Abstract
Although significant advances have been made over the last decade with respect to our understanding of stem cell biology, progress has been limited in the development of successful techniques for clinically significant ex vivo expansion of hematopoietic stem and progenitor cells. We here describe the effect of Notch ligand density on induction of Notch signaling and subsequent cell fate of human CD34+CD38– cord blood progenitors. Lower densities of Delta1ext-IgG enhanced the generation of CD34+ cells as well as CD14+ and CD7+ cells, consistent with early myeloid and lymphoid differentiation, respectively. However, culture with increased amounts of Delta1ext-IgG induced apoptosis of CD34+ precursors resulting in decreased cell numbers, without affecting generation of CD7+ cells. RNA interference studies revealed that the promotion of lymphoid differentiation was primarily mediated by Delta1 activation of Notch1. Furthermore, enhanced generation of NOD/SCID repopulating cells was seen following culture with lower but not higher densities of ligand. These studies indicate critical, quantitative aspects of Notch signaling in affecting hematopoietic precursor cell-fate outcomes and suggest that density of Notch ligands in different organ systems may be an important determinant in regulating cell-fate outcomes. Moreover, these findings contribute to the development of methodology for manipulation of hematopoietic precursors for therapeutic purposes.
Introduction
The widespread expression of Notch family members by hematopoietic cells, including stem cells, has led to speculation about their role in hematopoiesis. A critical role for Notch signaling in T versus B cell-fate decisions has been established in vivo with gain- and loss-of-function studies.1-3 Despite profound effects on lymphoid cell fates, these studies have failed to identify significant effects on myeloid differentiation. However, in contrast to these in vivo studies, in vitro studies in which the constitutively active intracellular domain of Notch1 was overexpressed did reveal inhibition of myeloid differentiation and enhanced generation of precursor cells in addition to promotion of early T-cell differentiation, indicating a potential role of Notch signaling on multipotent precursor cells.4-7
To exploit Notch signaling as a means of directing desired cell-fate outcomes or generating precursor cells from nontransduced stem cells, soluble or cell-expressed Notch ligands have been used by a number of laboratories. Initial studies met with only modest success, resulting in only a few-fold increase in progenitor cell number,4,5,7-12 but our more recent studies using an engineered Notch ligand Delta1 in immobilized form demonstrated profound effects on murine precursors with a multilog increase in the number of Sca-1+c-kit+ precursors with short-term lymphoid and myeloid repopulating ability.7 Based on these findings, we examined Notch signaling in human cord blood precursors because inadequate stem cell numbers in cord blood grafts have been associated with significantly delayed engraftment and, as a result, increased early transplant-related mortality from infection, thereby limiting the use of cord blood for hematopoietic cell transplantation (HCT) in adults and larger children. Our studies demonstrated an increase in early human hematopoietic reconstitution in NOD/SCID mice, indicating potential clinical importance in overcoming the delayed engraftment in umbilical cord blood transplants.5
We investigated whether quantitative differences in ligand-induced activation of Notch signaling could be the basis for the reported variability with Notch activation and cell fate outcomes of hematopoietic precursors and whether such quantitative differences might determine whether precursors self-renew or adopt a lymphoid cell fate. The importance of quantitative aspects of Notch signaling has been shown in Drosophila where different functions of Notch can require different thresholds of signaling. For example, Notch haploinsufficiency suffices to perform most functions of Notch indistinguishably from wild type, but causes improper specification of the dorsoventral margin of the wing, giving rise to the eponymous “notched wing” phenotype.13,14 In mammals, a reduction in Notch1 gene dosage in developing T cells favors the γδ T-cell fate over the αβ T-cell fate,15 and a dose-dependent effect of Delta1 on the determination of type 1 helper T cell (Th1) versus Th2 cell-fate decisions of activated CD4+ T cells has been demonstrated.16 These studies, therefore, suggest that a critical threshold of Notch signaling is required for inducing different cell-fate outcomes.
In the studies presented here, we cultured CD34+CD38– cord blood precursors with different densities of immobilized Notch ligand, Delta1ext-IgG. We found that relatively lower ligand densities of immobilized Delta1 led to submaximal induction of human C promoter binding factor, Drosophila melanogastersuppressor of Hairless and Caenorhabditis elegansLag-1 (CSL)–dependent Notch signaling, but promoted maximal generation of CD34+ precursor cells, including those with NOD/SCID repopulating cell activity. In contrast, whereas the generation of early lymphoid precursors was similar across all densities of ligand, higher densities were associated with decreased generation of myeloid cells and increased apoptosis of CD34+ and repopulating cells. Moreover, further lymphoid maturation was seen as the density of ligand increased. These studies indicate an important role for ligand density in the differential promotion of cell-fate outcomes and also suggest a physiologic role for the known in vivo variations of ligand density. Furthermore, these findings contribute significantly to the successful development of methods for ex vivo manipulation of hematopoietic precursor cell proliferation and differentiation for therapeutic application, methods that remain largely elusive, but with extensive therapeutic significance.
Materials and methods
Antibodies, reagents, and immunofluorescence studies
Immunofluorescence analysis was performed as previously described5 using fluorescein isothiocyanate (FITC)–labeled antibodies against annexin V, CD19, CD34, CD44, CD117, and T-cell receptor-αβ (TCR-αβ; Becton Dickinson, Sunnyvale, CA); phycoerythrin (PE)–labeled antibodies against CD38, CD3, CD10, CD11b, CD14, CD34 (Becton Dickinson), CD7 (8H8.1), CD19, CD25, CD33, CD56 (Immunotech, Marseille, France), or peridinin chlorophyll protein (PerCP)–labeled antibodies against CD45 (Becton Dickinson, San Jose, CA). FITC-, PE- or PerCP-conjugated, isotype-matched antibodies served as controls. Staining for cytoplasmic CD3ϵ was done by first permeabilizing cells using Permiflow (Invirion, Frankfort, MI).
Separation of CD34+CD38– cells
CD34+CD38– precursors were purified from human cord blood samples as previously described.5 Briefly, samples obtained from normal full-term deliveries were incubated in ammonium chloride red blood cell lysis buffer, washed, and suspended in phosphate-buffered saline (PBS) with 2% human type AB serum. Cells were incubated with anti-CD34 antibody 12.8 (generated in our laboratory), followed by immunomagnetic bead-conjugated goat anti–mouse immunoglobulin M (IgM) (Miltenyi Biotec, Bergisch Gladbach, Germany), purified using a Miltenyi AutoMACS magnetic bead cell sorter, and then frozen. CD34+ cells were thawed, and CD34+CD38– cells were isolated by fluorescence-activated cell sorting (FACS) after staining with anti-CD34 and anti-CD38 antibody.
Generation and immobilization of Delta1ext-IgG protein
Generation of the construct encoding the extracellular domain of Delta1 fused to the fc domain of human IgG1 and purification of Delta1ext-IgG protein from culture medium of NSO cells electroporated with the construct have been previously described.6 Wells of non–tissue culture-treated culture plates (Falcon, Becton Dickinson, Franklin Lakes, NJ) were incubated with increasing concentrations of Delta-1ext-IgG (1.25, 2.5, 5, 10, and 20 μg/mL) or control human IgG (HuIgG; designated in the figures as Delta1ext-IgG at 0 μg/mL) diluted in PBS together with 5 μg/mL fibronectin fragment CH-296 (Takara Shuzo, Otsu, Japan), incubated overnight at 4°C, washed extensively, and further incubated with a solution of 2% bovine serum albumin dissolved in PBS.
Cell cultures
Cells were cultured in serum-free medium (Stemspan Serum Free Expansion Medium; StemCell Technologies, Vancouver, BC, Canada) with 300 ng/mL human stem cell factor (SCF) and human Flt-3 ligand, 100 ng/mL each human interleukin 6 (IL-6) and thrombopoietin (TPO), and 10 ng/mL human IL-3 (5GF; Biosource, Camarillo, CA) and 20 μg/mL low-density lipoprotein (LDL; Sigma, St Louis, MO) as described.5 To obtain sufficient numbers of CD34+CD38– starting cells for each experiment, individual units of cord blood were pooled. The number of individual cord blood samples used per experiment varied, ranging from 3 to7 units used per experiment. After separation of CD34+CD38– cells as described (see “Separation of CD34+CD38– cells”), cultures were initiated in 24-well plates prepared as described at 6000 cells/well and replated after 5 to 7 days of culture to similarly prepared wells in 6-well plates containing fresh medium with the original concentration of cytokines, and further transferred to similarly coated flasks after 14 days to prevent overgrowth. Fresh medium with cytokines was added every 3 to 4 days.
Real-time RT-PCR studies of Hes1 expression
Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed as previously described.5 Briefly, total mRNA was extracted using the Absolutely RNA RT-PCR Miniprep kit (Stratagene, La Jolla, CA). For SYBR green RT-PCR, cDNA was synthesized with oligo-dT primer using ThermoScript RT-PCR System (Invitrogen, Carlsbad, CA). Quantitative PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Amplification was carried out in an ABI Prism 7700 Sequence Detector using Sequence Detection System version 1.9 (Applied Biosystems).
Transplantation of human hematopoietic cells into NOD/SCID mice
Sublethally irradiated (325 cGy) nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice (8-10/group) were infused with the progeny generated from 3 × 103 CD34+CD38– cord blood cells cultured in serum-free medium with fibronectin fragments CH296, 5GF, and increasing concentrations of immobilized Delta1ext-IgG or control HuIgG for 21 days. Repopulating ability (percent human CD45+ cells in marrow) was assessed at 3 and 6 weeks after transplantation using marrow removed from the knee joint of anesthetized recipient mice and at 10 weeks when the mice were humanely killed and cells from both femurs and tibias were obtained.
Generation and transduction of siRNA lentiviral constructs
To construct the short hairpin RNA (shRNA) oligos, target sequences throughout Notch1 or Notch2 mRNA were identified, checked for about 50% GC content, and Basic Local Alignment Search Tool (BLAST) searched (National Center for Biotechnology Information database) to ensure that the short interfering RNA (siRNA) sequences are specific for the gene of interest. Oligo sequences for shRNA Notch1-1-GCATGTGTAACATCAACATCG, for shRNA Notch1-2-GGAGCATGTGTAACATCAACA, for shRNA Notch2-GCAGAGGACTCTTCTGCTAAC were used and hairpins oligos designed with TTCAAGAGA were annealed and subcloned into LentiLox3.7 containing a U6 promoter and a cytomegalovirus promoter (gift from Luc Van Parijs, Massachusetts Institute of Technology, Cambridge, MA). Lentivirus were produced and concentrated as previously described.17 CD34+CD38– cord blood cells were transduced overnight with concentrated lentiviral vector stocks at a multiplicity of infection of 10 to 25 in the presence of 4 μg/mL protamine sulfate as well as Delta1ext-IgG. Infected, green fluorescent protein (GFP)–expressing cells were isolated by flow microfluorometry and replaced in culture; efficiency of gene silencing was assessed 5 to 7 days after transduction by Western blot analysis using anti-Notch1 monoclonal antibody MN-1 and anti-Notch2 monoclonal antibody BHN6 to assess levels of Notch and anti–β-actin antibody for measurement of a housekeeping protein.
Statistical analysis
For the in vivo engraftment studies, generalized estimating equations (GEEs) were used to compare percent engraftment between groups, and this model was adjusted for experiment and week of sampling. The GEEs allowed us to use all the data, that is, from each experiment and from each week, as the week-to-week and experiment-to-experiment data from a particular mouse are treated as clustered. The GEE uses this information to obtain an adjusted estimate of the variance of the percent engraftment, this estimate being different than what one would obtain if the within-mouse data were treated as independent observations.
Results
Linear relationship between density of Delta1ext-IgG in culture and the amount of induced Notch signaling
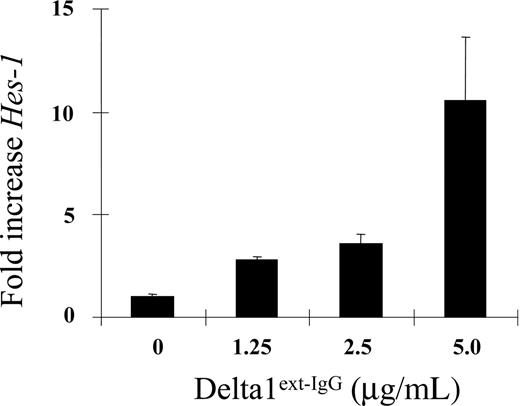
To determine whether there is a relationship between the amount of Delta1ext-IgG immobilized to the plastic tissue culture surface and resultant activation of the Notch pathway in cultured human hematopoietic progenitors, we measured expression of the Notch target gene, Hes1. CD34+CD38– umbilical cord blood progenitors were isolated by FACS and cultured in serum-free culture medium containing cytokines at concentrations previously shown to optimally support the growth of primitive human hematopoietic precursors.18-21 The engineered Notch ligand, Delta1ext-IgG, consisting of the extracellular domain of Delta1 fused to the Fc portion of human IgG1, was used to activate Notch signaling. Because we have previously demonstrated that immobilization of Notch ligand is required to induce endogenous Notch signaling in hematopoietic cells,8 CD34+CD38– cells were added to wells in which Delta1ext-IgG or a control construct consisting of the Fc portion of human IgG1 was immobilized on the plastic surface at varying concentrations. An enzyme-linked immunosorbent assay confirmed that the concentration of ligand plated on the tissue culture plastic surface correlated with the amount of ligand bound. A linear relationship of concentration plated and amount of ligand bound was demonstrated with an HRP-conjugated Fc-specific anti–human IgG antibody (Sigma) for detection of Delta1ext-IgG (R2 = 0.73; P = .007; data not shown).
After 6 hours in culture, cells were harvested and the expression of Hes1 was measured by quantitative RT-PCR. This early time point was chosen to establish whether a quantitative relationship existed between ligand density plated and activation of Notch signaling. There was a 10-fold increase in Hes1 expression in cultures incubated in wells coated with the highest density of ligand compared to wells coated with the control construct (Figure 1). The rapid increase seen within 6 hours likely results from a direct, CSL-dependent effect of Delta1-induced Notch signaling on the Hes1 promoter. After 7 days of culture, cells continued to show a Delta1ext-IgG density-dependent increase in Hes1 mRNA expression, reaching a maximum of 21-fold increase over control at a ligand concentration of 10 μg/mL (data not shown). However, this increase may have resulted, at least in part, as a consequence of differences in the cell populations generated in cultures initiated with different ligand densities.
Density-dependent activation of endogenous Notch signaling as indicated by quantitative RT-PCR of Hes1 mRNA. CD34+CD38– cord blood precursors were incubated with increasing densities of Delta1ext-IgG and expression of the Notch target gene, Hes1, was measured after 6 hours by quantitative RT-PCR (SYBR-green). Results indicate the fold increase in Hes1 levels normalized to control human IgG at increasing densities of Delta1ext-IgG. Hes1 levels were corrected for mRNA levels based on housekeeping gene expression (R = 0.92; 95% CI, 0.86-0.97; P < .001). Results are representative of 3 experiments ± SEM.
Density-dependent activation of endogenous Notch signaling as indicated by quantitative RT-PCR of Hes1 mRNA. CD34+CD38– cord blood precursors were incubated with increasing densities of Delta1ext-IgG and expression of the Notch target gene, Hes1, was measured after 6 hours by quantitative RT-PCR (SYBR-green). Results indicate the fold increase in Hes1 levels normalized to control human IgG at increasing densities of Delta1ext-IgG. Hes1 levels were corrected for mRNA levels based on housekeeping gene expression (R = 0.92; 95% CI, 0.86-0.97; P < .001). Results are representative of 3 experiments ± SEM.
Density-dependent effect of Delta1ext-IgG on the growth and differentiation of hematopoietic cells
We next evaluated whether the density of Delta1ext-IgG affects the growth and differentiation of umbilical cord blood progenitors. Cultures of CD34+CD38– umbilical cord blood cells were established in wells coated with Delta1ext-IgG at concentrations ranging from 1.25 to 20 μg/mL or HuIgG. Control HuIgG was plated at both low (1.25 μg/mL) and high (20 μg/mL) density; however, in all of the experiments, no difference was seen at these 2 densities. Therefore, control HuIgG is depicted in the figures as the absence of Delta1ext-IgG or Delta1ext-IgG at 0 μg/mL. Seven independent experiments demonstrated a significant Delta1ext-IgG density-dependent inhibition of proliferation with decreased total cell and CD34+ cell numbers generated after 21 days (Figure 2A). Cells cultured with ligand plated at 1.25 and 2.5 μg/mL produced similar total cell numbers when compared to control ligand (Delta1ext-IgG = 0 μg/mL), but showed an increase in the absolute number of CD34+ cells. In contrast, cells cultured with ligand plated at concentrations greater than 2.5 μg/mL resulted in significantly decreased total cell numbers and CD34+ cell numbers (Figure 2A).
We therefore determined whether the decreased generation of cells at higher ligand densities resulted from the loss of precursor cells during early culture periods. We found that an approximate 3-fold higher portion of CD34+ cells underwent apoptosis (defined as annexin V–positive, propidium iodide–negative, CD34+ cells by FACS) at higher compared to lower ligand densities after 3 to 6 days of culture (Figure 2B). Analysis of apoptosis by annexin V staining was carried out in these experiments at days 3, 6, 10, and 14, with maximal apoptosis seen at 3 days in one experiment and at 6 days in 2 experiments. Of note, the portion of CD34+ cells undergoing apoptosis in the presence of lower ligand densities was less than that found in control cultures, suggesting a survival signal provided by Delta1ext-IgG.
A direct relationship between inhibition of myeloid differentiation and ligand density was also seen. Myeloid differentiation examined by measurement of CD14 expression revealed near-complete inhibition in cultures with immobilized ligand plated at 5 μg/mL or more (Figure 2C). Assessment of apoptosis of CD14+ cells, however, revealed no increase in the proportion of CD14 cells undergoing apoptosis, with less than 3% of apoptotic cells also being CD14+ at any ligand density, suggesting that Delta1ext-IgG inhibits the generation of CD14+ cells by inhibiting their differentiation from CD34+ cells (data not shown).
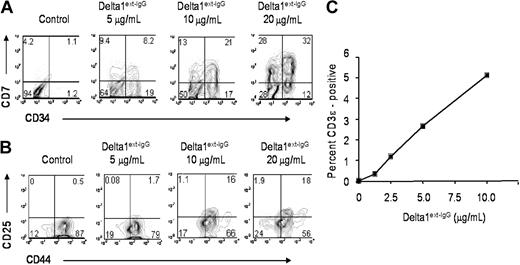
To assess the influence of ligand density on lymphoid differentiation, we determined the expression of CD7 and found an increase in the absolute number of CD7+ cells at all ligand densities tested. This effect, suggestive of early lymphoid development, is present at 7, 14, and 21 days, but is most pronounced by 21 days of culture, where up to a 5-fold increase in the number of CD7+ cells is seen at lower concentrations of ligand compared to control cultures (Figure 2D). Furthermore, there was a progressive increase in the proportion of CD7+ cells that were CD34– with increasing concentrations of Delta1ext-IgG (Figure 3A), indicating a ligand density-dependent promotion of early lymphoid maturation, because CD34–CD7+ cells are likely more mature than CD34+CD7+ cells. In addition, within 10 days of culture, there was a progressive increase in the generation of CD44+CD25+ cells, a phenotype associated with DN2 cells,22 with increased density of ligand (Figure 3B). Furthermore, cells incubated at higher densities expressed cytoplasmic CD3ϵ and after 14 days revealed a 5- to 10-fold increase in the percentage of cyCD3ϵ+ cells cultured with ligand immobilized at 20 μg/mL compared to lower ligand densities and control, consistent with density-dependent promotion of early T-cell differentiation (Figure 3C).
Delta1ext-IgG affects the number and apoptosis of CD34+ precursors and the number of myeloid and lymphoid precursors in a dose-dependent manner. CD34+CD38– cord blood precursors were incubated with increasing Delta1ext-IgG densities or human control IgG (designated in the figures as Delta1ext-IgG at 0 μg/mL). (A) Total number of cells (▪), and number of CD34+ cells (▴) generated following 21 days of culture. Results are the mean of 7 independent experiments ± SEM (R2 = 0.97; P = .002 for total cells and .001 for CD34). (B) Maximum percentage of CD34+ cells that are annexin V positive and propidium iodide negative] following 3 to 6 days in culture. Percent apoptosis was measured on days 3, 6, 10, and 14 in 3 independent experiments and maximum apoptosis of the CD34+ cells was seen at 3 and 6 days. Results are the mean of 3 independent experiments ± SEM. (C-D) Total number of CD14+ (R2 = 0.95; P = .004) and CD7+ cells, respectively, generated following 21 days of culture. Results are the mean of 7 independent experiments ± SEM.
Delta1ext-IgG affects the number and apoptosis of CD34+ precursors and the number of myeloid and lymphoid precursors in a dose-dependent manner. CD34+CD38– cord blood precursors were incubated with increasing Delta1ext-IgG densities or human control IgG (designated in the figures as Delta1ext-IgG at 0 μg/mL). (A) Total number of cells (▪), and number of CD34+ cells (▴) generated following 21 days of culture. Results are the mean of 7 independent experiments ± SEM (R2 = 0.97; P = .002 for total cells and .001 for CD34). (B) Maximum percentage of CD34+ cells that are annexin V positive and propidium iodide negative] following 3 to 6 days in culture. Percent apoptosis was measured on days 3, 6, 10, and 14 in 3 independent experiments and maximum apoptosis of the CD34+ cells was seen at 3 and 6 days. Results are the mean of 3 independent experiments ± SEM. (C-D) Total number of CD14+ (R2 = 0.95; P = .004) and CD7+ cells, respectively, generated following 21 days of culture. Results are the mean of 7 independent experiments ± SEM.
Dose-dependent induction of lymphoid differentiation. CD34+CD38– cord blood precursors were incubated with increasing Delta1ext-IgG densities or human control IgG and analyzed by FACS at day 21 for CD7 and CD34 expression (A), day 10 for CD25 and CD44 expression (B), and day 14 for intracellular CD3ϵ expression (C). Control human IgG is designated in the figures as control or Delta1ext-IgG at 0 μg/mL. Data are representative of 3 independent experiments. Numbers in corners of dot blots are the percentage of gated events within that quadrant (R2 = 0.95; P = .004).
Dose-dependent induction of lymphoid differentiation. CD34+CD38– cord blood precursors were incubated with increasing Delta1ext-IgG densities or human control IgG and analyzed by FACS at day 21 for CD7 and CD34 expression (A), day 10 for CD25 and CD44 expression (B), and day 14 for intracellular CD3ϵ expression (C). Control human IgG is designated in the figures as control or Delta1ext-IgG at 0 μg/mL. Data are representative of 3 independent experiments. Numbers in corners of dot blots are the percentage of gated events within that quadrant (R2 = 0.95; P = .004).
Notch1, but not Notch2, is required for promotion of lymphoid differentiation by Delta1ext-IgG
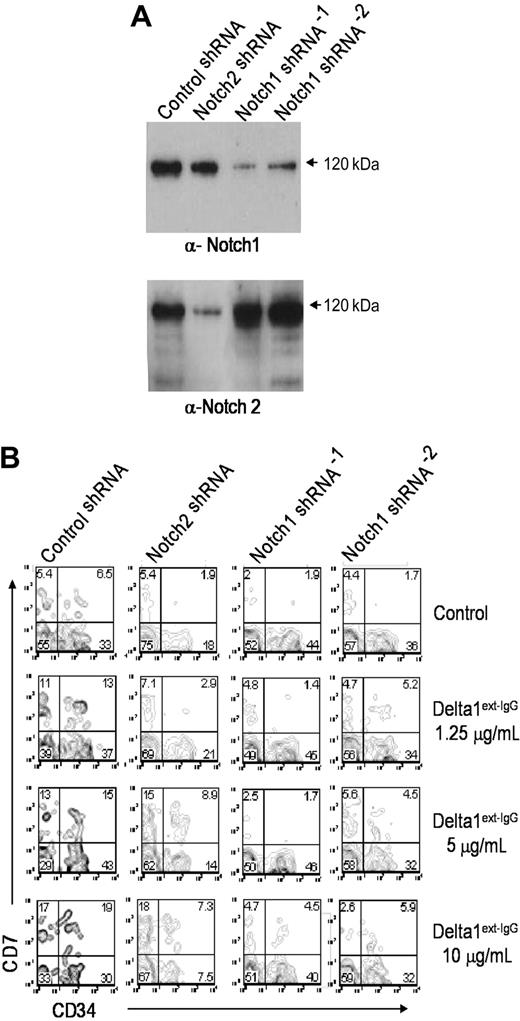
To determine whether the effects of different Delta1 densities were mediated by differential activation of Notch receptors and to distinguish whether the effects were mediated by Notch1 or Notch2, we designed shRNA to reduce Notch1 and Notch2 levels. Multiple primers were subcloned into a lentiviral vector that expressed GFP from a separate promoter and selected based on efficiency of Notch protein knockdown in Jurkat cells (data not shown). Freshly sorted CD34+CD38– cord blood progenitor cells were then transduced with Notch1, Notch2, or control shRNA constructs and cultured in the presence of increasing concentrations of immobilized Delta1ext-IgG. Four days after initial transduction, cells were sorted based on GFP expression and replaced in culture. Seven days later, cells were harvested for analysis of CD34 and CD7 expression by FACS as well as for preparation of cell lysates for Western blotting. Western blot analysis detected marked reduction in Notch1, but not Notch2, protein in cells expressing Notch1 shRNA and marked reduction in Notch2, but not Notch1, in cells expressing Notch2 shRNA (Figure 4A). Moreover, cells transduced with a control shRNA vector or nontransduced cells demonstrated an increased percentage of CD7-expressing cells at increased concentration of ligand, and similar CD7+ cell numbers, consistent with the described studies. In contrast, cells transduced with both Notch1 shRNA constructs showed decreased expression of CD7 at all concentrations of ligand. In addition, there was a marked decrease in formation of CD34–CD7+ cells, suggesting contribution of Notch1 to further promotion of lymphoid differentiation. Inhibition of Notch2 protein expression, however, only slightly abrogated CD7 expression, but did impair the generation of CD34+ cells at all tested ligand densities (Figure 4B). These findings suggest that in the absence of Notch1, Notch2 may promote generation of CD34+ progenitors but not lymphoid differentiation. Conversely, in the absence of Notch2, one can speculate that binding of Notch1 with Delta1 may be enhanced, resulting in increased Notch 1-mediated apoptosis of CD34+ cells and therefore decreased generation of CD34+ cell numbers. The influence of reduction of Notch1 or Notch2 protein on myeloid development was not apparent, with all groups expressing less than 4% CD14.
Notch1 but not Notch2 is primarily responsible for Notch-induced lymphoid differentiation. CD34+CD38– cord blood cells were transduced with either Notch1, Notch2, or control shRNA constructs and cultured in the presence of increasing concentrations of immobilized Delta1ext-IgG. After 4 days, cells were GFP sorted and replaced in culture. After 7 days, cells were harvested to prepare lysates or immunostain for FACS analysis. (A) Lysates were separated with sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunoblotted with the α-Notch1 monoclonal antibody, MN-1, stripped, and reimmunoblotted with the anti-Notch2 monoclonal antibody, BHN6. (B) Cells were assessed for CD7 and CD34 expression. Numbers in corners are the percentage of gated events within that quadrant.
Notch1 but not Notch2 is primarily responsible for Notch-induced lymphoid differentiation. CD34+CD38– cord blood cells were transduced with either Notch1, Notch2, or control shRNA constructs and cultured in the presence of increasing concentrations of immobilized Delta1ext-IgG. After 4 days, cells were GFP sorted and replaced in culture. After 7 days, cells were harvested to prepare lysates or immunostain for FACS analysis. (A) Lysates were separated with sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunoblotted with the α-Notch1 monoclonal antibody, MN-1, stripped, and reimmunoblotted with the anti-Notch2 monoclonal antibody, BHN6. (B) Cells were assessed for CD7 and CD34 expression. Numbers in corners are the percentage of gated events within that quadrant.
Dose-dependent effects of Delta1 on enhancement of repopulating ability
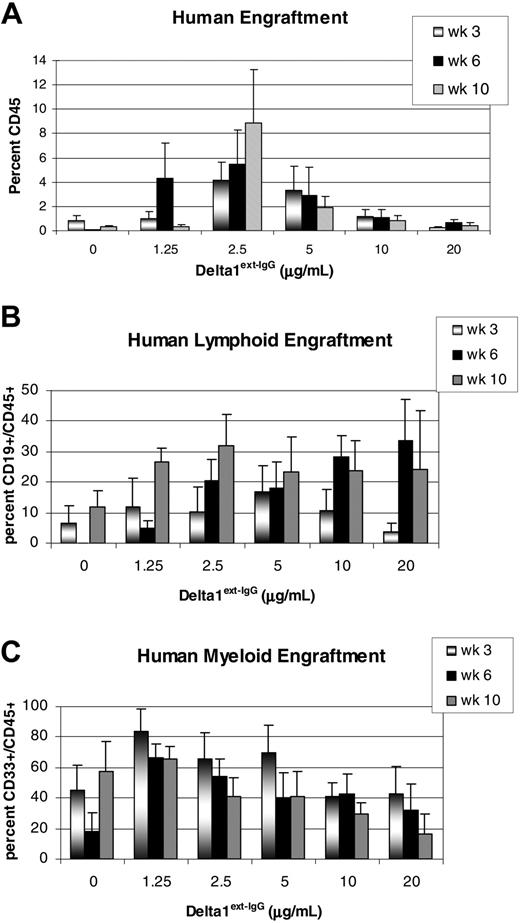
Having demonstrated a concentration-dependent effect of the Notch ligand, Delta1ext-IgG, on the in vitro generation of CD34+ progenitors, we next assessed the repopulating ability of these cultured cells in a NOD/SCID mouse model. Cells cultured for 21 days in the presence of varying concentrations of immobilized Delta1ext-IgG or control were infused into sublethally irradiated NOD/SCID mice (325 cGy). Human engraftment was assessed by the percentage of human CD45+ cells in the marrow of the NOD/SCID mice at 3, 6, and 10 weeks after transplantation. Mice were considered engrafted if they had at least 0.5% CD45+ cells present in the marrow. Significant enhancement of repopulating cell activity was seen in cells cultured with immobilized Delta1 at 1.25 μg/mL (2.7-fold, P = .01), 2.5 μg/mL (5.3-fold, P = .005), and 5 μg/mL (2.5-fold, P = .05) compared to control cultured cells or cells cultured with higher doses of Delta1 (Figure 5A). Notably, substantial longer-term (10 week) engraftment was demonstrated for cells cultured with relatively lower ligand densities. Furthermore, engraftment was seen in both lymphoid and myeloid lineages as detected as the proportion of CD45+/CD19+ and CD45+/CD33+ cells, respectively (Figure 5B-C).
Human engraftment in NOD/SCID mice of Delta1ext-IgG cultured cells. CD34+CD38– cord blood precursors were cultured for 3 weeks with increasing Delta1ext-IgG densities or human control IgG (designated in the figures as Delta1ext-IgG at 0 μg/mL) and transplanted into sublethally irradiated NOD/SCID mice. (A) Percent overall human engraftment (CD45+), (B) percent human lymphoid engraftment, and (C) percent human myeloid engraftment were assessed in bone marrow aspirates from mice at 3, 6, and 10 weeks. Data are the results of 5 independent experiments, with the mean ± SEM.
Human engraftment in NOD/SCID mice of Delta1ext-IgG cultured cells. CD34+CD38– cord blood precursors were cultured for 3 weeks with increasing Delta1ext-IgG densities or human control IgG (designated in the figures as Delta1ext-IgG at 0 μg/mL) and transplanted into sublethally irradiated NOD/SCID mice. (A) Percent overall human engraftment (CD45+), (B) percent human lymphoid engraftment, and (C) percent human myeloid engraftment were assessed in bone marrow aspirates from mice at 3, 6, and 10 weeks. Data are the results of 5 independent experiments, with the mean ± SEM.
Discussion
We have previously demonstrated the enhanced generation of murine and human hematopoietic progenitors and promotion of T-cell differentiation when multipotent hematopoietic progenitors are cultured with a single dose of the immobilized Notch ligand, Delta1.5,7 Herein, we demonstrate that the density of exogenous ligand can be varied to quantitatively regulate the induction of CSL-dependent Notch signaling and to differentially affect cell fate outcome of multipotent human hematopoietic precursors.
We observed that Delta1ext-IgG at all densities permitted CD7+ early lymphoid precursor development; however, the enhanced generation of CD34+ cells and cells capable of NOD/SCID reconstitution was seen only at lower densities of Delta1. In contrast, culture with higher density of ligand resulted in apoptosis of CD34+ progenitors, significantly decreased cells capable of NOD/SCID reconstitution, and further promoted lymphoid differentiation of CD34+CD7+ cells into CD34–CD7+ precursors. Furthermore, we show that this lymphoid promotion is primarily mediated by Notch1. These results are consistent with previous in vivo gain- and loss-of-function studies demonstrating a requirement for Notch1 in T-cell development.1-3
Previous studies of Notch signaling on hematopoietic stem cell fate have found variable effects, with differences seen primarily between overexpression studies versus those using exogenous ligand for Notch activation. For example, overexpression of constitutively active Notch1 or the Notch direct target gene Hes1 resulted in enhanced self-renewal of murine HSCs,23-25 and similarly, in human cord blood progenitors, overexpression of constitutively active Notch1 and Notch4 resulted in decreased differentiation as well as an increase in stem cell activity.25,26 In addition, these studies and others also showed that overexpressing constitutively active Notch1 or the Notch direct target genes Hes1 and Hes5 promoted T-cell differentiation. Thus, although these studies were crucial in demonstrating potential roles of Notch signaling in early hematopoiesis, they are confounded by hyperactivation of signaling and do not address whether physiologic levels of Notch signaling are an important aspect of stem cell maintenance and differentiation.
Subsequent studies in nontransduced cells with cell-bound, soluble, or immobilized Notch ligand forms have similarly shown enhanced generation of hematopoietic precursors as well as an influence of Notch signaling on cell fate outcome, including induction of T-cell differentiation of CD34+ progenitors cultured on OP9 stromal cells engineered to express Delta-like 1.5,9,10,27-29 However, the degree to which this effect was seen varied from laboratory to laboratory. These discrepancies indicate that there may be differences in the methods of ligand presentation, protein engineering, or amounts of ligand used by the different laboratories. In addition, cell-bound ligand expression systems are unable to quantitatively address the effects of ligand expression or how these cells that are altered to express ligand themselves contribute to the observed effects on cocultured cells. Other investigators, using cell-expressed Notch ligands, have shown differential effects of Delta versus Jagged, including the inhibition of B-cell differentiation of umbilical cord blood progenitors with Delta1 but not Jagged1,30 induction of naive CD4 T-helper cells to differentiation along a Th1 by Delta1 versus a Th2 lineage with Jagged1,31 and, more recently, the demonstration by Lehar et al32 that Delta1 and Jagged1 inhibit the differentiation of DN1 thymocytes into the B-cell lineage, but only the Delta1-expressing stromal cells promote the maturation of T-cell progenitors. The differential cell fate choices seen in these studies may result from distinct Notch signaling induced by Delta versus Jagged or may reflect decreased signal intensity from one or the other ligand. This is supported by observations that activation of Notch signaling by Jagged may result in decreased signal intensity, particularly in the presence of Fringe, a glycosylating enzyme thought to prevent Jagged1-induced activation of Notch1, but not Notch2.33 Thus, the dose-dependent effects of Delta1 on umbilical cord blood progenitors reported herein may be analogous to the modulation of Notch signaling achieved via different ligand-receptor pairing, both resulting in a potential quantitative effect of Notch signaling.
How differences in Notch ligand density lead to the observed different cell-fate outcomes of human hematopoietic progenitors remains elusive. It is possible that different levels of Notch signaling may induce different amounts of direct target gene expression, such as Hes1 or Hes5, in a single cell resulting in different cell-fate outcome. This possibility is supported by studies demonstrating intermediate levels of Hes1 or Hes5 overexpression produce different cell fate outcomes than high levels of overexpression.34 Alternatively, individual Notch target genes may require different thresholds of Notch signaling for induction, thereby regulating differentiation programs. Hence, our findings may result from differential induction of Notch target genes secondary to the different intensity levels of Notch signaling induced by culture with low versus high densities of ligand. Furthermore, it is possible that increasing density of ligand in these cultures increased the possibility of interaction with and activation of Notch receptors. However, although dimerization has not been shown to contribute to receptor activation, there is emerging evidence that receptor-ligand interaction induces clustering of Notch receptors with resultant effects on the accumulation and internalization of the receptor,35 but the contribution of clustering to Notch signaling remains unclear.
Physiologically, tight regulation of Notch signaling may be achieved in vivo by differences in ligand density between anatomic regions, such as observed within the thymus, or by differential activation of individual Notch receptors.36,37 The use of different Notch ligand-receptor combinations may induce Notch signals of different strength, particularly in the presence of intrinsic or extrinsic Notch modulators. Moreover, the restricted patterns of expression of Notch ligands throughout the hematopoietic system might reflect physiologically important effects of individual Notch ligands, whereby the expression of Jagged1 in the stem cell niche within the marrow is thought supportive of stem cell renewal and early precursor development, whereas the expression of relatively high amounts of Delta1 in the thymus may be important both for promotion of T cell development as well as for inhibiting myeloid development.
Our findings that relatively lower ligand densities enhanced the generation of cells able to reconstitute NOD/SCID mice, whereas higher densities resulted in decreased generation of these cells secondary to apoptosis indicate a quantitative aspect of the Notch signaling pathway in regulating cell-fate outcomes of human hematopoietic progenitors and provide a potential target for the manipulation of hematopoietic progenitors in vitro for clinical purposes. Although there is controversy as to whether the NOD/SCID mouse model is adequate for the detection of long-term repopulating cells,38 our studies clearly demonstrate enhanced short-term repopulation of cord blood progenitor cells cultured with Delta1. A limitation of this study is the lack of identification of the specific subpopulation of cells within the heterogeneous population of CD34+CD38– cord blood progenitor cells responsible for this enhanced short-term NOD/SCID repopulating ability. However, with respect to repopulating function, ligand effects here are necessarily on immature, multipotent repopulating cells as is required for the observed in vivo human cell repopulation of NOD/SCID mice. Thus, this is of particular interest in the area of umbilical cord blood transplantation (UCBT) because the generation of either short- or long-term hematopoietic repopulating cells is likely to improve the delayed myeloid engraftment encountered in patients undergoing UCBT, thereby improving overall survival.
Prepublished online as Blood First Edition Paper, June 23, 2005; DOI 10.1182/blood-2005-03-1131.
Supported by grants P50 HL54881, P30 DK56465, K23 HL0774446, and R24 HL74445 from the National Institutes of Health as well as an Amgen Career Development Award. I.D.B. is also supported as an American Cancer Society-F. M. Kirby Clinical Research Professor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Stacey Dozono, Steven Staats, David Flowers, Cynthia Nourigat, and Mariko Kawabori for expert technical assistance; Mari H. Dallas for her help in preparing the figures; and Ted Gooley for statistical analysis. We would also like to thank Dr Margaret Hutchinson and the other partners of the Seattle OB/GYN practice for collection of cord blood samples.

![Figure 2. Delta1ext-IgG affects the number and apoptosis of CD34+ precursors and the number of myeloid and lymphoid precursors in a dose-dependent manner. CD34+CD38– cord blood precursors were incubated with increasing Delta1ext-IgG densities or human control IgG (designated in the figures as Delta1ext-IgG at 0 μg/mL). (A) Total number of cells (▪), and number of CD34+ cells (▴) generated following 21 days of culture. Results are the mean of 7 independent experiments ± SEM (R2 = 0.97; P = .002 for total cells and .001 for CD34). (B) Maximum percentage of CD34+ cells that are annexin V positive and propidium iodide negative] following 3 to 6 days in culture. Percent apoptosis was measured on days 3, 6, 10, and 14 in 3 independent experiments and maximum apoptosis of the CD34+ cells was seen at 3 and 6 days. Results are the mean of 3 independent experiments ± SEM. (C-D) Total number of CD14+ (R2 = 0.95; P = .004) and CD7+ cells, respectively, generated following 21 days of culture. Results are the mean of 7 independent experiments ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/8/10.1182_blood-2005-03-1131/6/m_zh80200585560002.jpeg?Expires=1769094123&Signature=Gd54LoKcdjKgbvwmxtjaEZyH6sMzi-bdaDTX3ymV9mlMLeeZ-ep5PtjsQwYLuTIzEw9aoiNQh995HtKHJeuEfPdmoj4oQg41-qZkcjnfjPy8V0f8nIhU5BgzwbeeOK-lIbTgrz1dLL6uy~Pj2orIUoEOGJm7ebTcnWBA1vQtDvfLdCv7SuvXk9In5agNSkPlbRvF3pBSTfoZ6OSL5Jy-6qQxym-QoU9ADfyAsZSx-AwFpwc0wiASu4Z-X8GPnO9fUspVDX-RcRUfJPkcqXI4D9HZMj1RKvaub8PUiOSJfR1CYaUjTYzHtbJTLcisW8KTLZKU~1BorlDByx8m1WhPpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal