Abstract
Toll-like receptor (TLR) ligands lead to the induction of proinflammatory cytokines and are potent enhancers of specific immune responses. We show here that a single systemic dose of R-848, a ligand for TLR7, potently enhanced hapten sensitization during the induction of contact hypersensitivity (CHS). However, R-848 administration also resulted in a rapid and almost complete depletion of leukocytes from the blood. This effect was transient and was associated with general induction of endothelial adhesiveness. In response to R-848, endothelial cells up-regulated adhesion molecules in vitro and in vivo and leukocytes exhibited increased rolling on endothelia in R-848-treated animals. Adhesion molecule induction appeared to be a direct effect, because endothelial cells expressed TLR7 in vitro and in vivo. After R-848 treatment, the tissue residence time of leukocytes was markedly prolonged in all major peripheral organs. The resulting transiently reduced availability of peripheral-blood leukocytes (PBLs) (TRAP) significantly inhibited otherwise potent CHS responses until the effector cells returned. Thus, although TLR7 ligands are effective adjuvants for the induction of cell-mediated immunity, they can transiently inhibit the elicitation of localized immune responses, possibly due to a systemic endothelial activation throughout the vasculature. (Blood. 2005;106:2424-2432)
Introduction
Toll-like receptors (TLRs) are a family of mammalian proteins expressed on a variety of cell types of the immune system.1 TLRs are able to recognize specific patterns conserved in microorganisms. TLR triggering leads to the induction of inflammatory responses and induces the development of specific immunity.1 Consequently, specific TLR ligands, such as polyinosinic-polycytidylic acid (poly I:C), lipopolysaccharide (LPS), imidazoquinolines, or CpG oligodeoxynucleotides, have been employed as powerful immune adjuvants and may enhance specific antitumor and antiviral immunity.2,3
TLR7 is predominantly expressed by plasmacytoid dendritic cells (DCs), myeloid DCs,4 and B cells5 and recognizes ssRNA.6,7 R-848 and the structurally related compound imiquimod (the active substance of the drug Aldara; 3M Pharmaceuticals, St Paul, MN) are artificial ligands for TLR7.8,9 These imidazoquinolines are immune response modifiers that possess potent antiviral10 and antitumoral activity when applied topically to the skin.11 This activity is in part mediated by the induction of type I interferons and inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1α (IL-1α).12 Plasmacytoid DCs have been found to be major targets of R-848, producing large amounts of interferon-α and IL-12p40 as well as up-regulating activation markers in response to R-848 both in vitro13,14 and in vivo.14 In addition, topical imiquimod has been shown to induce emigration of resident LCs from murine skin, thereby amplifying allergic contact hypersensitivity (CHS) reactions15 as well as inducing the migration of immature human DCs into draining lymph nodes of cancer patients.16 Imidazoquinolines also strongly enhance T helper-1 (Th1)-type immune responses: They induce IL-12 in human Langerhans cells (LCs)17 and lead to production of high amounts of IFN-γ and to suppression of IL-4 and IL-5 production by T cells in cultures of human peripheral-blood leukocytes (PBLs) and murine spleen cells.18 Interestingly, peripheral-blood mononuclear cells (PBMCs) of human newborns have been found to exhibit a greatly reduced ability to respond toward most classic TLR ligands, which might explain the relative inefficiency of the newborn immune system to protect against infectious diseases.19 The only exception was R-848, which could induce normal levels of proinflammatory cytokines in newborn leukocytes.19
Thus, due to their in vitro Th1-inducing capacity and due to their clinical effectiveness for topical treatment of viral and neoplastic skin diseases, imidazoquinolines are attractive candidates as immune adjuvants for systemic use—for example, for tumor immunotherapy or vaccination against infectious diseases.19 However, studies on the systemic effect of imidazoquinolines are rare and have so far not addressed effector functions of specific immune reactions, which would be the ultimate target of therapy with these compounds.
Therefore, we investigated the effects of systemic R-848 on T-cell-mediated immune responses in vivo. A single dose of systemic R-848 strongly enhanced priming for hapten-specific CHS both for Th1- or Th2-prone haptens. However, R-848 treatment also induced a profound peripheral-blood leukopenia within minutes after dosing, presumably due to generalized induction of enhanced endothelial adhesiveness. This leukocyte depletion lasted about 24 hours and had a major influence on the effector phase of peripheral immune reactions, which were markedly suppressed during the period of leukopenia. Thus, due to rapid effects on leukocyte trafficking, systemic stimulation of TLR7 by R-848 was associated with transient local immune insufficiency.
Materials and methods
Mice
Female BALB/c mice were purchased from Bomholtgard (Ry, Denmark) and housed under specific pathogen-free conditions according to the guidelines of the regional animal care committee and used at 8 to 12 weeks of age.
R-848 treatment
R-848 and imiquimod salt were provided by 3M Pharmaceuticals. For in vivo studies, R-848 was applied by injecting 100 μL R-848 (25 μg/mL in phosphate-buffered saline [PBS]) intraperitoneally 1 hour before, directly after, and 1 hour after sensitization or challenge. This amounted to a dose of 100 μg R-848 per kilogram of body weight per injection.20
Contact hypersensitivity (CHS) reaction
Epicutaneous sensitization and challenge for allergic contact dermatitis against 2,4,-dinitrofluorobenzene (DNFB; Sigma Aldrich, Deisenhofen, Germany) or fluorescein isothiocyanate (FITC; Sigma) was performed as described.21 Briefly, mice were sensitized by painting 75 μL DNFB (0.5% in acetone-olive oil, 4:1) or 75 μL FITC (3% in acetone-dibutylphtalate-dimethyl sulfoxide [DMSO], 19:19:2) on the shaved abdomen. Suboptimal sensitization was performed using 1:10, 1:30, or 1:100 dilutions of the haptens. In some groups, animals were sensitized against FITC 1 day before sensitization with DNFB. Five days (FITC, 6 days) after sensitization mice were challenged on the right ear by applying 20 μL of 0.3% DNFB or 1% FITC. In some cases one ear was challenged with DNFB, while the other ear was challenged with FITC. The normal ear thickness of these animals had been measured before application of the haptens. Ear swelling was quantified with a spring-loaded micrometer (Mitutoyo, Kawasaki, Japan) 24 hours after challenge. CHS was determined as the thickness of the hapten-challenged ear minus the thickness of the untreated ear and was expressed in hundredths of a millimeter (mean ± SD). Mice that were ear-challenged without prior sensitization served as negative controls.
Immunohistochemistry
For immunohistochemistry of ears or organs tissue, samples were removed at the indicated time points and snap-frozen in liquid nitrogen. Cryosections were prepared and stained immunohistochemically using an indirect immunoperoxidase assay, as described elsewhere.22 For detection of antibodies (Abs), goat F(ab′)2 anti-rat immunoglobulin G (IgG) conjugated with peroxidase (Dianova, Hamburg, Germany) was used as secondary Abs, and 3-amino-9-ethyl-carbazol served as chromogen. Samples were analyzed using a Zeiss Axiophot microscope (Carl Zeiss, Oberkochen, Germany) with 20×/0.5 and 40×/0.7 dry objective lenses and 10× oculars. Images were acquired with a JVC KY-F4030 camera and Diskus software (Hidgers, Koenigsminter, Germany).
Quantification of PBLs
To analyze the effect of R-848 on PBLs, venous-blood samples were obtained from the tails of mice before, 1 hour, 24 hours, and 48 hours after intraperitoneal injection of PBS or R-848. After depletion of erythrocytes by osmotic shock, blood samples were FITC stained and analyzed on a Coulter EPICS-MCL flow cytometer (Coulter, Krefeld, Germany). By analyzing a defined volume of sample, the absolute amount of CD4+, CD8+, GR-1+, or B220+ cells per microliter of blood was determined.
In vivo selectin blockade
To assess the transit time of transferred leukocytes in peripheral organs, animals were pretreated with R-848 or PBS. Thirty minutes later 5 × 107 carboxyfluorescein diacetate succinimidyl ester (CFSE)-stained spleen leukocytes were adoptively transferred. Another 30 minutes later, the synthetic E-selectin blocker CGP69669A23 and the anti-P-selectin Ab RB40.324 were injected into the animals. This stopped further extravasation of lymphocytes into peripheral organs but allowed leukocyte emigration from peripheral tissues back into the bloodstream.23 Individual animals were killed 30 or 180 minutes after application of selectin blockers, and major peripheral organs as well as blood were analyzed for the presence of transferred cells by flow cytometry. RB40.3 was a kind gift from Dietmar Vestweber, University of Münster, Germany.
Staining of TLR7
Confluent layers of BEnd5 cells were detached by treatment with a solution containing 0.025% trypsin and 0.01% EDTA (ethylenediaminetetraacetic acid) in PBS (5 minutes, 37°C). In parallel, naive B cells from the spleens of BALB/c mice were isolated as described.25 The cells were fixed for 20 minutes in fix solution from the Fix/Perm kit of Becton Dickinson (Heidelberg, Germany). After 2 washes cells were resuspended in the permeabilization (perm)/wash solution of the kit containing 32 μg/mL primary anti-TLR7 monoclonal antibody (mAb) (Zmd.312; Zymed, San Francisco, CA). After 20 minutes cells were washed twice and then stained with a secondary antirabbit mAb (5 μg/mL in perm/wash; Pharmingen, Heidelberg, Germany) for 20 minutes. Controls were stained with secondary mAb alone. After 20 minutes, cells were washed and 20 000 cells were analyzed by flow cytometry. Immunofluorescence staining of liver and spleen was done on samples embedded in TISSUE TEC medium (Miles, Maperville, IL) and frozen at -20°C. Cryosections of these samples were incubated overnight with rat anti-CD31 (5 μg/mL) and rabbit anti-TLR7 (8 μg/mL). After washing, the Abs were detected with FITC-labeled antirabbit or Texas red-labeled antirat Ab (both at 1 μg/mL) for 2 hours. After extensive washing samples were analyzed on a Leica LSM confocal microscope using 20×/0.7 and 40×/0.8 dry objective lenses and Leica TCS software (Leica, Beusheim, Germany). Color bleed-through was controlled by exiting each fluorophore independently with the relevant laser lines, thereby checking the intensity in the irrelevant channel, which was negligible with both fluorochromes. Background staining with the secondary mAbs alone was also negligible.
Direct activation of endothelial cells by R-848
Confluent layers of the endothelial cell line BEnd5 26 were incubated with 25 μg/mL R-848 in the presence or absence of 200 000 spleen leukocytes or 5 μL heparinized peripheral blood. Controls were cells without stimulus (negative) or with 10 ng/mL murine TNF-α (positive). After 4 hours, cells were fixed in paraformaldehyde (PFA) and stained for detection of intercellular adhesion molecule-1 (ICAM-1), ICAM-2, vascular cell adhesion molecule-1 (VCAM-1), E-selectin, and P-selectin using a light fluorescence microscope (Olympus BX61, Hamburg, Germany) and an attached video camera.
Intravital imaging of leukocyte rolling
From 1 × 107 to 2 × 107 CFSE-stained spleen leukocytes were adoptively transferred into BALB/c animals. Subsequently animals were prepared for intravital imaging of CFSE-stained cells within lymph node vessels as described.25 Using videomicroscopy with mercury lamp illumination, the normal flow of transferred cells in a blood vessel overlying the inguinal lymph node was recorded for 1 hour at 1 frame per second. Subsequently the animal received 75 μg/kg R-848 in PBS by intraperitoneal injection. Then the change in cellular behavior within the same vessel was recorded for at least 1 more hour. Subsequently, the obtained movies were analyzed in a blinded fashion by 3 independent examiners (M.G., A.H., Sarah Schröter) for the presence of rolling cells. At the frame rate used, most cells were only visible for 1 frame. Cells that were visible for at least 2 frames were considered rolling.
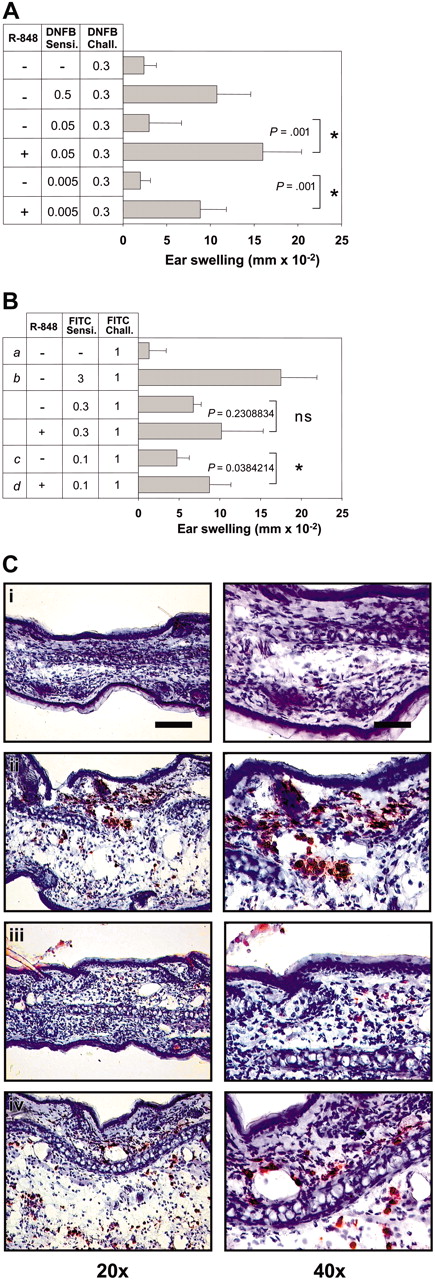
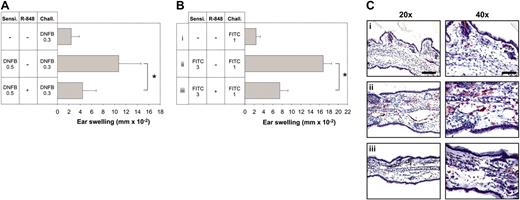
R-848 enhances the induction of CHS. Mice were sensitized by epicutaneous application of DNFB and FITC with the concentrations indicated (A and B, respectively). R-848 was injected intraperitoneally during the sensitization process. Six days (DNFB) and 7 days (FITC) later, the right ear was challenged with the respective hapten, and CHS was determined as increase in ear thickness compared with the vehicle-treated ear. Immediately after measuring ear thickness, ears were harvested for immunohistochemistry and stained with anti-Gr-1 (C). Corresponding groups in panels B and C are labeled i-iv. Data represent means of 5 to 7 mice per group. Each experiment was repeated at least 2 times. The bar represents 100 μm (left column) or 50 μm (right column). The P values for the relevant groups are indicated. Values in panels A and B represent the means ± standard deviation (SD) of all mice measured in each group. *Significant difference.
R-848 enhances the induction of CHS. Mice were sensitized by epicutaneous application of DNFB and FITC with the concentrations indicated (A and B, respectively). R-848 was injected intraperitoneally during the sensitization process. Six days (DNFB) and 7 days (FITC) later, the right ear was challenged with the respective hapten, and CHS was determined as increase in ear thickness compared with the vehicle-treated ear. Immediately after measuring ear thickness, ears were harvested for immunohistochemistry and stained with anti-Gr-1 (C). Corresponding groups in panels B and C are labeled i-iv. Data represent means of 5 to 7 mice per group. Each experiment was repeated at least 2 times. The bar represents 100 μm (left column) or 50 μm (right column). The P values for the relevant groups are indicated. Values in panels A and B represent the means ± standard deviation (SD) of all mice measured in each group. *Significant difference.
Statistical analysis
The ear-swelling response was measured in a blinded fashion. Data were analyzed by the Student t test, and differences were considered significant at P < .05.
Results
R-848 enhances the sensitization against haptens
It has been shown that hapten sensitization for CHS to DNFB can be enhanced by topical application of the TLR7 ligand imiquimod.15 We asked whether the TLR7 ligand R-848 would have the same effect after systemic application, in a setting where the substance is not directly applied to the site of immune activation. We also wanted to address whether R-848 has differential effects on the induction of Th1-type and Th-2 type immune responses. Therefore, in addition to the Th1-inducing hapten DNFB we also used the hapten FITC, which is considered to elicit a Th2 reaction.27 When R-848 was applied systemically to mice during the priming phase of CHS, it induced a strong enhancement of CHS to both DNFB and FITC (Figure 1A-B). This was particularly obvious when we reduced the sensitizing dose of haptens to a 10th or 100th (FITC, 10th to 30th) of the usual amount, where only very little response could be induced in mice without R-848 (Figure 1A-B). The enhancing effect could also be generated for both of these haptens simultaneously within a single animal (not shown). Upon challenge, only very few infiltrating Gr-1+ granulocytes were found in the ears of mice sensitized with suboptimal doses of hapten, whereas in ears from R-848-treated mice the infiltration with granulocytes was almost as strong as in control animals sensitized with the usual hapten dose (Figure 1C). Similar findings were obtained for CD8+ and CD4+ cells. Here, neither CD8 nor CD4 infiltrates could be detected by manual inspection of high-power fields (HPFs) in stained ear sections, while in R-848-treated animals 0.83 ± 0.41 CD8 and 2 ± 0 CD4 cells were detectable per HPF. This was almost the same as in animals sensitized with the full amount of hapten (1 ± 0 CD8 and 2 ± 0 CD4 cells per HPF). Thus, systemic R-848 was able to strongly enhance the sensitization phase of CHS against DNFB or FITC.
R-848 induces transiently reduced availability of PBLs (TRAP)
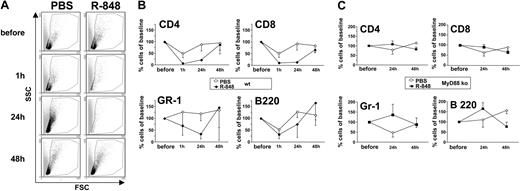
It has been shown that topical R-848 leads to an increase of LC migration.15 To test whether R-848 also affects recruitment of PBLs, we analyzed PBMCs of mice after treatment with R-848 or PBS. Surprisingly, however, R-848 strongly decreased the number of circulating PBMCs as early as 1 hour after dosing (not shown). This effect was maintained for 24 hours (Figure 2A). The PBMC count was restored to normal levels after 48 hours (Figure 2A-B). Although all major subtypes of PBMCs (CD4+, CD8+, Gr-1+, and B220+ cells) were decreased by the action of R-848, the most profound reduction was observed in CD4+ and CD8+ T cells (PBLs). One hour after administration of R-848, CD4 cells were reduced to 4.2% ± 1.2% (PBS, 47.2% ± 17.1%) and CD8 cells to 2.1% ± 9.9% (PBS, 49.8% ± 16%). After 24 hours the number of CD4 cells was at 21.7% ± 4.8% (PBS, 88.5% ± 28.3%) and CD8 at 13.6% ± 4.7% (PBS, 92.1% ± 27.2%) (Figure 2B; Table 1). Thus, R-848 leads to a transiently reduced availability of PBLs (TRAP). TRAP was dependent on TLR signaling, because the effect could not be induced by R-848 in mice deficient for MyD88 (Figure 2C), the major intracellular signaling molecule of TLR7.28
Absolute cell numbers for TRAP
Time after treatment, h, by treatment type . | Experiment no. 1 . | . | . | . | Experiment no. 2 . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CD4 (%) . | CD8 (%) . | GR-1 (%) . | B220 (%) . | CD4 (%) . | CD8 (%) . | GR-1 (%) . | B220 (%) . | ||||||
| PBS | ||||||||||||||
| 0 | 960 ± 39 (100) | 261 ± 37 (100) | 400 ± 42 (100) | 403 ± 49 (100) | 221 ± 49 (100) | 61 ± 15 (100) | 99 ± 46 (100) | 213 ± 73 (100) | ||||||
| 1 | 508 ± 149 (53) | 117 ± 19 (46) | 663 ± 83 (167) | 220 ± 66 (56) | 89 ± 38 (41) | 31 ± 9 (54) | 75 ± 15 (85) | 102 ± 36 (48) | ||||||
| 24 | 646 ± 42 (67) | 178 ± 7 (69) | 404 ± 81 (100) | 573 ± 41 (143) | 234 ± 13 (110) | 69 ± 12 (115) | 127 ± 42 (137) | 234 ± 73 (112) | ||||||
| 48 | 765 ± 59 (80) | 226 ± 14 (88) | 385 ± 94 (98) | 510 ± 69 (129) | 218 ± 103 (109) | 44 ± 15 (79) | 117 ± 75 (173) | 183 ± 69 (98) | ||||||
| R-848 | ||||||||||||||
| 0 | 848 ± 77 (100) | 214 ± 35 (100) | 369 ± 139 (100) | 11 ± 44 (100) | 257 ± 47 (100) | 80 ± 16 (100) | 136 ± 68 (100 | 222 ± 89 (100) | ||||||
| 1 | 35 ± 9 (4) | 18 ± 3 (9) | 306 ± 156 (88) | 66 ± 12 (21) | 311 ± 2 (4) | 9 ± 2 (12) | 48 ± 19 (36) | 91 ± 9 (45) | ||||||
| 24 | 195 ± 12 (23) | 29 ± 2 (14) | 151 ± 18 (45) | 349 ± 17 (113) | 52 ± 31 (20) | 11 ± 8 (13) | 21 ± 16 (14) | 37 ± 33 (15) | ||||||
| 48 | 609 ± 108 (72) | 139 ± 12 (66) | 536 ± 73 (172) | 566 ± 179 (190) | 286 ± 82 (110) | 53 ± 16 (65) | 134 ± 23 (108) | 283 ± 100 (129) | ||||||
Time after treatment, h, by treatment type . | Experiment no. 1 . | . | . | . | Experiment no. 2 . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | CD4 (%) . | CD8 (%) . | GR-1 (%) . | B220 (%) . | CD4 (%) . | CD8 (%) . | GR-1 (%) . | B220 (%) . | ||||||
| PBS | ||||||||||||||
| 0 | 960 ± 39 (100) | 261 ± 37 (100) | 400 ± 42 (100) | 403 ± 49 (100) | 221 ± 49 (100) | 61 ± 15 (100) | 99 ± 46 (100) | 213 ± 73 (100) | ||||||
| 1 | 508 ± 149 (53) | 117 ± 19 (46) | 663 ± 83 (167) | 220 ± 66 (56) | 89 ± 38 (41) | 31 ± 9 (54) | 75 ± 15 (85) | 102 ± 36 (48) | ||||||
| 24 | 646 ± 42 (67) | 178 ± 7 (69) | 404 ± 81 (100) | 573 ± 41 (143) | 234 ± 13 (110) | 69 ± 12 (115) | 127 ± 42 (137) | 234 ± 73 (112) | ||||||
| 48 | 765 ± 59 (80) | 226 ± 14 (88) | 385 ± 94 (98) | 510 ± 69 (129) | 218 ± 103 (109) | 44 ± 15 (79) | 117 ± 75 (173) | 183 ± 69 (98) | ||||||
| R-848 | ||||||||||||||
| 0 | 848 ± 77 (100) | 214 ± 35 (100) | 369 ± 139 (100) | 11 ± 44 (100) | 257 ± 47 (100) | 80 ± 16 (100) | 136 ± 68 (100 | 222 ± 89 (100) | ||||||
| 1 | 35 ± 9 (4) | 18 ± 3 (9) | 306 ± 156 (88) | 66 ± 12 (21) | 311 ± 2 (4) | 9 ± 2 (12) | 48 ± 19 (36) | 91 ± 9 (45) | ||||||
| 24 | 195 ± 12 (23) | 29 ± 2 (14) | 151 ± 18 (45) | 349 ± 17 (113) | 52 ± 31 (20) | 11 ± 8 (13) | 21 ± 16 (14) | 37 ± 33 (15) | ||||||
| 48 | 609 ± 108 (72) | 139 ± 12 (66) | 536 ± 73 (172) | 566 ± 179 (190) | 286 ± 82 (110) | 53 ± 16 (65) | 134 ± 23 (108) | 283 ± 100 (129) | ||||||
Zero hours after treatment indicates pretreatment count. Plus/minus values indicate absolute number of cells/μL blood in animals undergoing treatment either with PBS or R-848. Numbers of animals in each treatment category: experiment no. 1: PBS, n. = 3; R-848, n = 3; experiment no. 2: PBS, n = 3; R-848, n = 2. Data are based on 5 (PBS, 6) animals measured individually in 2 separate experiments. Values are presented as means ± SD of all animals measured in each experiment.
R-848 traps leukocytes in peripheral organs
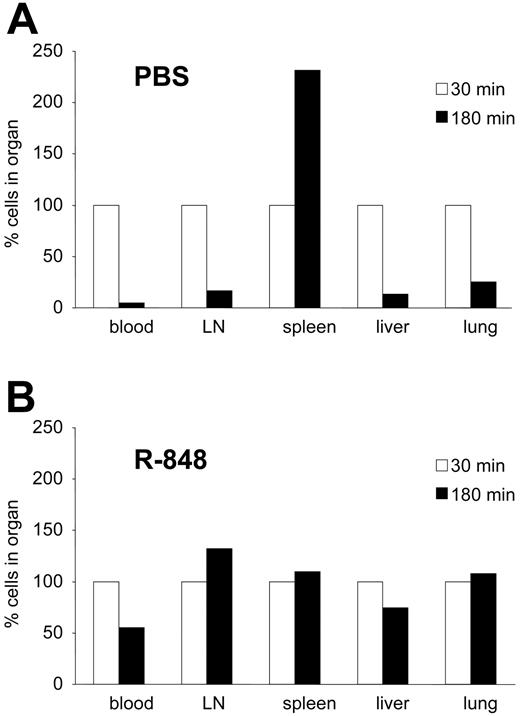
To get a better understanding of the phenomena involved in TRAP, we investigated the behavior of adoptively transferred leukocytes in animals under the influence of R-848 treatment. To this end, splenocytes from syngeneic mice were CFSE labeled and injected intravenously into animals pretreated with R-848 or NaCl. Within less than 30 minutes, almost all of the injected leukocytes had left the bloodstream. Initially, in untreated mice, cells could be detected in the lungs and liver, whereas at later time points they accumulated predominantly in the spleen, and no dramatic differences between R-848-treated and untreated mice were observed (data not shown). Thus, the turnover of blood leukocytes in mice appears to be fast (less than 30 minutes), and leukocyte emigration into peripheral tissues is not dramatically affected by R-848 treatment. Therefore, we next investigated whether the time of tissue residence of leukocytes was affected by systemic treatment with R-848. To investigate this, a combination of P- and E-selectin blockers was injected into R-848-treated and control animals 30 minutes after they had received CFSE-labeled splenocytes. As shown before,23,29 this blockade of endothelial selectins prevents further emigration of leukocytes into peripheral tissues and therefore inhibits repetitive recirculation of the transferred cells between blood and peripheral tissues. Thirty minutes or 180 minutes after this treatment, animals were killed and the peripheral blood as well as major organs were analyzed for the presence of transferred cells. Whereas most cells in control animals had left the peripheral organs and entered the spleen (Figure 3A), in R-848-pretreated animals cells remained in their original site of residence (Figure 3B). This suggested that leukocytes rapidly leave the bloodstream and remain in peripheral organs for prolonged periods in R-848-treated mice.
R-848 induces transiently reduced availability of PBLs (TRAP) in an MyD88-dependent fashion. Mice were bled from the tail vein immediately before injection of PBS or R-848. Between 24 hours and 48 hours after injection the same mice were bled again. Blood samples were cleared of erythrocytes by osmotic shock and the samples analyzed by flow cytometry for the presence of mononuclear cells (A). The same samples were also analyzed for the presence of CD4+, CD8+, GR-1+, or B220+ cells. Shown are time courses of the amount of these cell types in the blood relative to the amount present before injection of PBS or R-848 in wild-type (wt) (B) or MyD88 knock-out (ko) animals (C). Each point in panels B and C represents data of at least 3 individually measured animals (compare Table 1). Error bars indicate standard deviation.
R-848 induces transiently reduced availability of PBLs (TRAP) in an MyD88-dependent fashion. Mice were bled from the tail vein immediately before injection of PBS or R-848. Between 24 hours and 48 hours after injection the same mice were bled again. Blood samples were cleared of erythrocytes by osmotic shock and the samples analyzed by flow cytometry for the presence of mononuclear cells (A). The same samples were also analyzed for the presence of CD4+, CD8+, GR-1+, or B220+ cells. Shown are time courses of the amount of these cell types in the blood relative to the amount present before injection of PBS or R-848 in wild-type (wt) (B) or MyD88 knock-out (ko) animals (C). Each point in panels B and C represents data of at least 3 individually measured animals (compare Table 1). Error bars indicate standard deviation.
Endothelia directly react to R-848
The nonspecific trapping of PBMCs in peripheral organs suggested a general response of peripheral endothelia toward R-848. We therefore investigated whether endothelia became activated after exposure to R-848. As to be expected from a substance that releases proinflammatory cytokines in vivo, histologic examination and flow cytometry of lymph nodes, lung, liver, and spleen showed a marked increase of ICAM-1+ endothelial cells in response to R-848, which was of similar magnitude as LPS, a potent inducer of endothelial ICAM-1 30,31 (Figure S1; see the Supplemental Figures link at the top of the online article, at the Blood website). This could either be a direct effect of endothelia in response to TLR7 triggering, or the up-regulation of adhesion molecules might be mediated indirectly by the proinflammatory milieu induced by R-848. To address this question, we first investigated the expression of TLR7 by endothelial cells. The murine endothelial cell line BEnd5,26 which was used for these experiments, showed a pronounced expression of TLR7, which was comparable to that of naive splenic B cells (Figure 4B). In addition, splenic and liver endothelia were found to express TLR7 in vivo (Figure 4C; Figure S2). Thus, endothelial cells express TLR7 both in vivo and in vitro. We next addressed whether TLR7 expressed on endothelial cells was functional. Indeed, BEnd5 cells directly responded to stimulation with R-848 by up-regulation of several adhesion molecules in vitro (Figure 4A; Table 2). Remarkably, up-regulation of ICAM-2 by R-848 was even more pronounced than in response to TNF-α, a well-known stimulus for the up-regulation of adhesion molecules in endothelia.26 However, ICAM-1 could only be up-regulated by coincubation of BEnd5 with peripheral blood or spleen cells and R-848 (Table 2), suggesting that endothelia in vivo are subject to more complex interactions with factors produced by other cell types such as plasmacytoid DCs. Thus, endothelia of various organs, as well as an endothelial cell line, react to R-848 exposure with enhanced expression of adhesion molecules relevant for leukocyte attachment and transendothelial migration.
Expression of adhesion molecules on BEnd5 cells in vitro
BEnd5 cell culture treatment . | E-selectin . | P-selectin . | ICAM-1 . | ICAM-2 . | VCAM-1 . |
|---|---|---|---|---|---|
| R-848 | + | ++ | 0 | +++ | + |
| R-848 + spleen cells | ++ | 0 | ++ | ++ | 0 |
| R-848 + blood cells | + | + | ++ | +++ | + |
| TNF-α | ++ | +++ | +++ | ++ | + |
BEnd5 cell culture treatment . | E-selectin . | P-selectin . | ICAM-1 . | ICAM-2 . | VCAM-1 . |
|---|---|---|---|---|---|
| R-848 | + | ++ | 0 | +++ | + |
| R-848 + spleen cells | ++ | 0 | ++ | ++ | 0 |
| R-848 + blood cells | + | + | ++ | +++ | + |
| TNF-α | ++ | +++ | +++ | ++ | + |
Immunohistology of BEnd5 cells was examined for the expression level of the indicated molecule.
0 indicates no detectable expression; +++, very high expression.
R-848 leads to the retention of PBMCs in all peripheral organs. CFSE-stained spleen cells were adoptively transferred into animals that had been pretreated with PBS (A) or R-848 (B). Thirty minutes after transfer, blocking reagents to E- and P-selectin were injected, inhibiting further emigration to peripheral organs. An additional 30 (□) or 180 minutes (▪) later, animals were killed and the blood as well as peripheral organs were analyzed for the presence of adoptively transferred cells. The amounts found after 30 minutes were set to 100%, and the results after 180 minutes were calculated relative to this amount. In PBS-treated animals cells leave peripheral organs and home to the spleen, while in R-848-treated animals cells remain attached to all major peripheral organs with no obvious enrichment in the spleen.
R-848 leads to the retention of PBMCs in all peripheral organs. CFSE-stained spleen cells were adoptively transferred into animals that had been pretreated with PBS (A) or R-848 (B). Thirty minutes after transfer, blocking reagents to E- and P-selectin were injected, inhibiting further emigration to peripheral organs. An additional 30 (□) or 180 minutes (▪) later, animals were killed and the blood as well as peripheral organs were analyzed for the presence of adoptively transferred cells. The amounts found after 30 minutes were set to 100%, and the results after 180 minutes were calculated relative to this amount. In PBS-treated animals cells leave peripheral organs and home to the spleen, while in R-848-treated animals cells remain attached to all major peripheral organs with no obvious enrichment in the spleen.
R-848 induces rolling of PBLs on endothelia in vivo
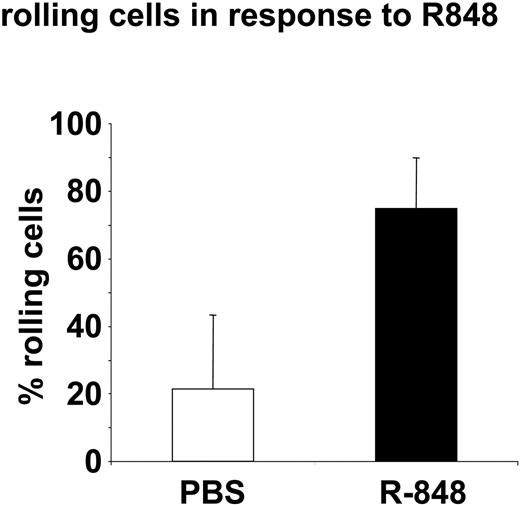
To investigate whether leukocytes exhibit enhanced adhesiveness to endothelia after R-848 treatment in vivo, we analyzed rolling and endothelial attachment of adoptively transferred leukocytes in blood vessels of live mice with intravital microscopy. Here we could observe a strong increase in the fraction of rolling cells within 1 hour after the injection of R-848, compared with animals that had received PBS (Figure 5). While in control animals, most cells were visible for only 1 frame, under the influence of R-848, cells were often visible for 10 or more frames or even sticking permanently to the endothelial wall. Thus, R-848 appears to induce an increased adhesiveness of endothelial cells in vivo, leading to increased rolling of leukocytes on endothelial walls.
TRAP inhibits peripheral immune responses
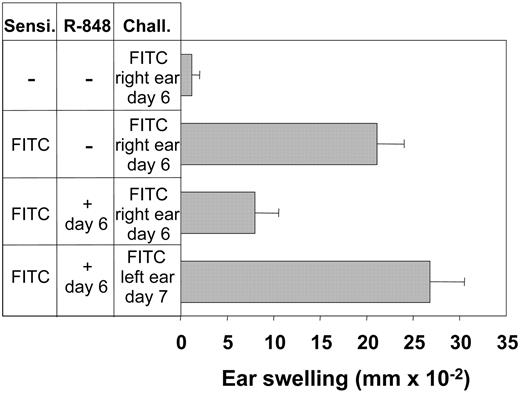
Is the observed TRAP effect an immunologically silent event due to its transient nature, or does it influence immune reactions? To clarify this question, we tested the effect of R-848 on elicitation of CHS reactions in hapten-sensitized mice. Indeed, systemic R-848 was found to strongly reduce the hapten-specific response of CHS. Ear swelling in response to both DNFB and FITC was significantly reduced when the drug was present (Figure 6A-B) The histologic examination of hapten-treated ears in animals that had received R-848 prior to hapten challenge showed almost no detectable infiltration with Gr-1+ cells (Figure 6C). Likewise, only 0.33 ± 0.52 CD4+ and 0 CD8+ cells HPF were detectable in the ear skin of mice treated with R-848 at the time of challenge. The CHS-suppressing effect of R-848 was transient, because animals that had no significant CHS reaction upon initial challenge performed at the time of R-848 application responded to a second challenge 24 hours later with a full ear-swelling reaction (Figure 7). This time course correlated well with the previously observed reduction of PBMC counts during TRAP (Figure 2). Thus, due to induction of generalized endothelial activation by R-848, PBLs had lost their ability to selectively home to inflamed tissues, resulting in locally decreased immune responses despite systemic immune activation.
Discussion
Systemic use of TLR ligands is a powerful means to stimulate immune reactions.32,33 TLR7 triggering via imiquimod or R-848 has proven its functionality as a potent and well-tolerated antiviral drug when applied locally.34-36 So far its systemic effects have been addressed mostly with regard to the induction of inflammatory molecules.37 From these studies it was suggested that the compound might be an attractive candidate for systemic use—for example, in inhibiting deleterious Th2-mediated effects or acting as adjuvant for induction of cell-mediated immune responses.37 Meanwhile, TLR7 ligands have been shown to synergize with CD40 stimulation in the long-term induction of specific CD8+ T cells in vivo, even in the absence of CD4, pointing to a direct effect on CD8+ T cells.38 Consequently, TLR7 ligands have been suggested as novel adjuvants for vaccination and immunotherapy.16,19 Despite a large set of data on the induction of cytokines and effector cells by R-848, the effect of the drug on effector-cell function has not been thoroughly addressed in vivo. Therefore, we were interested in the effects of systemic R-848 on the induction and effector phases of T-cell-mediated immune responses.
As a model system for the de novo induction of specific immune responses we chose CHS reaction toward haptens in mice,39 where the sensitization and effector phase is clearly defined and experimentally addressable. Analogous to topical imiquimod,15 systemic R-848 strongly boosted the sensitization phase of CHS (Figure 1). Especially at suboptimal hapten concentrations, a potent increase of the response against DNFB was noted, which was weaker but also clearly detectable against FITC (Figure 1). Because, by some investigators, FITC-induced CHS responses were found to be Th2 predominant,27 the weaker induction of an FITC reaction might reflect the preference of R-848 to induce a Th1-type of response,18 although the Th2 dependence of CHS responses to FITC is still a matter of debate.40 R-848 has a direct stimulating effect on B cells.41 Consequently, we also noted a profound induction of B-cell proliferation in R-848-treated mice leading to splenomegaly with large areas of confluent germinal centers within 1 week (data not shown). Possibly, this polyclonal activation of B cells, which leads to potent antigen-presenting cells,25 may also facilitate the induction of T-cell-mediated immunity against systemically available antigens.
R-848 leads to the induction of adhesion molecules on endothelial cells in vitro, and endothelia in situ express TLR7. (A) Confluent layers of the murine endothelial cell line BEnd5 were stimulated with R-848, PBS, or TNF-α for 4 hours and subsequently stained for the expression of the indicated adhesion molecules in the tissue culture. Red indicates adhesion molecules; blue, cell nuclei (DAPI [4,6 diamidino-2-phenylindole]). Bar indicates 25 μm. Data were comparable in 2 independent experiments. (B) Expression of TLR7 (gray peak) on BEnd5 cells and naive splenic B cells, which are known to express TLR7, as analyzed by flow cytometry. The white peak indicates the isotype control staining. (C) Coexpression of CD31 and TLR7 on endothelial cells of spleen tissue.
R-848 leads to the induction of adhesion molecules on endothelial cells in vitro, and endothelia in situ express TLR7. (A) Confluent layers of the murine endothelial cell line BEnd5 were stimulated with R-848, PBS, or TNF-α for 4 hours and subsequently stained for the expression of the indicated adhesion molecules in the tissue culture. Red indicates adhesion molecules; blue, cell nuclei (DAPI [4,6 diamidino-2-phenylindole]). Bar indicates 25 μm. Data were comparable in 2 independent experiments. (B) Expression of TLR7 (gray peak) on BEnd5 cells and naive splenic B cells, which are known to express TLR7, as analyzed by flow cytometry. The white peak indicates the isotype control staining. (C) Coexpression of CD31 and TLR7 on endothelial cells of spleen tissue.
Counterintuitively to the observed increase in the sensitization response we detected a transient but profound depletion of PBLs upon injection of R-848 in mice (Figure 2). Similar events have previously been observed with TLR9 ligands in vivo,42,43 but no immunologic consequence has been associated with this finding. As we show here, the R-848-induced leukodepletion (termed TRAP) has a major impact on the effector phase of both the reaction against DNFB and FITC. A normally profound swelling response was suppressed by R-848 to almost background levels (Figure 6). Because the effect was transient, the depletion of effector cells as a mechanism could be ruled out. An analysis of peripheral blood revealed that the reduction of leukocyte counts was especially pronounced in the CD4+ and CD8+ compartment (Figure 2). In mice, the induction of a CHS response has a critical time window of 2 to 6 hours after the application of the eliciting hapten, where CD4+ lymphocytes must enter the treated skin area. When the cells are not available during this period, no swelling response develops later.44 Therefore, the transient unavailability of CD4+ cells induced by R-848 might be the basis of an almost complete absence of a swelling response in treated mice.
R-848 leads to increased rolling of PBMCs on endothelia. CFSE-stained spleen cells were adoptively transferred to mice. Then the animals were subjected to intravital imaging of green cells within a blood vessel above the inguinal lymph node. After observation of the normal flow of cells, animals received injections of PBS followed by R-848 intraperitoneally. Then the observation was continued within the same vessel until 1 or 2 hours after each injection. Shown is the percentage of cells exhibiting reduced speed or rolling in the blood vessels between 40 to 60 minutes after injection of PBS or 88 ± 26 minutes after injection of R-848. Data represent the mean ± SD for 6 experiments performed with R-848 (5 experiments for PBS).
R-848 leads to increased rolling of PBMCs on endothelia. CFSE-stained spleen cells were adoptively transferred to mice. Then the animals were subjected to intravital imaging of green cells within a blood vessel above the inguinal lymph node. After observation of the normal flow of cells, animals received injections of PBS followed by R-848 intraperitoneally. Then the observation was continued within the same vessel until 1 or 2 hours after each injection. Shown is the percentage of cells exhibiting reduced speed or rolling in the blood vessels between 40 to 60 minutes after injection of PBS or 88 ± 26 minutes after injection of R-848. Data represent the mean ± SD for 6 experiments performed with R-848 (5 experiments for PBS).
R-848 inhibits the elicitation of CHS. Mice were sensitized with DNFB (A) and FITC (B), and challenge with the respective hapten was performed 5 and 6 days later. R-848 was injected during the challenge process. Twenty-four hours after challenge, CHS was determined as increase in ear thickness compared with the vehicle-treated ear. Immediately after measuring ear thickness, ears were harvested for immunohistochemical staining with anti-Gr-1 (C). Corresponding groups in panels B and C are labeled i-iii. Data represent means of 5 to 7 mice per group. Each experiment was repeated at least 2 times. The scale bar represents 100 μm (left column) or 50 μm (right column). Error bars indicate standard deviation. *Significant difference between the 2 bars.
R-848 inhibits the elicitation of CHS. Mice were sensitized with DNFB (A) and FITC (B), and challenge with the respective hapten was performed 5 and 6 days later. R-848 was injected during the challenge process. Twenty-four hours after challenge, CHS was determined as increase in ear thickness compared with the vehicle-treated ear. Immediately after measuring ear thickness, ears were harvested for immunohistochemical staining with anti-Gr-1 (C). Corresponding groups in panels B and C are labeled i-iii. Data represent means of 5 to 7 mice per group. Each experiment was repeated at least 2 times. The scale bar represents 100 μm (left column) or 50 μm (right column). Error bars indicate standard deviation. *Significant difference between the 2 bars.
The suppression of the effector phase of CHS by R-848 is transient. Mice were sensitized with FITC. Six days later one group of mice was treated with R-848, and challenge was performed on the right ear in all groups. On day 7, R-848-treated animals were rechallenged on the left ear. Twenty-four hours after challenge or rechallenge, CHS was determined as increase in ear thickness relative to the vehicle-treated ear or to the thickness of the ear before challenge. Data represent means of 5 to 7 mice per group. Error bars indicate mean ± SD.
The suppression of the effector phase of CHS by R-848 is transient. Mice were sensitized with FITC. Six days later one group of mice was treated with R-848, and challenge was performed on the right ear in all groups. On day 7, R-848-treated animals were rechallenged on the left ear. Twenty-four hours after challenge or rechallenge, CHS was determined as increase in ear thickness relative to the vehicle-treated ear or to the thickness of the ear before challenge. Data represent means of 5 to 7 mice per group. Error bars indicate mean ± SD.
The finding that TRAP is especially pronounced in T cells is remarkable, because T cells do not express TLR75 and do not respond directly to R-848 in the cell culture (data not shown). Thus, although the general effect of PBL depletion by R-848 is similar to the action of a well-known immunosuppressant, FTY720, the mechanisms involved must be different. FTY720 has been shown recently to inhibit the emigration of lymphocytes from lymphoid tissue by down-regulating a sphingosine-1-phosphate receptor, which lymphocytes need to leave lymphatic tissues.45 Instead, our data suggest that the endothelium is important for mediating TRAP. This assumption is based on the following observations: (1) In the presence of selectin blockade adoptively transferred leukocytes remained attached to the major vascularized organs (also nonlymphatic) in mice receiving R-848 (Figure 3), while in PBS-treated mice the leukocytes showed general homing to the spleen, which is known to be independent of selectin function.46 (2) Endothelial cells expressed TLR7 (Figure 4) and reacted to R-848 with the induction of adhesion receptors in vitro (Figure 4) and in vivo (not shown). (3) Intravital microscopy showed a strong increase in the fraction of leukocytes rolling on endothelia in mice within 1 hour after injection of R-848 (Figure 5). Therefore, we hypothesize that systemic R-848 leads to the induction of a “sticky” state on many, if not all, endothelia in mice, which mediates the nonspecific trapping of leukocytes on endothelial walls all around the body. It remains open as to whether leukocytes only attach to endothelial walls or also show an increased extravasation to the tissues. In addition, our finding of TLR7 on endothelial cells and its function in inducing the increased adhesiveness has similarly been described for another TLR7 family member, TLR9.47 This suggests that investigation into the function of TLRs in vivo should not exclusively be centered around immune cells alone.
In summary, we define 2 opposite effects of systemic R-848: long-term immunostimulation and transient inhibition to mount an immune response.
Physiologically, our findings shed new light on the events that might be active during viral infection. In early phases the local occurrence of viruses carrying TLR7 ligands (eg, influenza7 ) might have direct, locally restricted effects on the surrounding endothelium leading to an increased likelihood of leukocyte extravasation at the site of infection. This would be beneficial and help to resolve the infection. In the case of sepsis, however, the systemic availability of natural TLR7 ligands in the blood would lead to generalized induction of vascular adhesiveness, resulting in trapping of peripheral leukocytes either at the vessel wall (because there is no further chemotactic gradient beyond the blood vessel wall, if tissues are not acutely inflamed) or within peripheral tissues. If leukocytes adhere or extravasate diffusely, a dilution of immune effector cells ensues resulting in a limited availability of peripheral leukocytes, as has been observed in this study. This effect might lead to increased susceptibility to other infections. Indeed, leukopenia is a well-known feature of infections with influenza and other viruses both in mice48 and humans,49 as is a sharply increased susceptibility toward bacterial superinfection in influenza patients.50
In addition, our findings further substantiate the powerful role of R-848 as an adjuvant in a systemic setting in vivo, which might be of future clinical relevance. On the one hand, our data imply that approaches using TLR7 ligands systemically in the clinic should be accompanied by tight monitoring of PBMC levels and peripheral effector functions, at least for 24 hours around the application of the drug.19 On the other hand, one of the problems in tumor immunotherapy is the fact that tumor-specific T cells can be induced, but extravasation of these cells into tumor tissue is often poor. In this context, application of a TLR7 ligand may enhance leukocyte extravasation into all tissues (including tumor tissues, which are often noninflamed) and might therefore augment the effectiveness of tumor immunotherapy. Some of these hypotheses are being explored at present in our laboratories.
Prepublished online as Blood First Edition Paper, June 23, 2005; DOI 10.1182/blood-2005-01-0342.
Supported in part by research funding from 3M Medica (S.G.), and by grants of the German Research Association (S.G.) (SFB293/B1, SFB492/B2).
One of the authors (B.B.) is employed by a company (3M Medica) whose potential product was studied in the present work.
M.G., H.R., and Y.B. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr M. Tomai, 3M Pharmaceuticals, for critical review and valuable comments on the manuscript. Drs G. Varga, D. Faulhaber, and K. Loser are acknowledged for helpful discussions and help with CHS experiments. We also thank B. Engelhardt for providing the BEnd5 cell line, B. Holzmann for providing MyD88 ko animals, D. Vestweber for the anti-P-selectin Ab RB40.3, as well as A. Lengeling, B. Pasche, and H. Weich for critical discussions. The members of the Gunzer laboratory, especially Sarah Schröter and Mike Hasenberg, are acknowledged for technical support and comments on the manuscript.

![Figure 4. R-848 leads to the induction of adhesion molecules on endothelial cells in vitro, and endothelia in situ express TLR7. (A) Confluent layers of the murine endothelial cell line BEnd5 were stimulated with R-848, PBS, or TNF-α for 4 hours and subsequently stained for the expression of the indicated adhesion molecules in the tissue culture. Red indicates adhesion molecules; blue, cell nuclei (DAPI [4,6 diamidino-2-phenylindole]). Bar indicates 25 μm. Data were comparable in 2 independent experiments. (B) Expression of TLR7 (gray peak) on BEnd5 cells and naive splenic B cells, which are known to express TLR7, as analyzed by flow cytometry. The white peak indicates the isotype control staining. (C) Coexpression of CD31 and TLR7 on endothelial cells of spleen tissue.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-01-0342/6/m_zh80190584670004.jpeg?Expires=1769079376&Signature=niuoNT2QP8CamIgvsHdOGkVbyANQ2BoGSchlrReYrpe69yU~LvBTavcz9frYLXSGrjiLgm~pGN7hB3hzIFq1y~UmBLCXwK32CvpqKhmqXqZVhVVdMX1-G73Uk03CorehWCQ~nVPe9wvKDCk7hqQypxgy0zo46~bv4twMBqwe8M7S1zVi7SEQz4fEwte2vENBh3cSLdiEc9ALKUGdK5qzmIBHfhlZcFVU~OP-SqA1VvAXAZUpuBYV4FXl6tCiuhG~-mMH7I4JPcCXlBysBmRSR~WqLFLoULnih3VOYVyDnAM4CBkosBPt1cYtJnE0tHt4JVprNsEYe0WKjzY7-pfPyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal