Abstract
Interleukin-2 (IL-2) and IL-15 are structurally related cytokines that share receptor components but display markedly different effects in multiple in vivo model systems. Here we demonstrate that IL-15 but not IL-2 exacerbates xenogeneic graft-versus-host disease (X-GVHD) in severe combined immunodeficient murine recipients of human peripheral-blood lymphocytes (hu-PBL-SCID). Treatment of hu-PBL-SCID mice with IL-15 resulted in rapid fatality, lymphocytic infiltrations in the liver, lung, and spleen consistent with X-GVHD, and a marked expansion of human CD4+ and CD8+ T cells compared with controls. Depletion of human T cells in vivo abrogated the lethality of IL-15 treatment. To our knowledge, these data are the first to demonstrate in vivo activation and expansion of human T lymphocytes in response to IL-15 with concomitant exacerbation of human T-cell-mediated X-GVHD. (Blood. 2005;106:2433-2435)
Introduction
Xenogeneic graft-versus-host disease (X-GVHD) is the only experimental model of human allogeneic GVHD that incorporates human T cells and recapitulates the T-cell expansion and tissue destruction seen in patients. Severe combined immunodeficient (SCID) mice reconstituted with human peripheral-blood lymphocytes (hu-PBL-SCID) demonstrate, albeit infrequently, lethal X-GVHD associated with high levels of human T-cell engraftment and inflammation of the liver, kidneys, spleen, and lung.1 We have previously demonstrated that administration of continuous, low-dose recombinant human IL-2 (rhIL-2) to hu-PBL-SCID mice does not increase X-GVHD mortality.2 IL-15 is structurally similar to IL-2 and uses identical β and γ receptor subunits for signaling, but is produced by antigen-presenting cells and demonstrates in vivo activities distinct from IL-2. Recently, data from our laboratory and others have shown that endogenous or exogenous administration of IL-15 increases mortality in murine models of acute allogeneic GVHD.3,4 Therefore, we have hypothesized that exogenous rhIL-15 would enhance X-GVHD mortality in hu-PBL-SCID mice in contrast to the effects of rhIL-2.
Study design
Hu-PBL-SCID mouse model
Human PBLs (5 × 107) were obtained from healthy donors under an institutional review board (IRB)-approved protocol and injected (intraperitoneal) into 8- to 12-week-old female CB17 SCID mice (Taconic Farms, Germantown, NY) pretreated with murine IL-2Rβ antibodies.2,5 This regimen eliminates host natural killer cells6 and increases human T-cell chimerism.7 Mice were killed as described.3 All animal research was approved by The Ohio State University. Approval for the use of human subjects was obtained from The Ohio State University IRB. Informed consent was provided according to the Declaration of Helsinki.
Cytokines
Animals were dosed with 10 μg/d rhIL-15 (Amgen, Thousand Oaks, CA), rhIL-2 (Proleukin; Chiron, Emeryville, CA), or phosphate-buffered saline (PBS) containing 0.05% human albumin via subcutaneous Alzet mini-osmotic pumps (Model 1007D; DURECT, Cupertino, CA) beginning 1 day after injection of human PBLs.
In vivo antibody treatment
Animals were treated with anti-human CD3 (50 μg/mouse, Orthoclone-OKT3; Ortho Biotech Products, Raritan, NJ) or control mouse immunoglobulin G (IgG; Sigma, St Louis, MO) via intraperitoneal injection on days 1 to 5 after injection of human PBLs.8
Histopathology
Cytokine bead array
The Human Th1/Th2 Cytokine Bead Array (BD Pharmingen, San Diego, CA) was used to measure IL-4, IL-5, IL-10, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) in plasma samples as described.3
Statistics
Median survival times were compared using the log-rank test. Student t test and the exact Wilcoxon rank sum test were used for the remaining comparisons.
Results and discussion
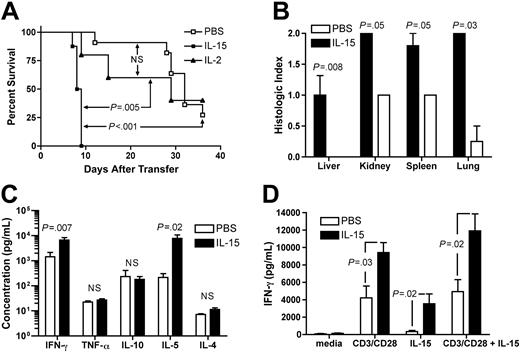
Administration of rhIL-15 to hu-PBL-SCID mice resulted in rapid morbidity and mortality compared with IL-2 (median survival time [MST], 8.4 days versus 25.0 days, P = .005; Figure 1A) or PBS control treatment (MST, 8.4 days versus 30.7 days, P < .001; Figure 1A). No statistically significant survival difference was observed between IL-2- and PBS-treated groups. Consistent with earlier reports, morbid mice developed hunched posture, inactivity, and labored breathing.1 Control SCID mice that received rhIL-15 (10 μg/d) but no human PBLs displayed 100% survival (data not shown). Blinded analysis of histopathology in mice treated with rhIL-15 revealed an increase in lymphocytic infiltrates in liver, kidney, spleen, and lung compared with PBS-treated mice (P = .008, P = .05, P = .05, and P = .03, respectively; Figure 1B). Tissue infiltrates were minimal to absent in IL-15-treated animals that did not receive hu-PBLs (data not shown). Early mortality in IL-15-treated mice was associated with elevations in serum human IFN-γ and IL-5 on day 10 after inoculation with human PBLs compared with PBS-treated mice (Figure 1C). Differences in serum human IL-10, IL-4, and TNF-α levels were not observed. Finally, in vitro stimulation of splenocytes isolated from IL-15-treated mice with rhIL-15 and/or anti-human CD3/CD28 cross-linking beads resulted in significantly higher levels of IFN-γ compared with similarly cultured splenocytes from PBS-treated control mice (Figure 1D).
Recombinant human IL-15 increases X-GVHD mortality, morbidity, and serum IFN-γ. (A) Hu-PBL-SCID mice treated with rhIL-15 demonstrated a median survival time of 8.4 days compared with 25 days for mice treated with rhIL-2 (P = .005) and 30.7 days for mice treated with PBS (P < .001). There was no statistically significant difference in survival between IL-2- and PBS-treated mice. N.S. indicates not significant. (B) Blinded analysis of X-GVHD histopathology revealed a significant increase in mononuclear-cell infiltration and tissue damage in the livers (P = .008), kidneys (P = .05), spleens (P = .05), and lungs (P = .03) of IL-15-treated mice compared with PBS-treated mice. (C) Serum was harvested from hu-PBL-SCID mice at day 10 and analyzed for the presence of human cytokines. Mice treated with rhIL-15 demonstrated increased serum human IFN-γ (P = .007) and IL-5 (P = .02) compared with PBS-treated mice. (D) Splenocytes were harvested from hu-PBL-SCID mice treated with either IL-15 or PBS and cultured (2 × 106/mL) in 24-well plates for 48 hours in the presence of anti-CD3/CD28 beads, rhIL-15 (5 ng/mL), both, or media alone. Human IFN-γ was measured in culture supernatants by cytometric bead array. Survival data were compared using the log-rank test, and all other data were compared using Student t test or the exact Wilcoxon rank sum test. Error bars indicate standard error of the mean.
Recombinant human IL-15 increases X-GVHD mortality, morbidity, and serum IFN-γ. (A) Hu-PBL-SCID mice treated with rhIL-15 demonstrated a median survival time of 8.4 days compared with 25 days for mice treated with rhIL-2 (P = .005) and 30.7 days for mice treated with PBS (P < .001). There was no statistically significant difference in survival between IL-2- and PBS-treated mice. N.S. indicates not significant. (B) Blinded analysis of X-GVHD histopathology revealed a significant increase in mononuclear-cell infiltration and tissue damage in the livers (P = .008), kidneys (P = .05), spleens (P = .05), and lungs (P = .03) of IL-15-treated mice compared with PBS-treated mice. (C) Serum was harvested from hu-PBL-SCID mice at day 10 and analyzed for the presence of human cytokines. Mice treated with rhIL-15 demonstrated increased serum human IFN-γ (P = .007) and IL-5 (P = .02) compared with PBS-treated mice. (D) Splenocytes were harvested from hu-PBL-SCID mice treated with either IL-15 or PBS and cultured (2 × 106/mL) in 24-well plates for 48 hours in the presence of anti-CD3/CD28 beads, rhIL-15 (5 ng/mL), both, or media alone. Human IFN-γ was measured in culture supernatants by cytometric bead array. Survival data were compared using the log-rank test, and all other data were compared using Student t test or the exact Wilcoxon rank sum test. Error bars indicate standard error of the mean.
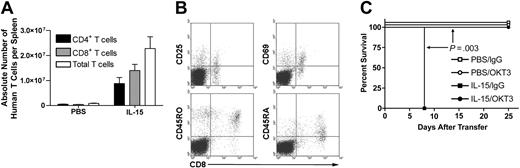
IL-15 increases T-cell engraftment in hu-PBL-SCID mice. (A) Spleens were harvested from hu-PBL-SCID mice treated with either rhIL-15 or PBS for 10 days. Mice receiving IL-15 demonstrated significantly higher engraftment with respect to the absolute number of both CD4+ and CD8+ T cells. (B) CD8+ T cells demonstrated an activated memory phenotype. Data from one representative mouse are shown. (C) Hu-PBL-SCID mice were treated with either PBS or rhIL-15 and then each group was randomized for in vivo human T-cell depletion with OKT3 antibody or control IgG. Depletion of human T cells completely abrogated IL-15-mediated X-GVHD lethality (P = .003). Survival data were compared using the log-rank test, and all other data were compared using Student t test. Error bars indicate standard error of the mean.
IL-15 increases T-cell engraftment in hu-PBL-SCID mice. (A) Spleens were harvested from hu-PBL-SCID mice treated with either rhIL-15 or PBS for 10 days. Mice receiving IL-15 demonstrated significantly higher engraftment with respect to the absolute number of both CD4+ and CD8+ T cells. (B) CD8+ T cells demonstrated an activated memory phenotype. Data from one representative mouse are shown. (C) Hu-PBL-SCID mice were treated with either PBS or rhIL-15 and then each group was randomized for in vivo human T-cell depletion with OKT3 antibody or control IgG. Depletion of human T cells completely abrogated IL-15-mediated X-GVHD lethality (P = .003). Survival data were compared using the log-rank test, and all other data were compared using Student t test. Error bars indicate standard error of the mean.
Analysis of chimerism in hu-PBL-SCID mice at day 10 after engraftment by flow cytometry revealed a significant increase in the absolute number of human T cells (P = .02; Figure 2A), human CD4+ T cells (P = .043; Figure 2A), and human CD8+ T cells (P = .012; Figure 2A) in the spleens of mice receiving rhIL-15 compared with mice receiving PBS. Consistent with the role of IL-15 in memory CD8+ T-cell activation and homeostasis,10,11 CD8+ human T cells in IL-15-treated mice displayed memory-activated phenotypes with expression of CD25, CD69, CD45RA(dim), and CD45RO (representative plots, Figure 2B). Given the significant expansion of human T cells with IL-15 treatment, we hypothesized that in vivo depletion of human T cells would protect these mice from X-GVHD mortality. In fact, treatment of hu-PBL-SCID mice with mouse anti-human CD3 (OKT3) antibody that depletes human T cells (data not shown) prevented IL-15-associated mortality compared with mice treated with rhIL-15 and control antibody (P = .003; Figure 2C). Animals treated with PBS and OKT3 also had 100% survival (Figure 2C).
To our knowledge, these experiments are the first to demonstrate that in vivo administration of rhIL-15 potently activates and expands pathogenic human T cells with the capacity to induce X-GVHD. Whether the memory T cells were selected from a pre-existing pool in the donor inoculum or whether they arose from a naive T-cell population12,13 is unclear.
It is remarkable that 100% of 27 IL-15-treated hu-PBL-SCID mice tested in 5 independent experiments developed lethal X-GVHD. This finding is in stark contrast to the historically low incidence of X-GVHD in the hu-PBL-SCID mouse, which is characteristically low due to low T-cell chimerism.1,7 Recent studies have demonstrated increased T-cell chimerism and X-GVHD when the more immunodeficient Rag2-/- γc-/- hosts are used, suggesting that the residual immune system in SCID mice presents a barrier to human T-cell engraftment.14 Our data suggest the possibility that a deficit of endogenous IL-15 production by the transplanted hu-PBLs or a failure of IL-15-producing cells to engraft may also contribute to low T-cell chimerism and the nominal level of GVHD in the absence of IL-15 treatment.
Recombinant human IL-15 increased human T-cell infiltration in the target organs of X-GVHD. Our results also show that IL-15-mediated X-GVHD can be prevented by complete T-cell depletion. These data support the recent findings of Alpdogan et al that exogenous IL-15 can be safely given in murine models of T-cell-depleted allogeneic bone marrow transplantation (BMT).4 We have now extended these findings to an in vivo xenogeneic model that incorporates elements of a competent human immune system. Exogenous IL-15 likely promoted the expansion of memory T cells that actively induced X-GVHD and/or promoted the maturation of murine and/or human dendritic cells, which in turn stimulated xenoreactive human T cells with greater efficiency.15
IL-2 and IL-15 are structurally related cytokines that share receptor components and display similar activity in vitro.16,17 However, the effect of IL-2 and IL-15 on T cells differs strikingly in vivo. IL-15-/- mice demonstrate profound lymphopenia,18 while IL-2-/- mice develop polyclonal T-cell expansions and lethal autoimmune disease, likely due to an absence of activation-induced cell death.19 IL-15 transgenic mice that overexpress a highly secreted form of IL-15 show polyclonal expansions of memory CD8+ T cells,20 while IL-2 transgenic mice demonstrate normal T-cell populations.21 Our results here show that administration of stoichiometrically equivalent doses of rhIL-2 and rhIL-15 result in significantly different survival rates of hu-PBL-SCID mice and extend the dichotomy of IL-2 and IL-15 function to a model of human GVHD.
The significant increase in human IFN-γ production in vivo seen in mice treated with rhIL-15 may provide one explanation for the rapid onset of X-GVHD seen in this model22-24 ; however, the biology of IFN-γ in this disease remains complex: in some models, the absence of donor-derived IFN-γ promotes acute GVHD.25
The results shown here demonstrate that xenografted human T cells respond to human IL-15 by expansion, activation, and tissue damage in SCID mice. As such, they strengthen the importance of murine transplantation models and further provide caution against the use of rhIL-15 as an immune adjuvant after T-cell-replete allogeneic BMT.3
Prepublished online as Blood First Edition Paper, June 23, 2005; DOI 10.1182/blood-2005-04-1597.
Supported by CA068458 and CA095426. S.R., B.W.B, and A.G.F. are recipients of a Medical Scientist Program Fellowship. R.A.B. is supported by National Institutes of Health (NIH) T32 CA09338.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Donna Bucci, Tamra Brooks, and Karen Feasel for assistance with acquisition of reagents and materials and submission of this article. We also thank Amgen for provision of IL-15.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal