Abstract
Thrombomodulin (TM) plays an essential role in the generation of activated protein C (APC), a mediator with both anticoagulant and anti-inflammatory properties, and is preferentially expressed in lungs. To investigate the role of TM in the coagulant and inflammatory response in the lung during tuberculosis, mice with a mutation in the TM gene (Thbd), which results in a minimal capacity for APC generation (TMpro/pro mice), were intranasally infected with live virulent Mycobacterium tuberculosis. Whereas pulmonary tuberculosis was not associated with activation of coagulation in either wild-type or TMpro/pro mice, 5 weeks after infection TMpro/pro mice displayed an uncontrolled inflammatory response in their lungs, as reflected by higher lung weights, a diminished ability to form well-shaped granulomas, elevated levels of proinflammatory cytokines, and concurrently reduced concentrations of anti-inflammatory cytokines. During a 36-week follow-up after infection with a lower dose of M tuberculosis, 35% of TMpro/pro mice died from week 28 onward versus none of the wild-type mice, and the surviving TMpro/pro mice displayed increased lung inflammation accompanied by higher mycobacterial loads in liver and spleen. These data suggest that a TM mutation that impairs APC generation results in uncontrolled lung inflammation during tuberculosis.
Introduction
Thrombomodulin (TM) is a widely expressed glycoprotein receptor that plays a pivotal role in the generation of the natural anticoagulant activated protein C (APC) by virtue of its capacity to bind thrombin with high affinity.1,2 Formation of the thrombin-TM complex limits the procoagulant and cellular-activating functions of thrombin and results in thrombin-mediated catalytic transformation of protein C (PC) into APC, an effect that is facilitated by the endothelial protein C receptor. APC acts as an anticoagulant, together with its cofactor protein S, by inactivating factors Va and VIIIa, and, in addition, exerts profibrinolytic effects through inhibition of plasminogen activator inhibitor type I, the main inhibitor of plasminogen activation. Moreover, several anti-inflammatory effects have been ascribed to APC, including inhibition of leukocyte activation, inhibition of E-selectin–mediated cell adhesion to the vascular endothelium, and reduction of tumor necrosis factor α (TNF-α) production.3-8
Evidence exists that exogenously administered APC can inhibit inflammatory responses in the pulmonary compartment. Intravenous infusion of APC protected against lung injury by inhibiting leukocyte activation in rats challenged with lipopolysaccharide (LPS) systemically,9 and direct intrapulmonary delivery of APC reduced allergic and bleomycin-induced lung inflammation in mice.10,11 Moreover, in a very recent study in healthy humans, intravenous administration of APC resulted in a reduced neutrophil influx into lung subsegments challenged with LPS.12 Considering that TM is abundantly expressed in the lungs,13-15 our laboratory recently investigated the influence of endogenous TM on acute lung inflammation induced by either live bacteria or LPS. Mice with a single amino acid substitution (Glu404Pro) in the gene for TM (TMpro/pro mice), which results in a profoundly reduced capacity to generate APC in both the circulation and in the alveolar space,15,16 were found to have an unaltered coagulant and inflammatory response in their pulmonary compartment after intranasal administration of Streptococcus pneumoniae (a Gram-positive pathogen that is the most common cause of community-acquired pneumonia), Klebsiella pneumoniae (a common Gram-negative respiratory pathogen), or LPS.15 Although this study indicated that the role of TM and endogenous APC in the regulation of acute lung inflammation is limited, we wondered whether the impaired capacity of TMpro/pro mice to generate APC would influence lung inflammation in a more chronic setting. One of the most dramatic manifestations of chronic lung inflammation is tuberculosis. Indeed, experimentally induced pulmonary tuberculosis in mice results in a gradually developing local inflammatory reaction characterized by the recruitment of mainly mononuclear cells and the formation of well-shaped granulomas at the site of the infection.17,18 In the present study we compared the coagulant and inflammatory responses in the lungs of TMpro/pro and normal wild-type (WT) mice intranasally infected with live virulent Mycobacterium tuberculosis.
Materials and methods
Animals
Mice with a single amino acid substitution (Glu404Pro) in the gene for TM, kindly provided by Dr R. D. Rosenberg (Massachusetts Institute of Technology, Cambridge, MA), were generated on a C57BL/6 (and B6D2F1) background, as previously described.16 Homozygous mutant TMpro/pro mice exhibit a decrease of approximately 1000-fold with respect to PC activation and approximately 100-fold with respect to binding of thrombin at physiologic levels of the enzyme.16 In addition, TMpro/pro mice produce less than 4% of APC in their alveolar space generated by WT mice upon intratracheal administration of PC and thrombin.15 Yet in contrast to TM gene (Thbd)–deficient mice, which die in the embryonic stage,19 TMpro/pro mice develop to term and possess normal reproductive performance.16 All mice were on a C57BL/6 background. The wild type of the TMpro/pro mice were derived from original littermates and in all studies mice from the same generation were used. All experiments were approved by the Committee on Use and Care of Animals of the Academic Medical Center, Amsterdam, the Netherlands.
Experimental infection
Pulmonary tuberculosis was induced exactly as described previously.20-22 Briefly, a virulent laboratory strain of M tuberculosis, H37Rv, was grown in liquid Dubos medium containing 0.01% Tween 80 for 4 days. A replicate culture was incubated at 37°C, harvested at mid–log phase, and stored in aliquots at –70°C. For each experiment, a vial was thawed and washed twice with sterile 0.9% NaCl. Mice were anesthetized by inhalation with isoflurane (Abbott Laboratories, Kent, United Kingdom) and infected with 105 or 104 live bacilli in 50 μL saline, as determined by viable counts on 7H11 Middlebrook agar plates (Gibson Laboratories, Lexington, KY). Mice were killed 2 or 5 weeks after infection with 105 bacilli (n = 8 per group at both time points) or 36 weeks after infection with 104 bacilli (experiment started with 14 mice per group). At these time points, mice were anesthetized with Hypnorm (Janssen Pharmaceutica, Beerse, Belgium) and midazolam (Roche, Meidrecht, the Netherlands) and exsanguinated by collecting blood from the inferior vena cava (from which heparinized plasma was prepared). Then lungs, one lobus of the liver, and (at 36 weeks) the spleen were removed aseptically without prior perfusion of the vasculature. Organs were homogenized with a tissue homogenizer (Biospec Products, Bartlesville, OK) in 5 volumes of sterile 0.9% NaCl, and 10-fold serial dilutions were plated on Middlebrook 7H11 agar plates to determine bacterial loads. Colonies were counted after 21-day incubation at 37°C. For cytokine measurements, lung homogenates were diluted 1:1 in lysis buffer (150 mM NaCl, 15 mM Tris, 1 mM MgCl2, 1 mM CaCl2, 1% Triton X-100, 100 μg/mL pepstatin A, 100 μg/mL leupeptin, and 100 μg/mL aprotinin) and incubated on ice for 30 minutes. Supernatants were sterilized using a 0.22-μm filter (Corning, Corning, NY) and frozen at –20°C until assays were performed.
Lung cell counts and differentiation
Pulmonary cell suspension was obtained using an automated disaggregation device (Medimachine System; DAKO A/S, Glostrup, Denmark) as described20-22 and resuspended in medium. Erythrocytes were lysed with ice-cold isotonic NH4Cl solution, and the remaining cells were washed twice with RPMI. Total leukocytes in pulmonary cell suspensions were counted using a hemacytometer and Turk solution (Merck KGaA, Darmstadt, Germany). The percentages of macrophages, neutrophils, and lymphocytes were determined using cytospin preparations stained with modified Giemsa stain (Diff-Quik; Baxter Healthcare, McGraw Park, IL).
Histologic examination
After 24-hour fixation of lungs in 10% formaline and embedding in paraffin, 4-μm–thick sections were stained with hematoxylin and eosin (H&E) and Ziehl Nielsen (ZN). All slides were coded and scored by a pathologist without knowledge of the genotype of mice or treatment. Staining for granulocytes, macrophages, fibrin(ogen), and TM was done exactly as decribed.15,23-25 In brief for TM staining, after quenching endogenous peroxidase activity and blocking nonspecific binding, slides were incubated with a rat anti–mouse TM monoclonal antibody (mAb; kindly provided by Dr S. J. Kennel, Oak Ridge National Laboratory, Oak Ridge, TN) followed by a biotinylated rabbit anti–rat polyclonal Ab (DAKO). Slides were then incubated in a streptavidin-biotin complex (streptABC) solution (DAKO) and developed using 1% H2O2 and 3.3′-diaminobenzidin-tetra-hydrochloride (Sigma, St Louis, MO) in Tris-HCl. The sections were mounted in glycerin gelatin. Images were obtained with an Olympus BX51 microscope (Olympus, Zoeterwoude, The Netherlands) using Olympus Plan objectives (4 ×/0.13 numeric aperture [NA], 10 ×/0.30 NA, 20 ×/0.50 NA, and 40 ×/0.85) and an Olympus DP70 camera. Sections were stained with H&E except those in Figure 3C-D, stained with ZN staining and immunostainings; images in Figures 1, 3C-J, 6E-F were also slightly counterstained with methyl green. Images were acquired with Olympus DPController software and were processed with Adobe Photoshop CS (Adobe Systems, San Jose, CA).
FACS analysis
For fluorescence-activated cell sorter (FACS) analysis pulmonary cell suspensions were obtained using an automated disaggregation device (Medimachine System; DAKO) and processed as described previously.20-22 Cells were brought to a concentration of 4 × 109 cells/L FACS buffer (phosphate-buffered saline supplement with 0.5% bovine serum albumin, 0.01% NaN3, and 100 mM EDTA [ethylenediaminetetraacetic acid]). Immunostaining for cell surface molecules was performed for 30 minutes at 4°C using directly labeled Abs against CD3, CD4, CD25, CD69, Ly-6 (Gr-1), and CD11b. All Abs were used in concentrations recommended by the manufacturer (PharMingen, San Diego, CA). To correct for aspecific staining, an appropriate control Ab (rat immunoglobulin G2; PharMingen) was used. Cells were fixed with 2% paraformaldehyde, and lymphocyte surface molecules were analyzed by gating of the CD3+ population. Expression of CD11b on neutrophils was analyzed by gating of the Gr-1 population. The number of positive cells was obtained by setting a quadrant marker for nonspecific staining.
Assays
Thrombin-antithrombin complexes (TAT-c's) were measured by enzyme-linked immunosorbent assay (ELISA) as described.15,24 As a “positive control” for TAT-c measurements in lung homogenates samples from mice intranasally infected with S pneumoniae [and with positive fibrin(ogen) staining of lung tissue] were used.15 TNF-α, interleukin 4 (IL-4), IL-6, IL-10, interferon γ (IFN-γ), and monocyte chemotactic protein 1 (MCP-1) were measured by cytometric bead array (PharMingen). Macrophage inflammatory protein 2 (MIP-2) and transforming growth factor β (TGF-β) were measured by ELISA (R & D Systems, Abingdon, United Kingdom). All assays were done according to the manufacturer's instructions.
Statistical analysis
Data are expressed as means plus or minus SEM, unless indicated otherwise. Comparisons between groups were conducted using the Mann Whitney U test. For comparison of survival curves, Kaplan-Meier analysis with a log rank test was used. P less than .05 was considered to represent a statistically significant difference.
Results
TM expression
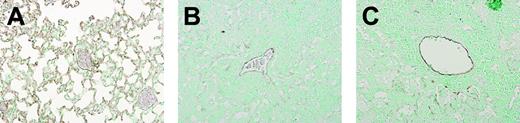
We previously reported that TM expression becomes downregulated in lung tissue during the acute inflammatory response to intranasally administered bacteria or LPS,15 which is in line with earlier findings of reduced TM expression in the dermal microvasculature of patients with severe bacterial sepsis.26 To obtain insight into the regulation of TM expression in the lung during chronic infection, we compared the expression of TM in lungs obtained from uninfected mice and from mice 5 weeks after intranasal infection with M tuberculosis using anti-TM immunostaining. As shown in Figure 1, a dramatic reduction of TM immunoreactivity was observed 5 weeks after infection in WT (Figure 1B) and TMpro/pro mice (Figure 1C) compared with uninfected mice (Figure 1A).
Reduced pulmonary TM expression during tuberculosis. Immunostaining for TM in lungs of WT mice without infection (A) and 5 weeks after M tuberculosis infection (B), and TMpro/pro mice 5 weeks after infection (C), showing a striking reduction in TM expression during tuberculosis in inflamed lungs. Slides are representative of 5 mice per group. Original magnification × 10.
Reduced pulmonary TM expression during tuberculosis. Immunostaining for TM in lungs of WT mice without infection (A) and 5 weeks after M tuberculosis infection (B), and TMpro/pro mice 5 weeks after infection (C), showing a striking reduction in TM expression during tuberculosis in inflamed lungs. Slides are representative of 5 mice per group. Original magnification × 10.
No evidence for coagulation activation in TMpro/pro mice
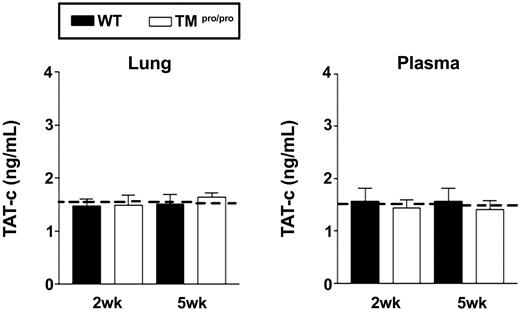
Tuberculosis can be associated with a modest procoagulant state, which occasionally can even result in disseminated intravascular coagulation.27,28 Since the TMpro/pro mutation results in a mild prethrombotic state,16 we wondered whether endogenous TM is important for maintaining normal hemostasis in the lung during chronic infection by M tuberculosis. For this, we measured TAT-c in lung homogenate and plasma 2 and 5 weeks after infection. Pulmonary tuberculosis was not associated with elevated TAT-c concentrations in lungs or plasma in either WT or TMpro/pro mice when compared with uninfected mice (Figure 2). In addition, lung tissue did not show deposition of fibrin in either mouse strain (Figure 3I-J). TAT-c concentrations were elevated in lung homogenates obtained from mice with pneumococcal pneumonia (4.0 ± 0.3 ng/mL), indicating that this assay is able to detect thrombin generation in these samples. Thus, these data argue against a role for TM-APC in preventing coagulation during tuberculosis.
TMpro/pro mice show uncontrolled pulmonary inflammation
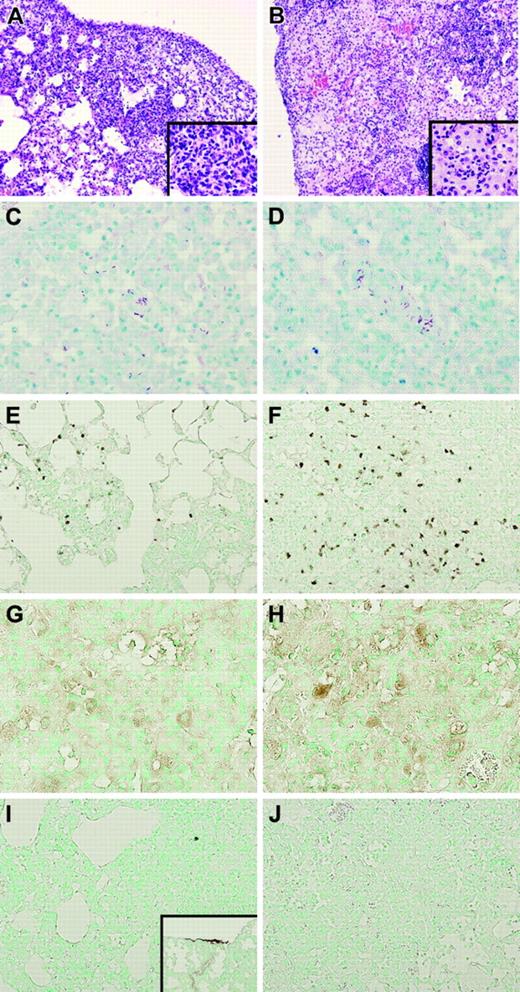
Next we evaluated whether the TMpro/pro mutation influenced the inflammatory response in the lung during tuberculosis. Remarkably, whereas at 2 weeks after infection lung weights were similar in both mouse strains (data not shown), at 5 weeks after inoculation with M tuberculosis the lung weights of TMpro/pro mice were much higher than those of WT mice (right lungs, 307 ± 10 versus 188 ± 19 mg, respectively; P < .05), indicative for enhanced inflammation and edema. In line with these findings, histopathologic examination of lung tissue did not reveal differences between TMpro/pro and WT mice at 2 weeks after infection (not shown). However, profound differences were found between TMpro/pro and WT mice at 5 weeks after infection (Figure 3). Indeed, at that time point, lungs of WT mice displayed granulomatous inflammatory infiltrates primarily located around small bronchi and vessels (Figure 3A) composed of lymphocytes and macrophages (Figure 3G) with few granulocytes (Figure 3E), confirming earlier observations.20-22 In contrast, lungs of TMpro/pro mice displayed more confluent areas of inflammation (Figure 3B) predominantly composed of neutrophils (Figure 3F) and foamy macrophages (Figure 3H). Moreover, edema and pleuritis were more pronounced in TMpro/pro than in WT mice.
The TMpro/pro mutation has no effect on coagulation during tuberculosis. TAT-c levels in plasma and lung homogenates. Mice were intranasally infected with 105 CFUs M tuberculosis and killed 2 and 5 weeks after infection. □ indicates WT mice; and □, TMpro/pro mice. Data are means ± SE of 8 mice per group. Dotted lines represent the mean values obtained from normal plasma and lung homogenate of mice without infection.
The TMpro/pro mutation has no effect on coagulation during tuberculosis. TAT-c levels in plasma and lung homogenates. Mice were intranasally infected with 105 CFUs M tuberculosis and killed 2 and 5 weeks after infection. □ indicates WT mice; and □, TMpro/pro mice. Data are means ± SE of 8 mice per group. Dotted lines represent the mean values obtained from normal plasma and lung homogenate of mice without infection.
Lungs of TMpro/pro mice show more confluent inflammation and an enhanced neutrophil influx. Representative histopathologic sections of lungs of WT (A) and TMpro/pro (B) mice 5 weeks after inoculation with M tuberculosis showing granulomatous inflammatory infiltrates in WT (original magnification × 10, A; × 40, inset) and confluent areas of inflammation in TMpro/pro mice (original magnification × 10, B; × 40, inset). Mycobacteria were easily found in ZN staining of lungs in both groups (original magnification × 40, C-D). Enhanced neutrophil influx was observed in TMpro/pro mice (original magnification × 20, F) compared with WT mice (original magnification × 20, E). Macrophages were observed in both groups (original magnification × 20, G-H). Fibrin(ogen) could not be detected in either group (original magnification × 20, I-J; inset I: positive control for fibrin(ogen) staining showing lung tissue 48 hours after induction of pneumococcal pneumonia, original magnification × 10). Figures are representative of n = 5 for each group.
Lungs of TMpro/pro mice show more confluent inflammation and an enhanced neutrophil influx. Representative histopathologic sections of lungs of WT (A) and TMpro/pro (B) mice 5 weeks after inoculation with M tuberculosis showing granulomatous inflammatory infiltrates in WT (original magnification × 10, A; × 40, inset) and confluent areas of inflammation in TMpro/pro mice (original magnification × 10, B; × 40, inset). Mycobacteria were easily found in ZN staining of lungs in both groups (original magnification × 40, C-D). Enhanced neutrophil influx was observed in TMpro/pro mice (original magnification × 20, F) compared with WT mice (original magnification × 20, E). Macrophages were observed in both groups (original magnification × 20, G-H). Fibrin(ogen) could not be detected in either group (original magnification × 20, I-J; inset I: positive control for fibrin(ogen) staining showing lung tissue 48 hours after induction of pneumococcal pneumonia, original magnification × 10). Figures are representative of n = 5 for each group.
TMpro/pro mice show increased neutrophil influx into lungs
To obtain more insight into the cellular composition of the pulmonary infiltrates in TMpro/pro and WT mice, we prepared whole lung cell suspensions at 2 and 5 weeks after infection (Table 1). At 2 weeks, TMpro/pro and WT mice had similar leukocyte numbers in their lungs, albeit lungs of the former mouse strain already contained more neutrophils (P < .05 versus WT). At 5 weeks after infection, the lungs of TMpro/pro mice contained more leukocytes (P = .07 versus WT), which was caused by a profound rise in the number of neutrophils (P < .05 versus WT). Interestingly, this enhanced neutrophil influx was accompanied by the presence of less lymphocytes and macrophages in lungs of TMpro/pro mice (both P < .05 versus WT). To determine whether the diminished influx of lymphocytes in TMpro/pro mice was restricted to a certain subset, we analyzed whole lung cell suspensions obtained 5 weeks after infection by FACS (Table 2). This revealed that the percentages of CD4+ and CD8+ T cells within the CD3+ population did not differ between TMpro/pro and WT mice. Moreover, in both strains the CD4+ and CD8+ T cells equally expressed the activation markers CD25 and CD69. Finally, we also evaluated the activation status of Gr-1+ neutrophils in the lungs by analyzing CD11b expression and found no differences between TMpro/pro and WT mice.
Leukocyte counts in lung homogenates
. | 2 weeks after administration . | . | 5 weeks after administration . | . | ||
|---|---|---|---|---|---|---|
. | WT . | TMpro/pro . | WT . | TMpro/pro . | ||
| Total cells | 0.9 ± 0.7 | 1.0 ± 0.5 | 11.9 ± 1.5 | 16.1 ± 2.4 | ||
| Neutrophils | 0.3 ± 0.7 | 0.7 ± 0.1* | 2.6 ± 0.7 | 12.4 ± 0.9* | ||
| Macrophages | 0.2 ± 0.9 | 0.2 ± 0.2 | 5.3 ± 0.3 | 2.2 ± 0.3* | ||
| Lymphocytes | 0.3 ± 0.1 | 0.1 ± 0.2 | 4.0 ± 0.8 | 1.5 ± 1.2* | ||
. | 2 weeks after administration . | . | 5 weeks after administration . | . | ||
|---|---|---|---|---|---|---|
. | WT . | TMpro/pro . | WT . | TMpro/pro . | ||
| Total cells | 0.9 ± 0.7 | 1.0 ± 0.5 | 11.9 ± 1.5 | 16.1 ± 2.4 | ||
| Neutrophils | 0.3 ± 0.7 | 0.7 ± 0.1* | 2.6 ± 0.7 | 12.4 ± 0.9* | ||
| Macrophages | 0.2 ± 0.9 | 0.2 ± 0.2 | 5.3 ± 0.3 | 2.2 ± 0.3* | ||
| Lymphocytes | 0.3 ± 0.1 | 0.1 ± 0.2 | 4.0 ± 0.8 | 1.5 ± 1.2* | ||
Data are mean numbers of cells × 109/L, ± SE (n = 8 mice per group for each time point) at 2 and 5 weeks after intranasal administration of M tuberculosis (105 CFUs). *P < .05 versus WT mice.
Cellular composition in the lungs at 5 weeks after infection
. | Cells positive 5 weeks after infection, % . | . | |
|---|---|---|---|
| Cell type . | WT . | TMpro/pro . | |
| CD4+ | 48.4 ± 4.2 | 47.8 ± 2.2 | |
| CD8+ | 37.3 ± 2.4 | 34.3 ± 2.1 | |
| CD4+/CD69+ | 15.1 ± 1.2 | 17.8 ± 2.0 | |
| CD4+/CD25+ | 2.3 ± 1.3 | 4.1 ± 2.1 | |
| CD8+/CD69+ | 12.9 ± 6.2 | 10.1 ± 3.2 | |
| CD8+/CD25+ | 4.9 ± 1.3 | 3.6 ± 1.4 | |
| Gr-1+/CD11b+ | 22.9 ± 2.1 | 19.5 ± 1.8 | |
. | Cells positive 5 weeks after infection, % . | . | |
|---|---|---|---|
| Cell type . | WT . | TMpro/pro . | |
| CD4+ | 48.4 ± 4.2 | 47.8 ± 2.2 | |
| CD8+ | 37.3 ± 2.4 | 34.3 ± 2.1 | |
| CD4+/CD69+ | 15.1 ± 1.2 | 17.8 ± 2.0 | |
| CD4+/CD25+ | 2.3 ± 1.3 | 4.1 ± 2.1 | |
| CD8+/CD69+ | 12.9 ± 6.2 | 10.1 ± 3.2 | |
| CD8+/CD25+ | 4.9 ± 1.3 | 3.6 ± 1.4 | |
| Gr-1+/CD11b+ | 22.9 ± 2.1 | 19.5 ± 1.8 | |
Lymphocyte typing was performed on pulmonary cell suspensions 5 weeks after infection as described in “FACS analysis.” Data are mean ± SE of 8 mice per group. FACS results of the lymphocytes are expressed as the percentage of CD4+, CD8+, CD25+, and CD69+ population; results of neutrophils as percentage CD11b+ cells within the Gr-1+ population. Differences between groups were not significant.
TMpro/pro mice have elevated levels of proinflammatory cytokines and reduced levels of anti-inflammatory cytokines in lungs
Cytokines and chemokines play a pivotal role in the regulation of the immune response to tuberculosis.18,29,30 Therefore, we measured the concentrations of proinflammatory (IFN-γ, TNF-α) and anti-inflammatory cytokines (IL-4, IL-10, TGF-β) in lung homogenates obtained 2 and 5 weeks after infection (Table 3). At 2 weeks after infection, the pulmonary concentrations of these mediators were similar in TMpro/pro and WT mice. However, at 5 weeks TMpro/pro mice displayed increased levels of IFN-γ and TNF-α in their lungs compared with WT mice (P < .05), whereas the lung levels of IL-4, IL-10, and TGF-β were lower in TMpro/pro mice at that time point. In light of the altered recruitment of inflammatory cells to the pulmonary compartment in TMpro/pro versus WT mice, we also measured MCP-1 (a prototypic CC chemokine) and MIP-2 (a major CXC chemokine with neutrophil-attracting properties).31 Both MCP-1 and MIP-2 concentrations were higher in TMpro/pro than in WT mice (P < .05).
Cytokine and chemokine concentrations in lung homogenates
. | 2 weeks after administration . | . | 5 weeks after administration . | . | ||
|---|---|---|---|---|---|---|
. | WT . | TMpro/pro . | WT . | TMpro/pro . | ||
| IFN-γ | 191.2 ± 34.5 | 215.3 ± 52.8 | 219.3 ± 18.8 | 445.4 ± 70.8* | ||
| IL-4 | 23.5 ± 10.5 | 37.6 ± 8.5 | 488.3 ± 28.5 | 381.8 ± 28.5* | ||
| TNF-α | 461.2 ± 89.8 | 480.3 ± 135.3 | 376.6 ± 46.3 | 496.3 ± 55.2* | ||
| IL-10 | 44.4 ± 12.5 | 55.2 ± 0.9 | 235.3 ± 22.1 | 42.0 ± 21.3* | ||
| TGF-β | 380.4 ± 85.1 | 337.3 ± 79.9 | 1306.4 ± 155.7 | 812.2 ± 63.5* | ||
| MCP-1 | 458.2 ± 99.2 | 478.1 ± 78.4 | 154.2 ± 55.7 | 189.8 ± 17.1* | ||
| MIP-2 | 1978.2 ± 68.2 | 1800.6 ± 89.0 | 1581.2 ± 469.1 | 2363.4 ± 125.9* | ||
. | 2 weeks after administration . | . | 5 weeks after administration . | . | ||
|---|---|---|---|---|---|---|
. | WT . | TMpro/pro . | WT . | TMpro/pro . | ||
| IFN-γ | 191.2 ± 34.5 | 215.3 ± 52.8 | 219.3 ± 18.8 | 445.4 ± 70.8* | ||
| IL-4 | 23.5 ± 10.5 | 37.6 ± 8.5 | 488.3 ± 28.5 | 381.8 ± 28.5* | ||
| TNF-α | 461.2 ± 89.8 | 480.3 ± 135.3 | 376.6 ± 46.3 | 496.3 ± 55.2* | ||
| IL-10 | 44.4 ± 12.5 | 55.2 ± 0.9 | 235.3 ± 22.1 | 42.0 ± 21.3* | ||
| TGF-β | 380.4 ± 85.1 | 337.3 ± 79.9 | 1306.4 ± 155.7 | 812.2 ± 63.5* | ||
| MCP-1 | 458.2 ± 99.2 | 478.1 ± 78.4 | 154.2 ± 55.7 | 189.8 ± 17.1* | ||
| MIP-2 | 1978.2 ± 68.2 | 1800.6 ± 89.0 | 1581.2 ± 469.1 | 2363.4 ± 125.9* | ||
Data are mean values (in pg/mL) ± SE (n = 8 mice per group for each time point) at 2 and 5 weeks after intranasal administration of M tuberculosis (105 CFUs).
P <.05 versus WT.
TM pro/pro mice have a modestly decreased bacterial outgrowth
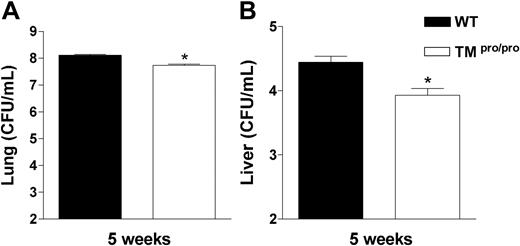
We next determined the role of the TMpro/pro mutation in containing the bacterial outgrowth. For this we compared the number of M tuberculosis colony-forming units (CFUs) in lungs and livers of TMpro/pro and WT mice 2 and 5 weeks after infection. While at 2 weeks mycobacterial loads were similar in lungs and livers of both mouse strains (data not shown), at 5 weeks both lungs and livers of TMpro/pro mice contained less mycobacteria than lungs and livers of WT mice (Figure 4; both P < .05).
TMpro/pro mice have less mycobacteria in lungs and liver 5 weeks after infection. Mycobacterial loads in CFUs/mL in lungs (A) and livers (B) at 5 weeks after intranasal infection with 105M tuberculosis. Data are means ± SE (n = 8 per group). *P < .05 versus WT mice.
TMpro/pro mice have less mycobacteria in lungs and liver 5 weeks after infection. Mycobacterial loads in CFUs/mL in lungs (A) and livers (B) at 5 weeks after intranasal infection with 105M tuberculosis. Data are means ± SE (n = 8 per group). *P < .05 versus WT mice.
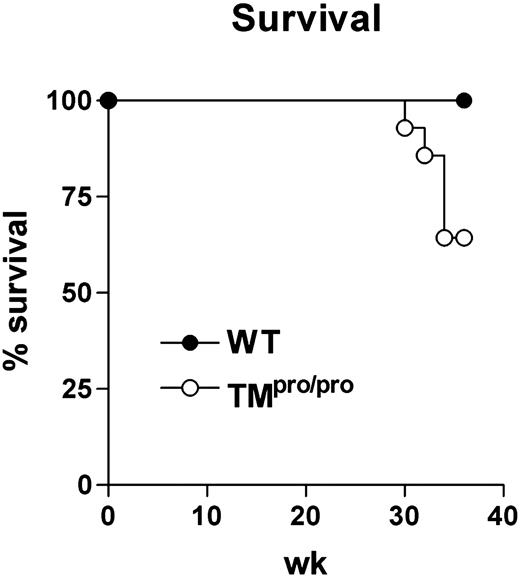
TMpro/pro mice succumb to prolonged mycobacterial infection
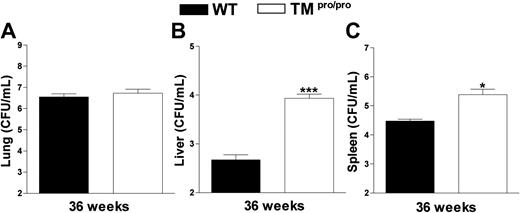
Having established that TMpro/pro mice display an uncontrolled inflammatory response in their lungs 5 weeks after infection with M tuberculosis, we next evaluated the consequences of the TMpro/pro mutation on the pulmonary inflammatory response to even more prolonged infection. For this, 14 TMpro/pro and 14 WT mice were infected with a lower inoculum of M tuberculosis (104 CFUs) and followed for 36 weeks. During the first 28 weeks of infection, none of the mice of either group died; however, during the subsequent 6 weeks, 5 TMpro/pro mice died versus none of the WT mice (Figure 5; P < .05). At 36 weeks after infection, all remaining mice were killed and lung inflammation was evaluated by histopathology. In line with the findings at 5 weeks after infection with a higher inoculum, lung weights were much higher in TMpro/pro mice than in WT mice (right lungs, 493 ± 37 versus 326 ± 16 mg, respectively; P < .05). Moreover, a profound difference was seen in the percentage of confluent inflammation of the lung, revealing more inflammation in the lungs of TMpro/pro mice than in the lungs of WT mice (84% ± 5% versus 57% ± 4%, respectively; P < .05; representative slides shown in Figure 6). Moreover, the inflammatory infiltrate consisted mostly of lymphocytes in WT mice versus mostly foamy macrophages in TMpro/pro mice (Figure 6C,D). In all surviving mice M tuberculosis could be cultured from lungs, livers, and spleens. Whereas the mycobacterial burden in lungs of TMpro/pro mice and WT mice was similar, livers and spleens of TMpro/pro mice contained significantly more mycobacteria than livers and spleens of WT mice (Figure 7).
TMpro/pro mice have a decreased survival during chronic infection. Groups of 14 WT and TMpro/pro mice were intranasally infected with 104 CFUs of live M tuberculosis and the animals were followed during 36 weeks after infection. • indicates WT mice; and ○, TMpro/pro mice. The difference in survival was statistically significant (P < .05).
TMpro/pro mice have a decreased survival during chronic infection. Groups of 14 WT and TMpro/pro mice were intranasally infected with 104 CFUs of live M tuberculosis and the animals were followed during 36 weeks after infection. • indicates WT mice; and ○, TMpro/pro mice. The difference in survival was statistically significant (P < .05).
Discussion
The central aim of this study was to examine the influence of TM, in particular the domain of TM that is crucial for the generation of APC, in the regulation of the pulmonary response to tuberculosis. In theory, a reduced capacity to produce APC could influence the local response to chronic infection with M tuberculosis via 2 major mechanisms: in light of the anticoagulant properties of APC, the TMpro/pro mutation could result in an increased tendency to form pulmonary thrombosis, whereas in light of the anti-inflammatory properties of APC, this TM mutation could lead to an enhanced proinflammatory reaction. We demonstrate here that TMpro/pro mice do not display a diminished capacity to maintain a normal hemostatic balance during tuberculosis but clearly have a reduced ability to control the inflammatory response to M tuberculosis infection.
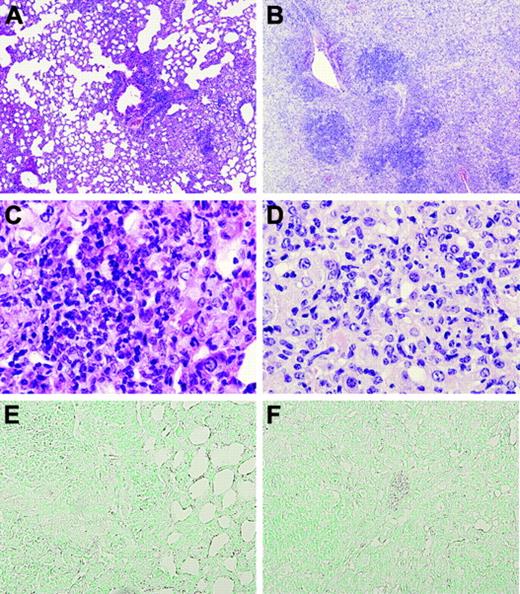
Lungs of TMpro/pro mice are diffusely inflamed 36 weeks after infection. Representative lung sections of WT (original magnification × 4, A) and TMpro/pro (original magnification × 4, B) mice 36 weeks after infection, showing more confluent and dense inflammation in TMpro/pro mice than in WT mice. Monocytes and lymphocytes are the dominant inflammatory cell types in WT mice (original magnification × 40, C) compared with large, “foamy” macrophages in TMpro/pro mice (original magnification × 40, D). At this late time point, fibrin(ogen) is still undetectable (original magnification × 20, E-F). Figures are representative of the remaining mice presented in Figure 4 (9/14 TMpro/pro mice and 14/14 WT mice).
Lungs of TMpro/pro mice are diffusely inflamed 36 weeks after infection. Representative lung sections of WT (original magnification × 4, A) and TMpro/pro (original magnification × 4, B) mice 36 weeks after infection, showing more confluent and dense inflammation in TMpro/pro mice than in WT mice. Monocytes and lymphocytes are the dominant inflammatory cell types in WT mice (original magnification × 40, C) compared with large, “foamy” macrophages in TMpro/pro mice (original magnification × 40, D). At this late time point, fibrin(ogen) is still undetectable (original magnification × 20, E-F). Figures are representative of the remaining mice presented in Figure 4 (9/14 TMpro/pro mice and 14/14 WT mice).
The role of TM in disease cannot be investigated using Thbd-deficient mice since these mice die in the embryonic phase.19 Therefore, we used TMpro/pro mice, which are viable yet have a strongly reduced capacity to produce APC. Indeed, TMpro/pro mice exhibit a 100-fold reduction with respect to binding of thrombin and a 1000-fold reduction with respect to PC activation in the circulation.16 Similarly, TMpro/pro mice display a strongly reduced capacity to generate APC in their alveolar space (a 25-fold reduction compared with WT mice).15 The incapacity of TMpro/pro mice to activate PC has been demonstrated using an indirect approach (ie, mice received an intravenous16 or intratracheal15 dose of human PC with or without thrombin, after which human APC levels were determined in plasma or bronchoalveolar lavage fluid, respectively, using an immuno-capture assay). To the best of our knowledge, APC measurements in mice have not been reported previously. In addition, the “direct” demonstration of reduced APC levels in TMpro/pro mice during tuberculosis will be very difficult for several reasons. In particular, tuberculosis is accompanied by low PC levels in plasma27 ; together with our present finding of reduced TM expression in lungs during murine tuberculosis, these data suggest that this infection per se leads to a reduced capacity to generate APC. In line with this assumption, patients with interstitial lung disease demonstrated indirect evidence of diminished PC activation in bronchoalveolar lavage fluid.32 Furthermore, patients with sepsis have low or undetectable APC concentrations in their circulation.26 Hence, infection and inflammation likely result in a relative insufficiency to produce APC,2,33 and a further reduction of the APC production capacity, as in TMpro/pro mice, apparently has a profound influence on the regulation of inflammation during chronic pulmonary infection such as produced by M tuberculosis.
TMpro/pro mice have more mycobacteria in liver and spleen 36 weeks after infection. Mycobacterial loads in CFUs/mL in lungs (A), livers (B), and spleens (C) at 36 weeks after intranasal infection with 104M tuberculosis. Data are means ± SE of the remaining mice presented in Figure 4 (9/14 TMpro/pro mice and 14/14 WT mice). *P < .05 versus WT mice.
TMpro/pro mice have more mycobacteria in liver and spleen 36 weeks after infection. Mycobacterial loads in CFUs/mL in lungs (A), livers (B), and spleens (C) at 36 weeks after intranasal infection with 104M tuberculosis. Data are means ± SE of the remaining mice presented in Figure 4 (9/14 TMpro/pro mice and 14/14 WT mice). *P < .05 versus WT mice.
TMpro/pro mice did not show any evidence for activation of coagulation during tuberculosis. In humans, tuberculosis can be accompanied by modest alterations in the coagulant-anticoagulant balance.27 Considering the preferential expression of TM in the lung,13,14 we argued that TM-mediated generation of APC may be important for inhibiting a sustained activation of coagulation at the site of chronic inflammation such as produced by M tuberculosis infection in mice. This hypothesis was further supported by studies from other groups, reporting that TMpro/pro mice display a prethrombotic state and an increased susceptibility to thrombosis.16,34 The current data clearly indicate that murine tuberculosis does not result in local activation of coagulation and that TM and APC do not contribute to prevention of thrombosis in this condition (ie, TMpro/pro mice had normal TAT-c levels in plasma and lungs and a complete absence of fibrin deposits in their pulmonary compartment). Sato et al35 recently reported modest diffuse fibrin(ogen) deposition in lungs of WT mice intratracheally infected with M avium. Of interest, mice deficient for tissue-type plasminogen activator, urokinase-type plasminogen activator, and especially plasminogen showed more dense and extensive fibrin(ogen) deposition. Although Sato et al35 used a different pathogen, these data suggest that mycobacterial infection can be associated with modest fibrin generation in lungs; the fact that mice with a deficient fibrinolysis showed clearly enhanced fibrin(ogen) deposition further supports the notion that the role of the TM in prevention thrombosis is not important. Our inability to detect activation of coagulation in mice with tuberculosis was not due to an inadequate sensitivity of the fibrin staining, considering that exactly the same antibodies and procedures were used in our earlier investigations showing positive fibrin(ogen) staining in lungs of mice with either pneumonia caused by Streptococcus pneumoniae (Figure 3I) or Klebsiella pneumoniae, with sterile lung inflammation caused by LPS, or with Escherichia coli peritonitis.15,24 In addition, TAT-c levels did not rise during tuberculosis, whereas elevated lung homogenate levels were found in mice with pneumococcal pneumonia.
In contrast to the lack of an effect on coagulation, the TMpro/pro mutation exerted a profound effect on the inflammatory reaction to M tuberculosis infection. Indeed, at 5 weeks after infection TMpro/pro mice displayed uncontrolled inflammation in their lungs, which was associated with a diminished capacity to form well-shaped granulomas, a reduced recruitment of lymphocytes and macrophages to the site of the infection, and an increased influx of neutrophils. Our current data do not provide direct insight into how the attraction of lymphocytes and macrophages is impaired in TMpro/pro mice. The mechanisms underlying cell recruitment and granuloma formation during lung tuberculosis are complex involving chemokines, chemokine receptors, and adhesion molecules.18 Thus, although the modestly higher MCP-1 concentrations in the lungs of TMpro/pro mice could potentially result in the recruitment of more mononuclear cells, such an effect apparently is overruled by other more dominant mechanisms. TMpro/pro mice had higher levels of proinflammatory cytokines (IFN-γ and TNF-α) and lower levels of anti-inflammatory cytokines (IL-4, IL-10, and TGF-β) in their lungs, reflecting a net shift toward a proinflammatory cytokine environment. Furthermore, in a separate study, in which mice were followed for 36 weeks after infection with a lower dose of M tuberculosis, 35% of TMpro/pro mice died from week 28 onward versus none of the WT mice, and the surviving TMpro/pro mice displayed increased lung inflammation. The fact that the TMpro/pro mutation influenced the inflammatory response only at later time points during the infection may indicate that this mutation disturbs the switch from innate to adaptive immunity. Alternatively, it may point out that TM is primarily important for the orchestration of chronic inflammation in general. In any case, the present findings are in line with several earlier studies examining the effect of TM and APC on inflammation. Exogenously administered TM and APC were both able to attenuate lung injury related to disseminated intravascular coagulation by inhibiting leukocyte activation,9,36-40 and intravenous infusion of APC in healthy humans attenuated the recruitment of neutrophils into lung segments challenged with LPS.12 Furthermore, intrapulmonary delivery of APC exerted anti-inflammatory effects in bleomycin-induced lung fibrosis and an experimental model of allergic asthma.10,11 Moreover, leukocyte activation was also inhibited by APC after renal and spinal cord injury in rats.6,7,41 In addition, APC can inhibit the production of TNF-α and other proinflammatory cytokines in vitro and in vivo.6-9 The majority of the anti-inflammatory effects of APC in these models were unrelated to its anticoagulant effects. Our current data are also in accordance with recent findings in heterozygous protein C–deficient mice, which demonstrated higher levels of proinflammatory cytokines and increased neutrophil invasion in their lungs after intraperitoneal injection of LPS.23
Notably, the present study contrasts with a previous investigation from our laboratory, in which the influence of the TMpro/pro mutation on the pulmonary coagulant and inflammatory response during acute lung inflammation models induced by intranasal administration with either bacterial respiratory pathogens or LPS was examined.15 In that study we did not detect any difference between TMpro/pro and WT mice in either of these models, although in pneumococcal pneumonia fibrin deposition was modestly increased in TMpro/pro mice.15 Thus, TM and APC apparently have a greater impact on the inflammatory reaction in the lung during chronic lung infection such as in murine tuberculosis. Alternatively, the influence of the TMpro/pro mutation on lung inflammation may be determined by the type of respiratory pathogen.
Conway et al42 recently reported several anti-inflammatory properties of endogenous TM that were unrelated to its anticoagulant properties. These authors generated mice that lack the NH2-terminal (lectin) domain of TM; these TMLeD/LeD mice were shown to have normal TM antigen levels and to retain the capacity to generate APC. TMLeD/LeD mice were found to have elevated circulating cytokine levels upon systemic challenge with LPS. Moreover, TMLeD/LeD mice had higher neutrophil counts in bronchoalveolar lavage fluid after exposure to LPS via a nebulizer. This investigation indicates that TM, and in particular its lectin domain, has anti-inflammatory properties that can be dissected from the anticoagulant properties of TM.42 It should be noted that although the TMpro/pro mice used here have an intact TM lectin domain, they have a diminished antigenic expression of TM.16 Hence, TMpro/pro mice not only have a severely impaired capacity to generate APC but also display an overall relative TM deficiency including the function of the TM lectin domain. Therefore, our data do not definitively establish that the phenotype of TMpro/pro mice in this model of chronic infection is solely attributable to a reduced capacity to generate APC. In addition, the fact that the TMpro/pro mutation has a profound impact on the lung inflammatory response in spite of the fact that the overall pulmonary TM expression was strongly reduced as a consequence of M tuberculosis infection suggests that even the presence of low TM levels is important for the regulation of lung inflammation during tuberculosis. Alternatively, and not mutually exclusive, TM at remote sites and/or in the systemic vasculature may affect lung inflammation, possibly by delivering APC. Another way by which TM deficiency may affect inflammatory reactions is by negatively influencing the activation of thrombin-activatable fibrinolysis inhibitor (TAFI). Indeed, TM is a critical cofactor for thrombin-mediated activation of TAFI.43 TAFI not only inhibits fibrinolysis by impeding the conversion of plasminogen into plasmin but also may exert anti-inflammatory effects by virtue of its ability to inactivate complement factors C3a and C5a.1,44 However, our own preliminary experiments suggest that TAFI does not play a major role in the phenotype of TMpro/pro mice in tuberculosis: at 5 weeks after infection with M tuberculosis TAFI gene (Cpb2)–deficient mice had lower lung weights than WT mice and total cell counts in whole lung cell suspensions were not different from WT mice at this time point; moreover, the cellular composition of lung suspensions (lymphocyte subsets, neutrophils) did not differ between TAFI-deficient and WT mice (C.W.W., J. C. M. Meijers, and T.v.d.P., unpublished observations, December 2004), which contrasts sharply with our current findings in TMpro/pro mice. These preliminary data strongly suggest that the altered inflammatory response in TMpro/pro mice is independent of a potential effect of TM deficiency on the activation of TAFI and, thereby, also independent of secondary (TAFI-mediated) effects on the complement system and the fibrinolytic system. Considering that TM may also influence fibrinolysis via APC (which may exert profibrinolytic effects by inhibition of plasminogen activator inhibitor type I45 ), we measured D-dimer concentrations in lung homogenates of infected TMpro/pro and WT mice and found no differences (data not shown).
Relative to the strongly altered inflammatory response, the TMpro/pro mutation modestly influenced the outgrowth of M tuberculosis. At 5 weeks after infection, TMpro/pro mice had less mycobacterial CFUs in lungs and livers than WT, in spite of the fact that they were less able to form well-defined granulomas. It is conceivable that the enhanced local concentrations of the protective cytokines IFN-γ and TNF-α played a role in the modestly reduced mycobacterial loads in TMpro/pro mice.29,30 In addition, splenocytes harvested from TMpro/pro mice displayed an enhanced protective antigen–specific type 1 response at 5 weeks after infection (data not shown). However, at 36 weeks after infection surviving TMpro/pro mice had similar mycobacterial loads in their lungs compared with WT mice, whereas mycobacteria had disseminated to a larger extent to liver and spleen. Conceivably, in the absence of a controlled granulomatous inflammatory response, such as observed in TMpro/pro mice, eventually the infection cannot be contained within the lung adequately. Nonetheless, we consider it likely that the premature deaths among TMpro/pro mice were primarily caused by the increased pulmonary inflammation rather than by the enhanced mycobacterial outgrowth in distant organs during the later phase of the infection.
Tuberculosis results in a chronic inflammatory reaction of the lungs that normally is not or to a very limited extent is associated with activation of the coagulation system. We here tested the hypothesis that the capacity to generate APC in the lung is important for inhibiting the coagulant and the inflammatory response during pulmonary tuberculosis. By using TMpro/pro mice we show that a strongly reduced ability to generate APC in the alveolar space does not result in enhanced activation of coagulation but that the coordinated inflammatory response characterized by the recruitment of lymphocytes and macrophages to the site of the infection and the formation of well-shaped granulomas is disturbed. These data suggest that the presence of functional TM in the lung is of importance for the regulation of the immune response to M tuberculosis.
Prepublished online as Blood First Edition Paper, July 12, 2005; DOI 10.1182/blood-2004-12-4623.
Supported by grants from the Dutch Association for Scientific Research (NWO; S.W.) and the Mr Willem Bakhuys Roozeboom Foundation (C.W.W.).
S.W. and C.W.W. performed the research, analyzed the data and took part in writing the manuscript. S.F. performed and analyzed the histopathology and took part in writing the manuscript. T.v.d.P. designed the research and wrote the final version of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank I. Kopp and J. B. Daalhuisen for expert technical assistance and N. Claessen for immunostaining.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal