Abstract
Systemic lupus erythematosus (SLE) is the most common disease associated with antiphospholipid syndrome (APS). We, therefore, evaluated 46 patients with refractory SLE treated by autologous hematopoietic stem cell transplantation (HSCT) for a history of APS prior to transplantation. The prevalence of SLE-related APS in our patient population was 61% (28 of 46 patients with refractory SLE). Nineteen of 28 patients with APS had lupus anticoagulant (LA) or high titers of anticardiolipin antibodies (ACLAs), either immunoglobulin (Ig)G or IgM, when evaluated at study entry. Six of 8 evaluable LA+ patients became and remained LA–; 5 of 7 initially ACLA IgG+ patients and 9 of 11 ACLA IgM+ patients demonstrated normalization of ACLA titers when followed after HSCT. Eighteen of 22 patients refractory to chronic anticoagulation discontinued anticoagulation therapy a median of 4 months after transplantation; 78% of them remained free of thrombotic events and in complete SLE remission for up to 78 months (median, 15 months) after HSCT. There was no treatment-related mortality. Autologous HSCT may be performed safely in patients with APS and appears to be effective therapy for eliminating ALPAs and preventing thrombotic complications in patients with SLE.
Introduction
Antiphospholipid syndrome (APS) is defined by the Sapporo criteria as the presence of antiphospholipid antibodies (APLAs), either lupus anticoagulant (LA) or anticardiolipin antibodies (ACLAs), and occurrence of either an acute thromboembolic event or fetal wastage.1,2 APS-related thrombotic events may be venous or arterial, whereas APS-related fetal morbidity includes premature births, spontaneous abortions, and fetal death. Other manifestations associated with APS but not part of the Sapporo criteria for definite APS include thrombocytopenia, hemolytic anemia, cardiac valve disease (Libman-Sacks endocarditis [LSE]), livedo reticularis (LR), and various neurologic manifestations including intractable headaches, migraines, seizures, chorea, transient ischemic attacks (TIAs), cerebrovascular accidents (CVAs), amaurosis fugax, dementia, psychosis, depression, transverse myelitis, and a multiple sclerosis-like disease.1-15 With the exception of anticoagulation, there is no standard therapy for APS. Current therapy for APS-related thrombosis is life-long anticoagulation with warfarin adjusted for an international normalization ratio greater than 2.0 to 3.0 to reduce the risk of recurrent thrombi despite the potential for serious bleeding complications.16-21 Some studies suggest there might be no significant protection against futureAPS-related thromboembolic events even with warfarin or aspirin anticoagulation.22,23
APS is classified into primary and secondary, the latter being associated with connective tissue disease. Systemic lupus erythematosus (SLE) is the most common cause of secondary APS, and the prevalence of APLAs, either LA or ACLAs, in patients with SLE is reported to be as high as 30% to 50%.24-29 Recently, a new approach to treating severe and refractory SLE with high-dose chemotherapy and autologous hematopoietic stem cell transplantation (HSCT) has been reported to induce remission of active SLE.30-35 We analyzed 46 patients with severe and refractory SLE treated by high-dose chemotherapy and autologous HSCT at Northwestern University with the objective to determine the prevalence of APS, the effect of this therapy on APLA positivity, recurrent thrombotic rates, and changes in APS-related clinical manifestations.
Patients and methods
Patients
During the 7 years from April 1997 through August 2004, a total of 46 patients from 20 different states of United States with SLE refractory to pulse cyclophosphamide were treated with autologous HSCT in a study approved by the Institutional Review Board of Northwestern University and the US Food and Drug Administration (Investigational New Drug [IND] 6559). Informed consent was obtained from each patient according to the Declaration of Helsinki. All patients had at least 4 of 11 American College of Rheumatology (ACR) criteria for SLE and required more than 20 mg prednisone daily or its equivalent despite monthly intravenous pulse cyclophosphamide (500-1000 mg/m2). Patient eligibility criteria included World Health Organization class III or IV glomerulonephritis, involvement of the lung (vasculitis, pneumonitis, shrinking lung syndrome) or central nervous system (CNS; cerebritis or transverse myelitis), transfusion-dependent autoimmune cytopenias, or APS. Nephritis required failure of 6 or more monthly pulses of cyclophosphamide. Other visceral organ involvement required at least 3 months of prior intravenous cyclophosphamide. APS eligibility required recurrent thrombi despite warfarin anticoagulation.
SLE disease activity
Remission of SLE is defined according to Responder Index for Lupus Erythematosus (RIFLE) criteria as requiring no immunosuppressive medications except physiologic doses of corticosteroids, that is, 10 mg or less of prednisone or corticosteroid equivalent per day.36
APS
APS according to the Sapporo criteria is defined by one clinical criteria of either vascular thrombosis or fetal morbidity and one immunologic criteria of either LA or ACLAs (termed “definite APS”).1 Vascular thrombosis may be arterial, venous, or small vessel confirmed with imaging, Doppler, or histopathology. ACLAs must be either immunoglobulin (Ig)G or IgM isotype. Other features of APS, such as LR, LSE, thrombocytopenia, hemolytic anemia, transverse myelitis, TIA, migraine, or other neurologic and psychiatric manifestations, are not currently designated as Sapporo criteria, although they are frequently encountered as a part of definite APS or are associated with APLA positivity.1,2 In our study, patients with SLE with a history of APLAs and any APS-related clinical features but without past thrombotic events were categorized as those with “probable APS” and were analyzed along with the definite APS group.
ACLAs (IgG and IgM) were determined in Northwestern Memorial Hospital (NMH; Chicago, IL) laboratory using a commercial enzyme-linked immunosorbent assay (ELISA) technique with purified cardiolipin antigen bound to wells of a polystyrene microwell plate (INOVA QUANTA Lite anticardiolipin IgG and IgM [horseradish peroxidase]; INOVA Diagnostics, San Diego, CA). Samples were also sent for testing to Specialty Laboratories (Santa Monica, CA), where an ELISA method was used as well. For ACLAs, cutoff values for positive were defined as follows. For IgG: more than 15 GPL U/mL (IgG phospholipid units per milliliter); 15 to 20 GPL, low positive; 21 to 40 GPL, moderate positive; and more than 40 GPL, high positive. For IgM: more than 12.5 MPL U/mL (IgM phospholipid units per milliliter); 12.5 to 20 MPL, low positive; 21 to 40 MPL, moderate positive; and more than 40 MPL, high positive. LA was tested at NMH and was detected by activated partial thromboplastin time (aPTT) and dilute Russell viper venom time (dRVVT) with failure of prolonged coagulation time to correct with platelet-poor plasma but shortening or correction with excess phospholipids. An aPTT- or dRVVT-to-normal ratio of 1.4 or greater was considered positive. The Staclot LA test using hexagonal phase phospholipids was a final assay confirming LA positivity.
Stem cell mobilization and conditioning regimen
Peripheral blood stem cells were mobilized with cyclophosphamide (2.0 gm/m2) and granulocyte colony-stimulating factor (G-CSF) at 5 μg/kg/d administered subcutaneously daily beginning 3 days later. Leukapheresis was initiated when the white blood cell count reached 1.0 × 109/L and continued daily until the number of stem cells exceeded 1.4 × 106 CD34+ cells/kg after positive selection using Isolex (Nexell, Irvine, CA) or Ceprate (Cellpro, Bothell, WA) stem cell concentrator. The conditioning regimen consisted of cyclophosphamide 50 mg/kg/d intravenously on days –5, –4, –3, and –2 (total dose 200 mg/kg) and equine anti–thymocyte globulin (ATG) 30 mg/kg/d intravenously on days –4, –3, and –2 (total dose 90 mg/kg).
Antibiotic prophylaxis
Prophylactic antibiotic regimen included aerosolized pentamidine (300 mg) once the day of admission, fluoroquinolone (ciprofloxacin or levofloxacin) changed to intravenous cefepime with the development of neutropenia, fluconazole or voriconazole, and acyclovir or valacyclovir daily. G-CSF (5 μg/kg/d) injections were started on day 0 (day of stem cell infusion) and continued until the resolution of neutropenia (absolute neutrophil count > 1.0×109/L). Patients continued to take prophylactic monthly pentamidine or sulfamethoxazole/trimethoprim 3 times per week, daily fluconazole or voriconazole for 6 months, and daily acyclovir or valacyclovir twice a day for a year after transplantation.
Anticoagulation prophylaxis
One to 2 weeks prior to admission, warfarin anticoagulation was discontinued and therapeutic anticoagulation initiated with either subcutaneous enoxaparin 1.0 mg/kg every 12 hours (reduced to once a day for creatinine clearance < 30 mL/h) or subcutaneous dalteparin 200 U/kg/d (reduced to 100 U/kg/d for creatinine clearance < 30 mL/h). After the conditioning regimen when platelet counts fell below 50×109/L, low-molecular-weight heparin (LMWH) was adjusted for a prophylaxis dose of either 40 mg/kg/d enoxaparin or 5000 U/d dalteparin.
Blood transfusions
Platelet transfusions were given to maintain platelet counts greater than 20×109/L to 30×109/L. Packed red blood cells (PRBCs) were transfused if the hemoglobin concentration was less than 80 g/L. Platelets and PRBCs were irradiated, cytomegalovirus safe, and leukocyte depleted.
Posttransplantation follow-up
After HSCT, patients visited transplant physicians at NMH for scheduled follow-up visits at 3, 6, and 12 months and then yearly thereafter. History, physical examination, serologic testing, and necessary imaging studies were performed during follow-up visits. Nine patients from other states at certain time points were not able to return for follow-up, and in such cases, medical records and laboratory blood samples were collected from the local physician or medical facility.
Statistical analysis
The Kendall τ coefficient of concordance was used for analysis of the relationship between nonparametric variables such as post-HSCT relapsed versus negative APLAs, thrombotic events, associated APS features, and active SLE using Statistica software (Tulsa, OK).
Results
Patient profile
Forty-six patients underwent autologous HSCT for SLE. This paper is restricted to the 28 patients (61%) with APS: 20 patients (44%) who fulfilled Sapporo criteria for definite APS and 8 patients (17%) who did not meet Sapporo criteria but demonstrated APLA positivity with other APS-associated manifestations such as thrombocytopenia, hemolytic anemia, cardiac valve disease, or dermatologic or neurologic manifestations (“probable APS” group; Table 1). One patient (2%) was APLA (ACLA IgG) positive but had no history of thrombosis or manifestations related to APS and is not discussed further. Of 28 patients with APS, 25 (89%) were women, and their mean age was 29 years (range, 16-52 years). The indications for HSCT in patients with APS included nephritis (18 patients), CNS disease such as cerebritis/myelitis (20 patients), pulmonary involvement including pneumonitis/alveolar hemorrhage/shrinking lung syndrome (8 patients), cutaneous vasculitis (9 patients), autoimmune hemolytic anemia (3 patients), immune thrombocytopenic purpura (3 patients), autoimmune hepatitis (1 patient), and myocarditis (1 patient). Two patients were eligible for HSCT only due to refractory APS. One patient, despite warfarin and aspirin anticoagulation, had recurrent arterial and venous thrombi including 2 myocardial infarctions, 2 cerebral infarcts, 2 deep venous thromboses, and pulmonary emboli. The second patient experienced pulmonary embolism twice, suffered from CVAs with residual hemiparesis, and continued to have refractory migraines while being managed with quadruple anticoagulation including warfarin, aspirin, LMWH, and pentoxifylline.
Profile of patients with SLE-related APS
. | . | . | . | ACLAs . | . | Thrombotic Events . | . | . | . | Coexisting thrombogenic risk factors . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Age, y . | Sex . | LA . | IgG . | IgM . | Arterial . | Venous . | Pregnancy morbidity . | Non-Sapporo manifestations . | HTN . | DM . | OCP . | Smoking . | Indications for HSCT . | |||||
| D-APS | |||||||||||||||||||
| 1 | 15 | F | PI | PI | — | — | UE DVT | None | LSE, diplopia/nystagmus | Yes | No | No | No | N, C, P | |||||
| 2 | 52 | F | — | PI | — | SMA + RCA | — | 5 first-trimester spontaneous abortions | None | Yes | No | No | No | V | |||||
| 3 | 30 | F | PT | — | — | — | LE DVT×2 | None | None | Yes | No | No | No | N | |||||
| 4 | 29 | F | — | PI | PI | — | UE DVT×3 | None | LR, thrombocytopenia, diplopia | No | No | Yes | No | C, V | |||||
| 5 | 20 | F | PT | — | PT | Splenic infarct | PE | None | None | No | No | No | Yes | AIH, AIHA | |||||
| 6 | 45 | F | — | PT | — | CNS infarct | LE DVT×2 | None | Myelitis/paraparesis | Yes | No | Yes | No | N, C | |||||
| 7 | 21 | F | PI | — | — | — | PE×2 + UE DVT×3 | None | LR, thrombocytopenia, hallucinations | Yes | No | Yes | No | N, C, V | |||||
| 8 | 21 | F | — | — | PT | Mesenteric artery | — | None | Calciphylaxis | No | No | No | No | N, calciphylaxis | |||||
| 9 | 48 | F | PT | PT | PT | — | PE + LE DVT×2 | 2 second-trimester spontaneous abortions | TIA×7 | Yes | No | Yes | No | C, P, APS | |||||
| 10 | 31 | F | — | — | PT | — | UE DVT | None | Seizures, thrombocytopenia | Yes | No | No | No | N, C, AIHA | |||||
| 11 | 38 | F | PT | PI | PT | LLE aa.×3 | PE×2 + LE DVT×2 | None | Multiple CVA | No | No | No | No | C, nonhealing LLE ulcer | |||||
| 12 | 36 | F | PI | — | — | CNS infarct | — | None | None | No | No | Yes | Yes | C, P | |||||
| 13 | 41 | F | — | PT | PT | — | UE DVT | Spontaneous abortion | Diplopia, calciphylaxis | No | No | No | No | N, C, P, calciphylaxis | |||||
| 14 | 27 | F | PI | — | — | — | LE DVT + PE | None | None | Yes | No | No | No | N, C, P | |||||
| 15 | 29 | M | PT | PI | PI | — | PE + LE DVT×2 | None | Seizures | Yes | No | No | No | N, C | |||||
| 16 | 22 | F | PT | — | — | — | PE + LE DVT | None | None | Yes | No | No | No | N, C, P | |||||
| 17 | 39 | M | PI | PI | PI | — | LE DVT×2 + UE DVT | None | Seizures, thrombocytopenia | No | No | No | No | C, thrombocytopenia | |||||
| 18 | 18 | F | — | PI | — | — | PE | None | Seizures, thrombocytopenia | Yes | No | No | No | N, C, myocarditis | |||||
| 19 | 23 | F | PT | — | — | CNS infarct × 2 | PE + LE DVT×2* | None | Multiple silent CVA, LR | Yes | No | No | No | CAPS, N, C | |||||
| 20 | 30 | F | — | PI | — | CNS infarct | PE×2 | None | Multiple TIA, intractable H/A | No | Yes | No | No | CAPS, C | |||||
| P-APS | |||||||||||||||||||
| 1 | 20 | F | — | — | PT | — | None | None | LR | Yes | No | No | No | P, V | |||||
| 2 | 27 | F | — | PT | PT | — | None | None | Seizures, myelitis | Yes | No | No | No | N, C, P, V | |||||
| 3 | 20 | F | — | PT | — | — | None | None | Cranial nerve palsies | No | No | No | No | N, C | |||||
| 4 | 29 | F | PT | PT | — | — | None | None | Myelitis | No | No | No | No | C | |||||
| 5 | 29 | F | PT | — | — | — | None | None | LSE, thrombocytopenia | No | No | Yes | No | N, V | |||||
| 6 | 37 | M | PI | — | PT | — | None | None | Transverse myelitis | No | No | No | No | N, myelitis | |||||
| 7 | 20 | F | PT | PT | PT | — | None | None | Intractable headache | No | No | No | Yes | N, C, V | |||||
| 8 | 16 | F | — | — | PT | — | None | None | Seizures | No | No | No | No | N, C | |||||
. | . | . | . | ACLAs . | . | Thrombotic Events . | . | . | . | Coexisting thrombogenic risk factors . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Age, y . | Sex . | LA . | IgG . | IgM . | Arterial . | Venous . | Pregnancy morbidity . | Non-Sapporo manifestations . | HTN . | DM . | OCP . | Smoking . | Indications for HSCT . | |||||
| D-APS | |||||||||||||||||||
| 1 | 15 | F | PI | PI | — | — | UE DVT | None | LSE, diplopia/nystagmus | Yes | No | No | No | N, C, P | |||||
| 2 | 52 | F | — | PI | — | SMA + RCA | — | 5 first-trimester spontaneous abortions | None | Yes | No | No | No | V | |||||
| 3 | 30 | F | PT | — | — | — | LE DVT×2 | None | None | Yes | No | No | No | N | |||||
| 4 | 29 | F | — | PI | PI | — | UE DVT×3 | None | LR, thrombocytopenia, diplopia | No | No | Yes | No | C, V | |||||
| 5 | 20 | F | PT | — | PT | Splenic infarct | PE | None | None | No | No | No | Yes | AIH, AIHA | |||||
| 6 | 45 | F | — | PT | — | CNS infarct | LE DVT×2 | None | Myelitis/paraparesis | Yes | No | Yes | No | N, C | |||||
| 7 | 21 | F | PI | — | — | — | PE×2 + UE DVT×3 | None | LR, thrombocytopenia, hallucinations | Yes | No | Yes | No | N, C, V | |||||
| 8 | 21 | F | — | — | PT | Mesenteric artery | — | None | Calciphylaxis | No | No | No | No | N, calciphylaxis | |||||
| 9 | 48 | F | PT | PT | PT | — | PE + LE DVT×2 | 2 second-trimester spontaneous abortions | TIA×7 | Yes | No | Yes | No | C, P, APS | |||||
| 10 | 31 | F | — | — | PT | — | UE DVT | None | Seizures, thrombocytopenia | Yes | No | No | No | N, C, AIHA | |||||
| 11 | 38 | F | PT | PI | PT | LLE aa.×3 | PE×2 + LE DVT×2 | None | Multiple CVA | No | No | No | No | C, nonhealing LLE ulcer | |||||
| 12 | 36 | F | PI | — | — | CNS infarct | — | None | None | No | No | Yes | Yes | C, P | |||||
| 13 | 41 | F | — | PT | PT | — | UE DVT | Spontaneous abortion | Diplopia, calciphylaxis | No | No | No | No | N, C, P, calciphylaxis | |||||
| 14 | 27 | F | PI | — | — | — | LE DVT + PE | None | None | Yes | No | No | No | N, C, P | |||||
| 15 | 29 | M | PT | PI | PI | — | PE + LE DVT×2 | None | Seizures | Yes | No | No | No | N, C | |||||
| 16 | 22 | F | PT | — | — | — | PE + LE DVT | None | None | Yes | No | No | No | N, C, P | |||||
| 17 | 39 | M | PI | PI | PI | — | LE DVT×2 + UE DVT | None | Seizures, thrombocytopenia | No | No | No | No | C, thrombocytopenia | |||||
| 18 | 18 | F | — | PI | — | — | PE | None | Seizures, thrombocytopenia | Yes | No | No | No | N, C, myocarditis | |||||
| 19 | 23 | F | PT | — | — | CNS infarct × 2 | PE + LE DVT×2* | None | Multiple silent CVA, LR | Yes | No | No | No | CAPS, N, C | |||||
| 20 | 30 | F | — | PI | — | CNS infarct | PE×2 | None | Multiple TIA, intractable H/A | No | Yes | No | No | CAPS, C | |||||
| P-APS | |||||||||||||||||||
| 1 | 20 | F | — | — | PT | — | None | None | LR | Yes | No | No | No | P, V | |||||
| 2 | 27 | F | — | PT | PT | — | None | None | Seizures, myelitis | Yes | No | No | No | N, C, P, V | |||||
| 3 | 20 | F | — | PT | — | — | None | None | Cranial nerve palsies | No | No | No | No | N, C | |||||
| 4 | 29 | F | PT | PT | — | — | None | None | Myelitis | No | No | No | No | C | |||||
| 5 | 29 | F | PT | — | — | — | None | None | LSE, thrombocytopenia | No | No | Yes | No | N, V | |||||
| 6 | 37 | M | PI | — | PT | — | None | None | Transverse myelitis | No | No | No | No | N, myelitis | |||||
| 7 | 20 | F | PT | PT | PT | — | None | None | Intractable headache | No | No | No | Yes | N, C, V | |||||
| 8 | 16 | F | — | — | PT | — | None | None | Seizures | No | No | No | No | N, C | |||||
HTN, hypertension; DM, diabetes mellitus; OCP, oral contraceptive pill; D-APS, definite APS; P-APS, probable APS; PI, positive in the past but not immediately before transplantation; — negative result; UE, upper extremity; DVT, deep venous thrombosis; N, nephritis; C, cerebritis; P, pneumonitis; SMA, superior mesenteric artery; RCA, right coronary artery; V, cutaneous vasculitis; PT, positive during pretransplant testing; LE, lower extremity; PE, pulmonary embolism; AIH, autoimmune hepatitis; AIHA, autoimmune hemolytic anemia; LLE aa, left lower extremity arteries; sp.-spontaneous; LSE-Libman-Sacks endocarditis; CAPS, catastrophic APS; H/A-headache. Other abbreviations are explained in the text.
Patient 19 also had 2 myocardial infarctions.
Definite APS
Twenty patients of a total 46 patients with SLE who underwent HSCT met Sapporo criteria for definite APS (44%; Table 1). The mean age and sex ratio of those patients meeting Sapporo criteria was 31 years old, 18 women to 2 men, respectively. Of 20 patients with definite APS, 11 had APLAs at the time of study entry; 7 patients had LA, and 7 patients had elevated titers of ACLAs, IgG or IgM. The remaining 9 patients with a history of APS had previously tested positive for ACLAs but were negative at enrollment. Eight patients had a history of arterial thrombi: 2 mesenteric, 1 coronary, 1 splenic, 3 lower extremity, 4 CNS (total of 12 arterial thrombotic events). Sixteen patients had prior venous thrombi/emboli: 13 pulmonary emboli, 16 lower extremity deep venous thromboses (DVTs), 10 upper extremity DVTs (total of 39 venous thrombotic events). Three patients had fetal wastage (total of 8 miscarriages), all of whom also had a history of arterial or venous thrombosis. Coexisting thrombotic risk factors included hypertension in 12 patients, diabetes mellitus in 1 patient, use of oral birth control pills in 5 patients, and smoking in 2 patients. In patients who met Sapporo criteria for APS, numerous other APLA-associated manifestations were present including thrombocytopenia (5), LR (2), LSE (1), seizures (4), silent CVAs (4), cranial nerve palsies (3), multiple TIAs (2), myelitis/paraparesis (1), ataxia (1), hallucinations (1), intractable headaches (1), and calciphylaxis (2).
Probable APS
Eight patients with positive APLA, either LA or ACLAs, and associated APS symptoms did not meet Sapporo criteria for definite APS, but for the purpose of this summary, were classified as having a “probable APS” (Table 1). APS manifestations within this group included LR (1), LSE (1), thrombocytopenia (1), myelitis/transverse myelitis (3), intractable headache (1), seizures (2), and cranial nerve palsies (1). All 8 patients with probable APS manifested positive APLAs at the enrollment. Three patients had LA, and 7 patients had elevated titers of ACLAs, IgG or IgM. Additionally, one patient with elevated ACLA IgM titer demonstrated positive LA in the past but not immediately before transplantation. Seven (88%) of 8 patients with probable APS were women. The mean age of patients in the probable APS group was 25 years. Although no history of thrombi was documented, coexisting thrombotic risk factors included hypertension in 2 patients, diabetes mellitus in 1 patient, and smoking in 1 patient.
Toxicity
There was no treatment-related mortality in any of the 28 patients with SLE-related APS who underwent autologous HSCT. Characteristics related to stem cell engraftment, including day of white blood cell and platelet engraftment after stem cell infusion (day 0), platelet and red blood cell transfusion requirements, total number of days spent in hospital, infections during hospitalization, and serious adverse events including need for dialysis and intubation, are described in Table 2. The mean times to absolute neutrophil count greater than 0.5× 109/L and platelet count above 20× 109/L were days 9 and 12, respectively. The mean hospitalized stay for HSCT was 20 days. The only patients who required dialysis were already on dialysis (1 peritoneal dialysis, 2 hemodialysis) prior to enrollment into the study. One patient required intubation secondary to pulmonary edema for 1 day. Peritransplantation-related infections included bacteremia (4 patients), candida fungemia (1 patient), candida peritonitis (1 patient), bacterial pneumonia (1 patient), bacterial urinary tract infection (2 patients), Clostridium difficile colitis (1 patient), Salmonella colitis (1 patient), cytomegalovirus (CMV) antigenemia (1 patient), and mucocutaneous herpes simplex (2 patients).
Stem cell engraftment-related hematologic and infectious characteristics and adverse events during immediate posttransplantation period in 28 patients with SLE-related APS
Patient no. . | ANC above 0.5×109/L, d after transplantation . | Platelet count above 20×109/L, d after transplantation . | No. RBC units . | No. platelet units . | Time in hospital, d . | Infections during hospitalization . | Dialysis . | Intubation . |
|---|---|---|---|---|---|---|---|---|
| D-APS | ||||||||
| 1 | 8 | 0* | 6 | 4 SDU | 21 | None | No | No |
| 2 | 9 | 17 | 5 | 8 SDU | 10 | None | No | No |
| 3 | 10 | 12 | 4 | 7 SDU | 19 | None | No | No |
| 4 | 8 | 7 | 3 | 3 SDU | 26 | None | No | No |
| 5 | 8 | 7 | 3 | 2 SDU | 19 | None | No | No |
| 6 | 13 | 13 | 6 | 9 SDU + 12 RDU | 20 | None | Enrolled on HD | No |
| 7 | 10 | 13 | 6 | 10 SDU | 19 | MRSA bacteremia (catheter related) | No | No |
| 8 | 11 | 13 | 6 | 10 SDU | 20 | CMV antigenemia | Enrolled on HD | No |
| 9 | 8 | 8 | 3 | 3 SDU | 18 | None | No | No |
| 10 | 10 | 11 | 5 | 7 SDU + 5 RDU | 18 | Candida peritonitis (PD catheter related) | Enrolled on PD | No |
| 11 | 10 | 14 | 8 | 9 SDU + 5 RDU | 21 | None | No | No |
| 12 | 9 | 10 | 9 | 7 SDU + 6 RDU | 21 | None | No | No |
| 13 | 8 | 0† | 4 | 0 | 18 | Staphylococcus aureus UTI | No | No |
| 14 | 11 | 22 | 15 | 7 SDU + 35 RDU | 31 | Klebsiella pneumoniae bacteremia and pneumonia, Candida glabrata fungemia, Enterococcus faecalis UTI | No | Yes |
| 15 | 9 | 10 | 10 | 6 SDU | 17 | None | No | No |
| 16 | 10 | 14 | 6 | 5 SDU + 15 RDU | 31 | MRSA and VRE bacteremia, HSV rash | No | No |
| 17 | 9 | 11 | 0 | 5 SDU + 5 RDU | 18 | None | No | No |
| 18 | 9 | 11 | 5 | 2 SDU | 17 | None | No | No |
| 19 | 9 | 13 | 5 | 6 SDU | 11 | HSV stomatitis, Clostridium difficile colitis | No | No |
| 20 | 11 | 13 | 6 | 11 SDU + 12 RDU | 21 | None | No | No |
| P-APS | ||||||||
| 1 | 10 | 12 | 5 | 5 SDU | 19 | None | No | No |
| 2 | 9 | 10 | 2 | 9 SDU | 19 | Salmonella enterocolitis | No | No |
| 3 | 7 | 0* | 1 | 0 | 16 | None | No | No |
| 4 | 9 | 10 | 4 | 14 SDU | 17 | None | No | No |
| 5 | 11 | 11 | 7 | 7 SDU | 18 | Streptococcus sanguis bacteremia | No | No |
| 6 | 8 | 9 | 2 | 0 | 17 | None | No | No |
| 7 | 9 | 15 | 4 | 11 RDU | 22 | None | No | No |
| 8 | 8 | 0‡ | 4 | 0 | 17 | None | No | No |
| Average | 9 | 12 | 5 | 6 SDU + 4 RDU | 20 | NA | NA | NA |
Patient no. . | ANC above 0.5×109/L, d after transplantation . | Platelet count above 20×109/L, d after transplantation . | No. RBC units . | No. platelet units . | Time in hospital, d . | Infections during hospitalization . | Dialysis . | Intubation . |
|---|---|---|---|---|---|---|---|---|
| D-APS | ||||||||
| 1 | 8 | 0* | 6 | 4 SDU | 21 | None | No | No |
| 2 | 9 | 17 | 5 | 8 SDU | 10 | None | No | No |
| 3 | 10 | 12 | 4 | 7 SDU | 19 | None | No | No |
| 4 | 8 | 7 | 3 | 3 SDU | 26 | None | No | No |
| 5 | 8 | 7 | 3 | 2 SDU | 19 | None | No | No |
| 6 | 13 | 13 | 6 | 9 SDU + 12 RDU | 20 | None | Enrolled on HD | No |
| 7 | 10 | 13 | 6 | 10 SDU | 19 | MRSA bacteremia (catheter related) | No | No |
| 8 | 11 | 13 | 6 | 10 SDU | 20 | CMV antigenemia | Enrolled on HD | No |
| 9 | 8 | 8 | 3 | 3 SDU | 18 | None | No | No |
| 10 | 10 | 11 | 5 | 7 SDU + 5 RDU | 18 | Candida peritonitis (PD catheter related) | Enrolled on PD | No |
| 11 | 10 | 14 | 8 | 9 SDU + 5 RDU | 21 | None | No | No |
| 12 | 9 | 10 | 9 | 7 SDU + 6 RDU | 21 | None | No | No |
| 13 | 8 | 0† | 4 | 0 | 18 | Staphylococcus aureus UTI | No | No |
| 14 | 11 | 22 | 15 | 7 SDU + 35 RDU | 31 | Klebsiella pneumoniae bacteremia and pneumonia, Candida glabrata fungemia, Enterococcus faecalis UTI | No | Yes |
| 15 | 9 | 10 | 10 | 6 SDU | 17 | None | No | No |
| 16 | 10 | 14 | 6 | 5 SDU + 15 RDU | 31 | MRSA and VRE bacteremia, HSV rash | No | No |
| 17 | 9 | 11 | 0 | 5 SDU + 5 RDU | 18 | None | No | No |
| 18 | 9 | 11 | 5 | 2 SDU | 17 | None | No | No |
| 19 | 9 | 13 | 5 | 6 SDU | 11 | HSV stomatitis, Clostridium difficile colitis | No | No |
| 20 | 11 | 13 | 6 | 11 SDU + 12 RDU | 21 | None | No | No |
| P-APS | ||||||||
| 1 | 10 | 12 | 5 | 5 SDU | 19 | None | No | No |
| 2 | 9 | 10 | 2 | 9 SDU | 19 | Salmonella enterocolitis | No | No |
| 3 | 7 | 0* | 1 | 0 | 16 | None | No | No |
| 4 | 9 | 10 | 4 | 14 SDU | 17 | None | No | No |
| 5 | 11 | 11 | 7 | 7 SDU | 18 | Streptococcus sanguis bacteremia | No | No |
| 6 | 8 | 9 | 2 | 0 | 17 | None | No | No |
| 7 | 9 | 15 | 4 | 11 RDU | 22 | None | No | No |
| 8 | 8 | 0‡ | 4 | 0 | 17 | None | No | No |
| Average | 9 | 12 | 5 | 6 SDU + 4 RDU | 20 | NA | NA | NA |
ANC indicates absolute neutrophil count; SDU, single donor units; RDU, random donor units; HD, hemodialysis; MRSA, methicillin-resistant Staphylococcus aureus; CMV, cytomegalovirus; PD, peritoneal dialysis; UTI, urinary tract infection; VRE, vancomycin-resistant enterococcus; HSV, herpes simplex virus. Other abbreviations are explained in the text and Table 1.
Platelet counts never below 20×109/L.
Platelet count never below 75×109/L.
Platelet count never below 30×109/L.
APLAs
Nineteen patients with SLE-related APS (11 from the definite APS group and 8 from the probable APS group) were positive for LA or ACLAs at the time of entry into study.
Lupus anticoagulant
10 patients had LA at the enrollment: 7 patients with definite APS and 3 patients with probable APS. Of 10 initially positive LA patients with SLE, 2 were not retested for LA after HSCT. Of the remaining 8 patients, 6 (75%) became and remained negative for LA during posttransplantation evaluations up to 30 months (median, 18 months). In the probable APS group, 1 patient was not retested, and 2 of remaining 2 patients (100%) became negative for LA. In the definite APS group, 1 patient was not retested, and 4 of remaining 6 patients (67%) became negative for LA. One patient became negative at 3 months, but LA returned to positive at 12 months. This patient remains in clinical remission and her severe cutaneous lesions have healed completely, although she did experience saphenous vein graft occlusion 1 year after HSCT. Another patient also became negative at 3 months, but had a relapse with positive LA at 6 months. Although she is in clinical SLE remission and free from thrombotic events, 3 months after transplantation she developed immune-mediated thrombocytopenia, which responded to intravenous gammaglobulin and resolved by 6 months after HSCT. Five patients with definite APS and 1 with probable APS were negative for LA immediately before transplantation, but had well-documented LA antibody in the past. Followed prospectively for up to 78 months (median, 12 months) after HSCT, 4 (3 from definite APS and 1 from probable APS group) have remained negative, 1 (definite APS) was not retested for LA, and 1 (definite APS) seroconverted to positive 4 years after transplantation (data not shown).
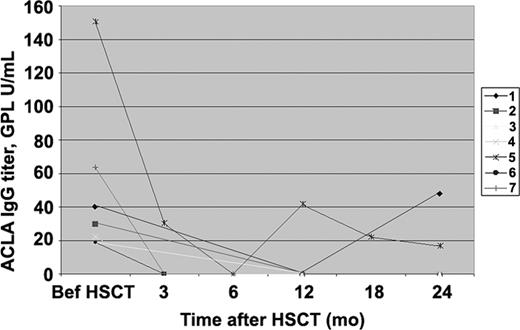
ACLA IgG antibodies
Before transplantation, 14 patients had high titers of ACLAs, IgG or IgM (3 patients, IgG; 7 patients, IgM; and 4 patients, both IgG and IgM). In patients positive for ACLA IgG followed for up to 24 months (median, 24 months), a gradual decline in antibody titers with normal end results occurred in all but 2 (71%; Figure 1). In the definite APS group, 3 patients had elevated ACLA IgG titers before transplantation. Two of them (67%) then had normalized titers. The titer in the remaining patient nevertheless showed a gradual and significant trend down from initial above 150 to almost negative 16.7. Also, her initially positive LA and high ACLA IgM titer became negative, and she is in clinical SLE remission and thrombosis-free. In the probable APS group, of 4 initially ACLA IgG+ patients, 3 (75%) had normalization of titers; the fourth patient's ACLA IgG became undetectable 12 months after transplantation, but reappeared with high titers at 24 months. Eight patients (all with definite APS) were known to have high ACLA IgG titers in the past, but not immediately before transplantation. Seven of them remained IgG– after HSCT with up to 60 months (median, 12 months) follow-up. One patient who had negative initial LA and undetectable titer of ACLA IgG seroconverted to positive (both, LA and ACLA IgG) 4 years after HSCT. Interestingly, she is the only APS patient who has become pregnant after HSCT (4 years after transplantation) and has had an elective abortion. Since then, she developed recurrence of proteinuria without additional SLE activity and recently has been experiencing refractory headaches.
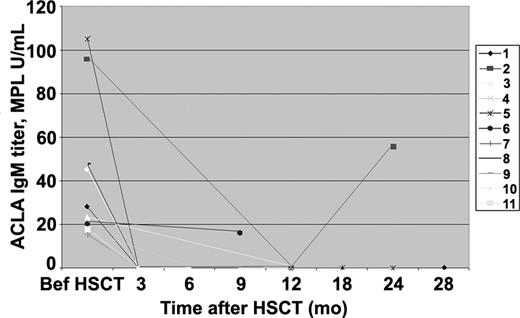
ACLA IgM antibodies
Of 11 ACLA IgM+ patients, antibody serial measurements up to 28 months (median, 12 months) after HSCT showed normalization of titers in 9 (82%; Figure 2). In the definite APS group, 6 patients were ACLA IgM+ before transplantation. Five of them (84%) had normalization of titers. One woman demonstrated elevated ACLA IgM titer at 9 months corresponding to persistence of active SLE. In the probable APS group, 5 patients were positive for ACLA IgM before HSCT, and 4 of them (80%) seroconverted to negative. One patient's IgM reappeared together with high IgG titer followed by seizure recurrence at 24 months. Three patients (all from the definite APS group) with a history of elevated ACLA IgM titers in the past but normal before transplantation remained seronegative with a median of 12 months of follow-up.
Thrombotic events
Before HSCT, the thromboembolic manifestations in patients with APS included 8 miscarriages, 13 arterial thrombi, 2 cardiac infarcts, and 39 venous thrombi (16 lower extremity DVTs, 13 pulmonary embolisms, 10 upper extremity DVTs; Table 1). Twenty-two of 28 patients with APS were on anticoagulation (warfarin, enoxaparin, aspirin, or clopidogrel) when referred for transplantation. In 18 (82%) of these 22 patients, anticoagulation was discontinued immediately to 30 months after transplantation (mean of 6 months, median of 4 months; Table 3). The decision to discontinue anticoagulation for each patient was made by the primary physician (usually in consultation with the transplantation physician) and was based on the general condition of the patient, severity of thromboembolic history before HSCT, absence of thrombotic events after the procedure, and APLA negativity. Four of 22 patients are still receiving anticoagulation therapy, including 2 patients just 6 months after transplantation, 1 patient with a history of severe debilitating APS complication (stroke) now 18 months after HSCT, and 1 patient with severe coronary artery disease with recurrent occlusions who underwent transplantation 60 months ago. Of 18 patients in whom anticoagulation therapy was discontinued, 14 (78%) have not experienced thromboembolic events from 6 to 78 months (median, 15 months) since stopping anticoagulation. Four patients who had discontinued anticoagulation had rethrombosis. Of note, their anticoagulation was stopped early, at a mean interval of 3 months (median, 3 months) after HSCT. One patient developed a splenic infarct (found accidentally by imaging study) and an upper extremity DVT at 10 months after HSCT with elevated ACLA IgM titer. Another patient who developed a saphenous vein graft thrombosis 10 months after transplantation also showed recurrence of LA (which was negative at 3 months after HSCT when LMWH was discontinued). Of 2 patients who developed upper extremity DVT due to indwelling central lines, 1 remained negative for LA and the second was not retested for APLAs after HSCT.
Post-HSCT course in 28 patients with SLE-related APS
. | . | ACLA . | . | . | . | . | Anticoagulation . | . | . | . | Immunosuppression . | . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | LA . | IgG . | IgM . | Follow-up, mos. . | Thrombotic events . | Non-Sapporo manifestations . | Before HSCT, Y/N . | After HSCT, Y/N . | When d/c, mo . | SLE remission (mo. of relapse) . | After HSCT, P mg/d . | When d/c, mo . | ||||||||||||
| D-APS | ||||||||||||||||||||||||
| 1 | Pos | Pos | — | 78 | None | No LSE, new H/A | Yes | No | 0 | Y | None | 12 | ||||||||||||
| 2 | — | Neg | — | 60 | LAD + LCX×2 | None | Yes | Yes | Continued | No (3) | — | — | ||||||||||||
| 3 | Neg | — | — | 42 | None | None | Yes | No | 30 | Y | 5 | NA | ||||||||||||
| 4 | — | Neg | Neg | 36 | None | No thrombocytopenia, LR same | Yes | No | 0 | No (3) | — | — | ||||||||||||
| 5 | NRT | — | Neg | 30 | None | None | Yes | No | 12 | Y | None | 6 | ||||||||||||
| 6 | — | Neg | — | 30 | None | Neurologically improved | No | No | NA | Y | None | 13 | ||||||||||||
| 7 | NRT | — | — | 24 | UE DVT×3 | LR and hallucinations improved, thrombocytopenia recurred | Yes | Yes | Restarted at 12 | No (12) | — | — | ||||||||||||
| 8 | — | — | Neg | 24 | UE DVT | Calciphylaxis resolved | Yes | Yes | Restarted at 14 | Y | None | 0 | ||||||||||||
| 9 | Neg | Pos | Neg | 28 | None | No TIA | Yes | No | 5 | Y | 5 + fludrocortisone (AI) | NA | ||||||||||||
| 10 | — | — | Pos | 11 | Splenic infarct, UE DVT | No seizures, thrombocytopenia recurred | Yes | Yes | Restarted at 10 | No (3) | — | — | ||||||||||||
| 11 | Pos | Neg | Neg | 24 | LE vein graft | No CVA, LLE ulcer healed | Yes | Yes | Restarted at 10 | Y | 2.5 | NA | ||||||||||||
| 12 | NRT | — | — | Lost to follow-up | None | No CVA | Yes | Yes | Continued | Y | — | — | ||||||||||||
| 13 | — | Neg | Neg | 18 | None | Calciphylaxis improving, diplopia stable | Yes | No | 0 | Y | None | 12 | ||||||||||||
| 14 | Neg | — | — | 21 | None | None | Yes | No | 3 | Y | Hydrocortisone (AI) | NA | ||||||||||||
| 15 | Neg | Neg | Neg | 24 | None | No seizures | Yes | No | 10 | Y | None | 12 | ||||||||||||
| 16 | Neg | — | — | 15 | None | None | Yes | No | 9 | Y | 5 | NA | ||||||||||||
| 17 | Neg | Neg | Neg | 6 | None | No seizures, no thrombocytopenia | No | No | NA | Y | 10 | NA | ||||||||||||
| 18 | — | Neg | — | 12 | None | No seizures, no thrombocytopenia | Yes | No | 1 | Y | None | 1 | ||||||||||||
| 19 | Pos | — | — | 6 | None | LR improving, no CVA, immune thrombocytopenia | Yes | Yes | Continued | Y | 5 | NA | ||||||||||||
| 20 | — | Neg | — | 6 | None | No TIA, H/A same | Yes | Yes | Continued | Y | 5 | NA | ||||||||||||
| P-APS | ||||||||||||||||||||||||
| 1 | — | — | Neg | 33 | None | LR persisted | No | No | NA | No (9) | — | — | ||||||||||||
| 2 | — | Pos | Pos | 36 | None | Neurologically improved, seizures recurred at 24 mo | Yes | No | 0 | No (30) | — | — | ||||||||||||
| 3 | — | Neg | — | 36 | None | Neurologically improved | No | No | NA | Y | None | 12 | ||||||||||||
| 4 | NRT | Neg | — | 18 | None | Neurologically improved | Yes | No | 6 | Y | 2.5 | NA | ||||||||||||
| 5 | Neg | — | — | 30 | None | No thrombocytopenia, no LSE | Yes | No | 3 | Y | None | 6 | ||||||||||||
| 6 | Neg | — | Neg | 12 | None | Neurologically improved, but TM recurred at 9 mo | No | No | NA | No (9) | — | — | ||||||||||||
| 7 | Neg | Neg | Neg | 12 | None | H/A improved | No | No | NA | Y | 5 | NA | ||||||||||||
| 8 | — | — | Neg | 12 | None | No seizures | Yes | No | 6 | Y | 10 | NA | ||||||||||||
. | . | ACLA . | . | . | . | . | Anticoagulation . | . | . | . | Immunosuppression . | . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | LA . | IgG . | IgM . | Follow-up, mos. . | Thrombotic events . | Non-Sapporo manifestations . | Before HSCT, Y/N . | After HSCT, Y/N . | When d/c, mo . | SLE remission (mo. of relapse) . | After HSCT, P mg/d . | When d/c, mo . | ||||||||||||
| D-APS | ||||||||||||||||||||||||
| 1 | Pos | Pos | — | 78 | None | No LSE, new H/A | Yes | No | 0 | Y | None | 12 | ||||||||||||
| 2 | — | Neg | — | 60 | LAD + LCX×2 | None | Yes | Yes | Continued | No (3) | — | — | ||||||||||||
| 3 | Neg | — | — | 42 | None | None | Yes | No | 30 | Y | 5 | NA | ||||||||||||
| 4 | — | Neg | Neg | 36 | None | No thrombocytopenia, LR same | Yes | No | 0 | No (3) | — | — | ||||||||||||
| 5 | NRT | — | Neg | 30 | None | None | Yes | No | 12 | Y | None | 6 | ||||||||||||
| 6 | — | Neg | — | 30 | None | Neurologically improved | No | No | NA | Y | None | 13 | ||||||||||||
| 7 | NRT | — | — | 24 | UE DVT×3 | LR and hallucinations improved, thrombocytopenia recurred | Yes | Yes | Restarted at 12 | No (12) | — | — | ||||||||||||
| 8 | — | — | Neg | 24 | UE DVT | Calciphylaxis resolved | Yes | Yes | Restarted at 14 | Y | None | 0 | ||||||||||||
| 9 | Neg | Pos | Neg | 28 | None | No TIA | Yes | No | 5 | Y | 5 + fludrocortisone (AI) | NA | ||||||||||||
| 10 | — | — | Pos | 11 | Splenic infarct, UE DVT | No seizures, thrombocytopenia recurred | Yes | Yes | Restarted at 10 | No (3) | — | — | ||||||||||||
| 11 | Pos | Neg | Neg | 24 | LE vein graft | No CVA, LLE ulcer healed | Yes | Yes | Restarted at 10 | Y | 2.5 | NA | ||||||||||||
| 12 | NRT | — | — | Lost to follow-up | None | No CVA | Yes | Yes | Continued | Y | — | — | ||||||||||||
| 13 | — | Neg | Neg | 18 | None | Calciphylaxis improving, diplopia stable | Yes | No | 0 | Y | None | 12 | ||||||||||||
| 14 | Neg | — | — | 21 | None | None | Yes | No | 3 | Y | Hydrocortisone (AI) | NA | ||||||||||||
| 15 | Neg | Neg | Neg | 24 | None | No seizures | Yes | No | 10 | Y | None | 12 | ||||||||||||
| 16 | Neg | — | — | 15 | None | None | Yes | No | 9 | Y | 5 | NA | ||||||||||||
| 17 | Neg | Neg | Neg | 6 | None | No seizures, no thrombocytopenia | No | No | NA | Y | 10 | NA | ||||||||||||
| 18 | — | Neg | — | 12 | None | No seizures, no thrombocytopenia | Yes | No | 1 | Y | None | 1 | ||||||||||||
| 19 | Pos | — | — | 6 | None | LR improving, no CVA, immune thrombocytopenia | Yes | Yes | Continued | Y | 5 | NA | ||||||||||||
| 20 | — | Neg | — | 6 | None | No TIA, H/A same | Yes | Yes | Continued | Y | 5 | NA | ||||||||||||
| P-APS | ||||||||||||||||||||||||
| 1 | — | — | Neg | 33 | None | LR persisted | No | No | NA | No (9) | — | — | ||||||||||||
| 2 | — | Pos | Pos | 36 | None | Neurologically improved, seizures recurred at 24 mo | Yes | No | 0 | No (30) | — | — | ||||||||||||
| 3 | — | Neg | — | 36 | None | Neurologically improved | No | No | NA | Y | None | 12 | ||||||||||||
| 4 | NRT | Neg | — | 18 | None | Neurologically improved | Yes | No | 6 | Y | 2.5 | NA | ||||||||||||
| 5 | Neg | — | — | 30 | None | No thrombocytopenia, no LSE | Yes | No | 3 | Y | None | 6 | ||||||||||||
| 6 | Neg | — | Neg | 12 | None | Neurologically improved, but TM recurred at 9 mo | No | No | NA | No (9) | — | — | ||||||||||||
| 7 | Neg | Neg | Neg | 12 | None | H/A improved | No | No | NA | Y | 5 | NA | ||||||||||||
| 8 | — | — | Neg | 12 | None | No seizures | Yes | No | 6 | Y | 10 | NA | ||||||||||||
D/c indicates discontinued; P, prednisone; Pos, positive; Neg, negative; NRT, not retested (Pos, Neg, and NRT: were positive during pretransplant testing); —, none; NA, not applicable; LAD, left descending coronary artery; LCX, left circumflex coronary artery; AI, adrenal insufficiency; TM, transverse myelitis. Other abbreviations are explained in the text or Tables 1 and 2.
Anticardiolipin IgG antibodies before and serially after stem cell therapy in 7 SLE patients with positive antibody before transplantation.
Anticardiolipin IgG antibodies before and serially after stem cell therapy in 7 SLE patients with positive antibody before transplantation.
Anticardiolipin IgM antibodies before and serially after stem cell therapy in 11 SLE patients with positive antibody before transplantation.
Anticardiolipin IgM antibodies before and serially after stem cell therapy in 11 SLE patients with positive antibody before transplantation.
Nonthrombotic APS-related manifestations
There was no difference in APLA-associated symptoms between those who did not meet Sapporo criteria and those who did. In all 28 patients with APS, the type and number of pre-HSCT nonthrombotic APS-associated features included LSE (2), LR (3), calciphylaxis (2), thrombocytopenia (6), seizures (6), cranial nerve palsies (4), myelitis/transverse myelitis (4), ataxia (1), hallucinations (1), intractable headaches (2), multiple TIAs (1), and silent CVAs (1; Table 1). After HSCT, most nonthrombotic APS-related symptoms improved or stabilized (myelitis [3], ataxia [1], cranial nerve palsies [4], hallucinations [1], headache [1], calciphylaxis [2], LR [1]) and have not recurred (LSE [2], TIA [1], seizures [5], and thrombocytopenia [3]; Table 3). One patient with intractable headache and 2 with LR continue to manifest these APS features. One patient developed new headache at 48 months after HSCT. Three patients developed thrombocytopenia at 3, 5, and 12 months after transplantation. One patient had a relapse with seizure at 24 months after HSCT. One patient had a recurrent transverse myelitis attack at 9 months after the stem cell transplant.
Outcome of SLE
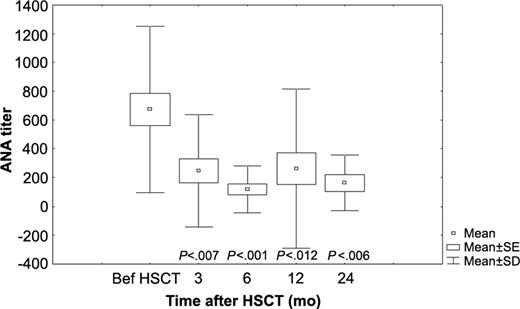
Twenty-one patients (75%) of those 28 with APS entered remission of SLE after HSCT. Nine patients (32%) were able to discontinue all immunosuppressive medications from immediately to 13 months after transplantation (median, 12 months) and stayed in remission from 12 to 66 months (median, 24 months) since stopping immunosuppression (Table 3). Analysis of antinuclear antibody (ANA) in the 28 patients with SLE-relatedAPS demonstrates a statistically significant decrease in mean of ANA titers after HSCT (Figure 3).
Correlation between post-HSCT APLAs versus thrombotic events, nonthrombotic APS-associated manifestations, and SLE disease activity
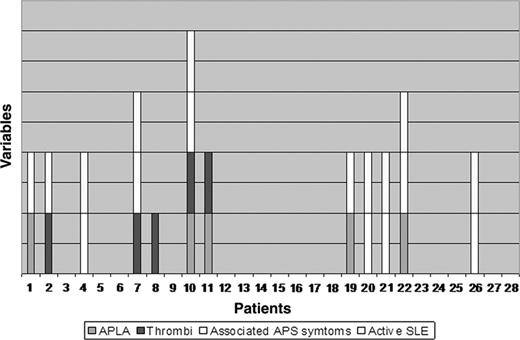
A detailed description of posttransplant APLA results, thrombotic events, associated APS features, outcome of SLE, and anticoagulation and immunosuppression history in each APS patient is shown in Table 3. Figure 4 illustrates the distribution of all positive APLA cases (5), recurrent thrombotic events (5), persistent or relapsed nonthrombotic APS-related manifestations (9), and active SLE disease (8) in each APS patient during the posttransplantation period. Of those 5 patients with positive APLAs, only 2 (40%) developed thrombotic events, although 4 of them (80%) had associated APS symptoms. Of 8 patients with active SLE, 3 (38%) had recurrence of thrombi and 7 (88%) demonstrated associated APS features.
Statistical analysis for nonparametric categorical values using the Kendall τ coefficient to test the relationship between posttransplant APLAs, thrombotic events, associated APS features, and SLE activity generated correlation coefficients (τ) for the variables as follows. In decreasing correlation: τ (SLE/associated APS manifestations) = 0.79 (P < .05); τ (APLA/associated APS manifestations) = 0.56 (P < .05); τ (SLE/thrombi) = 0.44 (P < .05); τ (APLA/thrombi) = 0.41 (P < .05); τ (APLA/SLE) = 0.27 (P < .05); τ (thrombi/associated APS manifestations) = 0.23 (not statistically significant).
Discussion
Our study is consistent with previous reports showing a high frequency of APLAs, LA or ACLAs, and secondary APS in patients with SLE.23-27 Controversy still exists as to which specific antibody, isotype, or their combination correlates with clinical deterioration.37,38 In addition to IgG and IgM ACLAs, some studies have found significant associations between persistently elevated ACLA IgA titers and APS manifestations such as progressive neurocognitive dysfunction.9,39 Because IgA ACLA isotype is not currently part of the Sapporo criteria for APS, we did not measure ACLA IgA levels in our patients. More recently, β2-glycoprotein I (β2-GPI) has been reported to be the clinically important APLA antigen associated with thrombosis in patients with autoimmune disease.40-50 However, in most laboratories, antibodies detected during standard ELISA testing are not specific for β2-GPI.50-52 Differentiation of “specific” anti–β2-GPI antibodies was not performed in our study, which was started 7 years ago, before the association of APS with β2-GPI.
Currently, Sapporo criteria do not include associated APS features (LR, LSE, thrombocytopenia, certain CNS manifestations) as part of the definition of APS.1,2 Our study is unique with respect to the SLE population studied because all the patients entering the study have had severe and refractory SLE and a large proportion of them have had significant histories of thromboembolic events as well as APS-related manifestations. Therefore, in addition to patients with definite APS we analyzed another group of patients who did not fulfill the Sapporo criteria, defined as probable APS. Before transplantation, these patients demonstrated positive APLA and had APS-related manifestations without a history of thrombosis. We observed similar effectiveness of HSCT on APS-related manifestations between patient groups with definite and probable APS. However, none of the patients in probable APS group experienced a thrombotic event after HSCT.
ANA titers of 28 patients with SLE-related APS before and serially after HSCT. Mean, SD, and SE are shown.
ANA titers of 28 patients with SLE-related APS before and serially after HSCT. Mean, SD, and SE are shown.
Patients with APS and positive APLAs at study enrollment generally became and remained negative for antibodies following HSCT. Of 8 patients who were positive for LA before HSCT, only 2 demonstrated reappearance of LA. Of 14 patients who were positive for ACLAs before HSCT enrollment, 3 patients manifested elevated ACLA titers after HSCT. Recurrent ACLAs were of the same isotype (LA, ACLA IgG or IgM) compared with before transplantation. Patients with APS who were negative for APLAs at enrollment before transplantation demonstrated sustained seronegativity when followed for up to 78 months after HSCT except one patient in whom recurrence of APLAs occurred in relation to pregnancy, an event associated with flare or initiation of SLE.53,54
With the exception of one patient with catheter-related thrombosis and one patient with recurrent coronary artery occlusions, no patient who maintained APLA seronegativity developed posttransplantation thromboembolic events. In contrast, relapsed LA or ACLAs tended to correlate with recurrent thrombotic or APS-related events. Of the 2 patients with relapsed LA, one experienced a saphenous vein graft thrombosis concurrently with reversion to seropositivity. Of 3 patients with elevated ACLAs following HSCT, one had a seizure and another developed a splenic infarct, upper extremity DVT, and thrombocytopenia.
Nonparametric statistical analysis showed significant correlation between ACLA/LA relapse, SLE recurrence, and APS-associated symptoms. The highest correlation was between active SLE and associated APS manifestations (τ= 0.79, P < .05), less so between positive APLAs and associated APS manifestations (τ= 0.56, P < .05). Thrombotic events had a moderate correlation with SLE activity (τ= 0.44, P < .05) and APLA positivity (τ= 0.41, P < .05). Active SLE disease correlated with both recurrent thrombotic events and associated APS manifestations, more so than seropositive APLAs. This finding supports multiple previous observations that ongoing inflammation in patients with lupus is involved in thrombogenesis, either by altering coagulation pathways in the presence of APLAs or by another procoagulant action of SLE, per se, other than that related to APLAs.50,55
Autologous HSCT in patients with SLE appears to be effective therapy in eliminating APLAs in most patients with APS, improving or stabilizing APS-related manifestations, and preventing further thromboembolic events. HSCT allowed the additional advantage of discontinuing anticoagulant therapy thereby preventing potential serious bleeding complications associated with prolonged anticoagulation. In developing autologous HSCT studies for SLE, APS was initially considered a possible contraindication to treatment due to lack of response of APS to immunosuppression and due to risk of thrombosis or bleeding during the procedure. These data indicate that HSCT may be performed safely in patients with APLA/APS without peritransplantation bleeding despite use of LMWH anticoagulation throughout the transplantation. Posttransplantation APS control with disappearance of APLAs and discontinuation of chronic anticoagulation in the majority of patients is a strong indication of the efficacy of this therapy for SLE-related APS. Whether autologous HSCT will result in long-term cure of some patients with APS is not currently known.
Our treatment induced SLE remission in 75% of the APS population. Similar remission rates as well as significant decrease in ANA titers were observed in the whole group of 46 patients with SLE treated by HSCT (data not shown; R.K.B., A.T., L.S., W. G. Barr, R. Rosa, J. Schroeder, L.V., N.K., K. Quigley, K.Y., M. Villa, M. Takahashi, Y.O. manuscript in preparation). None of the patients without definite or probable APS before the transplantation developed APS as a manifestation of relapse after HSCT.
There are several limitations to the current study. First, ACLA results were obtained in 2 different laboratories, and even if currently more standardized, variability in commercial ELISA kits is still a problematic issue.56 Second, the last few patients had relatively short period of follow-up, and durability of results and antibody and clinical relapse rate still need to be determined. Third, we did not have a control group, a significant argument considering common fluctuations in APLA titers. Fourth, anti–β2-GPI antibody, which at the start of this study (7 years ago) was not available, should be followed in future trials.
In summary, of 46 patients with severe and refractory SLE, the prevalence of APS was 61%. Ten patients had positive LA, and 14 patients had high titers of ACLA IgG or IgM immediately before the stem cell transplantation. LA disappeared in 75% of evaluable patients after HSCT; 71% had normalization of ACLA IgG titers, and 82% became and remained negative for ACLA IgM. Eighty-two percent of patients with SLE-relatedAPS discontinued anticoagulation a median of 4 months after the transplantation; 78% of them remained thrombosis-free for up to 78 months (median, 15 months) after HSCT. High-dose chemotherapy and autologous HSCT may be an efficient treatment modality in eliminating APLAs and preventing or diminishing thrombotic complications and APS-related manifestations in patients with SLE-related APS.
Casewise distribution of variables during the posttransplantation period. Shown are positive APLAs, thrombotic events, nonthrombotic APS-associated symptoms, and active SLE.
Casewise distribution of variables during the posttransplantation period. Shown are positive APLAs, thrombotic events, nonthrombotic APS-associated symptoms, and active SLE.
Prepublished online as Blood First Edition Paper, May 3, 2005; DOI 10.1182/blood-2005-01-0330.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal