Abstract
Dectin-1 is a lectin receptor for β-glucan that is important for innate macrophage recognition of fungi and contributes to phagocytosis, reactive oxygen production, and induction of inflammatory cytokines. The mechanisms by which Dectin-1 mediates intracellular signaling are just beginning to be defined. Spleen tyrosine kinase (Syk) is a protein tyrosine kinase that is critical for adaptive immune responses where it mediates signaling through B-cell receptors, T-cell receptors, and Fc receptors. Here we report that Dectin-1 activates Syk in macrophages and is important for Dectin-1-stimulated reactive oxygen production, but not for phagocytosis. Syk activation is restricted to a subpopulation of macrophages that is in equilibrium with cells that cannot activate the pathway. The proportion of macrophages using this signaling pathway can be modulated by cytokine treatment. Thus, Dectin-1 signaling reveals dynamic macrophage heterogeneity in inflammatory activation potential. (Blood. 2005;106:2543-2550)
Introduction
Innate immune receptors are germline-encoded receptors that bind microbial products to initiate inflammatory and antimicrobial responses.1 These innate immune responses are an early component of host defense and are important for initiating and shaping the development of productive adaptive immunity. Innate immune receptors include a variety of receptor families such as lectin receptors (such as the mannose receptor, DC-SIGN [dendritic cell specific intercellular adhesion molecule 3-grabbing nonintegrin 2], or Dectin-1), scavenger receptors (such as scavenger receptor I, CD36, or MARCO [macrophage receptor with collagenous structure]), and Toll-like receptors (TLRs). Of these receptors, much is known about signaling mechanisms activated by TLRs,2 whereas signaling mechanisms activated by lectin receptors and scavenger receptors are less well understood.
The 2 known spleen tyrosine kinase (Syk) family tyrosine kinases, Syk and zeta chain-associated protein kinase 70 kDa (Zap-70), are enzymes with well-established roles in developing and executing productive adaptive immune responses.3,4 Zap-70 is required for signaling by the T-cell receptor (TCR), and Syk is required for signaling by the B-cell receptor. Thus, the kinases are critical for development of T-cell and antibody responses. When antibodies are produced and immune complexes are formed, Syk is required by myeloid cells for crystallizable fragment (Fc) receptor signaling.5 Signaling through Syk kinases is typically activated by receptors or accessory proteins containing immunoreceptor tyrosine-based activation motifs (ITAMs). ITAM-containing proteins include the TCRζ and CD3γ/δ/ϵ chains of TCRs, the Igα/β chains of B-cell receptors, the FcRγ chain of Fc receptors, and DNAX activation protein 12 (DAP12) of activating natural killer (NK) receptors. Receptor clustering stimulates Src family kinases to phosphorylate ITAM motifs on 2 appropriately spaced tyrosine residues that then serve as docking sites for dual SH2 domains of Syk family kinases, leading to enzymatic activation of Syk kinases and downstream signal transduction.6 Aside from participating in adaptive immune processes, Syk plays a role in major histocompatibility complex class I tissue surveillance by NK cells and integrin signaling in neutrophils.7,8 To date, innate immune receptors have not been demonstrated to signal through Syk family kinases. In this report, we have demonstrated that an innate immune receptor of the lectin family, Dectin-1, activates macrophage inflammatory responses through Syk.
Dectin-1 is a C-type lectin receptor that was identified by Brown and Gordon in an expression cloning scheme for receptors that could mediate phagocytosis of zymosan, a β-glucan-rich cell wall particle prepared from Saccharomyces cerevisiae.9 The receptor binds β-glucan and is the primary receptor on macrophages for phagocytosis of various fungi.9-11 We and others have demonstrated that TLR signaling can be enhanced by cosignaling through Dectin-1.12,13 Phagocytosis of β-glucan particles by Dectin-1 also activates production of reactive oxygen species (ROSs).11 The receptor is a type II transmembrane protein with a short amino-terminal cytoplasmic tail and an extracellular region containing a single C-type lectin domain that is required for β-glucan recognition. This structure is similar to other lectinlike NK receptors found in the same chromosomal locus.14 The cytoplasmic tail of Dectin-1 contains a short motif similar to an ITAM, leading to the speculation that Dectin-1 may signal via an immunoreceptor pathway.15 Despite the presence of an ITAM-like motif in Dectin-1, Herre et al have reported that Syk is not strictly required for phagocytosis through Dectin-1.16 We have found that although the ITAM-like motif of Dectin-1 does not function as a true ITAM, the receptor does activate signaling through Syk, and this signaling is required for activation of antimicrobial reactive oxygen production. Surprisingly, Dectin-1 activation of Syk was restricted to a subpopulation of macrophages. This population was not irreversibly programmed for Syk activation and was in equilibrium with cells that do not use Syk. The proportion of cells using Syk could be modulated by cytokines that reflect the maturity of an ongoing immune response. Thus, the Syk signaling pathway, which is key to adaptive immune responses, is also used by the innate immune system and may be a focal point for cross-regulation of adaptive and innate immune responses.
Materials and methods
Reagents and mice
Unless noted, all reagents were from Sigma (Saint Louis, MO). 4-amino-5-(4-chlorophenyl)-7-(t-butyl)-pyrazolo[3,4-d]pyrimidine (PP2) and piceatannol were from Calbiochem (San Diego, CA). PAM3CSK4 was from EMC Microcollections (Tuebingen, Germany). Syk, phospho-specific Syk (Tyr525/526), and Src (Tyr416) antibodies were from Cell Signaling Technology (Beverly, MA). p47phox (NCF1) antibody was from Abcam (Cambridge, United Kingdom).
C57/Bl6 mice and gp91phox-/- mice were obtained from Jackson Laboratories (Bar Harbor, ME). CD18, DAP12, FcRγ (Taconic Farms, Germantown, PA), MyD88 (provided by Dr S. Akira, Osaka University, Osaka, Japan) and Syk-/- mice have been described.17-20 For preparation of Syk-/- and DAP12/FcRγ-/- macrophages, fetal liver cells from these mice were used to generate bone marrow chimeras as previously described.8 Mouse bone marrow-derived macrophages were grown in complete RPMI (RPMI supplemented with 10% fetal bovine serum [Hyclone, Logan, Utah], 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine) with 50 ng/mL recombinant human macrophage colony-stimulating factor (Chiron, Emeryville, CA).11 Cells were typically plated at a density of 200 000/well in a 24-well dish. Where indicated, the cells were treated overnight with recombinant murine interferon γ (IFN-γ; 25 U/mL) or interleukin 4 (IL-4; 20 ng/mL; PeproTech, Rocky Hill, NJ). Peritoneal macrophages were prepared by peritoneal lavage, and cells were plated in complete RPMI overnight before use.
Cell lines
RAW264.7 cells (American Type Culture Collection, Manassas, VA; ATCC no. TIB-71) were maintained in complete RPMI. The coding region for murine Dectin-1 was cloned into the retroviral vector pFB-Neo (Stratagene, La Jolla, CA) and tagged at the C-terminus with additional coding region for the following residues: SDEKTTGVWRGGHVVEGLAGELEQLRARLEHHPQQGQREPSSSGGSKLG. This tag includes an epitope from protein C recognized by the HPC4 monoclonal antibody (Amersham, Arlington Heights, IL) and the streptavidin binding peptide.21,22 Retrovirus was prepared according to the manufacturer's instructions, RAW264.7 cells were infected, and a G418-resistant population was obtained. HEK293 cells (ATCC no. CRL-1573) expressing green fluorescent protein (GFP)-tagged Dectin-1 were generated by cotransfecting cells with pTZ/GFP-Dectin-1 (an expression vector murine Dectin-1 fused to GFP at the amino terminus under control of a tetracycline regulatable promoter) and pTTIN, an expression vector directing production of a bicistronic mRNA coding for the tetracycline-regulated transcriptional activator (Tak, derived from pTetTak, Invitrogen, Huntsville, AL) and neomycin resistance under control of a tetracycline-regulated promoter. Stably transfected cell lines were selected with neomycin (in the absence of tetracycline) and cloned.
HEK293-cell transfection and phagocytosis assays
Variations on the tetracycline-inducible GFP-Dectin-1 vector were generated by polymerase chain reaction (PCR)-mediated mutation and confirmed by sequencing. Wild-type and mutant Dectin-1 constructs were transiently cotransfected into HEK293 cells together with pTTIN using Polyfect (Qiagen, Valencia, CA) according to the manufacturer's instructions. Cells were used 24 hours after transfection.
For phagocytosis assays, cells (transfected HEK293 cells or macrophages) were fed tetrarhodamine isothiocyanate (TRITC)-zymosan (50 μg/mL) for 30 minutes, washed with phosphate-buffered saline (PBS), and resuspended in PBS containing 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM sodium azide, and 2.5 U/mL proteinase K (Sigma). The suspension was incubated at room temperature for 20 minutes to remove all uninternalized zymosan particles. By flow cytometry, transfected Dectin-1-expressing HEK293 cells were identified (GFP), and internalization of TRITC-zymosan by these cells was recorded. Macrophages were gated by forward/side scatter (FSC/SSC), and internalization of TRITC-zymosan by these cells was recorded. Bound particles were efficiently removed by this protocol and therefore do not contaminate the phagocytosis measurements (Figure 4; and data not shown).
Immunofluorescence microscopy and flow cytometry
Cells grown on glass coverslips were fed zymosan or TRITC-labeled zymosan for 10 minutes at 37°C in the presence of 1 mM sodium orthovanadate, washed with cold PBS, and fixed with 10% formalin in PBS for 15 minutes. Cells were permeabilized and processed for immunofluorescence microscopy as previously described.23 Images were acquired using a Leica SP2 laser scanning confocal microscope equipped with a 63 ×/1.4 objective lens (Leica, Wetzlar, Germany). Images were cropped and placed on pages using Adobe Photoshop version 6.0 (Adobe Systems, Seattle, WA).
For intracellular staining and flow cytometry for phospho-Syk and phospho-Src, cells were stimulated with 50 μg/mL TRITC-zymosan in the presence of 1 mM sodium orthovanadate for the indicated periods of time. In some experiments Alexa Fluor 647-R-phycoerytherin (PE)-labeled zymosan was used. In this case, zymosan was biotinylated using EZ-link sulfo LCLC biotin (Pierce, Rockford, IL), washed thoroughly, and incubated with Alexa Fluor 647-R-phycoerytherin-labeled streptavidin (Molecular Probes, Eugene, OR). After stimulation, the cells were washed once with ice-cold PBS containing 1 mM sodium orthovanadate and lifted from their culture dishes in trypsin/EDTA containing 1 mM sodium orthovanadate. The cells were resuspended in 2.4G2 hybridoma supernatant containing sodium orthovanadate to block Fc receptors, fixed for 15 minutes with 10% formalin/PBS, and stained for 2 hours with the indicated antibodies in permeabilization buffer (PBS with 0.1% calf serum, 0.1% saponin, 1 mM sodium orthovandate). The cells were washed, stained with secondary goat anti-rabbit fluorescein isothiocyanate (FITC)-conjugated antibody (Jackson ImmunoResearch Labs, West Grove, PA) for 1 hour in permeabilization buffer, and analyzed by flow cytometry.
For detection of surface markers by flow cytometry, cells were harvested, Fc receptors were blocked by incubation in 2.4G2 supernatant, stained with FITC-anti-Dectin-1 (2A11, Serotec, Raleigh, NC), FITC-anti-MAC-1 (M1/70, PharMingen, San Diego, CA), or PE-anti-F4/80 (PharMingen), and analyzed by flow cytometry.
Detection of reactive oxygen production
The production of ROSs was assayed by luminol-enhanced chemiluminescence as described.12 Cells were plated at 100 000/well in a 96-well tissue-culture luminometer plate (Costar, Cambridge, MA) with IFN-γ (25 U/mL) 18 hours before use. Reactive oxygen production was also measured by flow cytometry using 2-[6-(4′-amino)phenoxy-3H-xanthen-3-on-9]benzoic acid (APF; Molecular Probes).24 Cells were stimulated for 1 hour with zymosan (50 μg/mL) and APF (10 μM) was added to the media for the final 30 minutes. Cells were lifted, kept on ice, and analyzed immediately by flow cytometry.
Stimulation with streptavidin-coated beads
In some experiments, RAW264.7 cells expressing streptavidin-binding peptide (SBP)/Dectin-1 were stimulated with streptavidin-coated beads (M280; Dynal, Oslo, Norway). Before use, the cells were washed and transferred into complete Dulbecco modified Eagle medium (DMEM) because RPMI contains biotin that interferes with the assays. For phagocytosis assays, cells were preincubated for 5 minutes with cytochalasin D (2.5 μM; Sigma) where indicated. The cells were fed streptavidin-coated beads diluted 1:60 for 30 minutes at 37°C, washed, and lifted off the dish for 20 minutes in PBS containing 1 mM EDTA, 1 mM sodium azide, and 2.5 U/mL proteinase K (Sigma). The cells were analyzed by flow cytometry, and bead internalization was detected by the natural red fluorescence of the paramagnetic particles, or by the strong side-scatter of cells' internalizing particles.
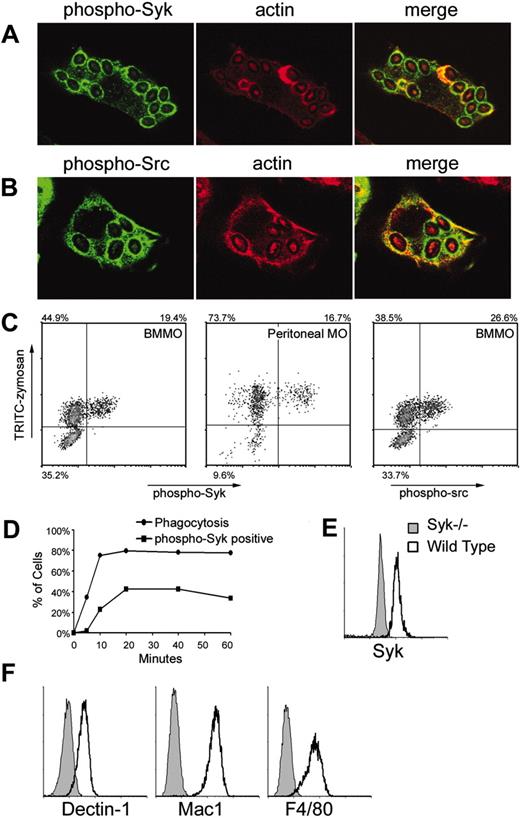
Src/Syk activation by zymosan. IFN-γ-primed bone marrow-derived macrophages were fed zymosan for 15 minutes in the presence of sodium orthovanadate and activation of Syk (A) and Src family (B) kinases was visualized by immunofluorescence microscopy using anti-phospho-Syk (Tyr519/520) and anti-phospho-Src (Tyr 416) antibodies, respectively. (C) Tyrosine phosphorylation of Syk (left panels) and Src family kinases (right panel) was measured by flow cytometry of bone marrow-derived (BMMO) or peritoneal macrophages (MO) stimulated as described with fluorescently labeled zymosan for 20 minutes. (D) Tyrosine phosphorylation of Syk was measured in IFN-γ-primed bone marrow-derived macrophages stimulated for the indicated times with zymosan. Phagocytosis was synchronized by centrifugation of zymosan particles onto the macrophages at 4°C, and then warming the cells to 37°C. (E) Syk expression was detected by intracellular staining and flow cytometry in IFN-γ-primed wild-type and Syk-/- bone marrow-derived macrophages. (F) Surface expression of Dectin-1, Mac-1 (CD11b), and F4/80 on IFN-γ-primed bone marrow-derived macrophages was measured by flow cytometry.
Src/Syk activation by zymosan. IFN-γ-primed bone marrow-derived macrophages were fed zymosan for 15 minutes in the presence of sodium orthovanadate and activation of Syk (A) and Src family (B) kinases was visualized by immunofluorescence microscopy using anti-phospho-Syk (Tyr519/520) and anti-phospho-Src (Tyr 416) antibodies, respectively. (C) Tyrosine phosphorylation of Syk (left panels) and Src family kinases (right panel) was measured by flow cytometry of bone marrow-derived (BMMO) or peritoneal macrophages (MO) stimulated as described with fluorescently labeled zymosan for 20 minutes. (D) Tyrosine phosphorylation of Syk was measured in IFN-γ-primed bone marrow-derived macrophages stimulated for the indicated times with zymosan. Phagocytosis was synchronized by centrifugation of zymosan particles onto the macrophages at 4°C, and then warming the cells to 37°C. (E) Syk expression was detected by intracellular staining and flow cytometry in IFN-γ-primed wild-type and Syk-/- bone marrow-derived macrophages. (F) Surface expression of Dectin-1, Mac-1 (CD11b), and F4/80 on IFN-γ-primed bone marrow-derived macrophages was measured by flow cytometry.
Results
Zymosan activates Syk in a subpopulation of macrophages
To determine if Syk is activated during phagocytosis of zymosan particles, we examined bone marrow-derived macrophages internalizing zymosan particles for 15 minutes by immunofluorescence microscopy. Mouse Syk is activated by tyrosine phosphorylation at tyrosines 519 and 520.25 Using a Tyr519/520 phospho-specific antibody, we detected activated Syk recruited to phagosomes during internalization of zymosan particles (Figure 1A). Activated Syk was present on phagosomes during early stages of phagocytosis while actin was present and after actin dissociated from the compartment (Figure 1A). Syk is typically activated downstream of Src family kinases, so we examined whether Src kinases were also activated and recruited to zymosan phagosomes. Using a phospho-specific antibody that recognizes activated Src family kinases, we detected strong activation and recruitment of the kinases to zymosan phagosomes (Figure 1B). Thus, Src/Syk signaling is activated during phagocytosis of zymosan.
Curiously, during these studies we observed that although nearly all macrophages internalized zymosan particles, only some cells were stained with anti-phospho-Syk or anti-phospho-Src (not shown). To quantitate this observation, we fed bone marrow-derived macrophages fluorescently labeled zymosan for 20 minutes in the presence of sodium orthovanadate to block tyrosine phosphatases, then assessed particle internalization and Syk activation by flow cytometry. We could detect phospho-Syk only in the presence of sodium orthovanadate, an observation that suggests that normally zymosan-activated Syk is rapidly deactivated by tyrosine phosphatases. Importantly, phospho-Syk could be detected only in the fraction of cells that bound and internalized the particles and not in neighboring cells that did not ingest particles (Figure 1C). The fluorescently labeled zymosan particles used in this study are uniformly labeled so that fluorescence intensity correlates very well with the number of particles ingested (data not shown). Thus, cells appeared to activate or not activate Syk independent of the number of zymosan particles internalized because we found no correlation between fluorescent zymosan intensity and Syk activation. Syk activation in only a subset of phagocytic cells occurred in both bone marrow-derived and peritoneal macrophages (Figure 1C). Like Syk, Src family kinases were phosphorylated in only a subset of macrophages ingesting zymosan (Figure 1C). A time-course analysis revealed that Syk activation lags behind zymosan internalization by 5 to 10 minutes and is complete within 20 minutes (Figure 1D). Due to the inclusion of sodium orthovanadate in the experiments to block tyrosine phosphatases, we conclude that cells that ingest zymosan but do not activate Syk will not activate Syk even at later time points.
We were surprised by this heterogeneity in signaling and therefore examined whether there was unappreciated heterogeneity in the macrophage population that we were studying. Syk is expressed uniformly by all bone marrow-derived macrophages (Figure 1E). We also examined expression of receptors expected to interact with zymosan and we found that Dectin-1 and Mac1 (CD11b) were expressed uniformly by all cells. Similarly, all cells were F4/80+ (Figure 1F). Thus, the macrophage population studied does not initially look unusually heterogeneous.
Syk activation through Dectin-1
We next explored the mechanism by which Syk becomes activated. Syk is typically activated by Src family kinases, so we examined whether PP2, a specific inhibitor of Src family kinases, could block zymosan-induced Syk activation. Treatment with PP2 strongly inhibited Syk activation but did not block internalization of zymosan by bone marrow-derived macrophages (Figure 2A). Zymosan stimulates macrophages, in part, through activation of TLR2. We therefore measured zymosan-induced Syk and Src activation in macrophages from mice deficient in MyD88, an adaptor required for signaling by TLRs including TLR2. We observed no defect in activation of Syk or Src (Figure 2B). Consistent with these data, we could not detect activation of Syk or Src by pure TLR agonists including PAM3CSK4 lipopeptide (TLR2) or lipopolysaccharide (LPS; TLR4; data not shown). To activate Syk, Src kinases target ITAM-containing signaling molecules. We therefore examined the role of DAP12 and the FcRγ chain, 2 ITAM-containing adaptor molecules that link surface receptors to Syk activation in macrophages. Macrophages doubly deficient in DAP12 and the FcRγ chain internalized zymosan and activated Syk and Src normally (Figure 2B), ruling out participation of a receptor associated with one of these molecules. CD18 has been demonstrated to activate Syk independent of DAP12/FcRγ chain, although the precise mechanism has yet to be elucidated.8 Because Mac1 (CD11b) associates with CD18 to form a surface dimer that has been implicated in zymosan recognition,26,27 we explored the role of CD18 in Syk activation. Macrophages from CD18-deficient mice internalized zymosan and activated Syk normally, indicating that CD18/CD11b is not required for these responses.
Requirements for zymosan activation of Src/Syk signaling. (A) Syk activation was assessed in IFN-γ-primed bone marrow-derived macrophages stimulated with zymosan in the presence of the Src kinase inhibitor, PP2 (25 μM), or the soluble β-glucan, laminarin (0.5 mg/mL). (B) Syk and Src activation was measured in IFN-γ-primed bone marrow-derived macrophages obtained from mice deficient in MyD88, DAP12/FcRγ chain, or CD18 as indicated.
Requirements for zymosan activation of Src/Syk signaling. (A) Syk activation was assessed in IFN-γ-primed bone marrow-derived macrophages stimulated with zymosan in the presence of the Src kinase inhibitor, PP2 (25 μM), or the soluble β-glucan, laminarin (0.5 mg/mL). (B) Syk and Src activation was measured in IFN-γ-primed bone marrow-derived macrophages obtained from mice deficient in MyD88, DAP12/FcRγ chain, or CD18 as indicated.
Dectin-1 signaling is sufficient to activate Syk in a subpopulation of macrophages. (A) Expression of SBP-tagged Dectin-1 in a stably transfected population of RAW264.7 macrophages was assessed by flow cytometry using an antibody to the epitope tag. (B) SBP-tagged Dectin-1 was localized by immunofluorescence microscopy in resting cells (left panel) or in cells fed streptavidin-coated beads (right panel). (C) RAW264.7 cells were incubated with streptavidin-coated beads in the presence of cytochalasin D (2.5 μM) or biotin (100 μM) as indicated, and internalization of the particles was measured by flow cytometry. (D) RAW264.7 cells expressing SBP-tagged Dectin-1 were incubated with streptavidin-coated beads (left panel) or zymosan (right panel) in the presence of laminarin (0.5 mg/mL) or biotin (100 μM) as indicated. ROS production was measured by luminol-enhanced chemiluminescence. (E) RAW264.7 cells expressing SBP-tagged Dectin-1 were stimulated with streptavidin-coated beads for 20 minutes in the presence of sodium orthovanadate, and activation of Syk was visualized by immunofluorescence microscopy as in Figure 1. (F) Activation of Syk in RAW264.7 cells expressing SBP-tagged Dectin-1 and stimulated with streptavidin-coated beads was assessed by flow cytometry.
Dectin-1 signaling is sufficient to activate Syk in a subpopulation of macrophages. (A) Expression of SBP-tagged Dectin-1 in a stably transfected population of RAW264.7 macrophages was assessed by flow cytometry using an antibody to the epitope tag. (B) SBP-tagged Dectin-1 was localized by immunofluorescence microscopy in resting cells (left panel) or in cells fed streptavidin-coated beads (right panel). (C) RAW264.7 cells were incubated with streptavidin-coated beads in the presence of cytochalasin D (2.5 μM) or biotin (100 μM) as indicated, and internalization of the particles was measured by flow cytometry. (D) RAW264.7 cells expressing SBP-tagged Dectin-1 were incubated with streptavidin-coated beads (left panel) or zymosan (right panel) in the presence of laminarin (0.5 mg/mL) or biotin (100 μM) as indicated. ROS production was measured by luminol-enhanced chemiluminescence. (E) RAW264.7 cells expressing SBP-tagged Dectin-1 were stimulated with streptavidin-coated beads for 20 minutes in the presence of sodium orthovanadate, and activation of Syk was visualized by immunofluorescence microscopy as in Figure 1. (F) Activation of Syk in RAW264.7 cells expressing SBP-tagged Dectin-1 and stimulated with streptavidin-coated beads was assessed by flow cytometry.
Recognition of β-glucan is required for macrophage responses to zymosan, and Dectin-1 is thought to be the major receptor for β-glucan expressed on macrophages. Macrophages treated with soluble β-glucan to block Dectin-1 fail to internalize zymosan and activate Syk (Figure 2A). Because blocking Dectin-1 blocks internalization, it is difficult to comment on the specific importance of Dectin-1 activation for Src/Syk signaling. To establish whether signaling through Dectin-1 is sufficient to activate Syk, we generated a model system for manipulation of Dectin-1 signaling. We made an expression vector for Dectin-1 fused at its extracellular C-terminus to a short peptide that strongly binds streptavidin (SBP).21,22 We stably expressed this receptor in RAW264.7 cells, a mouse macrophage cell line. The cell line uniformly expresses the SBP-Dectin-1 receptor at the cell surface (Figure 3A-B). Although parental RAW264.7 cells do not bind or ingest streptavidin-coated paramagnetic beads (not shown), the cells expressing the tagged receptor strongly bind and ingest the beads (Figure 3B-C). Phagocytosis of the beads is blocked by biotin, which binds to streptavidin with a higher affinity than the SBP tag (Figure 3C). This system provided us with a method for specifically triggering Dectin-1-mediated signaling and phagocytosis. We have previously implicated Dectin-1 in activation of ROS production triggered by zymosan.12 We observed that streptavidin-coated beads specifically triggered ROS production by RAW cells expressing the SBP-Dectin-1; this response was blocked by biotin, but not by laminarin (Figure 3D). ROS production triggered by zymosan was blocked only by laminarin. Thus, Dectin-1 signaling is sufficient to trigger ROS production.
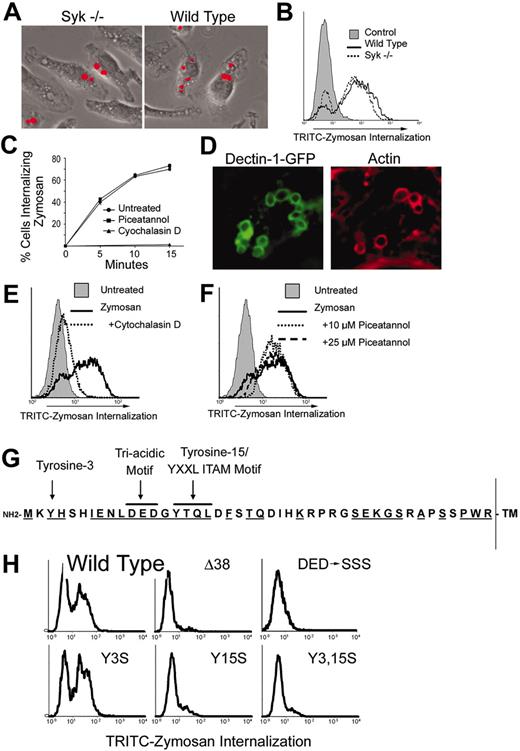
Syk is not required for phagocytosis of zymosan. Wild-type and Syk-/- bone marrow-derived macrophages were cultured with fluorescently labeled zymosan for 15 minutes. Phagocytosis of zymosan was detected microscopically (A) and quantified by flow cytometry (B). (C) Bone marrow-derived macrophages were cultured with fluorescently labeled zymosan for the indicated times in the presence or absence of the Syk inhibitor piceatannol (25 μM) or the actin-disrupting agent cytochalasin D (2.5 μM). Phagocytosis was measured by flow cytometry, and the percentage of cells ingesting particles is shown. (D) HEK293 cells expressing GFP-tagged Dectin-1 were cultured with zymosan particles for 15 minutes, and Dectin-1 and actin were detected by immunofluorescence microscopy. (E) GFP/Dectin-expressing HEK293 cells were cultured with fluorescently labeled zymosan for 30 minutes in the presence or absence of cytochalasin D (E) or piceatannol (F), and internalization was measured by flow cytometry. (G) The cytoplasmic tail of murine Dectin-1 is depicted showing the 2 tyrosine residues and the triacidic motif. Residues that are conserved between mouse and human Dectin-1 are underlined. (H) HEK293 cells were transiently transfected with GFP-tagged Dectin-1 (wild type and the indicated mutants). The cells were cultured with fluorescently labeled zymosan, and internalization was measured by flow cytometry. The data are gated on GFP+ (Dectin-1-expressing) cells.
Syk is not required for phagocytosis of zymosan. Wild-type and Syk-/- bone marrow-derived macrophages were cultured with fluorescently labeled zymosan for 15 minutes. Phagocytosis of zymosan was detected microscopically (A) and quantified by flow cytometry (B). (C) Bone marrow-derived macrophages were cultured with fluorescently labeled zymosan for the indicated times in the presence or absence of the Syk inhibitor piceatannol (25 μM) or the actin-disrupting agent cytochalasin D (2.5 μM). Phagocytosis was measured by flow cytometry, and the percentage of cells ingesting particles is shown. (D) HEK293 cells expressing GFP-tagged Dectin-1 were cultured with zymosan particles for 15 minutes, and Dectin-1 and actin were detected by immunofluorescence microscopy. (E) GFP/Dectin-expressing HEK293 cells were cultured with fluorescently labeled zymosan for 30 minutes in the presence or absence of cytochalasin D (E) or piceatannol (F), and internalization was measured by flow cytometry. (G) The cytoplasmic tail of murine Dectin-1 is depicted showing the 2 tyrosine residues and the triacidic motif. Residues that are conserved between mouse and human Dectin-1 are underlined. (H) HEK293 cells were transiently transfected with GFP-tagged Dectin-1 (wild type and the indicated mutants). The cells were cultured with fluorescently labeled zymosan, and internalization was measured by flow cytometry. The data are gated on GFP+ (Dectin-1-expressing) cells.
We next determined whether Dectin-1 triggering was sufficient to activate Syk. Macrophages expressing the SPB-Dectin-1 were fed streptavidin-coated beads, and phosphorylated Syk was detected by immunofluorescence microscopy (Figure 3E). Syk became activated in a subset of cells that internalized beads (Figure 3E-F). The data suggest that although Dectin-1 was bound and triggered phagocytosis in all the cells eating the beads, the signaling resulted in activation of Syk in only a subpopulation of those cells.
Syk is not required for phagocytosis by Dectin-1
We next explored the role of Syk signaling in Dectin-1-triggered macrophage responses to zymosan. Because Syk is not activated in many cells in which Dectin-1 is activated, the data predict that phagocytosis through Dectin-1 does not require Syk. Consistent with this prediction, we observed that Syk-/- macrophages ingest zymosan normally (Figure 4A-B) and that inhibition of Syk with the specific inhibitor piceatannol does not affect phagocytosis of zymosan (Figure 4C). We explored the possibility that Dectin-1 activates Syk for phagocytic signaling but that other phagocytic receptors compensate for the lack of Syk during phagocytosis of zymosan in macrophages. HEK293 cells do not express Dectin-1 and do not bind or ingest zymosan. Expression of Dectin-1 in these cells is sufficient to confer zymosan binding and phagocytosis (Figure 4D-E). Although HEK293 cells express Syk (data not shown), we observed that Syk is not activated in Dectin-1-expressing HEK293 cells (not shown) and that inhibition of Syk with piceatannol does not block phagocytosis of zymosan (Figure 4F).
It has been suggested that Dectin-1 possesses an ITAM-like motif in its cytoplasmic tail that could be required for signaling. We therefore explored the role of this motif in phagocytosis in HEK293 cells. An ITAM motif consists of 2 tyrosine residues arranged as YXXL/I X6-8 YXXL/I.28 Phosphorylation of both tyrosines by Src family kinases provides a binding site for dual SH2 domains on Syk. ITAMs usually occur in clusters; for example the FcRγ chain dimer has 2 ITAMs, and CD3ζ chain dimer has 6 ITAMs.29 Dectin-1 has no such cluster, although it is possible that the receptor exists as a dimer like the FcRγ and CD3ζ chains. The ITAM-like motif of Dectin-1 is not perfect because the amino terminal tyrosine (Tyr-3) is not in exactly the correct YXXL/I context, and a highly charged triacidic motif (DED) is located proximal to the second tyrosine (Tyr-15; Figure 4G). We evaluated the role of these various sites in Dectin-1-mediated phagocytosis in HEK293 cells. As noted above, when expressed in HEK293 cells, Dectin-1 mediates phagocytosis of zymosan particles. Truncation of the intracellular N-terminal 38 amino acids (Δ38) did not alter surface expression of the receptor but prevented phagocytosis (data not shown and Figure 4H). Mutation of the N-terminal tyrosine to serine (Y3S) did not affect signaling for phagocytosis. These findings are consistent with a previous report.16 However, mutation of the C-terminal tyrosine (Y15S) or mutation of the triacidic motif (DED-SSS) completely blocked phagocytic signaling without affecting surface expression (data not shown and Figure 4H). Taken together, the data demonstrate that Dectin-1 signaling for phagocytosis does not require a functional ITAM and does not require Syk signaling.
A role for Syk in Dectin-1 signaling
Although Syk is not required for Dectin-1-mediated phagocytosis, its activation suggests that it is required for some Dectin-1-triggered response. We observed that zymosan-stimulated ROS production was completely abolished in Syk-deficient macrophages (Figure 5A). As previously reported, the cells are also completely deficient in ROS production in response to IgG-opsonized sheep red blood cells5 (IgG-SRBCs; Figure 5A). It is possible that development of macrophages in the absence of Syk somehow disables the ROS production machinery; however, the cells respond normally to phorbol ester5 (Figure 5A). Similarly, ROS production triggered by zymosan in wild-type macrophages is inhibited by the Src family kinase inhibitor PP2 and by the Syk inhibitor piceatannol (Figure 5B). Zymosan-induced ROS production was normal in DAP12/FcRγ chain and in CD18 knockout macrophages (data not shown). The specificity of the chemiluminescence assay was confirmed by demonstrating that zymosan-induced ROS production was completely abrogated in macrophages from gp91phox knockout mice (Figure 5B).
Syk is required for zymosan-induced ROS production. (A) IFN-γ-primed wild-type or Syk-/- bone marrow-derived macrophages were stimulated with zymosan, IgG-opsonized sheep red blood cells (SRBCs), or phorbol myristate acetate (PMA) as indicated, and generation of reactive oxygen was measured by enhanced chemiluminescence. (B) IFN-γ-primed wild-type or gp91phox-/- bone marrow-derived macrophages were stimulated with zymosan in the presence or absence of the Syk inhibitor, piceatannol (Pic), or the Src family kinase inhibitor, PP2, and generation of reactive oxygen was measured. (C) IFN-γ-primed bone marrow-derived macrophages were fed zymosan for 15 minutes, and translocation of p47phox to phagosome membranes and Syk phosphorylation was observed by immunofluorescence microscopy. (D) RAW264.7 macrophages expressing SBP-tagged Dectin-1 were stimulated with fluorescently labeled zymosan for 1 hour, and ROS production was detected by flow cytometry using APF.
Syk is required for zymosan-induced ROS production. (A) IFN-γ-primed wild-type or Syk-/- bone marrow-derived macrophages were stimulated with zymosan, IgG-opsonized sheep red blood cells (SRBCs), or phorbol myristate acetate (PMA) as indicated, and generation of reactive oxygen was measured by enhanced chemiluminescence. (B) IFN-γ-primed wild-type or gp91phox-/- bone marrow-derived macrophages were stimulated with zymosan in the presence or absence of the Syk inhibitor, piceatannol (Pic), or the Src family kinase inhibitor, PP2, and generation of reactive oxygen was measured. (C) IFN-γ-primed bone marrow-derived macrophages were fed zymosan for 15 minutes, and translocation of p47phox to phagosome membranes and Syk phosphorylation was observed by immunofluorescence microscopy. (D) RAW264.7 macrophages expressing SBP-tagged Dectin-1 were stimulated with fluorescently labeled zymosan for 1 hour, and ROS production was detected by flow cytometry using APF.
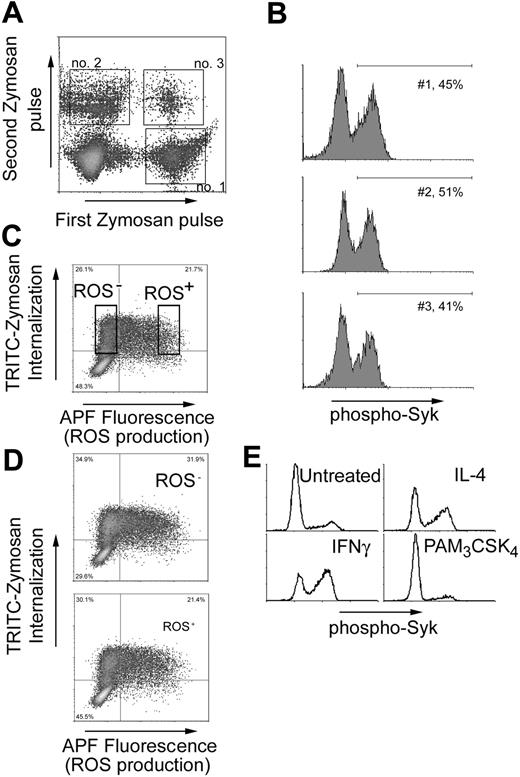
The ability of Dectin-1 to activate Syk and ROS production is a transient property of a macrophage. (A) IFN-γ-primed bone marrow-derived macrophages were stimulated for 20 minutes with TRITC-labeled zymosan (first zymosan pulse), washed, and then stimulated with a second round of R-PE-labeled zymosan for an additional 207 minutes (second zymosan pulse). Populations of cells internalizing zymosan in the first, second, and both periods were identified by flow cytometry. (B) Syk activation was measured in cells using the gates indicated in panel A. (C) RAW264.7 cells expressing SBP-tagged Dectin-1 were stimulated with TRITC-zymosan for 1 hour, and ROS production was measured by flow cytometry usingAPF. Cells internalizing zymosan but not activating ROS production (ROS-) and cells in which ROS production was activated (ROS+) were sorted and expanded for 5 days in culture. (D) The sorted cells from panel C were restimulated with TRITC-zymosan and ROS production was again measured by flow cytometry. (E) Bone marrow-derived macrophages were treated overnight with IFN-γ (25 U/mL), IL-4 (20 ng/mL), or PAM3CSK4 (100 ng/mL) as indicated, and zymosan-induced activation of Syk was measured by flow cytometry. Data are gated on cells that had eaten zymosan.
The ability of Dectin-1 to activate Syk and ROS production is a transient property of a macrophage. (A) IFN-γ-primed bone marrow-derived macrophages were stimulated for 20 minutes with TRITC-labeled zymosan (first zymosan pulse), washed, and then stimulated with a second round of R-PE-labeled zymosan for an additional 207 minutes (second zymosan pulse). Populations of cells internalizing zymosan in the first, second, and both periods were identified by flow cytometry. (B) Syk activation was measured in cells using the gates indicated in panel A. (C) RAW264.7 cells expressing SBP-tagged Dectin-1 were stimulated with TRITC-zymosan for 1 hour, and ROS production was measured by flow cytometry usingAPF. Cells internalizing zymosan but not activating ROS production (ROS-) and cells in which ROS production was activated (ROS+) were sorted and expanded for 5 days in culture. (D) The sorted cells from panel C were restimulated with TRITC-zymosan and ROS production was again measured by flow cytometry. (E) Bone marrow-derived macrophages were treated overnight with IFN-γ (25 U/mL), IL-4 (20 ng/mL), or PAM3CSK4 (100 ng/mL) as indicated, and zymosan-induced activation of Syk was measured by flow cytometry. Data are gated on cells that had eaten zymosan.
Because Syk is activated in only a fraction of Dectin-1-triggered cells, we were surprised at the absolute role for Syk in ROS production. ROS production during phagocytosis is triggered by assembly of the nicotinamide adenine dinucleotide phosphate (NADPH) phagocyte oxidase on the phagosomal membrane. We therefore examined recruitment of the cytosolic NADPH phagocyte oxidase component, p47phox, to zymosan phagosomes by immunofluorescence microscopy. In macrophages fed zymosan for 10 minutes, nearly all zymosan-containing phagosomes were strongly stained with an anti-p47phox antibody. However, Syk was activated in only some of these cells and was clearly not activated in some cells showing strong p47phox recruitment (Figure 5C). The data suggest that although some NADPH phagocyte oxidase assembly occurs on all zymosan phagosomes, ROS is produced in only a subset of the cells. We confirmed this by flow cytometry. RAW cells expressing Dectin-1 were fed fluorescently labeled zymosan in the presence of aminophenyl fluorescein (APF), a recently developed fluorescent probe that is significantly more selective for ROS than other commonly used probes.24 We observed strong oxidation of APF in only a subset of cells internalizing zymosan (Figure 5D). Consistent with the data in Figure 5 panels A and B, this oxidation was completely inhibited by Src and Syk inhibitors and by diphenyliodonium, a chemical inhibitor or the NADPH oxidase (data not shown).
Proportion of cells in which Dectin-1 activates Syk is dynamic
To explore whether there is simply a probability that Syk will be activated each time a zymosan particle is internalized, we designed an experiment in which a single population of macrophages was fed zymosan multiple times. Bone marrow-derived macrophages were fed PE-labeled zymosan for 20 minutes, washed, and then fed Alexa Fluor 647-R-PE-labeled zymosan for the next 20 minutes. Populations of cells that ate zymosan in the first interval, second interval, and in both intervals were easily distinguished by flow cytometry (Figure 6A). If each time a particle was eaten there was a chance that Syk would become activated, we would expect that Syk would be more likely to be activated in the population of cells that phagocytosed particles on 2 separate occasions. When Syk activation was compared in each of these populations, we found that the proportion of phospho-Syk-activated cells was the same in each (Figure 6B). This observation suggests that Syk activation is not simply a function of zymosan dose and that within the time frame of the experiment, some cells are poised for signaling through Syk, whereas others are set to be resistant to Syk activation.
The heterogeneity in activation of Syk by Dectin-1 could arise either from stable differentiation of macrophages into cells that do not trigger Src/Syk signaling and ROS production or to dynamic cell behavior in which cells move back and forth in sensitivity to activation of this pathway. If the phenotype is stable, cells that phagocytose zymosan but fail to activate Syk could be recovered and grown and should continue to not activate Syk. This direct experiment is not possible because we must kill the cells to measure Syk phosphorylation. However, because Syk is required for activation of ROS production we used cell sorting based on viable staining with APF to separate ROS+ versus ROS- cells following phagocytosis of zymosan (Figure 6C). Each population was then expanded for 5 days in culture and restimulated with zymosan. The same pattern of ROS production was observed in both populations of cells (Figure 6D). Thus, the ability to trigger or not trigger ROS production through Syk activation is not a stable phenotype of macrophage subpopulations.
We explored whether the cytokine environment of a macrophage population could influence the number of cells in which Dectin-1 signaling triggers Src/Syk activation. Treatment of bone marrow-derived macrophages with either IFN-γ or IL-4 increased Syk activation triggered by zymosan (Figure 6E). Neither cytokine enhanced the level of Syk phosphorylation as measured by fluorescence intensity. Rather, the number of cells opting to switch on Syk increased. Cytokines do not nonspecifically enhance Syk activation because stimulation with PAM3CSK4, which activates production of many cytokines and chemokines through TLR2, does not enhance Syk activation on subsequent stimulation with zymosan (Figure 6E). Taken together, the observations demonstrate that subsets of macrophages are not irreversibly programmed to activate or not activate Syk on Dectin-1 stimulation.
Discussion
Signaling by Dectin-1 in macrophages triggers phagocytosis and production of ROS. We have observed that Dectin-1 triggers activation of Syk and that Syk is not required for phagocytosis but is required for stimulation of ROS production. Further, we have demonstrated that Syk-dependent signaling occurs in a subset of macrophages in which Dectin-1 is engaged. The fraction of cells in which Syk signaling can be triggered is dynamic and does not appear to represent a stable alternatively differentiated macrophage.
As a key mediator of signaling through T-cell receptors, B-cell receptors, and Fc receptors, activation of Syk kinase is critical for development and execution of effective adaptive immune responses. Our finding that Syk kinase is required for ROS production activated by Dectin-1 demonstrates that the enzyme's signaling pathway is also harnessed by innate immunity. Because a major role of the Fc receptor expressed on macrophages is to coordinate phagocytosis of IgG-opsonized microbes, it is tempting to speculate that Dectin-1 taps into the same pathway for innate phagocytosis of microbes containing β-glucan. However, although Syk is required for phagocytosis through the Fc receptor,30 we and others have demonstrated that it is not required for phagocytosis through Dectin-1.16 The putative ITAM-like motif of Dectin-1 has several features that suggest that it does not actually function as an ITAM and, to date, no direct interaction with Syk has been detected. We have ruled out the possibility of Syk activation through Dectin-1 association with the ITAM-containing adaptor molecules, FcRγ chain and DAP12. Syk has been shown to be activated downstream of the β2-integrin, CD18,8 and we have observed that CD18 is not required for Dectin-1 activation of Syk. Like Dectin-1 activation of Syk, integrin activation of Syk is independent of FcRγ chain and DAP12, and it is possible that Dectin-1 and integrins share downstream mechanisms for tapping into the Syk signaling pathway.
In this study we have observed that Dectin-1 uses a Src/Syk pathway in a dynamic subset of cells for enhanced antimicrobial responses. Previous studies have suggested that epigenetic processes may contribute to macrophage heterogeneity. Macrophages stimulated with LPS release IL-6, IL-12, and monocyte chemoattractant protein 1 (MCP-1) as well as a host of other inflammatory mediators. However, when investigators measure this induction at the single-cell level by flow cytometry, it is common to observe that only subpopulations of primary peritoneal or bone marrow-derived macrophages produce these cytokines.31 More careful analyses using primary macrophages or macrophage cell lines have revealed that it is possible to select macrophage clones that more uniformly produce each of these mediators.32,33 Because they are clonable, these phenotypes are proposed to be due to DNA methylation patterns and chromatin structures that can be maintained through mitosis. We have observed that Dectin-1 signaling through Syk is not a clonable phenotype and, thus, is not likely to be controlled through transmissible DNA architecture. Further, this study has identified direct heterogeneity in the signaling mechanism prior to gene activation.
Recent studies on Fc receptor-mediated phagocytosis have revealed some level of heterogeneity in signaling on individual phagosomes. Using a fluorescent probe for localization of phosphatidylinositol 3-kinase (PI-3K) activity and RAW264.7 cells, Henry et al demonstrated that some phagosomes accumulate phosphatidylinositol 3-phosphate rapidly and lose it rapidly, whereas other phagosomes retain the phospho-lipid for a much longer period.34 The two types of phagosomes could be observed in the same macrophage, suggesting that the heterogeneity arises from local variability in signaling.34,35 Because activity is required for activation of reactive oxygen production by the NADPH phagocyte oxidase, it is attractive to propose that this same heterogeneity is reflected in Dectin-1 activation of Syk. However, such a model would predict that increasing the number of phagosomes in a macrophage would increase the likelihood of triggering Syk or ROS production, and we do not observe this relationship. Further, we observe by immunofluorescence microscopy and flow cytometry a distinct separation of cells that do and do not activate Syk. When multiple phagosomes are formed, they are either all associated with activated Syk (Figure 1A), or none are.
These observations led us to conclude that macrophages can exist in 2 interchangeable states, one that is permissive to Syk activation and ROS production, and one that is not. The cells that do not activate Syk are clearly responsive to the particle; they have bound and ingested it, and we have previously shown by flow cytometry that nearly all cells eating zymosan produce tumor necrosis factor α (TNF-α).23,36,37 Thus the phospho-Syk+ cells represent a specialized subpopulation primed for reactive oxygen production. Because reactive oxygen production itself has been implicated in coordination of other inflammatory responses in macrophages,38,39 it is likely that these cells are specialized for a broader program of inflammatory activity. This is currently under investigation. That the proportion of cells poised for activation of Syk/ROS production in response to zymosan is dynamic and can be modulated by cytokines suggests that net inflammatory activity may be coordinated at the level of macrophage population behavior as well as the inflammatory output of individual cells. Clinical control of macrophage population dynamics may prove to be a useful approach to regulating inflammatory processes prevalent in most human diseases.
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/blood-2005-03-1239.
Supported by National Institutes of Health grant RO1GM62995 (D.M.U.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal