Abstract
Multiple myeloma (MM) develops devastating bone destruction with enhanced bone resorption and suppressed bone formation. In contrast to enhanced osteoclastogenesis, little is known about the mechanism of impaired bone formation in MM. Because a canonical Wingless-type (Wnt) signaling pathway has recently been shown to play an important role in osteoblast differentiation, we examined whether MM cells affect a canonical Wnt pathway to suppress bone formation. Conditioned media from RPMI8226 and U266 MM cell lines and primary MM cells suppressed in vitro mineralization as well as alkaline phosphatase activity in osteoblasts induced by bone morphogenetic protein 2 (BMP-2). These cell lines constitutively produced a soluble Wnt inhibitor, secreted Frizzled-related protein 2 (sFRP-2), but not other Wnt inhibitors including sFRP-1, sFRP-3, and dickkopf 1 (DKK-1) at the protein level. Most MM cells from patients with advanced bone destructive lesions also expressed sFRP-2. Furthermore, exogenous sFRP-2 suppressed osteoblast differentiation induced by BMP-2, and immunodepletion of sFRP-2 significantly restored mineralized nodule formation in vitro, suggesting a predominant role for MM cell-derived sFRP-2 in the impairment of bone formation by MM. Thus, in addition to enhanced osteolysis, MM cells also suppress bone formation at least in part through an inhibition of the canonical Wnt pathway by secreting sFRP-2.
Introduction
Multiple myeloma (MM) almost exclusively develops and expands in the bone marrow (BM) and generates devastating bone destruction by osteoclasts (OCs). The bone destruction causes debilitating clinical symptoms including intractable bone pain, disabling multiple fractures, and hypercalcemia. The severity of bone disease correlates with the tumor burden and is one of the major parameters in the Durie and Salomon clinical staging system. Furthermore, the aggressive features of MM bone lesions have contributed significantly to its poor prognosis despite the recent development of intensive chemotherapeutic regimens.1,2 Therefore, elucidation of the molecular mechanism of bone destruction and tumor progression is essential for the development of effective therapies to improve survival as well as quality of life of patients with MM.
Interaction between receptor activator of nuclear factor-κB (RANK) expressed on the surface of cells of osteoclastic lineage and RANK ligand expressed on stromal cells plays a key role in the development and activation of OCs, whereas osteoprotegerin, a decoy receptor for RANK ligand secreted from stromal cells, inhibits RANK ligand-RANK signaling.3,4 MM cells stimulate osteoclastogenesis by triggering a coordinated increase in RANK ligand and decrease in osteoprotegerin in the BM.5-7 We and others have demonstrated that osteoclastogenic CC chemokines macrophage inflammatory protein 1α (MIP-1α) and MIP-1β are secreted from most MM cells and play a critical role in the development of MM bone lesions.8-12 These chemokines directly act on MM cells in an autocrine/paracrine fashion and enhance MM cell adhesion to stromal cells through activation of integrins including very late antigen 4. The interaction between MM cells and stromal cells induces RANK ligand expression by stromal cells, leading to OC differentiation and activation.8 Furthermore, OCs enhance MM cell growth and survival through a cell-to-cell contact-dependent mechanism that is partially mediated by OC-derived interleukin 6 (IL-6) and osteopontin.13,14 These observations suggest that interactions of MM cells and OCs form a vicious cycle leading to extensive bone destruction and MM cell expansion.
Along with enhanced bone resorption, mineralization is impaired in MM bone lesions. Radiographic examinations show radiolucent lesions without calcification known as “punched-out” lesions. Analyses of bone turnover in patients with MM by biochemical bone markers also suggested an imbalance of bone turnover with enhanced bone resorption and suppressed bone formation.15 However, the mechanisms of impaired bone formation in bone lesions of patients with MM remain poorly understood.
A canonical Wingless-type (Wnt) signaling pathway has been shown to play an important role in osteoblast differentiation. Wnts are secreted cysteine-rich glycoproteins, known as regulators of the differentiation of hematopoietic and mesenchymal cells as well as embryonic development.16-18 Wnt proteins bind to the Frizzled receptor and low-density lipoprotein receptor-related protein (LRP-5/6) complex and induce a canonical Wnt signaling pathway. Activation of Wnt signaling stabilizes β-catenin and causes its nuclear accumulation to cooperatively regulate target gene expression with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors.19,20 Loss of function mutations in the Wnt coreceptor LRP5 gene cause reduced bone mass in osteoporosis-pseudoglioma syndrome, and LRP-5–deficient mice have osteopenia with suppressed osteoblast proliferation.18,21 Conversely, activating mutations of LRP5 gene led to a hereditary syndrome of high bone mass.22,23 These findings indicate that the canonical Wnt signaling pathway positively regulates bone formation and thereby bone mass. Therefore, in the present study, we aimed to clarify mechanisms of suppressed bone formation by MM with particular focus on a canonical Wnt signaling pathway. We found that most of primary MM cells from patients with advanced bone lesions as well as MM cell lines produce a soluble Wnt antagonist, secreted Frizzled-related protein 2 (sFRP-2), which potently suppresses osteoblast differentiation and bone formation.
Patients, materials, and methods
Chemicals
Recombinant human (rh) bone morphogenetic protein 2 (BMP-2) and recombinant mouse sFRP-2 were obtained from R&D Systems (Minneapolis, MN). Goat polyclonal antibody against human noggin, chordin, sFRP-1, sFRP-3, and dickkopf 1(DKK-1), rabbit polyclonal antibody against human sFRP-2, and protein A– and protein G–Agarose beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). l-Ascorbic acid was obtained from Wako Pure Chemical (Osaka, Japan). β-Glycerophosphate was from Sigma (St Louis, MO).
Cells and cultures
Human MM cell lines, RPMI8226 and U266, and a human osteosarcoma cell line, MG63, were obtained from the American Type Culture Collection (Rockville, MD). A mouse preosteoblastic cell line, MC3T3-E1, was obtained from RIKEN (Tsukuba, Japan). BM mononuclear cells and peripheral blood mononuclear cells (PBMNCs) were isolated by Ficoll-Hypaque density gradient centrifugation (Pharmacia LKB Biotechnology, Uppsala, Sweden) from heparinized BM and peripheral blood drawn from patients with MM under written informed consent, according to the Declaration of Helsinki. MM cells were further purified from BM mononuclear cells with positive selection using CD138 (Syndecan-1) microbeads and the Miltenyi magnetic cell-sorting system (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. Primary stromal cells derived from fresh BM aspirates from patients with MM were isolated and cultured as we previously described.24 All procedures involving human specimens were performed according to the protocol approved by the Institutional Review Board for human protection.
Collection of conditioned media
MM cell lines and PBMNCs were cultured at 5 × 105 cells/mL in RPMI 1640 with 1% fetal bovine serum (FBS) for 2 days. Their conditioned media (CM) were collected, filtered with 0.22-μm filter systems (MILLEX-GV; Millipore, Bedford, MA), and stocked at –80°C until use.
Osteoblast differentiation
To examine osteoblast differentiation in vitro, MC3T3-E1 cells and BM-derived stromal cells were grown to confluence in 12- or 24-well culture plates in osteogenic medium, α-minimal essential medium (α-MEM) containing 10% FBS, β-glycerophosphate, and ascorbic acid. The medium was replaced every 3 days. For estimation of alkaline phosphatase (ALP) activity, after culturing for 10 days, the cells were washed twice with ice-cold phosphate-buffered saline (PBS), and incubated with 200 μL 1 × Passive Lysis Buffer (Promega, Madison, WI). After gentle shaking for 15 minutes at room temperature, the cells were scraped off and centrifuged at 1000g (12 000 rpm) for 5 minutes. ALP activity was determined using an ALP activity assay kit (Wako Pure Chemical) according to the manufacturer's instruction. Protein concentrations of the supernatants were assayed using Advanced Protein Assay Reagent (Cytoskeleton, Denver, CO).
For analyzing mineralized nodule formation, osteoblastic cells were cultured for up to 28 days and fixed with 10% neutral-buffered formalin and visualized by von Kossa staining as described previously.25
RNA analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). For reverse transcription–polymerase chain reaction (RT-PCR), 2 μg total RNA was reverse transcribed using SuperScript2 RNase H– reverse transcriptase (Invitrogen) and random primers (Promega, Madison, WI) in a 20-μL reaction solution for 50 minutes at 42°C. One microliter of reaction solution was used for subsequent PCR analysis using AmpliTaq gold DNA polymerase (Applied Biosystems, Foster City, CA) with cycles for 30 seconds at 95°C, for 30 seconds at 58°C to 60°C, and for 30 seconds at 72°C. Specific primers used were: 5′-cactacgacccaggcttcat-3′ and 5′-ctccgcagcttcttgcttag-3′ for human noggin; 5′-ctctgctcactctgcacctg-3′ and 5′-ccggtcaccatcaaaatagc-3′ for human chordin; 5′-tgccacctgagaaaggctac-3′ and 5′-acagacaggctcatccgact-3′ for human follistatin; 5′-tctacaccaagccacctcag-3′ 5′-cagtcaccccattcttcagg-3′ for human sFRP-1; 5′-ctcgctgctgctgctcttc-3′ and 5′-ggcttcacatacctttggag-3′ for human sFRP-2; 5′-atggtctgcggcagcccgg-3′ and 5′-ctgtcgtacactggcagctc-3′ for human sFRP-3; 5′-aggcgtgcaaatctgtctcg-3′ and 5′-tgcatttggatagctggtttagt-3′ for human DKK-1; and 5′-tgtcttcaccaccatggagaagg-3′ and 5′-gtggatgcagggatgatgttctg-3′ for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Amplified products were dissolved in a 2% agarose gel and visualized with ethidium bromide staining.
Western blot analysis
Total cell lysates were prepared with cell lysis buffer (Cell Signaling, Beverly, MA). For immunoprecipitation of human sFRP-2, MM cell lines were cultured at 5 × 105 cells/mL in RPMI 1640 without FBS for 2 days. Their CM were incubated with 10 μg/mL of specific antibodies overnight at 4°C. Protein A– or protein G–Agarose beads were added (25 μL packed beads/mL CM) and gently mixed for 1 hour. After centrifugation at 1000g (12 000 rpm) for 5 minutes at 4°C, immunoprecipitants were resuspended in sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris [tris(hydroxymethyl) aminomethane)]–HCl, pH 6.9, 5% β-mercaptoethanol, 3% SDS, 0.1% bromphenol blue, and 3.75% glycerol), and boiled at 100°C for 5 minutes. Equal amounts of protein samples were electrophoresed in 10% SDS–polyacrylamide gel electrophoresis gel and blotted onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). After blocking with 5% nonfat dry milk, the membranes were incubated with primary antibodies overnight at 4°C, followed by addition of a horseradish-peroxidase (HRP)–conjugated secondary antibody for 1 hour. The protein bands were visualized with an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, United Kingdom).
Immunohistochemistry
BM clot sections obtained from patients with MM were fixed in neutral-buffered formalin and embedded in paraffin. Paraffin-embedded tissue sections were deparaffinized and hydrated. After a 10-minute blocking process, the sections were incubated with anti–sFRP-2 antibody or control rabbit IgG overnight at 4°C or 1 hour at 37°C. After washing, immunoreactivity was detected by biotinylated secondary antibodies and HRP-conjugated streptavidin (Dako LSAB+ System; Dako, Carpinteria, CA) followed by diaminobenzidine substrate (Wako Pure Chemical) according to the manufacturer's instruction.
Immunocytochemistry
Isolated MM cells were prepared at 1 × 106 cells/mL and centrifuged at 19g (1000 rpm) for 10 minutes using a Cytospin 3 system (Shandon Scientific, Pittsburgh, PA). After fixing with 4% paraformaldehyde, the cells were washed and fixed with methanol containing 0.3% H2O2 for 15 minutes at room temperature. After washing and a 10-minute blocking process, the cells were then incubated with primary antibody for 1 hour at 37°C. Immunoreactivity was detected by Dako LSAB+ System and diaminobenzidine substrate.
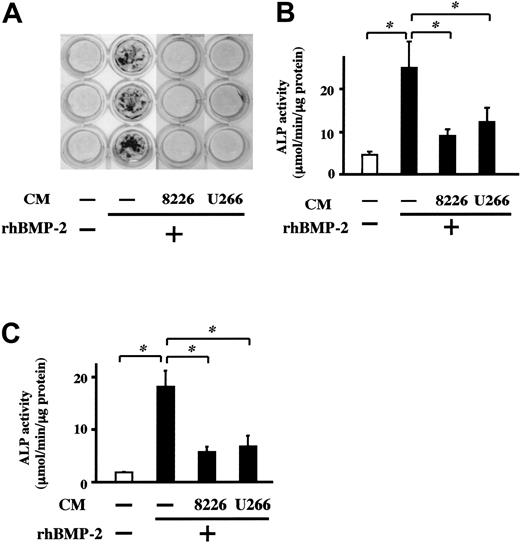
MM cell line CM suppresses BMP-2–induced mineralized nodule formation and ALP activity in osteoblasts. MC3T3-E1 cells (A-B) and BM-derived osteoblasts (C) were cultured at 5 × 105 cells/mL in 24-well culture plates in the osteogenic medium described in “Patients, materials, and methods.” CM from the indicated cell lines or control media were added at 20% in triplicate in the presence or absence of rhBMP-2 (50 ng/mL). Culture media were changed every 3 days. Mineralized nodules were visualized at day 14 by von Kossa staining (A). The cells were harvested at day 10, and ALP activity of cell lysates was measured (B-C). Results are expressed as means ± SEM of triplicate experiments. * indicates significantly different by Student t tests, P < .05.
MM cell line CM suppresses BMP-2–induced mineralized nodule formation and ALP activity in osteoblasts. MC3T3-E1 cells (A-B) and BM-derived osteoblasts (C) were cultured at 5 × 105 cells/mL in 24-well culture plates in the osteogenic medium described in “Patients, materials, and methods.” CM from the indicated cell lines or control media were added at 20% in triplicate in the presence or absence of rhBMP-2 (50 ng/mL). Culture media were changed every 3 days. Mineralized nodules were visualized at day 14 by von Kossa staining (A). The cells were harvested at day 10, and ALP activity of cell lysates was measured (B-C). Results are expressed as means ± SEM of triplicate experiments. * indicates significantly different by Student t tests, P < .05.
Immunodepletion of sFRP-2
MM cell CM was incubated for overnight at 4°C with constant mixing with either 20 μg/mL rabbit anti–human sFRP-2 antibody or normal rabbit IgG. Protein A–Agarose beads (50 μL packed beads) were then added and further incubated for 1 hour, followed by centrifugation at 12 000 rpm for 5 minutes at 4°C. Supernatants were harvested, filtered with 0.22-μm filter systems, and stocked at –80°C before use.
Dual-luciferase assay
Primary osteoblasts were prepared from calvariae of newborn ICR mice. Osteoblasts in 12-well culture plates at 50% to 60% confluence were cotransfected with 1 μg TCF reporter plasmid (TOPflash; Upstate Biotechnology, Lake Placid, NY) and 0.025 μg pRL-TK Renilla luciferase plasmids (Promega, San Luis Obispo, CA) using GenePorter 2 Transfection Reagents (GenLantis, San Diego, CA) in opti-MEM (Invitrogen, Grand Island, NY) with 1% FBS, followed by replacement by opti-MEM with 5% FBS. After incubation for 24 hours, luciferase activities were measured with luminometer (ATTO, Tokyo, Japan) by mixing 50 μL luciferase substrate solution (Promega, San Luis Obispo, CA) with 10 μL lysates. Transcriptional activity was normalized for Renilla luciferase activity.
Statistical analysis
Statistical significance was determined by Student t tests. The minimal level of significance was a P value equal to .05.
Results
MM cell CM suppressed osteoblast differentiation induced by BMP-2
To clarify whether MM cells can suppress osteoblast differentiation, we first examined the effects of CM from MM cell lines on mineralized nodule formation induced by BMP-2.26-29 When MC3T3-E1 osteoblastic cells were cultured in osteogenic medium with BMP-2, mineralized nodules were formed at day 14 (Figure 1A). Addition of CM from RPMI8226 and U266 cells almost completely suppressed mineralized nodule formation in the presence of BMP-2 (Figure 1). These results suggest that soluble factors secreted from MM cells inhibit the mineralization process under BMP-2 stimulation. Because BMP-2 can stimulate osteoblast differentiation from early to terminal stages,26-29 we next examined the effects of these CM on the induction of ALP, a marker of relatively early osteoblast differentiation. CM from RPMI8226 and U266 cells substantially suppressed ALP activity enhanced by BMP-2 in both MC-3T3E1 cells (Figure 1B) and BM-derived osteoblasts (Figure 1C). Thus, MM-cell–derived soluble factors block osteoblast differentiation.
MM cells produce soluble Wnt inhibitors
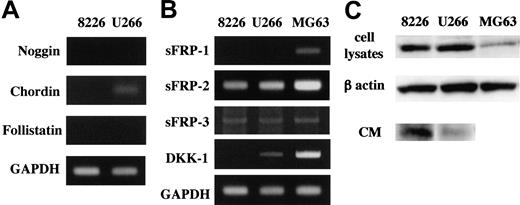
From these observations, we hypothesized that MM cells secrete soluble factors that block BMP-2 actions. Noggin, chordin, and follistatin are among potent soluble antagonists of BMP-2 that interfere with BMP-2 binding to its cognate receptors.30-32 However, none of these proteins were detected in CM from RPMI8226 and U266 cells, although only chordin was observed in U266 cells at the mRNA level (Figure 2A). Thus, it was unlikely that MM cells blocked osteoblast differentiation via secretion of these BMP antagonists.
We next focused on a canonical Wnt pathway that plays an important role in osteoblast differentiation. Activation of the Wnt pathway induces ALP activity in cells of osteoblastic lineage,33-35 and its blockade suppresses ALP activity and mineralization induced by BMP-2.34 Furthermore, BMP-2 induces expression of Wnt-3A,33,34,36 and Wnt-3A overexpression abrogates inhibitory effects of noggin on ALP induction.34 Recently, soluble inhibitors of a canonical Wnt pathway have been identified, including sFRP and DKK family members.37 Therefore, we next examined expression of soluble Wnt antagonists including sFRP-1, sFRP-2, and sFRP-3 as well as DKK-1 in MM cell lines. Both RPMI8226 and U266 cells expressed sFRP-2 and sFRP-3 mRNA. However, no cell lines expressed sFRP-1, and DKK-1 was expressed only in U266 cells at the mRNA level (Figure 2B). We next examined production and secretion of these proteins by Western blotting. We found strong immunoreactivity of sFRP-2 in both CM and total cell lysates of these cells (Figure 2C). However, no other factors were detected at the protein level. These results suggest that sFRP-2 secreted by MM cells plays a role in the suppression of osteoblast differentiation by MM cell CM.
Expression of soluble BMP-2 and Wnt antagonists by MM cell lines. mRNA expression of antagonists for BMP-2 (A) and Wnts (B). Total RNA (2 μg) extracted from the indicated cell lines was reverse transcribed, and 1 μL of the 20-μL reaction was used in PCR analyses as described in “Patients, materials, and methods.” BMP-2 antagonists noggin, chordin, and follistatin (A) and Wnt antagonists sFRP-1, sFRP-2, sFRP-3 and DKK-1 (B) and GAPDH mRNA expression were analyzed. The MG63 osteosarcoma cell line was used as a positive control. (C) sFRP-2 protein was detected in total cell lysates extracted from the indicated cell lines (top lane) and immunoprecipitants of their CM using sFRP-2 antibody and protein A–Agarose beads (bottom lane) by Western blot analysis. β-actin was shown as a protein loading control of total cell lysates (middle lane).
Expression of soluble BMP-2 and Wnt antagonists by MM cell lines. mRNA expression of antagonists for BMP-2 (A) and Wnts (B). Total RNA (2 μg) extracted from the indicated cell lines was reverse transcribed, and 1 μL of the 20-μL reaction was used in PCR analyses as described in “Patients, materials, and methods.” BMP-2 antagonists noggin, chordin, and follistatin (A) and Wnt antagonists sFRP-1, sFRP-2, sFRP-3 and DKK-1 (B) and GAPDH mRNA expression were analyzed. The MG63 osteosarcoma cell line was used as a positive control. (C) sFRP-2 protein was detected in total cell lysates extracted from the indicated cell lines (top lane) and immunoprecipitants of their CM using sFRP-2 antibody and protein A–Agarose beads (bottom lane) by Western blot analysis. β-actin was shown as a protein loading control of total cell lysates (middle lane).
sFRP-2 expression by primary MM cells
To investigate whether sFRP-2 is expressed by primary MM cells in the BM, we next examined sFRP-2 expression by RT-PCR and immunocytochemistry in MM cells purified from patients with bone destruction. sFRP-2 was expressed by 3 of 4 primary MM cells at the mRNA level (Figure 3A) and its immunoreactivity was observed in parallel with the mRNA expression (Figure 3B). sFRP-2 expression was further confirmed in MM cells in BM clot samples from patients with advanced bone disease. Immunoreactivity of sFRP-2 was observed in MM cells in 10 of 14 BM specimens including one from a patient with plasma cell leukemia (data not shown). However, sFRP-2 was detected in only one of 5 BM samples from patients without apparent bone lesions (data not shown).
sFRP-2 alone can suppress BMP-2–induced osteoblast differentiation
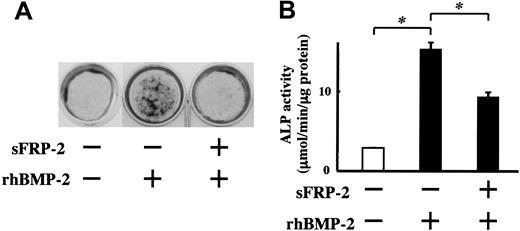
sFRP family members are demonstrated to act as decoy receptors to interfere with Wnt binding to its receptor, Frizzled, and sFRP-1 has been shown to suppress osteoblast differentiation similar to DKK-1.37-40 However, the effect of sFRP-2 on osteoblast differentiation has not been previously examined. To clarify the biologic effects of sFRP-2 on osteoblast differentiation, we added recombinant sFRP-2 to MC3T3-E1 cell cultures in the presence of BMP-2. sFRP-2 partially but significantly suppressed ALP activity induced by BMP-2. In addition, mineralized nodule formation was markedly inhibited by sFRP-2 (Figure 4). These results indicate that sFRP-2 is able to suppress osteoblast differentiation induced by BMP-2.
sFRP-2 is expressed by MM cells of patients with bone destruction. (A) Expression of sFRP-2 mRNA by MM cells isolated from patients with advanced bone disease was detected by RT-PCR. Total RNA (2 μg) extracted from primary cells was reverse transcribed, and 1 μL of the 20-μL reaction was used in PCR analysis for sFRP-2 and GAPDH mRNA. (B) The isolated MM cells were cytospun and stained with anti–sFRP-2 antibody or nonimmune IgG as described in “Patients, materials, and methods.” Cells were visualized under an Olympus BX50 microscope equipped with a UMplanFI 40 ×/0.75 objective lens (Olympus, Tokyo, Japan) to achieve an original magnification of 400 ×. Images were recorded with an Olympus SC35 CCD camera and Viewfinder Lite software (Pixera, Los Gatos, CA), and were digitally processed with Adobe Photoshop software (Adobe Systems, San Jose, CA).
sFRP-2 is expressed by MM cells of patients with bone destruction. (A) Expression of sFRP-2 mRNA by MM cells isolated from patients with advanced bone disease was detected by RT-PCR. Total RNA (2 μg) extracted from primary cells was reverse transcribed, and 1 μL of the 20-μL reaction was used in PCR analysis for sFRP-2 and GAPDH mRNA. (B) The isolated MM cells were cytospun and stained with anti–sFRP-2 antibody or nonimmune IgG as described in “Patients, materials, and methods.” Cells were visualized under an Olympus BX50 microscope equipped with a UMplanFI 40 ×/0.75 objective lens (Olympus, Tokyo, Japan) to achieve an original magnification of 400 ×. Images were recorded with an Olympus SC35 CCD camera and Viewfinder Lite software (Pixera, Los Gatos, CA), and were digitally processed with Adobe Photoshop software (Adobe Systems, San Jose, CA).
Recombinant sFRP-2 suppresses ALP activity and mineralization induced by BMP-2 in MC3T3-E1 cells. MC3T3-E1 cells were cultured in the osteogenic medium. Recombinant mouse sFRP-2 was added at 1 μg/mL in the presence of rhBMP-2 (50 ng/mL). (A) Mineralized nodules were visualized at day 14 by von Kossa staining. (B) ALP activity was determined at day 10. Results are expressed as mean ± SEM of triplicate experiments. * indicates significantly different by Student t test, P < .05.
Recombinant sFRP-2 suppresses ALP activity and mineralization induced by BMP-2 in MC3T3-E1 cells. MC3T3-E1 cells were cultured in the osteogenic medium. Recombinant mouse sFRP-2 was added at 1 μg/mL in the presence of rhBMP-2 (50 ng/mL). (A) Mineralized nodules were visualized at day 14 by von Kossa staining. (B) ALP activity was determined at day 10. Results are expressed as mean ± SEM of triplicate experiments. * indicates significantly different by Student t test, P < .05.
sFRP-2 immunodepletion can restore mineralization suppressed by MM cell CM
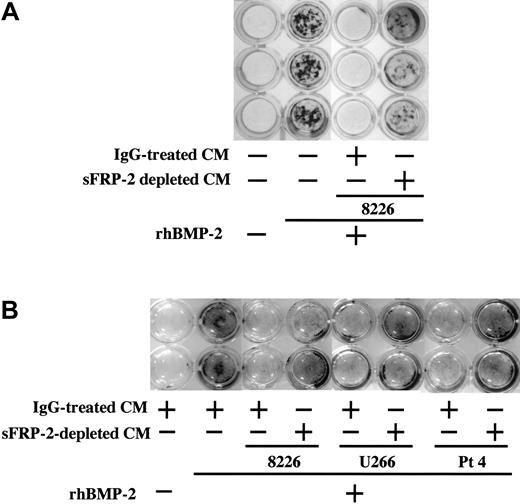
To further establish the role of sFRP-2 in the suppression of osteoblast differentiation in MM, we depleted sFRP-2 from CM of RPMI8226 and U266 MM cell lines as well as primary MM cells from patient no. 4 by immunoprecipitation with a specific antibody and immunobeads. Mineralized nodule formation induced by BMP-2 was almost completely suppressed by addition of these MM cell CM treated with control IgG and immunobeads (Figure 5A-B). In contrast, sFRP-2 depletion from CM substantially restored mineralized nodule formation in MC3T3-E1 cells (Figure 5A) and human BM-derived osteoblasts (Figure 5B). These results are consistent with the notion that MM-cell–derived sFRP-2 is a predominant factor responsible for the suppression of osteoblast differentiation by MM cells.
sFRP-2 immunodepletion restores suppression of mineralization by MM cell CM. MC3T3-E1 cells (A) and human BM-derived osteoblasts (B) were cultured in the osteogenic medium in 24-well culture plates in the presence or absence of rhBMP-2 (50 ng/mL). Control IgG-treated or sFRP-2–depleted CM from RPMI8226 and U266 cell lines as well as primary MM cells from patient no. 4 were added at 20% to the indicated wells. Mineralized nodules were visualized at day 14 and 21 by von Kossa staining in MC3T3-E1 cells and human BM-derived osteoblasts, respectively.
sFRP-2 immunodepletion restores suppression of mineralization by MM cell CM. MC3T3-E1 cells (A) and human BM-derived osteoblasts (B) were cultured in the osteogenic medium in 24-well culture plates in the presence or absence of rhBMP-2 (50 ng/mL). Control IgG-treated or sFRP-2–depleted CM from RPMI8226 and U266 cell lines as well as primary MM cells from patient no. 4 were added at 20% to the indicated wells. Mineralized nodules were visualized at day 14 and 21 by von Kossa staining in MC3T3-E1 cells and human BM-derived osteoblasts, respectively.
sFRP-2 immunodepletion restores suppression of TCF reporter activity in osteoblasts by MM cell CM. Osteobalsts were cotransfected with TCF reporter plasmid and pRL-TK Renilla luciferase plasmids as described in “Patients, materials, and methods.” The transfected osteoblasts were then treated with RPMI8226 CM with or without sFRP-2 immunodepletion at 20% in the presence of 50 ng/mL rhBMP-2. Data were corrected for Renilla luciferase and are expressed as a mean of 6 independent experiments with an error bar of SD in arbitrary units. * indicates significantly different by Student t test, P < .05.
sFRP-2 immunodepletion restores suppression of TCF reporter activity in osteoblasts by MM cell CM. Osteobalsts were cotransfected with TCF reporter plasmid and pRL-TK Renilla luciferase plasmids as described in “Patients, materials, and methods.” The transfected osteoblasts were then treated with RPMI8226 CM with or without sFRP-2 immunodepletion at 20% in the presence of 50 ng/mL rhBMP-2. Data were corrected for Renilla luciferase and are expressed as a mean of 6 independent experiments with an error bar of SD in arbitrary units. * indicates significantly different by Student t test, P < .05.
TCF reporter assays
To confirm that MM-cell–derived sFRP-2 suppresses a canonical Wnt–β-catenin pathway, we constructed sensitive TCF reporter assays (TOPflash) responding to nuclear translocation of β-catenin and its binding to TCF transcription factors in osteoblasts by stimulation of a canonical Wnt–β-catenin pathway. RPMI8226 CM suppressed luciferase reporter activity in the presence of BMP-2, indicating the inhibition of β-catenin nuclear translocation (Figure 6). However, sFRP-2–depleted RPMI8226 CM could not suppress the luciferase reporter activity (Figure 6). These results suggest that MM cells suppress a canonical Wnt–β-catenin pathway in osteoblasts in an MM cell-derived sFRP-2–dependent fashion.
Discussion
In the present study, we demonstrated that primary MM cells from patients with advanced bone lesions as well as MM cell lines secrete soluble factors that inhibit osteoblast differentiation and mineralized nodule formation (Figures 1 and 5), and that, among soluble inhibitors of BMP-2–induced osteoblast differentiation tested, a Wnt antagonist, sFRP-2, is secreted from most of these MM cells (Figures 2, 3). Addition of sFRP-2 markedly inhibited mineralized nodule formation in MC3T3-E1 cells in the presence of BMP-2 (Figure 4A), whereas the induction of ALP activity was only partially suppressed (Figure 4B). These results may imply that sFRP-2 inhibits osteoblastic differentiation at multiple steps and that the inhibition of a canonical Wnt signaling pathway inhibits not only early osteoblastic differentiation to express ALP but also terminal differentiation to acquire mineralizing properties. Such an assumption is consistent with our previous clinical observations that a late-stage bone formation marker, serum osteocalcin, was relatively low compared to serum ALP levels in many patients with MM showing “punched-out” bone lesions with defective calcification on roentgenograms.15
Tian et al41 demonstrated that MM cells potently inhibit a canonical Wnt pathway to cause suppression of osteoblast differentiation. They found that DKK-1, an inhibitor of a Wnt coreceptor, LRP-5, is secreted by MM cells from patients with bone lesions and suppresses ALP induction in osteoblasts in the presence of BMP-2. However, they also reported that DKK-1 is preferentially overexpressed in a phenotypically mature type of MM cells but not in plasmablastic or immature types of MM cells. These observations suggest that MM cell-derived inhibitors other than DKK-1 play a role in the impairment of bone formation, especially in advanced clinical stages or aggressive types of MM in which plasmablastic or immature types of MM cells overwhelm. In addition, we were unable to detect DKK-1 protein in MM cell lines established from patients with terminal stages of MM, except for U266 expressing DKK-1 mRNA (Figure 2B). In contrast, these cell lines expressed sFRP-2 at both protein and mRNA levels and sFRP-3 at mRNA levels. We further demonstrated that sFRP-2 protein was widely expressed by MM cells from patients with advanced or terminal stages of MM including plasma cell leukemia. These results are consistent with the assumption that MM cell-derived sFRP-2 plays an important role in the suppression of bone formation in advanced stages of MM. However, relative roles of sFRP-2 and DKK-1 in the suppression of osteoblastic differentiation by MM cells remain to be elucidated. Because MM cells secrete not only sFRP-2 but also DKK-1,41 IGF-BP4,42 tumor necrosis factor α, and IL-3,43 which are implicated as inhibitors of differentiation or proapoptotic factors of osteoblasts, MM cell-derived sFRP-2 may also act in combination with these factors to suppress bone formation.
Aberrant activation of a Wnt–β-catenin signaling pathway is associated with tumor progression in a variety of human cancers.44,45 The Wnt pathway perturbations have also been found to be involved in MM cell growth control.46,47 Derksen et al47 reported that MM cells overexpress β-catenin. They have shown that stimulation of Wnt signaling promotes proliferation of MM cells, whereas disruption of Wnt signaling by dominant-negative TCF/LEF inhibits MM cell proliferation. Given the oncogenic potential of Wnt signaling, sFRPs secreted from MM cells could be considered as tumor suppressors. However, Yaccoby et al48 reported that mature osteoblasts suppress MM cell growth, whereas immature stromal cells support MM cell growth and survival. Therefore, suppression of osteoblast maturation by Wnt antagonists secreted from MM cells may provide favorable conditions for MM growth. In addition, sFRP-2 expression has been reported in malignant glioma cells,49 suggesting that sFRP-2 expression is not specific to MM.
In addition to its effect on bone formation, a Wnt pathway is essential for self-renewal of hematopoietic stem cells50 and erythropoiesis,51,52 as well as early T- and B-cell development.53 Aberrant overproduction of soluble Wnt antagonists by MM cells in the BM microenvironment may therefore impair not only bone formation but also normal processes of hematopoiesis. Such versatile effects of Wnt antagonists in the BM microenvironment of patients with MM may also contribute to the wide spectrum of clinical features including anemia, suppression of immunoglobulin production, and reduced T-cell function.
Prepublished online as Blood First Edition Paper, July 19, 2005; DOI 10.1182/blood-2004-12-4940.
Supported by a Grant-in-Aid for Scientific Research on Priority Areas (T.M.; no. 12137207) and Grants-in-Aid for Scientific Research (T.M.; nos. 13557082 and 14370329) and (M.A.; no. 15591010) from the Ministry of Education, Culture, Science, and Sports of Japan.
M.A., T.O., and T.M. designed the research; T.O., M.A., J.A., T. Hara, K.K., E.S., Y.T., H.S., T. Hashimoto, S.O., and S.K. performed the research; J.A., T. Hara, K.K., E.S., Y.T., and H.S. collected and isolated primary myeloma cells; T.O., M.A., T. Hashimoto, S.O., S.K., D.I., and T.M. analyzed data; and M.A., T.O., D.I., and T.M. wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Momoko Nitta, Hiroe Amou, and Asuka Oda for their expert technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal