telomere homeostasis but also in cellular survival, DNA repair, and genetic stability. In a previous study, we described that tumor necrosis factor-×α (TNF×α) induced in the leukemic KG1 cells a senescence state characterized by decreased hTERT activity followed by prolonged growth arrest, increased× β-galactosidase activity, telomere shortening, and major chromosomal instability. Interestingly, granulocyte-macrophage colony-stimulating factor (GM-CSF) abrogated all these events. In the present study, we show for the first time that TNF×α acts by inhibiting the hTERT gene in both normal CD34×+ cells and fresh leukemic cells. Using KG1 cells as a representative cellular model, we show that TNF×α induced sphingomyelin hydrolysis, ceramide production, and c-Jun N-terminal kinase (JNK) activation, all of which are critical components of TNF×α signaling, resulting in hTERT gene inhibition. Moreover, we provide evidence that the protective effect of GM-CSF is related to its capacity to interfere with both ceramide generation and ceramide signaling. Negative regulation of the hTERT gene may represent one mechanism by which TNF×α interferes with normal hemopoiesis.
Introduction
Telomerase is a large ribonucleoprotein complex containing 2 major subunits contributing to enzymatic activity: a RNA component (hTR) that serves as a template for the polymerase activity of the enzyme and a catalytic subunit with reverse transcriptase activity (hTERT). Previous studies have documented that hTERT represents the rate-limiting step in telomerase function.1 For this reason, and because this enzyme has been found to play an essential role in the regulation of telomere elongation and cellular protection, hTERT regulation has been the subject of intense investigation in recent years. From these studies, it appears that hTERT is tightly regulated both at transcriptional and posttranscriptional levels. For example, the cloning and characterization of the hTERT 5′ gene regulatory elements have identified more than 20 transcription factor binding sites acting as activators or repressors (for a review, see Poole et al2 ).
Despite increasing knowledge about hTERT transcription regulation, much less is known about which type of internal or external stimuli could interfere with hTERT gene regulation. Recent studies have described that, in epithelial cells, oncogenes, such as Her2/Neu,3 growth factors, such as epidermal growth factor (EGF),4 or steroids5 could activate hTERT at the transcriptional level. In contrast, transforming growth factor-β (TGFβ) acts as a repressor of hTERT gene transcription in a variety of cellular models.6-8
In a previous study, we described that, in leukemic myeloid KG1 cells, tumor necrosis factor-α (TNFα) induced premature senescence, characterized by cellular growth arrest, increased β-galactosidase activity, reduced hTERT activity, telomeric disturbances (shortening, losses, fusions), and intense chromosomal instability.9 Moreover, increased β-galactosidase activity was also found in normal CD34+ as well as in fresh acute myeloid leukemia (AML) cells treated with TNFα.9 To the best of our knowledge, the role of TNFα in hTERT regulation in hematopoietic cells had not been previously documented. This question may be of great significance. Indeed, hTERT plays a critical role not only in telomere homeostasis but also in cellular survival, DNA repair, and genetic stability, whereas TNFα accumulates in a number of pathologic situations, including not only chronic inflammation and neoplastic disorders but also leukemia, myelodysplasia, and aplastic anemia.10-13
The present study was aimed at evaluating whether TNFα could interfere with hTERT expression in normal progenitor cells and in leukemic cells derived from patients with AML. In this study, we also investigated the signaling pathways by which TNFα influences hTERT gene regulation or which could interfere with TNFα.
Materials and methods
Normal progenitor purification and culture
Normal bone marrow CD34+ hematopoietic progenitor cells (HPCs) were obtained from discarded fragments of hematologically healthy patients undergoing hip surgery after informed consent was provided per the Declaration of Helsinki. Mononuclear cells from bone marrow were obtained by Ficoll-Hypaque density gradient centrifugation after which isolation of HPCs was performed by positive selection of CD34-expressing cells. Briefly, CD34+/HPCs were magnetically labeled using magnetic-activated cell sorting (MACS) CD34 microbeads then isolated by positive selection through medium-size (MS) separation column (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the CD34+ cells was evaluated by flow cytometry using anti-CD34 and anti-CD45 monoclonal antibodies and reached 85% to 98%. Human progenitors (5 × 105 cells/mL) were cultured in Iscoves modified Dulbecco medium (IMDM) containing 10% of a commercial mixture of bovine serum albumin (BSA), insulin, and transferrin (BIT9500; StemCell Technologies, Grenoble, France) supplemented with 100 ng/mL stem-cell factor, 1 U/mL interleukin 3, 100 ng/mL fms-like tyrosine kinase type 3 ligand (FLT3-L), and 5 pg/mL thrombopoietin (R&D Systems, Oxon, United Kingdom). Human recombinant TNFα (PeproTech-TEBU, Rocky Hill, NJ) was added to fresh medium containing cytokines every 2 days.
Patient sample culture
Approval for these studies was obtained from the Institut National de la Santé et de la Recherche Médicale (INSERM) U563 institutional review board. Fresh AML cells were obtained from patients diagnosed at the Hematology Department of Toulouse University Medical Center (France), after informed consent was provided, per the Declaration of Helsinki. AML cells were isolated from bone marrow by Ficoll-Hypaque density gradient centrifugation and were cryopreserved in IMDM medium with dimethyl sulfoxide (10% final concentration) and fetal calf serum (FCS; 50% final concentration) or immediately processed for assays. Leukemic cells (1 × 106 cells/mL) were maintained in IMDM containing 10% 5637-conditioned medium (CM-5637) (5637 is a bladder carcinoma cell line). Human recombinant TNFα was added to fresh medium containing CM-5637 every 2 days.
Cell line
The human leukemic cell line KG1 was purchased from American Type Culture Collection (ATCC, Rockville, MD), cultured in IMDM medium supplemented with 20% FCS (Invitrogen, Gibco, Cergy Pontoise, France), and incubated in a humidified incubator containing 5% CO2 at 37°C. The cells were split with fresh medium every 2 or 3 days and maintained at 4 × 105 cells/mL. Cell viability was assessed by trypan blue exclusion. Cells were treated with TNFα (20 ng/mL), human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems), or cell-permeant exogenous ceramide, C6-ceramide (10 μM; Sigma Aldrich, Saint Quentin Fallavier, France).
Immunophenotypic analysis
Anti-CD34 and anti-CD45 antibodies (Beckman Coulter, Villepinte, France) were used to examine surface antigen expression according to the manufacturer's recommendations. Analysis was performed with an EPICS XL-MCL (Beckman Coulter).
Quantitative RT-PCR
Total RNA were isolated by using Trizol Reagent (Invitrogen, Cergy Pontoise, France). Quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) was performed using the LightCycler TeloTAGGGhTERT quantification kit (Roche Diagnostics, Mannheim, Germany) as indicated by the manufacturer. Briefly, hTERT mRNA was reverse transcribed and amplified with specific primers in a one-step RT-PCR reaction. The amplicon was detected by fluorescence, using a specific pair of probes that hybridize to an internal sequence of the amplified fragment during the annealing phase of the amplification cycle. The emitted fluorescence was measured by the LightCycler instrument. In a separate one-step RT-PCR, mRNA encoding for porphobilinogen deaminase (PBGD) was used as a housekeeping gene. Results were expressed as the ratio between hTERT and PBGD transcripts normalized to untreated cells as previously described.9
Telomerase activity
Quantitative determination of telomerase activity was performed using the Telomeric Repeat Amplification Protocol (TRAP) with teloTAGGG telomerase PCR enzyme-linked immunosorbent assay (ELISA) kit (Roche Diagnostics) following the manufacturer's instructions as previously described.9 The relative telomerase activity (RTA) was determined as absorbance of the sample compared with absorbance of the control template.
Western blot
Cells were harvested and lysed with Laemmli buffer, sonicated, and boiled for 5 minutes at 95°C. Samples were separated on 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred onto nitrocellulose membrane, and immunostained. The following primary antibodies were used: anti-ERK (extracellular signal-regulated kinase), anti-JNK (c-jun N-terminal kinase) (Santa Cruz Biotechnology, Le Perray en Yvelines, France), anti–phospho-MAPK (mitogen-activated protein kinase), anti–phospho-JNK, anti–phospho-p38, anti-p38, anti–phospho-MAPKAPK-2 (MAPK-activated protein kinase 2), anti–MAPKAPK-2 (Cell Signaling, Ozyme, Saint Quentin en Yvelines, France), and horseradish peroxidase–labeled antimouse and antirabbit antibodies. Proteins were visualized using the electrochemiluminescence (ECL) detection system (Pierce, Rockford, IL).
Metabolic cell labeling and ceramide quantitation
Total cellular ceramide quantitation was performed by labeling cells to isotopic equilibrium with 1 μCi/mL [0.037 MBq] [9, 10-3H] palmitic acid (Amersham Biosciences, Orsay, France) for 48 hours in complete medium as previously described.14 Cells were then washed and resuspended in complete medium for time-course experiments. Lipids were extracted and resolved by thin-layer chromatography. Ceramide was scraped and quantified by liquid scintillation spectrometry.
Statistics
Results are expressed as mean values ± standard deviations. Statistical analysis of the data was performed by the Student t test. Differences were considered as significant for P values less than .05.
Results
Effect of TNFα on hTERT gene expression in CD34+ normal cells
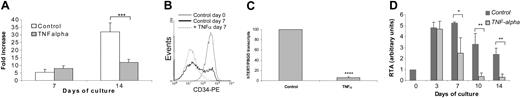
In a first set of experiments, we tested whether TNFα altered hTERT expression in normal bone marrow progenitors. Normal marrow CD34+ cells were cultured in medium containing various cytokines (stem-cell factor [SCF], interleukin-3 [IL3], FLT3-L, and thrombopoietin [TPO]) in the presence or not of TNFα for 14 days. According to a previous study, we used the dose of 20 ng/mL.15 In control cells, this mixture of cytokines induced 5.4-fold and 32-fold expansion at 7 and 14 days, respectively. As expected from previous studies using a similar mixture of cytokines,15 we found that TNFα reduced cellular expansion compared with controls. However, this difference became significant only after 14 days of culture (Figure 1A). We did not observe apoptosis in TNFα-treated cells (data not shown). Moreover, TNFα was found to facilitate differentiation as reflected by reduced CD34 expression compared with control cells after 7 days of culture (Figure 1B), whereas CD34 became undetectable after 14 days of culture in both TNFα-treated cells and control cells (data not shown). In parallel, as shown in Figure 1C, treatment with TNFα resulted in a dramatic decrease in hTERT gene expression compared with untreated cells as revealed by real-time quantitative PCR (93.8% ± 3.4%) followed by a time-dependent reduction in hTERT activity which became almost undetectable after 10 days of culture (Figure 1D). These results show that TNFα inhibits hTERT gene expression in normal myeloid lineage.
Influence of TNFα on normal bone marrow progenitors. Fresh CD34+ were cultured in medium containing various cytokines as described in “Materials and methods” in the presence or absence of TNFα (20 ng/mL) for 7 to 14 days. (A) Viable cells were counted by trypan blue dye exclusion. Results represent the mean ± SD of 5 independent experiments. ***P < .001. (B) Differentiation was analyzed by flow cytometry using anti-CD34 monoclonal antibody at 7 days of culture. Results are representative of 3 independent experiments. (C) Transcripts of hTERT were quantified using the LightCycler TeloTAGGGhTERT quantification kit as described in “Materials and methods.” hTERT/PBGD represents the ratio between hTERT and PBGD transcripts normalized to untreated control cells at 7 days of culture. They are expressed as the mean hTERT/PBGD values from 3 independent experiments ± SDs; ****P < .001. (D) Telomerase activity was measured by using the TeloTAGGG telomerase PCR ELISA kit as described in “Materials and methods.” Results are expressed as mean RTA values ± SDs of 3 independent experiments. *P = .014; **P = .002 and .004.
Influence of TNFα on normal bone marrow progenitors. Fresh CD34+ were cultured in medium containing various cytokines as described in “Materials and methods” in the presence or absence of TNFα (20 ng/mL) for 7 to 14 days. (A) Viable cells were counted by trypan blue dye exclusion. Results represent the mean ± SD of 5 independent experiments. ***P < .001. (B) Differentiation was analyzed by flow cytometry using anti-CD34 monoclonal antibody at 7 days of culture. Results are representative of 3 independent experiments. (C) Transcripts of hTERT were quantified using the LightCycler TeloTAGGGhTERT quantification kit as described in “Materials and methods.” hTERT/PBGD represents the ratio between hTERT and PBGD transcripts normalized to untreated control cells at 7 days of culture. They are expressed as the mean hTERT/PBGD values from 3 independent experiments ± SDs; ****P < .001. (D) Telomerase activity was measured by using the TeloTAGGG telomerase PCR ELISA kit as described in “Materials and methods.” Results are expressed as mean RTA values ± SDs of 3 independent experiments. *P = .014; **P = .002 and .004.
Effect of TNFα on hTERT gene expression in leukemic cells
We next investigated whether TNFα could also regulate hTERT gene expression in myeloid leukemic cells. These experiments were performed with both KG1 cells9 and in fresh AML cells cultured in CM-5637 for 7 days. They revealed that, as for normal myeloid cells, 7 days of exposure to TNFα resulted in a dramatic reduction in hTERT gene expression in KG1 cells.9 However, in these cells, hTERT inhibition was detectable as soon as 2 hours, with a maximum at 6 hours and remained stable over 7 days.9 Dose-effect analysis revealed a maximum reduction of hTERT gene at 20 ng/mL (data not shown). In initial experiments, we obtained similar results with fresh AML cells but experimental conditions based on 7-day exposure were often questionable because of the loss of viability in control cells. For this reason, further experiments were conducted with freshly thawed AML cells or with cells immediately harvested from patients with AML and incubated in short-term liquid culture (2-6 hours) in the presence of CM-5637 with or without TNFα. As depicted in Table 1, treatment with TNFα resulted in rapid hTERT gene reduction in virtually all cases, hTERT transcripts becoming undetectable in 2 of 10 cases. These results show that, as in myeloid normal cells, TNFα negatively influences hTERT expression in myeloid leukemic cells.
Influence of TNFα on hTERT gene expression in AML cells
Sample no. . | hTERT/PBGD-untreated cells . | hTERT/PBGD TNFα, 2 h . | hTERT/PBGD TNFα, 6 h . |
|---|---|---|---|
| 1 | 100 | ND | 60.0 |
| 2 | 100 | 16.1 | Undetectable |
| 3 | 100 | 73.1 | ND |
| 4 | 100 | 51.9 | Undetectable |
| 5 | 100 | ND | 4.4 |
| 6 | 100 | 77.7 | 35.0 |
| 7 | 100 | 107.9 | 57.0 |
| 8 | 100 | 112.0 | 70.6 |
| 9 | 100 | ND | 41.5 |
| 10 | 100 | 92.2 | 79.7 |
| KG1* | 100 | 49.4 ± 9.8† | 27.2 ± 2.1‡ |
Sample no. . | hTERT/PBGD-untreated cells . | hTERT/PBGD TNFα, 2 h . | hTERT/PBGD TNFα, 6 h . |
|---|---|---|---|
| 1 | 100 | ND | 60.0 |
| 2 | 100 | 16.1 | Undetectable |
| 3 | 100 | 73.1 | ND |
| 4 | 100 | 51.9 | Undetectable |
| 5 | 100 | ND | 4.4 |
| 6 | 100 | 77.7 | 35.0 |
| 7 | 100 | 107.9 | 57.0 |
| 8 | 100 | 112.0 | 70.6 |
| 9 | 100 | ND | 41.5 |
| 10 | 100 | 92.2 | 79.7 |
| KG1* | 100 | 49.4 ± 9.8† | 27.2 ± 2.1‡ |
Fresh AML cells and KG1 leukemia cell line were treated or not with TNFα (20 ng/mL) for 2 to 6 hours. hTERT transcripts were quantified as described in “Materials and methods.” Results are expressed as undetectable or as the ratio between hTERT and PBGD transcripts normalized to untreated cells.
ND indicates not done.
For KG1, results are the mean ± SEM of 10 independents experiments
P = .006
P > .001
Role of MAPK in TNFα-induced inhibition of hTERT in KG1 cells
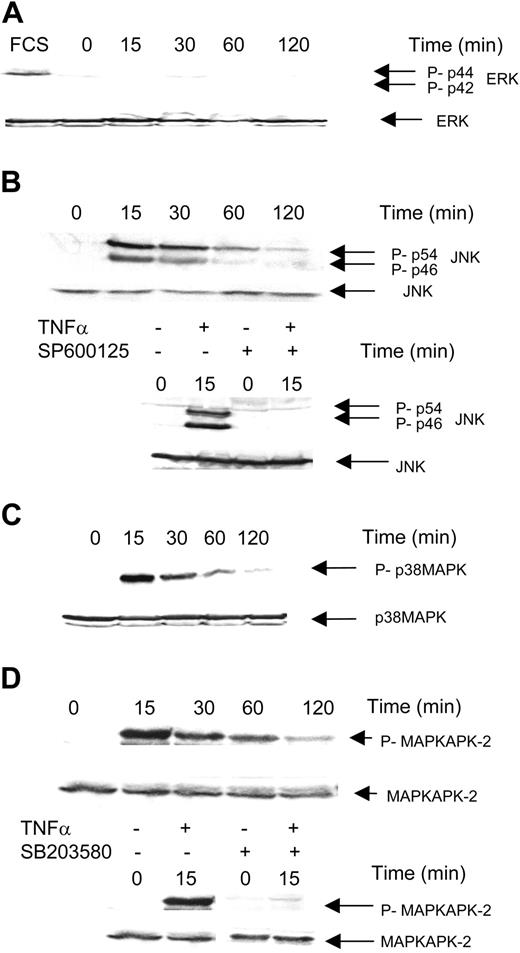
To investigate the signaling pathway regulating hTERT, we used KG1 cell line as a representative model of myeloid cells. Previous studies have described that TNFα activates a complex network of signaling pathways among which MAPK may represent an important functional component through regulation of a wide variety of genes. Therefore, we hypothesized that ERK, JNK, or p38MAPK might play an important role in mediating the inhibitory effect of TNFα on hTERT gene regulation. As a matter of fact, we found that, in KG1 cells, treatment with TNFα induced a rapid increase (as soon as 15 minutes) in JNK and p38MAPK, but not ERK phosphorylation as revealed by immunoblotting with antibodies directed against phosphorylated forms of p44/p42ERK, p56/p44JNK, and p38MAPK (Figure 2A,B,C, respectively). Moreover, we found that JNK activation was abrogated by pretreatment (one hour) with the JNK inhibitor SP600125 (20 μM), whereas the p38MAPK inhibitor SB203580 (1 μM) abrogated the activation of MAPKAPK-2, a substrate of p38MAPK (Figure 2B,D). Then, we investigated whether the JNK or p38MAPK pathway inhibition may interfere with TNFα-induced hTERT gene inhibition. As shown in Table 2, we found that pretreatment with the JNK inhibitor SP600125 (20 μM) abrogated the inhibitory effect of TNFα on hTERT, whereas pretreatment with the p38MAPK inhibitor SB203580 (1 μM) had no effect. These results suggest that JNK is specifically involved in TNFα-mediated hTERT gene regulation.
Influence of MAPK inhibitors on TNFα-induced inhibition of hTERT in KG1 cells
Cells . | hTERT/PBGD . |
|---|---|
| KG1 | 100 |
| KG1 + TNFα | 49.9 ± 9.8† |
| KG1 + SP600125 | 108.1 ± 18.1 |
| KG1 + TNFα + SP600125 | 98.5 ± 23.1* |
| KG1 + SB203580 | 33.1 ± 4.5 |
| KG1 + TNFα + SB203580 | 34.2 ± 11.6 |
Cells . | hTERT/PBGD . |
|---|---|
| KG1 | 100 |
| KG1 + TNFα | 49.9 ± 9.8† |
| KG1 + SP600125 | 108.1 ± 18.1 |
| KG1 + TNFα + SP600125 | 98.5 ± 23.1* |
| KG1 + SB203580 | 33.1 ± 4.5 |
| KG1 + TNFα + SB203580 | 34.2 ± 11.6 |
KG1 cells were pretreated or not with SP600125 (20 μM) or SB203580 (1 μM) for 1 hour, then treated with TNFα (20 ng/mL) for 2 to 6 hours. hTERT transcripts were quantified as described in “Materials and methods.” hTERT/PBGD represents the ratio between hTERT and PBGD transcripts normalized to untreated control cells. Results are expressed as mean hTERT/PBGD values ± SDs from 3 independent experiments.
P = .014
P = .006
Role of ceramide in TNFα-induced inhibition of hTERT in KG1 cells
Previous studies have documented that, in myeloid leukemic cells, TNFα stimulates the JNK pathway by activating the sphingomyelin cycle.16 This pathway consists in the stimulation of a neutral Mg2+-dependent sphingomyelinase responsible for sphingomyelin hydrolysis and subsequent generation of ceramide that activates the JNK cascade through a redox-dependent mechanism.17 Moreover, independent studies have shown that, at least in nonhematopoietic cellular models, ceramide interferes with hTERT gene expression.18,19 For all these reasons, we speculated that ceramide could mediate the effect of TNFα on hTERT expression in KG1 cells. As shown in Figure 3A, labeling studies revealed that, in KG1 cells, intracellular ceramide concentration increased on TNFα stimulation with a maximum of 145% at 3 to 5 minutes, and then returned to basal line. These results suggest that TNFα may indeed activate the sphingomyelin cycle in KG1 cells. To evaluate the functional consequence of endogenous ceramide release, we treated KG1 cells with cell-permeant exogenous ceramide (C6-ceramide) (10 μM) for 2 hours and hTERT transcripts were measured using real-time quantitative PCR (RQ-PCR). As shown in Figure 3B, treatment with C6-ceramide resulted in a 40% decrease in hTERT PCR products. Ceramide-mediated hTERT inhibition was therefore in a same range as that observed with TNFα. The fact that C6-ceramide mimicked the effect of TNFα strongly suggests that TNFα-induced ceramide production is responsible for hTERT regulation. Moreover, the effect of C6-ceramide was inhibited by the JNK inhibitor SP600125, suggesting that, like for TNFα, JNK mediated the effect of C6-ceramide on hTERT (Figure 3B). These results suggest that ceramide plays an important role in mediating the effect of TNFα on hTERT through a JNK-dependent mechanism.
Role of MAPK in TNFα-induced inhibition of hTERT in KG1 cells. KG1 cells were treated or not with TNFα for 15 to 120 minutes after overnight serum deprivation. MAPK expression was evaluated by Western blot analysis as described in “Materials and methods.” (A) Phospho-p42/44 ERK and p42/44 ERK expression. FCS was used as positive control. (B) Phospho-p46/54 JNK and p46/54 JNK expression. (C) Phospho-p38 and p38 expression. (D) Phospho-MAPKAPK-2 and MAPKAPK-2 expression.
Role of MAPK in TNFα-induced inhibition of hTERT in KG1 cells. KG1 cells were treated or not with TNFα for 15 to 120 minutes after overnight serum deprivation. MAPK expression was evaluated by Western blot analysis as described in “Materials and methods.” (A) Phospho-p42/44 ERK and p42/44 ERK expression. FCS was used as positive control. (B) Phospho-p46/54 JNK and p46/54 JNK expression. (C) Phospho-p38 and p38 expression. (D) Phospho-MAPKAPK-2 and MAPKAPK-2 expression.
Role of ceramide in TNFα-induced activation of JNK. (A) KG1 cells were prelabeled with [3H]-palmitic acid for 48 hours, washed, and treated with TNFα (20 ng/mL) at the indicated time. Intracellular levels of ceramide were analyzed as described in “Materials and methods.” Results are expressed as the mean values from 3 independent experiments ± SDs. *P < .05. (B) KG1 cells were preincubated or not for 1 hour with JNK inhibitor SP600125 (20 μM) then treated or not with C6-ceramide (10 μM) for 2 hours. Transcripts of hTERT were quantified using the LightCycler TeloTAGGGhTERT quantification kit as previously described. Results are expressed as mean ± SD from 4 independent experiments. ****P < .001; *P = .03.
Role of ceramide in TNFα-induced activation of JNK. (A) KG1 cells were prelabeled with [3H]-palmitic acid for 48 hours, washed, and treated with TNFα (20 ng/mL) at the indicated time. Intracellular levels of ceramide were analyzed as described in “Materials and methods.” Results are expressed as the mean values from 3 independent experiments ± SDs. *P < .05. (B) KG1 cells were preincubated or not for 1 hour with JNK inhibitor SP600125 (20 μM) then treated or not with C6-ceramide (10 μM) for 2 hours. Transcripts of hTERT were quantified using the LightCycler TeloTAGGGhTERT quantification kit as previously described. Results are expressed as mean ± SD from 4 independent experiments. ****P < .001; *P = .03.
Influence of GM-CSF on TNFα effects
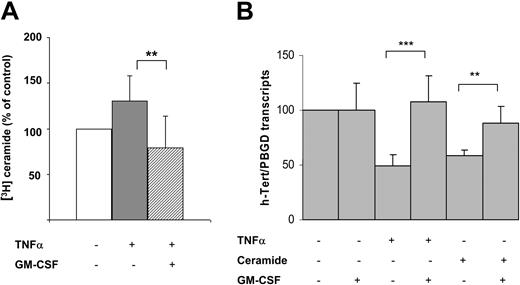
In further experiments, we considered the possibility that GM-CSF could interfere with the inhibitory effect of TNFα on the hTERT gene. This hypothesis was supported by the fact that GM-CSF has been found to negatively regulate stress-induced ceramide production.20 For this reason, KG1 cells were cotreated with TNFα (20 ng/mL) and GM-CSF (13 ng/mL), and ceramide production was investigated. These experiments revealed that GM-CSF inhibited TNFα-induced ceramide generation as illustrated in Figure 4A. Moreover, GM-CSF inhibited the effect of both TNFα and ceramide on the hTERT gene (Figure 4B), suggesting that GM-CSF acted both upstream and downstream of ceramide production.
Discussion
This study shows that TNFα regulates telomerase in both normal and leukemia cells. This regulation involves ceramide and JNK and is inhibited by GM-CSF.
Concerning CD34+ normal progenitors, previous studies have demonstrated that telomerase activity is detectable at low levels in hematopoietic progenitor cells and is up-regulated in response to cytokine stimulation.21,22 Our study suggests that, in hematopoietic cells, regulation of hTERT enzymatic activity is under the control of the hTERT gene as described in other cellular models, although we cannot rule out complementary posttranscriptional mechanisms. Our study shows for the first time that, in vitro, TNFα inhibited hTERT gene and enzymatic activity in normal myeloid progenitors. Although the functional consequence of this regulation remains uncertain, it is possible that hTERT inhibition mediates the negative effect of TNFα in cellular expansion,23 through 2 hypothetic mechanisms. First, based on the protective function of hTERT in many tissues,24 it is conceivable that TNFα-induced hTERT inhibition results in an increase in cell loss along myeloid differentiation even though we found no increase in apoptosis. Second, it is possible that hTERT plays by itself an important function in differentiation commitment as recently suggested.25
Influence of GM-CSF on ceramide pathway. (A) Intracellular ceramide variations measured at the peak of stimulation in KG1 cells treated with TNFα (20 ng/mL) and GM-CSF (13 ng/mL). Results are expressed as mean ± SD from 5 independent experiments. **P = .008. (B) KG1 cells were incubated with or without TNFα, C6-ceramide (10 μM), or GM-CSF for 2 hours. Transcripts of hTERT were quantified using the LightCycler TeloTAGGGhTERT quantification kit as previously described. Results are expressed as mean ± SD from 4 independent experiments. ***P < .001; **P = .004.
Influence of GM-CSF on ceramide pathway. (A) Intracellular ceramide variations measured at the peak of stimulation in KG1 cells treated with TNFα (20 ng/mL) and GM-CSF (13 ng/mL). Results are expressed as mean ± SD from 5 independent experiments. **P = .008. (B) KG1 cells were incubated with or without TNFα, C6-ceramide (10 μM), or GM-CSF for 2 hours. Transcripts of hTERT were quantified using the LightCycler TeloTAGGGhTERT quantification kit as previously described. Results are expressed as mean ± SD from 4 independent experiments. ***P < .001; **P = .004.
To investigate the signaling pathway regulating hTERT, we used the KG1 cell line as a representative model of myeloid cells. We show that TNFα induces early and transient ceramide generation in KG1 cells. Others and we have documented that, in other AML cell lines such as U937 cells, TNFα induced ceramide generation due to stimulation of a neutral sphingomyelinase and subsequent sphingomyelin (SM) hydrolysis (SM cycle).26,27 The kinetics and the magnitude of ceramide production observed in KG1 cells are very similar to that of TNFα-treated U937 cells. These results suggest that TNFα activated the SM cycle in KG1 cells. Previous studies showed that ceramide inhibited hTERT in epithelial tumor cells.18,19 Therefore, we hypothesized that, in KG1 cells, ceramide could mediate the effect of TNFα. Actually, cell-permeant ceramide inhibited hTERT gene expression in KG1 cells. This result suggests that the SM cycle is an important component of TNFα signaling leading to hTERT inhibition. If this is the case, it can be assumed that any negative regulators of this pathway, including increased protein kinase C activity or enhanced antioxidative defenses,28 may confer a significant protection of AML cells toward TNFα.
In additional studies, we investigated the effect of TNFα on MAPK. Indeed, previous studies have documented that TNFα may activate the 3 MAPK modules, that is, ERK/MAPK, JNK/SAPK (stress-activated protein kinase), and p38MAPK in various AML cellular models. Moreover, p38MAPK has been implicated in cellular senescence.29 For this reason, we used specific pharmacologic inhibitors to investigate the respective contribution of these pathways in TNFα-induced hTERT regulation. We found that JNK, but not ERK or p38MAPK, is involved in TNFα signaling leading to hTERT gene inhibition. This result contrasts with other studies, which showed that, at least in epithelial cells, JNK is an activator of the hTERT gene,30,31 suggesting that the role of JNK in hTERT regulation is cell specific. Further studies are needed to identify the regulatory mechanisms, which operate downstream of JNK and interfere with hTERT regulation in myeloid cells.
GM-CSF was found to protect cells from hTERT inhibition by TNFα. GM-CSF is not a unique growth factor capable to positively regulate hTERT. Indeed, it has been reported that insulin-like growth factor 1 (IGF-1) or IL6 abrogated dexamethasone-induced down-regulation of hTERT activity in multiple myeloma cells, and that phosphatidylinositol 3-kinase (PI3K) and nuclear factor κB (NF-κB) pathways mediated protective effects of IGF-1 and IL6.32 In KG1 cells, we found that GM-CSF inhibited ceramide generation. Moreover, this growth factor inhibits C6-ceramide inhibition of hTERT. These results suggest that GM-CSF exerts a dual mechanism that involves both abrogation of ceramide generation induced by TNFα and interruption of ceramide signaling.
In summary, our study shows that TNFα down-regulates the hTERT gene in normal and leukemic cells through the ceramide-JNK pathway. Hematopoietic growth factors efficiently counterregulate this signaling. On the basis of the role of hTERT in cellular protection, chromosome stability, and perhaps differentiation, the negative effect of TNFα may have important functional consequences, including abnormal regulation of hematopoietic cell differentiation, genetic instability, and marrow insufficiency.
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-04-1386.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Association Laurette Fugain, the Association Cent pour Sang La Vie and by a fellowship from the Ligue Nationale contre le Cancer (N.P.-H.).
N.P.-H., O.B.-R., and V.M.-D.M. performed the research and analyzed the data; J.A. provided the discarded fragment from hip surgery; C.R. and C.D. provided the fresh AML cells; G.L., O.B.-R., and V.M.-D.M. designed the research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Monique Laroche and Nicole Lhermie for technical assistance and Jean-Pierre Jaffrézou for improving the English manuscript.

![Figure 3. Role of ceramide in TNFα-induced activation of JNK. (A) KG1 cells were prelabeled with [3H]-palmitic acid for 48 hours, washed, and treated with TNFα (20 ng/mL) at the indicated time. Intracellular levels of ceramide were analyzed as described in “Materials and methods.” Results are expressed as the mean values from 3 independent experiments ± SDs. *P < .05. (B) KG1 cells were preincubated or not for 1 hour with JNK inhibitor SP600125 (20 μM) then treated or not with C6-ceramide (10 μM) for 2 hours. Transcripts of hTERT were quantified using the LightCycler TeloTAGGGhTERT quantification kit as previously described. Results are expressed as mean ± SD from 4 independent experiments. ****P < .001; *P = .03.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2005-04-1386/6/m_zh80210586240003.jpeg?Expires=1769213718&Signature=OGXEplpwKCJUwlc63zHx8-nlA9lEdKpv70MNrzRgN53FCrkSaOmBwtQ-mu9n2CaynbHWcIeVUOnzd-AHdgq3vUKNgonOqSPpnzUiq-MR9~ooBEnHb4IQ4eC9nWaMhkjJAoqlHgXxJXxPM~UQ49Vx8cXhzSpYE5haQql~wkHSs2KQV~MlUs6gV9W4O9tzJc5TPBhYGOfe7EmVNWEbJC3cMinqtScVAT1FPBTo8BwUvg5Ao2M75Zvs~Dg5CAefX7e0x9~xJ6OqaQ94Ikp5R6owleWKWHhKlvQzjk9XhdxVqvkzqQJ3YpAGpby4nkX2WC4lVmviFnpBAUfBqDWUJH0SOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal