Abstract
Innate immune responses to bacteria require cooperative interactions between host recognition molecules and phagocytes. The peptidoglycan recognition proteins (PGRPs) are a large group of proteins found in insects and mammals that bind to bacterial peptidoglycan (PGN). PGRP-S is located with other antimicrobial proteins, such as lysozyme, in the granules of human neutrophils. Whereas both PGRP-S and lysozyme recognize PGN, the exact binding specificity of human PGRP-S, its functional activity, and its potential synergy with other neutrophil-derived bactericidal proteins such as lysozyme have not been determined. Here we show that human PGRP-S binds to and inhibits the growth of Staphylococcus aureus (containing lysine-type PGN) and Escherichia coli (containing mesodiaminopimelic acid-type PGN). The binding affinity and thus antimicrobial activity of PGRP-S is determined by the third amino acid in the PGN stem peptide. Furthermore, the antimicrobial effect of PGRP-S against E coli is synergistic with lysozyme, and lysozyme and PGRP-S colocalize in neutrophil extracellular traps (NETs), suggesting that these granule-derived proteins act together to kill bacteria trapped in the NETs. Taken together, these results indicate that human PGRP-S plays a role in innate immunity in the context of neutrophils by contributing to the killing of intracellular and extracellular bacteria. (Blood. 2005;106:2551-2558)
Introduction
The innate immune system is a host defense mechanism, evolutionarily conserved from insects to humans, that mediates recognition and control of invading microorganisms.1,2 The basis of innate immune response lies in the ability of the host to recognize conserved products of microbial metabolism that are unique to microorganisms and are not produced by the host. The best known examples of such molecules, called pathogen-associated molecular patterns (PAMPs), include lipopolysaccharide (LPS) of Gram-negative bacteria, DNA sequences containing unmethylated CpG dinucleotides (CpG DNA), and peptidoglycan (PGN) present in Gram-positive and Gram-negative bacteria.3
PGN recognition proteins (PGRPs) are a family of pattern-recognition receptors (PRRs) that bind to, and in some cases hydrolyze, PGNs of bacterial cell walls.4-10 These molecules are highly conserved from insects to mammals, and all share a conserved 160-amino acid domain (the PGRP domain) with notable sequence similarity to N-acetylmuramyl-l-alanine amidases.11 The essential role of PGRPs in the Drosophila immune response is revealed by characterization of immunodeficient mutants. Two genes that encode PGRPs, PGRP-SA and PGRP-LC, are required in 2 different branches of the immune response. PGRP-SA is a circulating PGRP through which Gram-positive organisms activate the Toll pathway,12 whereas PGRP-LC is a transmembrane PGRP through which Gram-negative organisms activate the Imd/Relish pathway.13-15 Activation of these pathways leads to the production of antimicrobial peptides and to the activation of the phenoloxidase-melanin cascade. A recent study has determined that different forms of PGN (lysine type vs meso-diaminopimelic acid type) confer this Gram-positive versus Gram-negative specificity.16 Therefore, Drosophila PGRPs mediate direct interactions with distinctive PGN moieties in bacterial cell walls as a proximal mechanism upstream of Toll and Imd activation. The molecular basis of this specificity is unknown, but it is postulated that variations in the peptide bridges of PGN may be sufficient to confer specificity.
PGRPs are conserved in mice and humans, and a combined genomic and experimental approach has led to the identification of 4 human PGRPs.17 Analysis of human PGRP mRNA expression patterns revealed predominant expression of PGRP-L in liver, PGRP-Iα and -Iβ in esophagus, and PGRP-S in bone marrow and neutrophils.17 Little is known about the function of human PGRPs, though their role in innate immunity is inferred from studies in insects and mice. The best-characterized mammalian PGRP to date is PGRP-S, originally identified and characterized as a novel cytokine named Tag7.18 Murine PGRP-S, which is present in neutrophil tertiary granules, inhibits the growth of certain Gram-positive bacteria in culture media and participates in the intracellular killing of bacteria in neutrophils.19 Mice deficient in PGRP-S showed increased susceptibility to infection by low pathogenicity Gram-positive bacteria such as Bacillus subtilis and Micrococcus luteus.20 However, the PGRP-S-deficient mice were as susceptible as wild-type mice when infected with pathogenic bacteria such as Staphylococcus aureus and Escherichia coli. These results led Dziarski et al20 to postulate a role for PGRP-S in preventing nonpathogenic bacteria from causing disease, as Alexander Fleming21 did for lysozyme some 80 years ago. There are, however, qualitative and quantitative differences between murine and human neutrophils.22 To date there are no definitive data on the binding specificity and functional activity of human PGRP-S. Encouraged by recent data that showed bovine PGRP-S has bacteriostatic or bactericidal activity against Gram-positive and Gram-negative bacteria,23 we set out to investigate the function of human PGRP-S. Furthermore, we postulated that human PGRP-S might act synergistically with lysozyme, thereby extending the host range to include pathogenic and nonpathogenic bacteria.
In this study we show that recombinant human PGRP-S (rhPGRP-S) binds to and inhibits the growth of Gram-positive S aureus (containing Lys-type PGN) and Gram-negative E coli (containing DAP-type PGN). We used synthetic PGN fragments and BIAcore analysis to define molecular requirements for binding to human PGRP-S. The binding affinity and thus antimicrobial activity of human PGRP-S are determined by the third amino acid in the stem peptide of bacterial PGN. We also demonstrate that PGRP-S has a synergistic antibacterial effect with lysozyme against E coli, suggesting that neutrophil granular contents may collaborate in host defense. In support of this idea, we show colocalization of PGRP-S with lysozyme in the neutrophil extracellular traps (NETs) formed on neutrophil activation. These results suggest that these granule-derived proteins contribute to the killing of bacteria trapped in the NETs.
Materials and methods
Protein expression and purification
cDNA encoding full-length human PGRP-S (excluding the N-terminal signal sequence) was amplified by PCR with the primers 5′-GAAGATCTCAGGAGACAGAAGACCCGG-3′ and 5′-CCGCTCGAGGGGGGAGCGGTAGTGTGGC-3′ from human neutrophil cDNA. The PCR fragment was digested with BglII and XhoI (restriction sites are underlined in the primers) and then ligated into expression vector pMT/BiP/V5-His A (Invitrogen, Carlsbad, CA). This vector contains a copper-inducible metallothionein promoter, a BiP secretion signal, and C-terminal peptide containing the V5 epitope and polyhistidine tag. The vector pCoBlast encoding a blasticidin resistance gene was used for selection. Transfection was performed using the calcium-phosphate method, and a stable clone was selected in Schneider Drosophila medium (Invitrogen) supplemented with 10% fetal bovine serum and blasticidin (25 μg/mL). Transformed cells were adapted to serum-free medium (Invitrogen Drosophila-SFM) and grown in suspension culture at 27°C. At a concentration of 3 × 106 cells/mL, the cells were induced with CuSO4 (500 μM) and grown for 5 days. Cell-free supernatant was assayed for protein by Western blot analysis. Proteins in the medium were precipitated with 70% saturated ammonium sulfate overnight at 4°C. The precipitate was spun down (13 000g, 20 minutes, 4°C), and the pellet was dissolved in 30 mL binding buffer (10 mM imidazole, 500 mM NaCl, 50 mM Tris, pH 7.9). The suspension was dialyzed against 3 L binding buffer with 2 changes of buffer over a 24-hour period. Proteins were applied to a nickel-charged ProBond column (Invitrogen) at 0.4 mL/min. The column was washed with 60 mM imidazole, 500 mM NaCl, 50 mM Tris, pH 7.9, and elution was performed with 250 mM imidazole, 500 mM NaCl, 50 mM Tris, pH 7.9. The eluted protein was repurified by size exclusion on a Superdex 75HR column (Pharmacia, Uppsala, Sweden) pre-equilibrated in 100 mM NaCl, 20 mM Tris-HCl, pH 7.9. The purified protein was essentially free from other proteins, which was confirmed with a Coomassie Brilliant Blue-stained 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel. This purified rhPGRP-S was verified by N-terminal amino acid sequence analysis. The yield of purified rhPGRP-S was approximately 400 μg/L. For bacterial binding and antibacterial activity assays, the C-terminal peptide containing the V5 epitope and polyhistidine tag (approximately 3 kDa) was cleaved from the expressed protein by incubation with trypsin (Sigma, Saint Louis, MO) at a molar ratio of 1:100 (trypsin/expressed protein) at 4°C for 4 hours. The cleavage of C-terminal peptide was confirmed by gel filtration and N-terminal amino acid sequencing. The extracellular PGRP domain of Drosophila PGRP-LCx was also expressed using the method described.
Bacterial binding assay
Biotinylation of proteins was performed with EZ-Link Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) according to the manufacturer's protocol. Bacterial binding was detected as described by Park et al24 with slight modifications. S aureus CP525 and E coli K12 and CP9.13726 were used for assays. Bacteria (2 × 106) were incubated with biotinylated proteins in 10 mM sodium phosphate buffer (NaPB), pH 7.4, at 37°C for 30 minutes. After incubation, cells were washed with 10 mM NaPB and immobilized on glass coverslips treated with 0.01% polylysine, and biotin-labeled proteins were visualized using 1 μg/mL streptavidin-Alexa Fluor 488 (Molecular Probes, Eugene, OR). Coverslips mounted in Immu-mount (Thermo Shandon, Pittsburgh, PA) were observed by fluorescence microscopy using an inverted microscope (TE2000-U; Nikon, Tokyo, Japan) equipped with a Plan Apo 100×/1.40 objective lens (Nikon) and a Hamamatsu C4742 camera (Hamamatsu, Hamamatsu City, Japan).
PGN binding assay
Lys-type PGN from S aureus and DAP-type PGN from E coli were obtained from InvivoGen (San Diego, CA). The PGN binding assay was performed according to the procedure of Takehana et al.27 Purified rhPGRP-S or rdPGRP-LC (0.5 μg) were incubated with 0.32 mg insoluble Lys-type PGN or DAP-type PGN. Unbound protein isolated from the soluble fraction and bound protein recovered after washing the PGN with Tris-maleate buffer containing 1 M NaCl and 1 M NaCl plus 0.2% Tween 20 were examined by Western blot analysis using anti-His(C-term) antibody (Invitrogen).
BIAcore analysis
MurNAc-l-Ala-d-isoGln and GlcNAc-MurNAc-l-Ala-d-isoGln were obtained from Sigma. Other PGN fragments used in the study (Table 1) were synthesized based on the methods previously described.28 For calculation of association constants (KD) for rhPGRP-S, biotinylated rhPGRP-S was immobilized onto a streptavidin-coated biosensor chip SA (SA chip; BIAcore AB, Neuchâtel, Switzerland), and each PGN fragment in 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, containing 150 mM NaCl at a concentration of 500 nM to 80 μM was passed over the surface of the sensor chip at a flow rate of 25 μL/min. The interaction was monitored as the change of surface plasmon resonance (SPR) at 25°C with BIAcore 3000 (BIAcore AB). After 3 minutes of monitoring, the same buffer was introduced onto the sensor chip in place of the PGN fragment solution to initiate dissociation. At the end of each cycle, regeneration of the chip was accomplished by washing away the surface-bound PGN fragment with 12.5 μL of 1 M NaCl. Both the association rate constant (Ka) and the dissociation rate constant (Kd) were obtained from the SPR signal-binding data and calculated using the BIA-Evaluation software (version 3.2; BIAcore AB). The association constant (KD) was subsequently determined by Kd/Ka.
Binding specificity for the interaction between human PGRP-S and synthetic PGN fragments
. | Association constant Kd (M) . | . | |
|---|---|---|---|
| PGN fragment . | rhPGRP-S . | rdPGRP-LC . | |
| (GlcNAc-MurNAc-l-Ala-d-iso Gln-l-Lys-d-Ala)2 | 3.69 × 10−6 ± 2.65 × 10−6 | NO | |
| (GlcNAc-MurNAc-l-Ala-d-isoGln-l-Lys)2 | 5.50 × 10−8 ± 3.13 × 10−8 | NO | |
| (GlcNAc-MurNAc-l-Ala-d-isoGln)2 | NO | NO | |
| GlcNAc-MurNAc-l-Ala-d-isoGln | NO | NO | |
| MurNAc-l-Ala-d-isoGln-l-Lys | NO | NO | |
| MurNAc-l-Ala-d-isoGln | NO | NO | |
. | Association constant Kd (M) . | . | |
|---|---|---|---|
| PGN fragment . | rhPGRP-S . | rdPGRP-LC . | |
| (GlcNAc-MurNAc-l-Ala-d-iso Gln-l-Lys-d-Ala)2 | 3.69 × 10−6 ± 2.65 × 10−6 | NO | |
| (GlcNAc-MurNAc-l-Ala-d-isoGln-l-Lys)2 | 5.50 × 10−8 ± 3.13 × 10−8 | NO | |
| (GlcNAc-MurNAc-l-Ala-d-isoGln)2 | NO | NO | |
| GlcNAc-MurNAc-l-Ala-d-isoGln | NO | NO | |
| MurNAc-l-Ala-d-isoGln-l-Lys | NO | NO | |
| MurNAc-l-Ala-d-isoGln | NO | NO | |
Each value was determined as described in “Materials and methods” and is expressed as the mean ± SD of 3 independent experiments.
NO indicates Kd value not obtained because of weak binding of the tested PGN fragment at concentrations up to 80 μM.
Antibacterial activity assay
Inhibition of bacterial growth was assessed using a radial diffusion method, as described by Lehrer et al.29 Briefly, the underlay consisted of 1% agarose and 1/100 dilution trypticase soy broth (TSB) in 10 mM NaPB, pH 7.4, and the overlay consisted of 2 × TSB (6% wt/vol) and 1% agarose. In some experiments, the underlay gels were supplemented with up to 150 mM NaCl. Bacteria (2 × 106) were mixed with 10 mL underlay gel solutions kept molten at 48°C and poured into 100-cm2 Petri dishes. A series of 3-mm—diameter wells were punched after the agarose solidified, and 5 μL sample was added to designated wells. Plates were incubated at 37°C for 3 hours to allow for peptide diffusion. The microbe-laden underlay was then covered with 10 mL molten overlay, and the plates were allowed to harden. Antibacterial activity was identified as a clear zone around the well absent of microbial growth after 18-hour incubation at 37°C.
The importance of PGRP-S/PGN interactions in the antimicrobial activity of rhPGRP-S was assessed by suspension assays. Bacteria were grown to mid-log phase in 1 × TSB (3% wt/vol; 1 × TSB contains 0.5% NaCl [approximately 86 mM]) at 37°C. Ten-microliter aliquots of cells were then added to 2 mL TSB. Purified rhPGRP-S was added to a final concentration of 25 μg/mL, either alone or supplemented with 100 μg/mL S aureus PGN or E coli PGN (InvivoGen). The tubes were shaken at 300 rpm for 5 hours, and bacterial density was monitored by measurement of optic density (OD) at 600 nm at 1-hour intervals. To minimize the effect of bacterial aggregation on OD, the cell suspensions were stirred for 1 minute before each measurement.
Lysis of lysozyme-treated E coli cells
The assay was performed by measuring the decrease in turbidity of E coli cells. Just before the assay, freshly grown E coli cells were suspended in 10 mM NaPB, pH 7.4, and were treated with 50 μg/mL human neutrophil lysozyme (Calbiochem, Darmstadt, Germany) for 5 minutes at room temperature. After the addition of rhPGRP-S or Polymyxin B nonapeptide (PMBN; Sigma), the change in absorbance at 600 nm was measured for 10 minutes.
Immunofluorescence microscopic analysis
Immunofluorescence assays were performed as described by Brinkmann et al30 with slight modifications. Human neutrophils were isolated from peripheral blood of healthy donors under a protocol approved by the Institutional Review Board of Massachusetts General Hospital. Informed consent was provided according to the Declaration of Helsinki. Neutrophils (1 × 105) were seeded on glass coverslips treated with 0.01% polylysine, allowed to settle, and treated with 100 nM phorbol 12-myristate 13-acetate (PMA) or left unstimulated. Cells were fixed with 4% paraformaldehyde (PFA), blocked overnight (5% normal donkey serum, 1% saponin in PBS), and incubated with primary antibodies (anti-PGRP-S from Immunogenex, La Jolla, CA; anti-lysozyme from Calbiochem) detected with secondary antibodies coupled to Cy2 or Cy5 (Jackson ImmunoResearch Laboratories, West Grove, PA). Controls were made with isotype-matched antibodies. For DNA detection, Hoechst 33342 (Sigma) was used. Fluorescence pictures were taken on an inverted Nikon TE2000-U microscope using a Plan Apo 60 ×/1.40 objective lens and a Hamamatsu C4742 camera. Images were processed with Openlab 4 software (Improvision, Lexington, MA).
Results
Human PGRP-S binds to Gram-positive S aureus and Gram-negative E coli and their PGNs
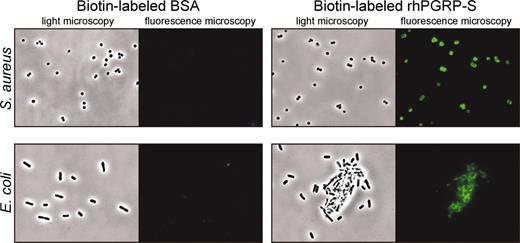
rhPGRP-S was overexpressed in Schneider 2 cells (Figure 1). To facilitate purification, a C-terminal His6 tag was added. Sequential nickel affinity and gel-filtration purification yielded 400 μg pure protein from 1 L culture medium. For bacterial binding and antibacterial activity assays, the C-terminal tag was cleaved from the expressed protein by trypsin, which resulted in 20-kDa protein (data not shown). To determine bacterial binding of rhPGRP-S, biotin-labeled rhPGRP-S was incubated with bacteria and visualized with streptavidin-Alexa Fluoro 488. rhPGRP-S bound to both S aureus and E coli, and binding of biotin-labeled rhPGRP-S was competed with a 10-fold excess of unlabeled rhPGRP-S (Figure 2; also see Supplemental Figure S1, available at the Blood website [click on the Supplemental Figures link at the top of the online article]). In contrast, no bacterial binding was observed by bovine serum albumin (BSA) controls. rhPGRP-S did not bind to Saccharomyces cerevisiae or Candida albicans (data not shown).
Expression and purification of recombinant human PGRP-S. (A) Cell culture supernatant and cell lysates were tested for expression by Western blot with anti-His(C-term) antibody. (B) Coomassie Blue-stained 12% SDS-PAGE gel showing affinity-purified rhPGRP-S. Molecular markers are shown on the left. (C) Gel filtration chromatogram showing elution of rhPGRP-S from a Superdex 75HR column at a flow rate of 0.5 mL/min. Based on elution volumes of molecular weight standards, rhPGRP-S elutes as a monomer of approximately 23 kDa. (D) fast protein liquid chromatography (FPLC) fractions containing rhPGRP-S were subjected to 12% SDS-PAGE.
Expression and purification of recombinant human PGRP-S. (A) Cell culture supernatant and cell lysates were tested for expression by Western blot with anti-His(C-term) antibody. (B) Coomassie Blue-stained 12% SDS-PAGE gel showing affinity-purified rhPGRP-S. Molecular markers are shown on the left. (C) Gel filtration chromatogram showing elution of rhPGRP-S from a Superdex 75HR column at a flow rate of 0.5 mL/min. Based on elution volumes of molecular weight standards, rhPGRP-S elutes as a monomer of approximately 23 kDa. (D) fast protein liquid chromatography (FPLC) fractions containing rhPGRP-S were subjected to 12% SDS-PAGE.
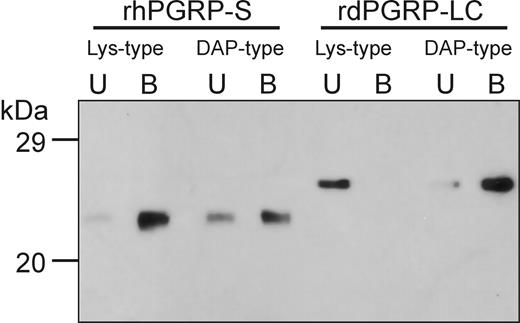
Insect PGRPs are known to bind selectively to DAP-type PGN or Lys-type PGN,16 but rhPGRP-S bound both Lys-type PGN-containing S aureus and DAP-type PGN-containing E coli. We, therefore, analyzed the PGN binding of rhPGRP-S (Figure 3), which was incubated with insoluble Lys-type PGN from S aureus and DAP-type PGN from E coli. After extensive washing, rh-PGRP-S was retained by both Lys-type PGN from S aureus and DAP-type PGN from E coli. In contrast, the extracellular PGRP domain of Drosophila PGRP-LCx (rdPGRP-LC) was retained only by DAP-type PGN from E coli. These results indicate that rhPGRP-S acts as a pattern-recognition molecule for both Lys-type and DAP-type PGNs. Although the sensitivity of this assay is insufficient to quantitate the differential binding of rhPGRP-S to Lys-type and DAP-type PGNs, we noticed that rhPGRP-S binds more avidly to Lys-type PGN.
Human PGRP-S binds to S aureus CP5 and E coli K12. Bacteria (2 × 106) were incubated with biotin-labeled rhPGRP-S (10 μg/mL) and BSA (40 μg/mL), respectively, at 37°C for 30 minutes and visualized with streptavidin-Alexa Fluor 488.
Human PGRP-S binds to S aureus CP5 and E coli K12. Bacteria (2 × 106) were incubated with biotin-labeled rhPGRP-S (10 μg/mL) and BSA (40 μg/mL), respectively, at 37°C for 30 minutes and visualized with streptavidin-Alexa Fluor 488.
Binding affinity of human PGRP-S is determined by the third amino acid in the stem peptide of bacterial PGN
We next examined interactions between defined synthetic PGN fragments and rhPGRP-S using SPR. Biotinylated rhPGRP-S was fixed onto the surface of a streptavidin-immobilized biosensor chip, and each synthetic PGN fragment with peptide or glycan chains of various sizes were passed over the biosensor chip (Table 1). Lys-type PGN fragment (GlcNAc-MurNAc-l-Ala-d-isoGln-l-Lys)2 and (GlcNAc-MurNAc-l-Ala-d-isoGln-l-Lys-d-Ala)2 bound to rhPGRP-S in a time- and dose-dependent fashion. However, no specific binding could be detected with (GlcNAc-MurNAc-l-Ala-d-isoGln)2 or MurNAc-l-Ala-d-isoGln-l-Lys at concentrations up to 80 μM. These results demonstrate that a minimum of 3 amino acids in the stem peptide and both GlcNAc and MurNAc in the glycan backbone are required for optimal binding to rhPGRP-S. We found that the association constant (KD) of (GlcNAc-MurNAc-l-Ala-d-isoGln-l-Lys)2 was 70 times higher than that of (GlcNAc-MurNAc-l-Ala-d-isoGln-l-Lys-d-Ala)2. These results suggest that the third amino acid in the stem peptide of bacterial PGN determines the binding affinity to rhPGRP-S and that the fourth d-Ala residue may act to hinder optimal interactions between rhPGRP-S and the l-Lys residue at position 3 in the peptide chain.
Human PGRP-S binds to Lys-type and DAP-type PGNs. Purified rhPGRP-S or rdPGRP-LC (0.5 μg) were incubated with insoluble Lys-type or DAP-type PGN, and bound protein on the insoluble PGN was separated from unbound protein, as described in “Materials and methods.” One tenth of unbound protein (lane U) and one fifth of bound protein (lane B) were analyzed by Western blot analysis using anti-His(C-term) antibody. Molecular markers are indicated on the left.
Human PGRP-S binds to Lys-type and DAP-type PGNs. Purified rhPGRP-S or rdPGRP-LC (0.5 μg) were incubated with insoluble Lys-type or DAP-type PGN, and bound protein on the insoluble PGN was separated from unbound protein, as described in “Materials and methods.” One tenth of unbound protein (lane U) and one fifth of bound protein (lane B) were analyzed by Western blot analysis using anti-His(C-term) antibody. Molecular markers are indicated on the left.
Human PGRP-S inhibits the growth of S aureus and E coli
We next tested whether human PGRP-S has antimicrobial activity against human pathogenic bacteria. Of note, we found that rhPGRP-S causes the aggregation of E coli (Figure 2), so we did not use dilution assays to assess killing because these might lead to spurious results. Instead the ability of rhPGRP-S to inhibit the growth of bacteria was determined in radial diffusion assays to exclude the potential effects of aggregation in antimicrobial activity (Figure 4A). Wells were bored into agar impregnated with bacteria. Proteins were introduced into wells (0.1-5 μg/well), and the plates were incubated overnight at 37°C. rhPGRP-S inhibited the growth of S aureus and E coli but was more active against S aureus. The salt sensitivity of rhPGRP-S was tested by including NaCl (up to 150 mM) in underlay gels. The antimicrobial activity of rhPGRP-S diminished gradually (as evidenced by decreasing zones of inhibition) as the concentration of NaCl increased and was ablated when 150 mM NaCl was present. A zone of inhibition was still apparent, however, at a NaCl concentration of 100 mM (data not shown). We then examined rhPGRP-S/PGN interactions in the antimicrobial activity of rhPGRP-S (Figure 4B). The inclusion of excess S aureus PGN (100 μg/mL) completely blocked the rhPGRP-S (25 μg/mL)-mediated growth inhibition of S aureus, whereas rhPGRP-S (25 μg/mL)-mediated growth inhibition of E coli was partially reversed with excess E coli PGN (100 μg/mL). The effect of rhPGRP-S was similar with both E coli K12 (rough strain; Figure 4) and CP9.137 (smooth strain; Supplemental Figure S2). Neither S aureus PGN nor E coli PGN affected growth in the absence of rhPGRP-S. These results indicate that rhPGRP-S inhibits the growth of bacteria by a mechanism that includes PGN-dependent binding.
Human PGRP-S has a synergistic antimicrobial effect with lysozyme against E coli and colocalizes with lysozyme in neutrophil extracellular traps
Various antimicrobial substances and hydrolytic enzymes are distributed within the cytoplasmic granules of neutrophils, and efficient killing of bacteria usually requires concerted action of these molecules, which often act synergistically.31 It has been postulated that murine PGRP-S20 and human lysozyme,21 2 proteins that target PGN, act predominantly to limit the disease-causing ability of nonpathogenic bacteria. Whereas lysozyme has been shown to have bactericidal activity against some Gram-negative bacteria, its predominant role is against Gram-positive bacteria with more exposed PGNs.32 We tested whether rhPGRP-S has a synergistic antibacterial effect with lysozyme against Gram-negative bacteria. Lysozyme (50 μg/mL) alone was not bactericidal against the strain of E coli we tested, but lysozyme-treated E coli cells were rapidly lysed by subinhibitory concentrations of rh-PGRP-S (Figure 5A), and the growth of E coli was completely inhibited by the combined treatment of lysozyme and rhPGRP-S (Figure 5B). These results suggest that neutrophil granular contents may collaborate in host defense functions against human pathogenic bacteria.
Human PGRP-S inhibits the growth of S aureus CP5 and E coli K12. (A) Radial diffusion assay. Wells were bored into underlay gel (1% agarose in 0.01 × TSB, 10 mM NaPB, pH 7.4) impregnated with bacteria. Proteins were introduced into wells (0.1-5 μg/well), and the plates were overlaid with 1% agarose in 2 × TSB (6% wt/vol) and incubated overnight at 37°C. (B) Suspension assay. Bacteria were incubated in 1 × TSB (3% wt/vol) with 25 μg/mL rhPGRP-S, either alone (•) or supplemented with 100 μg/mL S aureus PGN or E coli PGN (▴), PGN only (▦) or no additives (⋄). Tubes were shaken at 300 rpm for 5 hours, and bacterial density was monitored by measurement of optic density (OD) at 600 nm at 1-hour intervals. Data represent the mean ± SD of 3 independent experiments.
Human PGRP-S inhibits the growth of S aureus CP5 and E coli K12. (A) Radial diffusion assay. Wells were bored into underlay gel (1% agarose in 0.01 × TSB, 10 mM NaPB, pH 7.4) impregnated with bacteria. Proteins were introduced into wells (0.1-5 μg/well), and the plates were overlaid with 1% agarose in 2 × TSB (6% wt/vol) and incubated overnight at 37°C. (B) Suspension assay. Bacteria were incubated in 1 × TSB (3% wt/vol) with 25 μg/mL rhPGRP-S, either alone (•) or supplemented with 100 μg/mL S aureus PGN or E coli PGN (▴), PGN only (▦) or no additives (⋄). Tubes were shaken at 300 rpm for 5 hours, and bacterial density was monitored by measurement of optic density (OD) at 600 nm at 1-hour intervals. Data represent the mean ± SD of 3 independent experiments.
Human PGRP-S shows synergistic antibacterial effect with lysozyme against E coli K12. (A) Lysis of lysozyme-treated E coli cells. Lysozyme-treated E coli cells (⋄) were incubated in 10 mM NaPB, pH 7.4, with rhPGRP-S (4 μg/mL, [▴]; 8 μg/mL, [•]) or PMBN (2.5 μg/mL, [▦]), and the change in OD at 600 nm was monitored for 10 minutes. Data represent the mean ± SD of 3 independent experiments. (B) Growth inhibition of E coli cells. E coli cells were incubated in 1 × TSB (3% wt/vol) with 50 μg/mL lysozyme (▦), 25 μg/mL rhPGRP-S (▴), 8 μg/mL rhPGRP-S and 50 μg/mL of lysozyme (•), or no protein (⋄). Tubes were shaken at 300 rpm for 5 hours, and bacterial density was monitored by measurement of optic density (OD) at 600 nm at 1-hour intervals. Data represent the mean of duplicate samples from 1 of 2 similar experiments.
Human PGRP-S shows synergistic antibacterial effect with lysozyme against E coli K12. (A) Lysis of lysozyme-treated E coli cells. Lysozyme-treated E coli cells (⋄) were incubated in 10 mM NaPB, pH 7.4, with rhPGRP-S (4 μg/mL, [▴]; 8 μg/mL, [•]) or PMBN (2.5 μg/mL, [▦]), and the change in OD at 600 nm was monitored for 10 minutes. Data represent the mean ± SD of 3 independent experiments. (B) Growth inhibition of E coli cells. E coli cells were incubated in 1 × TSB (3% wt/vol) with 50 μg/mL lysozyme (▦), 25 μg/mL rhPGRP-S (▴), 8 μg/mL rhPGRP-S and 50 μg/mL of lysozyme (•), or no protein (⋄). Tubes were shaken at 300 rpm for 5 hours, and bacterial density was monitored by measurement of optic density (OD) at 600 nm at 1-hour intervals. Data represent the mean of duplicate samples from 1 of 2 similar experiments.
Colocalization of PGRP-S with lysozyme in the NETs. Neutrophils were activated with 100 nM PMA for 30 minutes and stained for DNA (A), PGRP-S (B), and lysozyme (C). For DNA detection, Hoechst 33342 (blue) was used. PGRP-S was detected with anti-human PGRP-S monoclonal antibody (mouse immunoglobulin G [IgG]) and Cy2-conjugated donkey anti-mouse IgG (green). Lysozyme was detected with anti-human lysozyme polyclonal antibody (sheep IgG) and Cy5-conjugated donkey anti-sheep IgG (red). The merged image (D) clearly shows colocalization of PGRP-S with lysozyme in the NETs.
Colocalization of PGRP-S with lysozyme in the NETs. Neutrophils were activated with 100 nM PMA for 30 minutes and stained for DNA (A), PGRP-S (B), and lysozyme (C). For DNA detection, Hoechst 33342 (blue) was used. PGRP-S was detected with anti-human PGRP-S monoclonal antibody (mouse immunoglobulin G [IgG]) and Cy2-conjugated donkey anti-mouse IgG (green). Lysozyme was detected with anti-human lysozyme polyclonal antibody (sheep IgG) and Cy5-conjugated donkey anti-sheep IgG (red). The merged image (D) clearly shows colocalization of PGRP-S with lysozyme in the NETs.
Brinkmann et al30 recently reported that neutrophils release granule proteins and chromatin on activation that together form extracellular fibers and that these neutrophil extracellular traps (NETs) bind Gram-positive and Gram-negative bacteria and display antibacterial activity. We postulated that human PGRP-S and lysozyme may localize in these NETs and tested this by immunofluorescence microscopic analysis. Triple immunostaining of neutrophils activated with PMA (100 nM) confirmed the presence of extracellular fibrous material. As expected, we found nuclear staining of DNA (Figure 6A) and granular staining for human PGRP-S and lysozyme (Figure 6B-C). The merged image (Figure 6D) clearly shows colocalization of human PGRP-S with lysozyme in the NETs, suggesting that granule-derived proteins such as human PGRP-S and lysozyme contribute to the killing of bacteria trapped in the NETs.
Discussion
Although PGRP proteins are highly conserved across species, individual species differ in their number of PGRP genes and in their ligand-binding specificities. Four human PGRP genes (PGRP-S, PGRP-L, PGRP-Iα and PGRP-Iβ) have been identified,17 but the activity of their gene products remains relatively undefined. In insects, PGRP-S is a soluble pattern-recognition molecule whose ligation leads to prophenoloxidase-mediated melanization,4 antimicrobial peptide induction,12 or amidase-mediated cleavage of PGN.8 In mammals (murine19 and bovine23 ), PGRP-S has been described as an antibacterial protein active within neutrophil granules and lacking in amidase activity. We chose to focus on human PGRP-S and its interaction with the pathogenic bacteria S aureus and E coli. We felt it important to study human PGRP-S because neutrophils are a more dominant mechanism in human host defense than they are in murine host defense.22,33 We reasoned that the activities of human PGRP-S may be different from those reported in mice. The 2 bacteria were chosen as prototypical Gram-positive (containing Lys-type PGN) and Gram-negative (containing DAP-type PGN) organisms because of their clinical significance in human infections.
Our results indicate that human PGRP-S binds to both Gram-positive S aureus and Gram-negative E coli. Interestingly, human PGRP-S bound more avidly to S aureus than E coli but was able to agglutinate intact E coli (but not S aureus). The mechanism of binding to E coli (in which the PGN layer is situated beneath the outer membrane leaflet) and the mechanism of agglutination are of great interest. Ongoing studies are aimed at investigating that phenomenon and exploring the range of Gram-negative bacteria that might be recognized by human PGRP-S.
We were able to confirm the recognition by human PGRP-S of both Lys-type (as found in S aureus) and DAP-type PGN (as found in E coli). We show that human PGRP-S is indeed able to ligate both types of PGN, binding more avidly in this assay to the Lys-type PGN. The specificity of this assay was confirmed by replicating the known inability of Drosophila PGRP-LC to bind to Lys-type PGN while binding avidly to DAP-type PGN.34 Human PGRP-S thus has affinity for both main types of PGN. In this respect, human PGRP-S has broader ligand specificity than reported for other mammalian PGRP-S molecules, though the assays used to determine binding specificity are not all the same. We did not assay other mammalian PGRP-S molecules.
We analyzed the molecular requirements for Lys-type PGN binding by using synthetic PGN fragments and SPR. These assays indicate a requirement for backbone glycans and peptide stems in high-affinity binding by human PGRP-S. Specificity was confirmed in this assay by the inability of Drosophila PGRP-LC to bind to any of the PGN fragments tested. The presence of GlcNAc and MurNAc in the glycan backbone was needed to attain high-affinity binding. Although muramyl tripeptide (up to 80 μM) did not bind to human PGRP-S, the same tripeptide coupled to GlcNAc-MurNAc gave rise to high-affinity binding. Guan et al35 recently reported the crystal structure of the C-terminal PGN-binding domain of human PGRP-Iα (PGRP-IαC) in complex with a muramyl tripeptide. In contrast to our finding, they observed the binding of muramyl tripeptide to PGRP-IαC, albeit at a high concentration (100-800 μM). They also suggested, however, that both GlcNAc and MurNAc in the glycan backbone are needed to attain high-affinity binding. Similarly, and in accordance with binding data reported by others,35,36 we note that at least 3 amino acids in the stem peptide are required before high-affinity binding of PGRP-S is attained. Interestingly, and in contrast to the speculation of Guan et al,35 ligand-binding affinity was decreased more than 70-fold by the addition of a fourth amino acid to the peptide stem. We speculate that this terminal/fourth d-Ala residue may act to hinder optimal interactions between PGRP-S and the l-Lys residue at position 3 in the peptide stem. Unfortunately, the analogous synthetic PGN fragments from Gram-negative bacteria (containing DAP at the third position in the peptide stem) are not yet available for us to test by SPR. It is worth noting that whereas the PGRP residues that interact with PGN are highly conserved across species, binding specificity for different PGN types must be conferred indirectly by nonconserved residues or perhaps by different tertiary or quaternary PGRP structures.
We were most interested in the antibacterial effects of human PGRP-S. In a standard in vitro radial diffusion assay, rhPGRP-S produced dose-dependent zones of inhibition around S aureus and E coli. When the bacterial inhibition studies were performed in liquid culture, rhPGRP-S, at a dose of 25 μg/mL, was able to inhibit the growth of both these bacterial types, inhibiting the growth of S aureus more completely than that of E coli. When cognate PGN was added to the cultures at 100 μg/mL, Lys-type PGN completely reversed the inhibitory effects of rhPGRP-S on S aureus growth, whereas DAP-type PGN had a partial (approximately 50%) effect on the inhibitory effect of rhPGRP-S on E coli growth. These data indicate that rhPGRP-S inhibits the growth of bacteria by a mechanism that includes PGN-dependent binding, and the binding affinity and thus antimicrobial activity of rh-PGRP-S is determined by the third amino acid in the stem peptide of bacterial PGN. However, we could not exclude the possibility that rhPGRP-S may achieve its antibacterial effect on E coli by interacting with residues other than PGN. Indeed, recent studies indicate that PGNs may not be the sole microbial surface determinants recognized by PGRPs. For example, the unexpected finding that bovine PGRP-S is microbicidal for some organisms that lack PGN (Cryptococcus neoformans), or in which PGN is obscured by LPS (Salmonella typhimurium), suggests that envelope components other than PGN may interact, directly or indirectly, with certain PGRPs.23 Similarly, recombinant PGRP-S proteins from the large beetle Holotrichia diomphalia were found to specifically bind both 1,3-β-d-glucan and PGN.37 Direct binding studies are required to test this hypothesis.
The antibacterial effects we observed, coupled with the location of PGRP-S within neutrophil granules, led us to speculate that PGRP-S may collaborate with other phagocyte-derived antimicrobial effectors. In particular, we were interested in determining whether PGRP-S would act synergistically with other host defense molecules that target PGN. Lysozyme is a well-characterized enzyme with N-acetylmuramoylhydrolase activity that can hydrolyze PGN and contribute to host defense by bacterial lysis. The enzyme is present in all granule subsets of neutrophils, in macrophages, and in numerous mucosal secretions. Gram-negative bacteria are normally insensitive to the lytic effects of lysozyme because the outer membrane hinders access to the peptidoglycan substrate. Lysozyme does, however, bind through hydrophobic interactions to LPS in the Gram-negative outer membrane.38 This interaction reportedly diminishes the enzymatic activity of lysozyme, decreases the biologic activity of LPS, and perturbs the outer membrane structure, but it does not decrease Gram-negative bacterial viability. Substances that permeabilize the Gram-negative outer membrane (eg, EDTA [ethylenediaminetetraacetic acid], PMBN, poly-L-lysine, defensins, bactericidal/permeability-increasing protein [BPI], and lactoferrin) enhance the susceptibility of Gram-negative organisms to lysozyme.39 Furthermore, the addition of a hydrophobic pentapeptide to the C-terminus of lysozyme has been reported to confer antibacterial activity against E coli on otherwise inactive lysozyme.40 In assays of bacterial lysis and bacterial growth in vitro, we confirmed that lysozyme alone has no effect on E coli. When lysozyme was combined with rhPGRP-S, however, marked bacterial lysis and growth inhibition were apparent. Our experiments do not provide a mechanism for this synergistic interaction, but we speculate that rhPGRP-S may permeabilize the outer membrane of E coli, thereby providing lysozyme with access to the previously hidden PGN. The ability of rhPGRP-S to bind to the surface of intact E coli bacteria (Figure 2) supports this hypothesis, but additional studies are needed to elucidate the exact mechanism.
The synergy we observed between lysozyme and rhPGRP-S led us to hypothesize that these antibacterial proteins might colocalize spatially in neutrophils whose antimicrobial capabilities had been activated. Recent studies have described the existence of NETs—physical extracellular structures composed of DNA and histones that act as localized concentrators of microbes and neutrophil-derived antimicrobial products.30 These NETs are reportedly required for optimal microbial killing, but the exact biochemical composition of the NET microenvironment remains undefined. Using cytochemical staining for DNA and immunostaining for PGRP-S and lysozyme, we showed that both of these antimicrobial proteins are localized in the NETs associated with phorbol ester-activated neutrophils (Figure 6). Interestingly, the NETs appeared to harbor immunoreactive intact granules and nongranule-associated proteins. These data provide evidence that human PGRP-S and lysozyme, which exhibits synergistic antibacterial activity against E coli in vitro, are spatially colocalized where antibacterial activity is anticipated. We speculate that different combinations of neutrophil-derived antimicrobial effectors might be optimally effective against different microorganisms and that the NETs provide a physical structure for the optimal display of these effectors to microorganisms that have become entrapped. Like many other antimicrobial peptides, the antibacterial activity of PGRP-S shows sensitivity to elevated salt concentrations.41,42 Although we do not know the salt concentration of the NET microenvironment, our data indicate that human PGRP-S retains at least some antimicrobial activity at NaCl concentrations as high as 86 to 100 mM. Future studies will be required to elucidate the exact biochemical composition of the NET microenvironment and its effect on the various antimicrobial activities of neutrophils. Taken together, our results indicate human PGRP-S functions in the innate immunity of neutrophils by contributing to the killing of intracellular and extracellular bacteria.
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/blood-2005-02-0530.
Supported by National Institutes of Health program grant PO1 AI 44220.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank L. Shi for assistance with BIAcore analysis, C. Kocks for assistance with microscopy, V. Brinkmann for advice with NET preparation, and members of the laboratory of Developmental Immunology at Massachusetts General Hospital for helpful discussion and critical reading of this manuscript.



![Figure 5. Human PGRP-S shows synergistic antibacterial effect with lysozyme against E coli K12. (A) Lysis of lysozyme-treated E coli cells. Lysozyme-treated E coli cells (⋄) were incubated in 10 mM NaPB, pH 7.4, with rhPGRP-S (4 μg/mL, [▴]; 8 μg/mL, [•]) or PMBN (2.5 μg/mL, [▦]), and the change in OD at 600 nm was monitored for 10 minutes. Data represent the mean ± SD of 3 independent experiments. (B) Growth inhibition of E coli cells. E coli cells were incubated in 1 × TSB (3% wt/vol) with 50 μg/mL lysozyme (▦), 25 μg/mL rhPGRP-S (▴), 8 μg/mL rhPGRP-S and 50 μg/mL of lysozyme (•), or no protein (⋄). Tubes were shaken at 300 rpm for 5 hours, and bacterial density was monitored by measurement of optic density (OD) at 600 nm at 1-hour intervals. Data represent the mean of duplicate samples from 1 of 2 similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-02-0530/6/m_zh80190584830005.jpeg?Expires=1763514859&Signature=FzpciiIbNwFt0grKKsZ4s5s2syTw~IFIPDEi3g-mqXhhwyxa2vAr8jYCYkxe~i2jAyUqO-92Z5rIiheA66gUffAKihWDp0Ycrlz3pQ2ILnrcHaTj2jQ1CKLcwAjIN-7KlfgbHF1afkUGsjaIl9NhuHgQOHKccVOZdQceGeir9qGEEuCQkwCttepDnQmEYTS8s7CIPPWmhXkASFFT7INJepMqSRSuamRlG~ggDGQdBiyxFMA8RK4p0esY8zJfq6yb220WnVNJgHzFyuvwjy6FB-QRuaJSwgX9QjGMyI8uZg-OPogO7xAPi0OhJOl91jGrYbWubuESM~2ooU55UEC1Mg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Colocalization of PGRP-S with lysozyme in the NETs. Neutrophils were activated with 100 nM PMA for 30 minutes and stained for DNA (A), PGRP-S (B), and lysozyme (C). For DNA detection, Hoechst 33342 (blue) was used. PGRP-S was detected with anti-human PGRP-S monoclonal antibody (mouse immunoglobulin G [IgG]) and Cy2-conjugated donkey anti-mouse IgG (green). Lysozyme was detected with anti-human lysozyme polyclonal antibody (sheep IgG) and Cy5-conjugated donkey anti-sheep IgG (red). The merged image (D) clearly shows colocalization of PGRP-S with lysozyme in the NETs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-02-0530/6/m_zh80190584830006.jpeg?Expires=1763514859&Signature=wEAzUMICQ7wmq6DjyVvo64w4oVA9bmx7M8nbOJtNyGLXbV9Td~Cdj-kleNxmEy-~2WmPk1x2wSPTDx9DUqVBPj0ZelEt0qlUxrDrTeRYPQjOx3SpJNWcVxLXkEIPTnpyYCnyvwHeiYd7Ng64jgGrxiS51-2IRhn5k02Bc2gZx14a8gH~xbdI4qY5kXu1oNSquI288Zy9bEt1bWdJGkK9HAXfXcJjSUB70SdYWuN3J5wYKRZbNcJTHXP7Tjn~ITljDIQDGWCPnJXacpl6~TRd2p6tWHMYRUO~DW5SewnKtWWlV6WzQpaL9KaCH7mhu~b0tUmRAxCGFYuO79E-Vy6xXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal