Abstract
Human Fcα receptor (FcαR; CD89), the receptor for the crystallizable fragment (Fc) of immunoglobulin A (IgA), is expressed exclusively in myeloid cells, including granulocytes and monocytes/macrophages, and is considered to define a crucial role of these cells in immune and inflammatory responses. A 259-base pair fragment of the FCAR promoter is sufficient to direct myeloid expression of a reporter gene and contains functionally important binding sites for CCAAT/enhancer-binding protein α (C/EBPα) (CE1, CE2, and CE3) and an unidentified Ets-like nuclear protein. Here, we show that the Ets-binding site is bound by a heterodimer composed of GA-binding protein α (GABPα), an Ets-related factor, and GABPβ, a Notch-related protein. Cotransfection of GABP increased FCAR promoter activity 3.7-fold through the Ets-binding site. GABP and C/EBPα synergistically activated the FCAR promoter 280-fold. Consistent with these observations, in vitro binding analyses revealed a physical interaction between the GABPα subunit and C/EBPα. This is the first report demonstrating both physical and functional interactions between GABP and C/EBPα and will provide new insights into the molecular basis of myeloid gene expression. (Blood. 2005;106:2534-2542)
Introduction
Bone marrow stem cells can differentiate into various hematopoietic lineages. Myeloid cells, including granulocytes and monocytes, are known as phagocytes and antigen-presenting cells that play crucial roles in infectious and inflammatory responses. During the differentiation from bone marrow precursors, myeloid cells express characteristic genes that are required for their roles as immune effector cells. Expression of these genes is regulated by combinatorial actions of lineage-restricted and widely expressed transcription factors (reviewed by Tenen et al1 ), which directly or indirectly involve protein-protein interactions.
CCAAT/enhancer-binding protein α (C/EBPα) was initially shown to regulate the terminal differentiation of adipocytes and hepatocytes.2-6 However, in the hematopoietic system, C/EBPα is expressed exclusively in myeloid lineage,7,8 and it has recently been recognized as a key regulator of granulopoiesis. Cebpαa knockout mice lack the entire granulocytic lineage, but they develop the other blood cell lineages, including monocytes.9 Enforced expression of C/EBPα in immature myeloid cell lines induces granulocytic differentiation8,10 and blocks monocytic differentiation.8 These lineage commitment decisions have been proposed to involve the functional inactivation of the myeloid master regulator PU.111 and/or its coactivator c-Jun12 through their physical interactions with C/EBPα. However, relatively little is known about how C/EBPα interacts with other nuclear proteins to activate myeloid-specific transcription.
Human Fcα receptor (FcαR; CD89) is a receptor for the crystallizable fragment (Fc) portion of immunoglobulin A (IgA). IgA-immune complexes activate the cells on which FcαR is expressed and trigger their immune effector functions, including phagocytosis,13 antibody-dependent cell-mediated cytotoxicity,14 superoxide production,15,16 the release of inflammatory mediators and cytokines,17-19 and internalization and presentation of IgA-complexed antigen.20 FcαR is expressed exclusively in myeloid cells associated with mucosal surfaces, including neutrophils,21,22 eosinophils,23 monocytes,21,22 and macrophages.24,25 A 259-base pair (bp) fragment of the FCAR promoter is sufficient to direct myeloid expression of a reporter gene26,27 and contains 3 functionally important binding sites for C/EBPα at -139, -127, and -74 relative to the translation ATG codon (hereafter we designate these sites as CE1, CE2, and CE3, respectively; Figure 1).27 We have shown that C/EBPα strongly activates the FCAR promoter.27 Although these observations suggest a role of C/EBPα as the major granulocyte regulator of the FCAR gene, the FCAR promoter region also contains an Ets-binding site whose mutation reduced promoter activity by 80%.27 The Ets-binding site is located only 10 bp upstream of the CE3 site (Figure 1). We previously demonstrated that artificial spacing introduced between these sites significantly reduces FCAR promoter activity, suggesting a functional interaction of C/EBPα with a nuclear factor that binds to the Ets site.27
Among Ets-related factors expressed in myeloid cells, PU.1 is implicated as the myeloid master regulator as demonstrated by a block of myeloid development in Pu1 null mice.28,29 In fact, in vitro studies have suggested that almost all characterized myeloid promoters are regulated by PU.1 (reviewed by Tenen et al1 ). However, supershift electrophoretic mobility shift assay (EMSA) showed that a DNA-protein complex formed on the FCAR Ets-binding site does not contain PU.1.27 In this context, there are also reports demonstrating novel regulatory mechanisms for myeloid transcription involving several other Ets-related factors, including Ets-2,30 E74-like factor 1 (Elf-1),31 and GA-binding protein (GABP).32-38 Among them, GABP is unique because it is the only known multimeric Ets-related factor, which consists of GABPα and GABPβ.39,40 Despite its wide expression patterns, there is increasing evidence that GABP is a key regulator of myeloid gene expression (reviewed by Rosmarin et al41 ). To date, GABP has been reported to regulate myeloid expression of the CD18,32,33 lysozyme,34 neutrophil elastase,35,36 and tumor necrosis factor α genes.37 In addition, GABP activates myeloid transcription synergistically with other transcription factors, including lineage-restricted PU.132 and C/EBPα,35 and widely expressed c-Myb35 and specificity protein 1 (Sp1).33,36 More recently, physical interaction of GABP with the transcriptional coactivator p300 has been demonstrated in relation to the responsiveness of the CD18 promoter to retinoic acid,38 which plays critical roles in granulocyte differentiation. Although these reports point to crucial roles of GABP in the regulation of genes required for the development and function of myeloid cells, molecular mechanisms accounting for how the widely expressed GABP controls myeloid gene expression remain unknown.
Here, we reveal that GABP binds to the FCAR Ets-binding site and cooperates with C/EBPα to strongly activate the FCAR promoter. Consistent with these observations, we demonstrate physical interaction between GABPα and C/EBPα. To our knowledge, this is the first report demonstrating both functional and physical interactions of GABP with the lineage-restricted regulator C/EBPα and will provide new insights into the molecular basis of myeloid gene expression regulated by widely expressed transcription factors.
Materials and methods
Cell culture
The human promonocytic U937, the T-cell leukemia Jurkat, and the epithelioma HeLa cell lines were cultured at 37°C in a 5% CO2 humidified incubator and maintained in RPMI1640 (Sigma-Aldrich, St Louis, MO) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Sigma-Aldrich), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Plasmids
The promoterless-firefly luciferase reporter plasmid pGL3-dBH and 259-bp FCAR promoter-luciferase construct pGL-259 were previously described.26,27 Constructs with a mutated Ets-binding site on antisense strand at -87 to -90 from GGAA to TTAA, with mutated C/EBP-binding sites at -139 to -131 (CE1) from TGAGGCAAT to TGAGCACCA and at -127 to -119 (CE2) from TGTGGAAAT to TGTGCACCA, and with an extra 10 bp (TCTAAGGTAC) between the adjacent CE3 and Ets-binding sites (at -80/-79) were previously described (pGLmETS-259 and pGLmCE12-259)27 or newly generated by 2-step polymerase chain reaction (PCR) mutagenesis (pGLmCE12mETS-259, and pGLmCE12INS10).
The human C/EBPα expression vector hCMV-C/EBPα was kindly provided by Dr Gretchen J. Darlington (Baylor College of Medicine, Houston, TX),42 and the corresponding empty vector pCMV-Empt was previously described.27 To construct pD3C/EBPαKoz, a BamHI DNA fragment containing the CEBPA gene from hCMV-C/EBPα was subcloned into the BamHI site downstream of the T7 promoter in pcDNA3.1(+) (Invitrogen, Carlsbad, CA), and subsequently the extra sequence upstream of the CEBPA gene was eliminated and the translation initiation codon was changed to the perfect Kozak sequence (CCACCATGG)43 by 2-step PCR mutagenesis. cDNA of human C/EBPβ, pBluehNF-IL6(610SalI),44 was provided by DNA bank, RIKEN BioResource Center (Ibaraki, Japan), and subcloned into pcDNA3.1(+) to generate pD3NF-IL6. To generate expression vectors for GABPα and GABPβ (pC3E4TF1-60S and pC3E4TF1-53S, respectively), cDNAs of human GABPα (E4TF1-60)45 and GABPβ1 (E4TF-1-53)45 were amplified from total RNA of HeLa cells by reverse transcriptase-polymerase chain reaction (RT-PCR), and cloned down-stream of the cytomegalovirus (CMV) promoter in pCR3.1 by TA cloning (Invitrogen). The corresponding empty vector pCR3.1E was generated by digestion of pCR3.1 with EcoRI followed by self-ligation. Expression vectors of GABP deletion mutants pC3GAα318-454 and pC3GAαΔETS, which contains only the Ets and GABPβ-binding domains and which lacks the Ets domain, respectively, were generated by PCR or 2-step PCR mutagenesis. To create the pGST-GABPα and its mutant plasmids, in which glutathione S-transferase (GST) is fused to their amino terminus, the fragments of the GABPA gene were amplified by PCR from pC3E4TF1-60 and subcloned into the SalI-NotI site of pGEX-4T3 (Amersham Biosciences, Piscataway, NJ) in flame. To create the pGST-GABPβ plasmid, in which GST is fused to the amino terminus of GABPβ, a HindIII-NotI DNA fragment containing most of the open reading frame of the GABPB gene from pC3E4TF1-53 and a synthetic oligonucleotide of the remaining 5′ end of the coding sequence (Invitrogen) were inserted into the BamHI/NotI site in pGEX-4T3 in flame. The pC3HA-GAα plasmid, in which hemagglutinin (HA) is fused to the amino terminus of GABPα, was generated by 2-step PCR method.
Transfection
Plasmids were purified using a Maxiprep kit (Qiagen, Hilden, Germany) and transfected into cells using Superfect transfection reagent (Qiagen) as described,27 except that HeLa cells were plated at 4 × 104 cells per well of a 24-well plate the day before transfection and transfected with 1 μg DNA using 5 μL reagent. To normalize for transfection efficiency, the pRL-CMV or pRL-SV40 plasmid (Promega, Madison, WI), which contains the Renilla luciferase gene driven by the CMV immediate early promoter or the simian virus 40 (SV40) early promoter, respectively, was included in transfections. All transfections were performed in duplicate. Cells were harvested 24 hours after transfection and assayed for firefly and Renilla luciferases as described.27
EMSAs
Nuclear extracts were prepared as described.27 In vitro-translated proteins were prepared with 1 μg supercoiled pC3E4TF1-60S and pC3E4TF1-53S using the TNT coupled reticulocyte lysate system (Promega). EMSAs were performed as described.27 Antibodies used for supershift EMSAs were rabbit antiserum against human Elf-1 (sc-631X; Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit antiserum raised against chicken GABPβ (kindly provided by Dr Seikichi Toku, University of the Ryukyus, Okinawa, Japan), which supershifts DNA-protein complexes generated by in vitro-translated human GABPα and GABPβ (T.S., C.R., and Seikichi Toku, unpublished observations, December 22, 2004).
Renaturation and EMSA of DNA-binding proteins fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Nuclear extract (200 μg protein) was loaded onto a 7.5% polyacrylamide gel containing 0.1% SDS. After electrophoresis, the gel was cut crosswise in fractions on the basis of molecular weight, and proteins were eluted and renatured according to Ossipow et al.46 Briefly, the gel pieces containing the fractionated proteins were homogenized in 3 volumes of elution-renaturation buffer (ER buffer) containing 1% Triton X-100, 20 mM Tris (tris(hydroxymethyl)aminomethane)-HCl, pH 7.6, 1 mM ETDA (ethylenediaminetetraacetic acid), 100 mM NaCl, 2 mg/mL bovine serum albumin, 2 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, and 5 μg/mL aprotinin and incubated at 37°C for 4 hours. After the polyacrylamide residues were sedimented by centrifugation, each supernatant was analyzed by EMSA as described in “EMSAs,” except that protein-DNA binding reactions were performed in 20-μL reaction mixture containing 50% ER buffer, 0.5 μg poly dI-dC·(deoxyinosinic-deoxycytidylic acid), 50 mM KCl, 12.5 mM Tris-HCl, pH 7.6, 0.25 mM dithiothreitol, 1 mM MgCl2, 0.5 mM spermidine, and 5% glycerol.
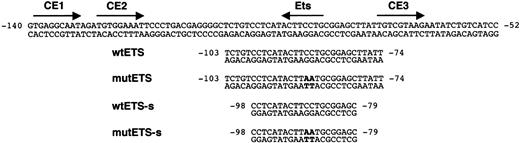
Transcription factor-binding motifs in the FCAR promoter and oligonucleotides used for EMSA. Binding sequences for Ets and C/EBP family members (CE1, CE2, and CE3) in the regulatory region of the FCAR gene are indicated by arrows. The numbers indicate nucleotide positions relative to the translation initiation ATG at +1. Wild-type oligonucleotides between -103 and -74 (wtETS) and between -98 and -79 (wtETS-s) used in EMSA contain an Ets-binding site. Specific base pair substitutions used to mutate the Ets-binding site in mutant oligonucleotides (mutETS and mutETS-s) are indicated in bold.
Transcription factor-binding motifs in the FCAR promoter and oligonucleotides used for EMSA. Binding sequences for Ets and C/EBP family members (CE1, CE2, and CE3) in the regulatory region of the FCAR gene are indicated by arrows. The numbers indicate nucleotide positions relative to the translation initiation ATG at +1. Wild-type oligonucleotides between -103 and -74 (wtETS) and between -98 and -79 (wtETS-s) used in EMSA contain an Ets-binding site. Specific base pair substitutions used to mutate the Ets-binding site in mutant oligonucleotides (mutETS and mutETS-s) are indicated in bold.
Cell line and chromatin immunoprecipitation (ChIP)
U937/HA-GAα cells, a U937 cell line that stably expresses HA-tagged GABPα, were established by electroporation (950 μF and 240 V) with 10 μg ScaI-linearized pC3HA-GAα as described.47 The cells were selected with G418 (0.5 mg/mL) and subjected to cloning by limiting dilution. ChIP assays were performed using the ChIP assay kit as described by the manufacturer (Upstate Biotechnology, Lake Placid, NY) with slight modifications. Briefly, U937/HA-GABPα cells (1 × 107/assay) were crosslinked with 1% formaldehyde at 37°C for 10 minutes. After the crosslinking reaction was quenched with 0.125 M glycine, the cells were washed with cold phosphate-buffered saline containing protease inhibitors (5 μg/mL each antipain, aprotinin, leupeptin, soybean trypsin-chymotrypsin inhibitor, and pepstatin A, and 1 mM each benzamidine and phenylmethylsulfonyl fluoride), lysed with SDS lysis buffer (1% SDS, 10 mM EDTA, and 50 mM Tris-HCl, pH 8.0) containing the above-listed protease inhibitors and sonicated to shear the genomic DNA to an average length of 500 to 1000 bp. The sonicated lysate was diluted 10-fold in ChIP dilution buffer (0.01% SDS, 1% Triton X-100, 150 mM NaCl, 15 mM Tris-HCl, pH 8.0) containing the above-listed inhibitors and subjected to immunoprecipitation with specific antibody and salmon sperm DNA/protein A agarose. The antibodies used were rabbit polyclonal antisera against HA and human C/EBPα (sc-805 and sc-61, respectively; Santa Cruz Biotechnology). The precipitated complexes were washed, eluted with buffers from the manufacturer, and heated at 65°C to reverse the crosslinks. The immunoprecipitated DNA was treated with proteinase K, followed by purification using the QIAquick PCR purification kit (Qiagen), and then amplified by PCR using primers specific for the FCAR promoter region from -143 to +8 (5′ primer, 5′-GCTGTGAGGCAATAGATGTGGAA-3′;3′ primer, 5′-GGGTCCATCGTGCTGACAC-3′) and a region within the FCAR EC1 exon (5′ primer, 5′-CATAAAAAACTCCACGTACCGAGAG-3′; 3′ primer, 5′-GCCCTATCCTATATTGGCACTGATA-3′), which locates approximately 6 kilobase (kb) downstream of the FCAR promoter.48
Protein interaction assays
35S-labeled proteins were prepared by in vitro translation in the presence of L-[35S]methionine (Perkin-Elmer, Boston, MA) as described in “EMSAs.” Escherichia coli BL21 or JM109 expressing GST-fused proteins was suspended in NETN-100 buffer (50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 100 mM NaCl, and 0.5% Nonidet P-40 [(Octylphenoxy)polyethoxyethanol]), lysed by sonication, and centrifuged at 20 000g at 4°C for 10 minutes. The supernatants containing GST-fused proteins were immobilized on glutathione-Sepharose 4B beads (Amersham Biosciences) equilibrated with NETN-100 buffer. The beads were washed 3 times with NETN-100 buffer and subsequently 3 times with NETN-50 buffer (50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 50 mM NaCl, and 0.5% Nonidet P-40). The relative amount of proteins bound to the beads was determined by SDS-PAGE of proteins eluted from the beads and staining with Coomassie blue using bovine serum albumin as a standard. For interaction assays, 5-μL beads bound by 10 μg GST-fused protein were incubated with 35S-labeled protein in 200 μL NETN-50 buffer containing 100 μg/mL ethidium bromide49 at room temperature for 1 hour. The beads were then washed with 300 μL NETN-50 buffer 6 times in the presence of 100 μg/mL ethidium bromide and subsequently 2 times in its absence. Bound proteins were eluted by boiling in SDS sample buffer and resolved by SDS-PAGE using a 4% to 20% gradient gel. (Daiichi Pure Chemicals, Tokyo, Japan).
Results
The FCAR Ets-binding site is required for the transcriptional activity of C/EBPα
The functional binding sites for Ets-like factor and C/EBPα (CE3) within the FCAR essential promoter region at -102 to -64 are separated by only 10 bp (Figure 1). We previously reported that artificial spacing introduced between them significantly decreases promoter activity in FcαR-expressing U937 cells.27 Because this observation suggests a functional Ets-C/EBP factor interaction, we first examined the effect of the artificial spacing between the CE3 and Ets-binding sites on transactivation of the FCAR promoter by C/EBPα. In these experiments, we used Jurkat T-cell line, in which the nuclear factor that binds to the FCAR Ets site is abundant,27 whereas C/EBPα is not expressed.8 In addition, 2 functional C/EBP-binding sites (CE1 and CE2; Figure 1) upstream of the CE3 site have shown to compensate the effect of CE3 mutation on transcriptional activity of C/EBPα.27 Therefore, to investigate only the effect on CE3-mediated activation, we used the mutagenized FCAR promoter constructs in which both the CE1 and CE2 sites but not the CE3 site are mutated.
As shown previously,27 the FCAR promoter containing the mutated CE1 and CE2 sites (pGLmCE12-259) was activated 2.6-fold by cotransfected C/EBPα (Figure 2B). However, 10-bp separation of the adjacent CE3 and Ets-binding sites (pGLmCE12INS10) significantly reduced the CE3-mediated activation by C/EBPα to 1.5-fold. This is consistent with the observation that artificial spacing between the CE3 and Ets-binding sites decreased FCAR promoter activity in U937 cells. Moreover, mutagenesis of the Ets-binding site (pGLmCE12mETS-259) resulted in complete loss of the CE3-mediated activation. These results strongly suggest that C/EBPα regulates FCAR gene expression in cooperation with a nuclear factor that binds to the Ets site. We also assessed the activity of C/EBPβ, another C/EBP family member, and observed a similar Ets site-dependent transactivation (Figure 2C).
HEL-NF1 is not detectable after fractionation by SDS-PAGE
We previously showed that Elf-1, an Ets-related factor, could bind to the FCAR Ets-binding site with a low affinity.27 However, the major part of the complexes forming on this site in U937 nuclear extracts are neither supershifted by anti-Elf-1 antibody nor affected by competitor oligonucleotide to which Elf-1 binds. This binding complex is detectable in a wide variety of cell lines but preferentially in cells of hematopoietic origin. Therefore, we referred to the major Ets-binding factor as the hematopoietic Ets-like nuclear factor 1 (HEL-NF1).27
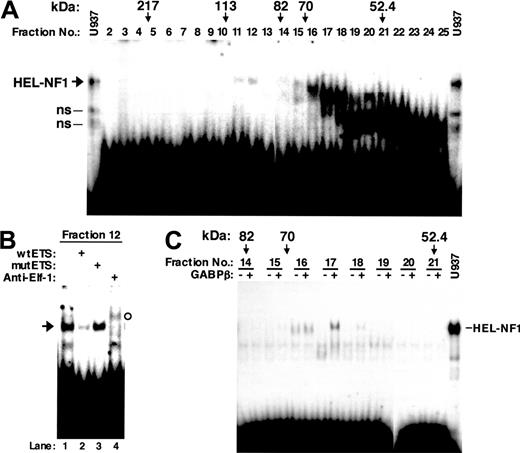
To attempt to determine the identity of HEL-NF1, we first fractionated U937 nuclear proteins by SDS-PAGE. The gel was cut crosswise in fractions on the basis of molecular weight. Proteins were eluted from the gel pieces and assayed for binding to the labeled oligonucleotide containing the FCAR Ets site between -103 and -74 (wtETS; Figure 1). Although we observed in the fractions a number of complexes that interact with this oligonucleotide, a complex comigrating with HEL-NF1 was detected only in fractions 11 and 12 (Figure 3A). Abrogation of this species by competition with self-oligonucleotide (Figure 3B, lane 2) but not with mutant oligonucleotide (lane 3) indicates that it specifically recognizes the FCAR Ets site. However, the complex observed in fraction 12 was supershifted by anti-Elf-1 antibody (lane 4). Similar results were also obtained using fraction 11 (data not shown). Elf-1 has been reported to migrate as a protein with an apparent molecular mass of 98 kDa in SDS-PAGE,50 which fits the molecular size range of fractions 11 and 12 (90-110 kDa). Thus, we concluded that the observed binding complex is Elf-1, and no binding complex representing HEL-NF1 could be detected when fractionated by SDS-PAGE.
Effect of artificial spacing between the adjacent Ets- and C/EBP-binding sites or mutation in the Ets-binding site on transactivation of the FCAR promoter by C/EBPα and C/EBPβ. (A) A schematic representation for the structure of the wild-type FCAR upstream-luciferase (Luc) construct (pGL-259) and the mutated FCAR upstream-luciferase constructs. The numbers indicate nucleotide positions relative to the translation initiation ATG at +1. The major transcription start site (-197) identified by de Wit et al48 is indicated as bent arrow. The 3 C/EBP-binding sites at -139, -127, and -74 and an Ets-binding site at -92 are indicated. X indicates mutated site. (B-C) Jurkat cells were transfected with 0.7 μg luciferase reporter constructs containing the indicated FCAR promoter mutant in the absence or presence of 0.1 μg expression vector for C/EBPα (hCMV-C/EBPα) (B) or C/EBPβ (pD3NF-IL6) (C), along with 0.03 μg pRL-SV40; each sample was transfected with the additional corresponding empty vector pCMV-Empt (B) or pcDNA3.1(+) (C) to bring the amount of transfected DNA in each sample to 1 μg. Firefly and Renilla luciferase activities were determined 24 hours after transfection. Values are corrected for transfection efficiency and presented as the fold stimulation of each construct by C/EBPα (B) or C/EBPβ (C), compared with the promoter activity seen without the expression vector. Thin bars represent the SE from 3 to 4 transfections. *Significant decrease (P < .01) compared with pGLmCE12-259 by 2-tailed t test.
Effect of artificial spacing between the adjacent Ets- and C/EBP-binding sites or mutation in the Ets-binding site on transactivation of the FCAR promoter by C/EBPα and C/EBPβ. (A) A schematic representation for the structure of the wild-type FCAR upstream-luciferase (Luc) construct (pGL-259) and the mutated FCAR upstream-luciferase constructs. The numbers indicate nucleotide positions relative to the translation initiation ATG at +1. The major transcription start site (-197) identified by de Wit et al48 is indicated as bent arrow. The 3 C/EBP-binding sites at -139, -127, and -74 and an Ets-binding site at -92 are indicated. X indicates mutated site. (B-C) Jurkat cells were transfected with 0.7 μg luciferase reporter constructs containing the indicated FCAR promoter mutant in the absence or presence of 0.1 μg expression vector for C/EBPα (hCMV-C/EBPα) (B) or C/EBPβ (pD3NF-IL6) (C), along with 0.03 μg pRL-SV40; each sample was transfected with the additional corresponding empty vector pCMV-Empt (B) or pcDNA3.1(+) (C) to bring the amount of transfected DNA in each sample to 1 μg. Firefly and Renilla luciferase activities were determined 24 hours after transfection. Values are corrected for transfection efficiency and presented as the fold stimulation of each construct by C/EBPα (B) or C/EBPβ (C), compared with the promoter activity seen without the expression vector. Thin bars represent the SE from 3 to 4 transfections. *Significant decrease (P < .01) compared with pGLmCE12-259 by 2-tailed t test.
DNA binding of renatured nuclear proteins fractionated by SDS-PAGE. U937 nuclear proteins were separated by SDS-PAGE. The gel was cut crosswise in fractions on the basis of molecular weight, and the nuclear proteins were eluted and renatured. (A) Distribution of DNA-binding activity in gel size-fractionated nuclear proteins. Renatured proteins (10 μL) from different molecular weight fractions were analyzed by EMSA using the labeled FCAR Ets oligonucleotide (wtETS). The molecular weights indicated correspond to molecular weight size markers (Prestained SDS-PAGE Standard; Bio-Rad, Hercules, CA) run alongside the nuclear extract. U937, one third of the binding reaction with 6 μg U937 nuclear extract was applied to the electrophoresis. ns indicates nonspecific bands identified previously.27 (B) DNA-binding specificity and supershift EMSA of size-fractionated nuclear factors that bind to the FCAR Ets site. Renatured proteins (6 μL) from fraction 12 were analyzed by EMSA using the labeled FCAR Ets oligonucleotide (wtETS) in the absence (lane 1) or presence of 100-fold molar excess of unlabeled wild-type (lane 2) and mutant FCAR Ets oligonucleotide competitors (mutETS) (lane 3), or 2 μg antibody against Elf-1 (lane 4). Arrow indicates the binding complex comigrating with HEL-NF1. The supershifted band is indicated by open circle. (C) DNA binding of gel size-fractionated nuclear proteins in the presence of the GABPβ subunit. The indicated SDS-PAGE fractions (10 μL) were incubated with the labeled wtETS-s oligonucleotide in the absence (-) or presence of 1 μL in vitro-translated GABPβ (+). U937, one third of the binding reaction with 6 μg U937 nuclear extract was applied to the electrophoresis.
DNA binding of renatured nuclear proteins fractionated by SDS-PAGE. U937 nuclear proteins were separated by SDS-PAGE. The gel was cut crosswise in fractions on the basis of molecular weight, and the nuclear proteins were eluted and renatured. (A) Distribution of DNA-binding activity in gel size-fractionated nuclear proteins. Renatured proteins (10 μL) from different molecular weight fractions were analyzed by EMSA using the labeled FCAR Ets oligonucleotide (wtETS). The molecular weights indicated correspond to molecular weight size markers (Prestained SDS-PAGE Standard; Bio-Rad, Hercules, CA) run alongside the nuclear extract. U937, one third of the binding reaction with 6 μg U937 nuclear extract was applied to the electrophoresis. ns indicates nonspecific bands identified previously.27 (B) DNA-binding specificity and supershift EMSA of size-fractionated nuclear factors that bind to the FCAR Ets site. Renatured proteins (6 μL) from fraction 12 were analyzed by EMSA using the labeled FCAR Ets oligonucleotide (wtETS) in the absence (lane 1) or presence of 100-fold molar excess of unlabeled wild-type (lane 2) and mutant FCAR Ets oligonucleotide competitors (mutETS) (lane 3), or 2 μg antibody against Elf-1 (lane 4). Arrow indicates the binding complex comigrating with HEL-NF1. The supershifted band is indicated by open circle. (C) DNA binding of gel size-fractionated nuclear proteins in the presence of the GABPβ subunit. The indicated SDS-PAGE fractions (10 μL) were incubated with the labeled wtETS-s oligonucleotide in the absence (-) or presence of 1 μL in vitro-translated GABPβ (+). U937, one third of the binding reaction with 6 μg U937 nuclear extract was applied to the electrophoresis.
The binding complex representing HEL-NF1 is generated in the presence of the GABPβ subunit
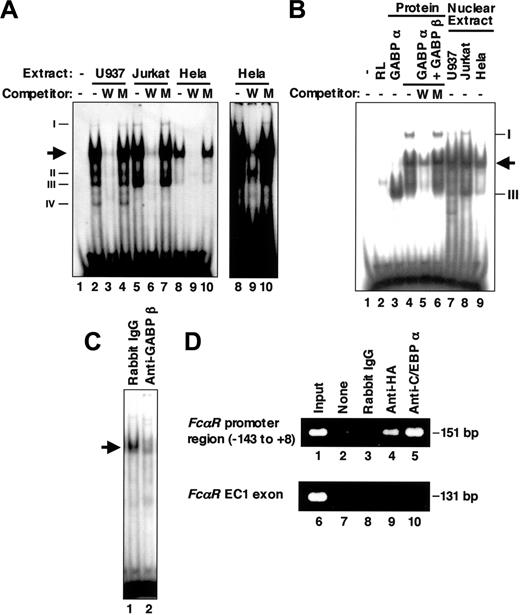
Because several nonspecific binding complexes were observed in the EMSA for HEL-NF1 (Figure 3A),27 we performed EMSA using a shorter probe containing the FCAR Ets site between -98 and -79 (Figure 1; wtETS-s). Nuclear extracts prepared from U937, Jurkat, and HeLa cells generated several complexes with this probe (Figure 4A). Consistent with the previous results obtained using the longer wtETS probe, HEL-NF1-binding activity was present predominantly in hematopoietic cell lines, including U937 and Jurkat cells (lanes 2 and 5), but relatively less abundant in nonhematopoietic HeLa cells (lane 8). Additional minor binding complexes that we had not previously detected were complexes I, II, III, and IV. Complexes I and III were observed predominantly in Jurkat cells (lane 5), but they were also detectable in other cell lines (lanes 2 and 8). Complex IV was detected only in U937 cells (lane 2). These binding complexes specifically recognize the FCAR Ets-binding site, because they were abolished by competition with cold self-oligonucleotide (lanes 3, 6, and 9) but not by oligonucleotide containing mutated Ets-binding site (lanes 4, 7, and 10).
As described in “HEL-NF1 is not detectable after fractionation by SDS-PAGE,” we could detect no binding complex representing HEL-NF1 when nuclear extracts were fractionated by SDS-PAGE (Figure 3A). Although we cannot exclude the possibility that the DNA-binding ability of HEL-NF1 was inactivated during the fractionation, another possibility is that HEL-NF1 consists of multiple subunits. If it is the case, GABP is the strongest candidate because this transcription factor is the only known multimeric Ets family member. GABP is composed of GABPα, an Ets-related factor, and GABPβ, a Notch-related protein.39,40 GABPβ itself has no DNA-binding activity but is required for efficient DNA binding of GABPα and for transcriptional activation. When the SDS-PAGE fractions were assayed for binding to the FCAR Ets-binding site (wtETS-s) in the presence and absence of in vitro-translated GABPβ, we detected a DNA-protein complex comigrating with HEL-NF1 in fractions 17 only when GABPβ was added to the binding reaction (Figure 3C). This fraction corresponds to 60 to 65 kDa, which fits the apparent molecular mass of GABPα on the basis of SDS-PAGE (60 kDa).40 Moreover, GABPα has been reported to be the only Ets-related factor that is recognized by GABPβ.51 These data strongly support that HEL-NF1 consists of GABPα and GABPβ.
GABP binds to the FCAR promoter Ets-binding site. (A) EMSA of the FCAR promoter Ets-binding site using nuclear extracts. The labeled double-stranded oligonucleotide corresponding to the FCAR promoter sequence from -98 to -79 (wtETS-s) was incubated without (lane 1) and with 6 μg nuclear extracts from U937 (lanes 2-4), Jurkat (lanes 5-7), and HeLa cells (lanes 8-10) in the absence (lanes 1, 2, 5, and 8) or presence of 100-fold molar excess of unlabeled wild-type (wtETS-s; W) (lanes 3, 6, and 9) and mutant FCAR Ets oligonucleotide competitors (mutETS-s; M) (lanes 4, 7, and 10). Arrow indicates the location of HEL-NF1. Newly detected binding species (complexes I, II, III, and IV) are also indicated on left. Right panel shows long exposure of lanes 8 to 10 to show complex I. (B) EMSA of the FCAR promoter Ets-binding site using in vitro-translated GABP. The labeled double-stranded oligonucleotide corresponding to the sequence from -98 to -79 (wtETS-s) was incubated without (lane 1) and with 1 μL unprogrammed reticulocyte lysate (lane 2), 1 μL in vitro-translated GABPα (lane 3), 1 μL each of in vitro-translated GABPα and GABPβ (lanes 4-6), or 6 μg nuclear extracts from U937 (lane 7), Jurkat (lane 8), and HeLa cells (lane 9) in the absence (lanes 1-4, 7-9) or presence of 100-fold molar excess of unlabeled wild-type (wtETS-s; W) (lane 5) and mutant FCAR Ets oligonucleotide competitors (mutETS-s; M) (lane 6). Arrow indicates the location of HEL-NF1. Complexes I and III detected in nuclear extracts are also indicated on right. (C) Supershift EMSA of U937 nuclear extracts using the FCAR promoter Ets-binding site. The labeled double-stranded oligonucleotide corresponding to the sequence from -98 to -79 (wtETS-s) was incubated with 6 μg nuclear extracts from U937 cells in the presence of rabbit IgG (lane 1) or 1 μL antiserum against GABPβ (lane 2). Arrow indicates the location of HEL-NF1. (D) ChIP assay of HA-tagged GABPα binding in vivo. Crosslinked protein-DNA complexes from U937/HA-GAα cells were immunoprecipitated without antibody (lanes 2 and 7), with control rabbit IgG (lanes 3 and 8), and with antibodies against HA (lanes 4 and 9) and C/EBPα (lanes 5 and 10), and they were analyzed by PCR using primers corresponding to the FCAR promoter region from -143 to +8 (lanes 1-5) and a region within the FCAR EC1 exon (lanes 6-10). Positive control is chromatin prior to immunoprecipitation (lanes 1 and 6).
GABP binds to the FCAR promoter Ets-binding site. (A) EMSA of the FCAR promoter Ets-binding site using nuclear extracts. The labeled double-stranded oligonucleotide corresponding to the FCAR promoter sequence from -98 to -79 (wtETS-s) was incubated without (lane 1) and with 6 μg nuclear extracts from U937 (lanes 2-4), Jurkat (lanes 5-7), and HeLa cells (lanes 8-10) in the absence (lanes 1, 2, 5, and 8) or presence of 100-fold molar excess of unlabeled wild-type (wtETS-s; W) (lanes 3, 6, and 9) and mutant FCAR Ets oligonucleotide competitors (mutETS-s; M) (lanes 4, 7, and 10). Arrow indicates the location of HEL-NF1. Newly detected binding species (complexes I, II, III, and IV) are also indicated on left. Right panel shows long exposure of lanes 8 to 10 to show complex I. (B) EMSA of the FCAR promoter Ets-binding site using in vitro-translated GABP. The labeled double-stranded oligonucleotide corresponding to the sequence from -98 to -79 (wtETS-s) was incubated without (lane 1) and with 1 μL unprogrammed reticulocyte lysate (lane 2), 1 μL in vitro-translated GABPα (lane 3), 1 μL each of in vitro-translated GABPα and GABPβ (lanes 4-6), or 6 μg nuclear extracts from U937 (lane 7), Jurkat (lane 8), and HeLa cells (lane 9) in the absence (lanes 1-4, 7-9) or presence of 100-fold molar excess of unlabeled wild-type (wtETS-s; W) (lane 5) and mutant FCAR Ets oligonucleotide competitors (mutETS-s; M) (lane 6). Arrow indicates the location of HEL-NF1. Complexes I and III detected in nuclear extracts are also indicated on right. (C) Supershift EMSA of U937 nuclear extracts using the FCAR promoter Ets-binding site. The labeled double-stranded oligonucleotide corresponding to the sequence from -98 to -79 (wtETS-s) was incubated with 6 μg nuclear extracts from U937 cells in the presence of rabbit IgG (lane 1) or 1 μL antiserum against GABPβ (lane 2). Arrow indicates the location of HEL-NF1. (D) ChIP assay of HA-tagged GABPα binding in vivo. Crosslinked protein-DNA complexes from U937/HA-GAα cells were immunoprecipitated without antibody (lanes 2 and 7), with control rabbit IgG (lanes 3 and 8), and with antibodies against HA (lanes 4 and 9) and C/EBPα (lanes 5 and 10), and they were analyzed by PCR using primers corresponding to the FCAR promoter region from -143 to +8 (lanes 1-5) and a region within the FCAR EC1 exon (lanes 6-10). Positive control is chromatin prior to immunoprecipitation (lanes 1 and 6).
The FCAR promoter is activated by GABP. (A) A schematic representation for the structure of the wild-type and mutant FCAR upstream-luciferase (Luc) constructs. X indicates Ets site mutation. (B) HeLa cells were transfected with 0.7 μg luciferase reporter constructs containing the indicated FCAR promoter in the absence or presence of 0.1 μg each of expression vector(s) for GABPα and/or GABPβ, along with 5 ng pRL-CMV; each sample was transfected with the additional corresponding empty vector pCR3.1E to bring the amount of transfected DNA in each sample to 1 μg. Firefly and Renilla luciferase activities were determined 24 hours after transfection. Values are corrected for transfection efficiency and presented as the fold stimulation of each construct by GABPα and/or GABPβ, compared with the promoter activity seen without the expression vector(s). Thin bars represent the SE from 3 transfections.
The FCAR promoter is activated by GABP. (A) A schematic representation for the structure of the wild-type and mutant FCAR upstream-luciferase (Luc) constructs. X indicates Ets site mutation. (B) HeLa cells were transfected with 0.7 μg luciferase reporter constructs containing the indicated FCAR promoter in the absence or presence of 0.1 μg each of expression vector(s) for GABPα and/or GABPβ, along with 5 ng pRL-CMV; each sample was transfected with the additional corresponding empty vector pCR3.1E to bring the amount of transfected DNA in each sample to 1 μg. Firefly and Renilla luciferase activities were determined 24 hours after transfection. Values are corrected for transfection efficiency and presented as the fold stimulation of each construct by GABPα and/or GABPβ, compared with the promoter activity seen without the expression vector(s). Thin bars represent the SE from 3 transfections.
GABP complexes bind to the FCAR promoter
To determine whether GABP actually binds to the FCAR Ets-binding site, we performed EMSA using in vitro-translated GABPα and GABPβ. GABPα clearly formed a large amount of DNA-protein complex with the FCAR Ets-binding site (Figure 4B, lane 3). Furthermore, GABPβ and GABPα together generated 2 additional complexes (lane 4). These complexes were abolished or considerably reduced by competition with self-oligonucleotide (lane 5) but not by oligonucleotide containing mutated Ets-binding site (lane 6), indicating that GABP actually binds to the FCAR Ets-binding site in a sequence-specific manner.
The complexes generated by in vitro-translated GABPα together with GABPβ (Figure 4B, lane 4) appeared to correspond to the heterodimeric GABPα/GABPβ and heterotetrameric GABPα2/GABPβ2 species demonstrated previously.52,53 The former was the major binding complex and comigrated with HEL-NF1 observed in nuclear extracts (compare lane 4 with lanes 7-9). The latter comigrated with complex I (compare lane 4 with lanes 7-9). In addition, the complex generated by GABPα alone comigrated with complex III (compare lane 3 with lanes 7-9). Thus, all the complexes that were generated with the FCAR Ets-binding site by in vitro-translated GABP proteins comigrated with the binding species detected in nuclear extracts. In addition, the generation of HEL-NF1 in U937 nuclear extracts was considerably reduced by anti-GABPβ antibody (Figure 4C), confirming that HEL-NF1 is GABP.
To establish the in vivo binding of GABPα to the FCAR promoter, crosslinked chromatin from U937/HA-GAα cell line, a U937 transfectant that stably expresses HA-tagged GABPα, was immunoprecipitated with an anti-HA antibody. PCR using primers specific for the FCAR promoter region from -143 to +8 demonstrated that the immunoprecipitated chromatin contained the FCAR promoter (Figure 4D, lane 4). This interaction is specific because of the absence of amplification when used with no antibody (lane 2) or control IgG (lane 3), or primers specific for the FCAR EC1 exon region, which locates approximately 6 kb downstream of the FCAR promoter48 (lane 9). Chromatin containing the FCAR promoter but not the EC1 region was also immunoprecipitated with anti-C/EBPα antibody (lanes 5 and 10). These results establish that GABPα as well as C/EBPα binds to the FCAR promoter region in vivo.
GABP transactivates the FCAR promoter
We next examined whether GABP functionally activates the FCAR promoter (Figure 5). Cotransfection of both expression vectors for GABPα and GABPβ into HeLa cells with the wild-type FCAR promoter-luciferase construct pGL-259 increased FCAR promoter activity 3.7-fold (Figure 5B). However, GABP did not activate pGLmETS-259, in which the Ets-binding site is mutated. In addition, either GABPα or GABPβ alone failed to activate the FCAR promoter. These results demonstrate that GABP functionally activates the FCAR promoter through the Ets-binding site, and this activation requires both GABPα and GABPβ subunits.
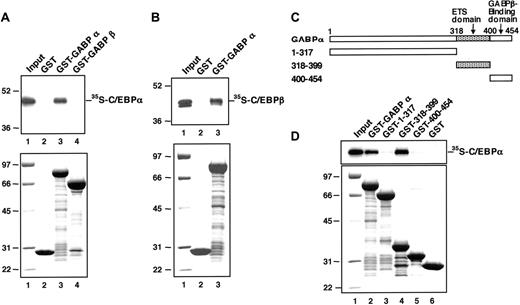
C/EBP proteins physically interact with GABPα. (A) In vitro-translated 35S-C/EBPα protein was mixed with GST alone (lane 2), GST-fused GABPα protein (lane 3), and GST-fused GABPβ protein (lane 4). The proteins bound to each GST-fusion protein beads were analyzed by SDS-PAGE and autoradiography (top); lane 1 shows 5% of the input 35S-C/EBPα; the molecular weight markers (Prestained SDS-PAGE Standard; Bio-Rad) are shown (left). The input GST-fused proteins were analyzed by SDS-PAGE and Coomassie blue staining (bottom); the molecular weight markers are shown in lane 1. (B) In vitro-translated 35S-C/EBPβ protein was mixed with GST alone (lane 2) and GST-fused GABPα protein (lane 3). The proteins bound to each GST-fusion protein beads were analyzed by SDS-PAGE and autoradiography (top): lane 1 shows 5% of the input 35S-C/EBPβ; the molecular weight markers (Prestained SDS-PAGE Standard; Bio-Rad) are shown (left). The input GST-fused proteins were analyzed by SDS-PAGE and Coomassie blue staining (bottom); the molecular weight markers are shown in lane 1. (C) Schematic structure of GST-fused GABPα deletion mutants. ETS domain indicates E26 transformation-specific domain. (D) In vitro-translated 35S-C/EBPα protein was mixed with GST-fused GABPα protein (lane 2), the indicated GST-fused GABPα deletion mutants (lanes 3-5), and GST alone (lane 6). The proteins bound to each GST-fusion protein beads were analyzed by SDS-PAGE and autoradiography (top); lane 1 shows 5% of the input 35S-C/EBPα. The input GST-fused proteins were analyzed by SDS-PAGE and Coomassie blue staining (bottom); the molecular weight markers are shown in lane 1.
C/EBP proteins physically interact with GABPα. (A) In vitro-translated 35S-C/EBPα protein was mixed with GST alone (lane 2), GST-fused GABPα protein (lane 3), and GST-fused GABPβ protein (lane 4). The proteins bound to each GST-fusion protein beads were analyzed by SDS-PAGE and autoradiography (top); lane 1 shows 5% of the input 35S-C/EBPα; the molecular weight markers (Prestained SDS-PAGE Standard; Bio-Rad) are shown (left). The input GST-fused proteins were analyzed by SDS-PAGE and Coomassie blue staining (bottom); the molecular weight markers are shown in lane 1. (B) In vitro-translated 35S-C/EBPβ protein was mixed with GST alone (lane 2) and GST-fused GABPα protein (lane 3). The proteins bound to each GST-fusion protein beads were analyzed by SDS-PAGE and autoradiography (top): lane 1 shows 5% of the input 35S-C/EBPβ; the molecular weight markers (Prestained SDS-PAGE Standard; Bio-Rad) are shown (left). The input GST-fused proteins were analyzed by SDS-PAGE and Coomassie blue staining (bottom); the molecular weight markers are shown in lane 1. (C) Schematic structure of GST-fused GABPα deletion mutants. ETS domain indicates E26 transformation-specific domain. (D) In vitro-translated 35S-C/EBPα protein was mixed with GST-fused GABPα protein (lane 2), the indicated GST-fused GABPα deletion mutants (lanes 3-5), and GST alone (lane 6). The proteins bound to each GST-fusion protein beads were analyzed by SDS-PAGE and autoradiography (top); lane 1 shows 5% of the input 35S-C/EBPα. The input GST-fused proteins were analyzed by SDS-PAGE and Coomassie blue staining (bottom); the molecular weight markers are shown in lane 1.
C/EBPα physically interacts with GABPα
Because the transactivation experiment of C/EBPα (Figure 2) strongly suggests a functional interaction between C/EBPα and the nuclear factor that binds to the FCAR Ets site, we determined whether C/EBPα physically interacts with GABP. 35S-labeled C/EBPα prepared by in vitro translation was mixed with GST-fused GABPα, GST-fused GABPβ, or GST alone, which were bound to glutathione-coated Sepharose beads. To exclude the possibility that the interaction is mediated by the binding of interacting factors to contaminant DNA in the preparations, the binding assays were performed in the presence of 100 μg/mL ethidium bromide.49 We observed that 4% of the input 35S-labeled C/EBPα was bound by GST-GABPα beads (Figure 6A, lane 3) but not retained by GST-GABPβ or GST beads (lanes 2 and 4). Thus, C/EBPα physically and specifically interacts with GABPα.
C/EBPβ was also bound by GST-GABPα (Figure 6B). This suggests that GABPα binds to the basic region-leucine zipper domain of each C/EBP factor, which is highly conserved between C/EBPα and C/EBPβ. On the other hand, when GST-fused GABPα deletion mutants (Figure 6C) were applied to binding analysis, deletion mutants lacking the Ets domain, known as a DNA-binding motif, could not efficiently bind C/EBPα (Figure 6D, lanes 3 and 5). Moreover, the Ets domain alone was sufficient to bind C/EBPα (lane 4). These results demonstrate that GABPα physically interacts with C/EBPα through its DNA-binding Ets domain.
GABP cooperates with C/EBPα to strongly activate the FCAR promoter
We investigated whether GABP functionally cooperates with C/EBPα to activate the FCAR promoter. Cotransfection of pGL-259 into HeLa cells with expression vectors for GABPα and GABPβ resulted in 4-fold activation (Figure 7B). C/EBPα alone activated the FCAR promoter 107-fold. Moreover, cotransfection of GABPα/β in addition to C/EBPα resulted in roughly multiplicative effect on pGL-259 (280-fold activation), indicating that these factors synergistically activate the FCAR promoter. In contrast, the activation by C/EBPβ was modest, and cotransfection with GABP in addition to C/EBPβ activated the FCAR promoter only 8-fold (Figure 7C). However, this is more than an additive effect, indicating that C/EBPβ also cooperates with GABP to activate the FCAR promoter.
Cooperative effect of GABP and C/EBPα was also assessed on CE3-mediated transcription. Cotransfection of pGLmCE12-259 with expression vectors for GABPα and GABPβ resulted in 2-fold activation (Figure 7E). At the low dose used (3 ng), C/EBPα alone activated the FCAR promoter through the CE3 site only 2-fold. However, the combination of GABPα/β and C/EBPα resulted in 26-fold activation, indicating that these factors synergistically and strongly activate the FCAR promoter. Extending the distance between the adjacent Ets and CE3 sites (pGLmCE12INS10) significantly reduced the cooperative activation by GABPα/β and C/EBPα without affecting the transactivation by GABP alone. Moreover, mutation of the Ets-binding site (pGLmCE12mETS-259) prevented most of cooperative activation by GABPα/β and C/EBPα. These results are consistent with the observation in Jurkat cells that the transactivation through the CE3 site by C/EBPα requires the Ets-binding site (Figure 2). In addition, GABPα deletion mutant lacking the Ets domain (GABPαΔETS) could not cooperate with C/EBPα to activate CE3-mediated transcription. Moreover, a considerable synergy (17-fold activation) was still observed with GABPα deletion mutant containing only the Ets and GABPβ-binding domains (GABPα318-454). Thus, the synergy between GABP and C/EBPα through the CE3 site requires the DNA-binding Ets domain of GABPα that is important for the direct physical interaction with C/EBPα.
GABP cooperates with C/EBPα to strongly activate the FCAR promoter. (A,D) A schematic representation for the structure of the FCAR upstream-luciferase (Luc) constructs. The numbers indicate nucleotide positions relative to the translation initiation ATG at +1. The major transcription start site is indicated as bent arrow. X indicates mutated site. (B-C,E) HeLa cells were transfected with 0.7 μg indicated FCAR upstream-luciferase constructs in the absence or presence of 0.1 μg each of expression plasmids for GABPβ (pC3E4TF1-53S) and GABPα (pC3E4TF1-60S) or its deletion mutant, GABPαΔETS (pC3GAαΔETS) or GABPα318-454 (pC3GAα318-454), along with 3 ng C/EBPα expression plasmid (hCMV-C/EBPα) or the empty vector (pCMV-Empt) (B,E), or with 3 ng C/EBPβ expression plasmid (pD3NF-IL6) or the empty vector [pcDNA3.1(+)] (C); each sample was transfected with 5 ng pRL-CMV and the additional empty vector pCR3.1E to bring the amount of transfected DNA in each sample to 1 μg. Firefly and Renilla luciferase activities were determined 24 hours after transfection. Values are corrected for transfection efficiency and presented as the fold stimulation of each construct by the indicated transcription factor(s), compared with the promoter activity seen without the expression vector(s). Thin bars represent the standard errors (SE) from 3 to 6 transfections. *Significant decrease (P < .05) compared with pGLmCE12-259 by one-tailed t test.
GABP cooperates with C/EBPα to strongly activate the FCAR promoter. (A,D) A schematic representation for the structure of the FCAR upstream-luciferase (Luc) constructs. The numbers indicate nucleotide positions relative to the translation initiation ATG at +1. The major transcription start site is indicated as bent arrow. X indicates mutated site. (B-C,E) HeLa cells were transfected with 0.7 μg indicated FCAR upstream-luciferase constructs in the absence or presence of 0.1 μg each of expression plasmids for GABPβ (pC3E4TF1-53S) and GABPα (pC3E4TF1-60S) or its deletion mutant, GABPαΔETS (pC3GAαΔETS) or GABPα318-454 (pC3GAα318-454), along with 3 ng C/EBPα expression plasmid (hCMV-C/EBPα) or the empty vector (pCMV-Empt) (B,E), or with 3 ng C/EBPβ expression plasmid (pD3NF-IL6) or the empty vector [pcDNA3.1(+)] (C); each sample was transfected with 5 ng pRL-CMV and the additional empty vector pCR3.1E to bring the amount of transfected DNA in each sample to 1 μg. Firefly and Renilla luciferase activities were determined 24 hours after transfection. Values are corrected for transfection efficiency and presented as the fold stimulation of each construct by the indicated transcription factor(s), compared with the promoter activity seen without the expression vector(s). Thin bars represent the standard errors (SE) from 3 to 6 transfections. *Significant decrease (P < .05) compared with pGLmCE12-259 by one-tailed t test.
Discussion
Although C/EBPα has been recognized as the inducer of granulocytic differentiation,8,9 how this lineage-restricted regulator interacts with other transcription factors to activate myeloid transcription is still poorly understood. Various myeloid genes regulated by C/EBPα also possess functional Ets-binding site(s) (reviewed by Tenen et al1 ). These include genes involved in the development and/or function of myeloid cells, such as the granulocyte colony-stimulating factor receptor54,55 and neutrophil elastase genes.56 PU.1 is a hematopoietic Ets-related factor essential for myeloid development28,29,57 and has been reported to bind to these promoters and activate transcription.54,56 However, it has been shown that C/EBPα physically interacts with PU.1 and inactivates its transcriptional activity.11 Thus, other Ets-related factor(s) could cooperate with C/EBPα and play an important role in activation of myeloid transcription. In fact, it has been demonstrated that the neutrophil elastase promoter is also bound by GABP and synergistically activated by GABP and C/EBPα.35
A 259-bp fragment of the FCAR promoter, which is sufficient to direct myeloid expression of a reporter gene,26,27 contains 3 functionally important binding sites for C/EBPα (CE1, CE2, and CE3) and an unidentified Ets-like factor.27 The Ets-binding site is located only 10 bp upstream of the CE3 site. We previously suggested a functional interaction between C/EBPα and the factor that binds to this Ets site by demonstrating decreased activity of the FCAR promoter containing artificial spacing between the adjacent CE3 and Ets-binding sites.27 Here, we have shown that the FCAR Ets-binding site is required for the CE3-mediated transactivation of the FCAR promoter by C/EBPα (Figure 2) and is bound by a heterodimer composed of GABPα and GABPβ (Figures 3, 4). Consistent with the previous observation27 and present data (Figure 2) that suggest their functional interaction, C/EBPα physically interacted with GABPα (Figure 6A) and cooperated with GABP to activate the FCAR promoter through the CE3 site dependently on the Ets-binding site (Figure 7E). This synergy required the DNA-binding Ets domain of GABPα (Figure 7E) that was important for its direct physical interaction with C/EBPα (Figures 6D). Functional synergy by GABP and C/EBPα was previously reported as mentioned above.35 However, this is the first report demonstrating physical interaction between GABP and C/EBPα to account for their functional synergy, although their in vivo physical interaction remains to be demonstrated.
A major question raised in our studies is how the widely expressed GABP controls myeloid-specific gene expression. One possible mechanism could involve specific posttranslational modification of GABP. We identified HEL-NF1 as an Ets-like factor that is predominantly detected in cells of hematopoietic origin in EMSAs using nuclear extracts (Figure 4A).27 It has been reported that the non-DNA-binding subunit GABPβ is required for translocation of GABPα into nuclei.58 Thus, cell type-specific modification of GABPβ at the posttranslational level might control the nuclear GABP levels, which in part contributes to the myeloid-specific transcriptional activation.
An alternative possible mechanism by which the widely expressed GABP mediates myeloid-specific gene expression is through its interaction with lineage-specific nuclear factors. One of such candidate factors may be Sp1. GABP cooperates with Sp1 to activate myeloid expression of the CD1833 and neutrophil elastase genes36 and has been demonstrated to physically interact with Sp1.59 Despite its ubiquitous expression patterns, Sp1 has been shown to contact with the CD11B promoter60 and the CD11C promoter61 in a myeloid-specific manner by in vivo footprinting, suggesting that it can act as a lineage-specific regulator in this context. We have now shown that GABP physically interacts with the more lineage-restricted factor C/EBPα (Figure 6A). This suggests that GABP may mediate myeloid gene expression by binding to C/EBPα and thereby acting as a myeloid regulator with combinatorial functions. GABP is a target of Ras-Raf-ERK (Ras-Raf-extracellular signal-regulated kinase)62-65 and JNK/SAPK (c-Jun N-terminal kinase/stress-activated protein kinase) cascades66 : both GABPα and GABPβ are phosphorylated by ERK1/262,64,65 and 3 JNK/SAPK isoforms (SAPKαI, SAPKβ, and SAPKγ).66 Behre et al67 have proposed a model where Ras signaling phosphorylates the transactivation domain of C/EBPα, resulting in an enhancement of the transcriptional activity of C/EBPα to activate the granulocyte colony-stimulating factor receptor promoter, which contributes to the induction of granulocytic differentiation. Through the interaction with C/EBPα, GABP might serve as an integrator that converges additional signals arising from different extracellular stimuli, leading to the appropriate change in the patterns of myeloid gene expression. It would be interesting to address the role of phosphorylation of C/EBPα and GABP with respect to their physical interaction. Although further analyses are needed to demonstrate the means by which these factors cooperate to control myeloid transcription, our results provide the first evidence that the widely expressed Ets-related factor GABP regulates myeloid-specific transcription through direct interaction with lineage-specific C/EBPα.
Prepublished online as Blood First Edition Paper, May 31, 2005; DOI 10.1182/blood-2004-06-2413.
Supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan to promote advanced scientific research; a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science; a grant from Nihon University School of Medicine; and a grant from the Uehara Memorial Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Gretchen J. Darlington (Baylor College of Medicine, Houston, TX) for the C/EBPα expression vector; Dr Seikichi Toku (University of Ryukyus, Okinawa, Japan) for antibody against GABPβ; Dr Ko Sato (Yokohama City University School of Medicine, Yokohama, Japan) for advice on GST-pull down assay; Dr Azuma Watanabe (Department of Medicine, Nihon University School of Medicine) for experimental help.



![Figure 7. GABP cooperates with C/EBPα to strongly activate the FCAR promoter. (A,D) A schematic representation for the structure of the FCAR upstream-luciferase (Luc) constructs. The numbers indicate nucleotide positions relative to the translation initiation ATG at +1. The major transcription start site is indicated as bent arrow. X indicates mutated site. (B-C,E) HeLa cells were transfected with 0.7 μg indicated FCAR upstream-luciferase constructs in the absence or presence of 0.1 μg each of expression plasmids for GABPβ (pC3E4TF1-53S) and GABPα (pC3E4TF1-60S) or its deletion mutant, GABPαΔETS (pC3GAαΔETS) or GABPα318-454 (pC3GAα318-454), along with 3 ng C/EBPα expression plasmid (hCMV-C/EBPα) or the empty vector (pCMV-Empt) (B,E), or with 3 ng C/EBPβ expression plasmid (pD3NF-IL6) or the empty vector [pcDNA3.1(+)] (C); each sample was transfected with 5 ng pRL-CMV and the additional empty vector pCR3.1E to bring the amount of transfected DNA in each sample to 1 μg. Firefly and Renilla luciferase activities were determined 24 hours after transfection. Values are corrected for transfection efficiency and presented as the fold stimulation of each construct by the indicated transcription factor(s), compared with the promoter activity seen without the expression vector(s). Thin bars represent the standard errors (SE) from 3 to 6 transfections. *Significant decrease (P < .05) compared with pGLmCE12-259 by one-tailed t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2004-06-2413/6/m_zh80190584710007.jpeg?Expires=1765960623&Signature=31Tr0dxvQKgCCfhhpzuKw8qWI-gUmeDtN4tBPIx~pUDZCpKbKCMqDZCs6jqXaqvTVPlxOph8IV6G4-Dg~0RtZk4SO5n8YoQkQ3b~~E6dIv1qwlOyal-9riS-eEU8IBHT~XexEjNCy~uwh7mNFEpKAI0J8-Z8oq~M1vx9zaQcTba5~SeHsqZvvdAlYjoge6oynvQ6wNSXi2fyZN8DG1qV8dqYFeqDO3DzvkJZK6zbfzTnDM6if8JMb7tAcGhVH9FQPIFdggIyFpXG-5TL~DYaQxFrukCQdJiU2eg9RFz-VIzYL2H~vP3iiTVi0Slh3YSeVYa~0ymbomullpvtFKFmjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal