Comment on Tan et al, page 2936
Dendritic cells with low expression of B7 molecules tend to induce tolerance, but it has been difficult to show that the observations are related. A new study suggests that they may be.
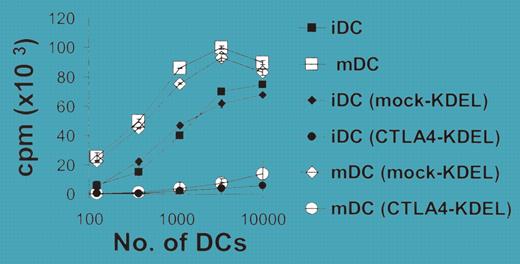
Recently, it has become clear that “resting” (unactivated or immature) dendritic cells (DCs) can induce immunologic tolerance in responding T cells.1 This pathway may be important in maintaining tolerance toward antigens in the periphery. However, it has been less clear exactly why resting DCs are tolerogenic.2 One leading hypothesis (reviewed in Redmond and Sherman3 ) has focused on the fact that these DCs express low levels of the costimulatory molecules B7-1/CD80 and B7-2/CD86. Since costimulation is important for successful T-cell activation, it has been proposed that the lack of B7-1/B7-2 is the direct, mechanistic cause of anergy and tolerance induced by resting/immature DCs. However, there has been relatively little firm experimental evidence available to directly support this hypothesis.2 FIG1
Transfection of DCs with CTLA4-KDEL. See the complete figure in the article beginning on page 2936.
Transfection of DCs with CTLA4-KDEL. See the complete figure in the article beginning on page 2936.
Attempts to answer the question using B7-1/B7-2 knock-out mice have been somewhat ambiguous, due to the multiple developmental immunologic defects (including abnormalities in both immunity and tolerance) present in these mice. A second strategy has attempted to mask or block B7 molecules using a soluble fusion protein incorporating the high-affinity B7-binding site of the cytotoxic T lymphocyte antigen 4 (CTLA4) molecule. Experimentally, this CTLA4-immunoglobulin construct (CTLA4-Ig) can indeed induce immunosuppression and tolerance in certain settings, although the results have been variable. Interpretation has been further complicated by the fact that CTLA4-Ig can also induce the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO).4
To help clarify this rather complex situation, Tan and colleagues now bring the novel strategy of binding and destroying the B7 molecules in DCs before they have a chance to reach the cell surface. The authors used the B7-binding portion of CTLA4 but modified it to include a retention signal that targets it to the endoplasmic reticulum. When this construct was transfected into human monocyte-derived DCs, it markedly reduced the cell-surface expression of B7-1 and B7-2, and—just as theory would predict—rendered the DCs tolerogenic in vitro. This was accomplished without inducing IDO in the DCs (unlike CTLA4-Ig) and apparently also without inducing other changes to the basic DC biology (at least not obvious ones).
Thus, this study offers novel support for the hypothesis that it is simply the absence of B7 expression that renders the DCs tolerogenic. This interpretation of the authors' data remains still somewhat speculative and will need to be more directly tested in the future (eg, by artificially ligating the appropriate costimulatory counter-receptor on the T cells to test whether this bypasses the tolerogenic effects of the transfected DCs). It will also be important to definitively rule out that the formation and degradation of intracellular CTLA4/B7 complexes do not somehow alter the basic biology of the DCs. But these questions notwithstanding, from a therapeutic standpoint the results of Tan and colleagues suggest a novel and intriguing strategy that might render human monocyte-derived DCs stably tolerogenic in vivo. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal