Comment on Cao et al, page 3234
Bacterial lipopolysaccharide induces macrophages to migrate from inflamed tissues to lymph nodes using the adhesion molecule Mac-1.
Cell migration requires attachments to cells and matrix by adhesion molecules. Mac-1, also known as CD11b/CD18, αMβ2 integrin, or complement receptor 3, is an adhesion molecule expressed by a variety of phagocytes including macrophages in inflammatory sites. In the present issue, Cao and colleagues demonstrate that Mac-1 mediates a bacterial lipopolysaccharide (LPS)–induced migration of macrophages out of inflamed tissues and into the lymph nodes.FIG1
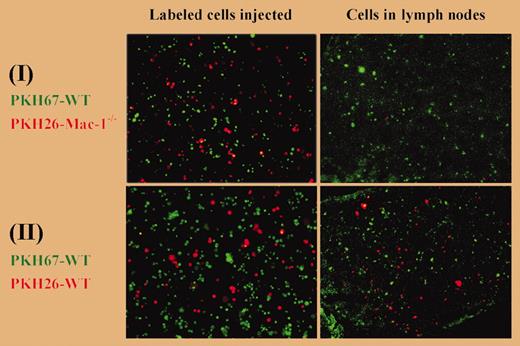
Macrophage migration from the peritoneum to the lymph nodes. See the complete figure in the article beginning on page 3234.
Macrophage migration from the peritoneum to the lymph nodes. See the complete figure in the article beginning on page 3234.
Proinflammatory stimuli including LPS can render macrophages difficult to extract from body cavities using lavage. In the present studies, Cao and colleagues observed that LPS decreased the numbers of macrophages that could be lavaged from thioglycollate-inflamed wild-type (WT) mice, but LPS did not affect the numbers of thioglycollate-elicited macrophages lavaged from Mac-1–deficient mice. Therefore, Mac-1 is necessary to whatever makes thioglycollate-elicited macrophages refractory to lavage after LPS stimulation.
To determine how LPS affected such macrophages, Cao and colleagues transferred fluorescent thioglycollate-elicited macrophages to thioglycollate-inflamed peritoneal spaces and tracked them after injecting saline or LPS. LPS but not saline induced the fluorescent macrophages to adhere to peritoneal surfaces within 5 minutes. However, by lavage time (4 hours after LPS injection), these cells were no longer present in either the peritoneal lavage fluid or on the peritoneal surfaces; instead, they were in the draining lymph nodes and the circulating blood. When Mac-1 was inhibited in these WT mice by a soluble antagonist of Mac-1 (neutrophil inhibitory factor), more macrophages could be recovered by lavage and fewer macrophages appeared in the lymph nodes. When fluorescent Mac-1–deficient macrophages were injected into Mac-1–deficient hosts, LPS failed to induce the adhesion of fluorescent cells to the peritoneal surfaces and fluorescent cells did not appear in the lymph nodes or blood. Labeling thioglycollate-elicited WT macrophages green and thioglycollate-elicited Mac-1–deficient macrophages red allowed these different cells to be mixed together and then studied within the same WT mice with thioglycollate-induced peritonitis. After LPS injection, red Mac-1–deficient macrophages but not green WT macrophages were recovered by lavage, whereas green WT but not red Mac-1–deficient macrophages appeared in the lymph nodes (see figure). Altogether, these data make a compelling argument for Mac-1–mediated migration of macrophages to the lymph nodes in this inflammatory setting.
Mechanisms by which macrophages migrate to the lymph nodes are beginning to be elucidated.1 LPS must induce changes in chemokines, adhesion molecules, and other factors to mediate this transit of inflammatory macrophages. The initial tight adhesion to peritoneal surfaces mediated by Mac-1 may be critical to the ensuing migration. This initial adhesion occurs within 5 minutes after LPS injection, suggesting that it does not require new gene expression. The Mac-1 ligands essential for attachment to peritoneal surfaces and migration to the lymph nodes, and the mechanisms by which LPS rapidly alters their availability or function, remain to be determined.
Agents targeting Mac-1 (via CD11b or CD18) and Mac-1 ligands are planned and ongoing in clinical trials.2 The findings of Cao and colleagues suggest that therapies inhibiting Mac-1 interaction with ligands could compromise macrophage migration from inflammatory sites to lymph nodes for some patients, which could influence the resolution of inflammation and/or antigen presentation and adaptive immunity. Thus, the discovery that inflammatory macrophages can be stimulated to migrate to lymph nodes using Mac-1–dependent pathways has implications for experimental medicine as well as fundamental immunology. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal