Abstract
Donor treatment with granulocyte-colony-stimulating factor (G-CSF) attenuates the ability of donor T cells to induce acute graft-versus-host disease (aGVHD) but increases the severity of chronic GVHD (cGVHD). We investigated the role of the regulatory cytokine transforming growth factor β (TGF-β) in this paradox in well-established murine models of aGVHD and cGVHD wherein recipients undergo transplantation with splenocytes from donors treated with G-CSF. Neutralization of TGF-β after stem-cell transplantation (SCT) significantly increased the severity of aGVHD, and the concurrent prevention of interleukin-10 (IL-10) production further exaggerated this effect. Early after SCT, donor T cells were the predominant source of TGF-β and were able to attenuate aGVHD in a TGF-β-dependent fashion. Although the neutralization of TGF-β augmented the proliferation and expansion of donor T cells after SCT, it paradoxically impaired cellular cytotoxicity to host antigens and associated graft-versus-leukemia (GVL) effects. In cGVHD, neutralization of TGF-β from day 14 after SCT attenuated histologic abnormalities, and CD11b+ mononuclear cells infiltrating sclerodermatous skin produced 50-fold more TGF-β than corresponding T cells. Thus, though the production of TGF-β by donor T cells early after transplantation attenuates aGVHD and is required for optimal GVL, the production of TGF-β late after SCT is preferentially from mononuclear cells and mediates cGVHD. These data have important implications for the timing of therapeutic TGF-β neutralization to prevent cGVHD after allogeneic SCT. (Blood. 2005;106:2206-2214)
Introduction
Allogeneic stem cell transplantation (SCT) is the treatment of choice for a variety of hematologic, neoplastic, and genetic disorders. However, a significant limitation to the efficacy of allogeneic SCT is the occurrence of graft-versus-host disease (GVHD). Acute GVHD (aGVHD), developing within the first 100 days of SCT, occurs in most SCT recipients and targets the skin, gastrointestinal (GI) tract, and liver. Chronic GVHD (cGVHD), developing more than 100 days after SCT, typically results in cutaneous and hepatic fibrosis. The use of peripheral-blood stem cells (PBSCs) obtained from granulocyte-colony-stimulating factor (G-CSF)-mobilized donors has led to improved rates of immune and hemopoietic reconstitution and to improved leukemia eradication after SCT.1 It has recently become clear that donor pretreatment with G-CSF augments donor regulatory responses and attenuates aGVHD in murine models of allogeneic SCT.2 However, the incidence of extensive cGVHD is paradoxically increased in recipients of G-CSF-mobilized PBSCs, both clinically and experimentally.3,4
Transforming growth factor-β (TGF-β) is a pleiotropic cytokine with unique and potent immunoregulatory properties that has been implicated in the induction of alloantigen-specific tolerance.5 G-CSF is known to increase the production of TGF-β from CD4+ donor T cells.6 Furthermore, TGF-β has been suggested as a mediator of fibrosis in murine models of scleroderma.7 We investigated the role of TGF-β in 2 well-established murine models of aGVHD and cGVHD, specifically in the setting of allogeneic SCT rather than bone marrow transplantation. We demonstrate that TGF-β separates aGVHD and graft-versus-leukemia (GVL) after allogeneic SCT but conversely augments the severity of cGVHD. Therefore, the production of TGF-β from differential sources after allogeneic SCT provides an explanation for the opposing effects of G-CSF mobilization on the severity of aGVHD versus cGVHD after allogeneic SCT and the potential to separate GVHD and GVL.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b, Ly-5.2+, CD45.2+), B6 PTPRCA Ly-5a (H-2b, Ly-5.1+, CD45.1+), Balb/c (H-2d, Ly-9.1-), B10.D2 (H-2d, Ly-9.1+), and B6D2F1 (F1, H-2b/d, Ly-5.2+, CD45.2+)8 mice were purchased from the Animal Resource Centre (Perth, Western Australia, Australia). C57BL/6 interleukin-10-/- (IL-10-/-) mice were supplied by the Australian National University (Canberra, Australia). Mice were housed in sterilized microisolator cages and received acidified autoclaved water (pH 2.5) for the first 2 weeks after transplantation.
Cytokine treatment
Recombinant human G-CSF (Amgen, Thousand Oaks, CA) was diluted in 0.9% normal saline before injection. Mice were injected subcutaneously with human G-CSF (10 μg/dose and 2 μg/dose for aGVHD and cGVHD experiments, respectively) from day -6 to day -1, and spleens were harvested on day 0.
Stem-cell transplantation
Mice underwent transplantation according to a standard protocol previously described.4,9,10 Briefly, in aGVHD experiments, on day -1, B6D2F1 mice received 1100 cGy total body irradiation (cesium Cs 137 [137Cs] source at 108 cGy/min), split into 2 doses separated by 3 hours to minimize GI toxicity. Donor C57Bl/6 splenocytes (107 per inoculum) were injected intravenously into recipients. T-cell depletion (TCD) and T-cell purification by nylon wool enrichment were performed as previously described.11 In cGVHD experiments, on day -1, Balb/c recipient mice received 600 cGy total body irradiation (137Cs source at 108 cGy/min), which was split into 2 doses. Donor B10.D2 spleens from G-CSF or control antibody-treated donors were mashed, and then whole, unseparated spleen cells (12.5 × 107 per inoculum) were resuspended in 0.5 mL Leibovitz L-15 media (Gibco BRL, Gaithersburg, MD) and injected intravenously into recipients. All animals were monitored daily, and each recipient's body weight and GVHD clinical score were measured weekly and then more frequently once GVHD developed (see “Assessment of GVHD”). In GVL experiments, animals underwent transplantation with T-cell-replete or T-cell-depleted spleens from G-CSF-treated donors in the presence of anti-TGF-β or control antibody and 5 × 104 host-type P815 cells. Animals were monitored daily, and the presence of hind limb paralysis or characteristic nodular hepatosplenomegaly after death was deemed leukemic death, as previously described.12,13 In the absence of these features of leukemia and in the presence of GVHD as defined by clinical scores higher than 4, death was deemed to have been caused by GVHD. In some experiments, lower doses of P815 (2 × 104) transfected with a luciferase reporter gene were transferred, and animals were imaged after SCT with the IVIS 100 imaging system (Xenogen; Alameda, CA), as previously described.14
TGF-β neutralization
SCT recipients in the aGVHD model were treated with anti-TGF-β antibody (1D11) or an irrelevant isotype-matched control antibody (13C4) by intraperitoneal injection at day 0 and then 3 times weekly. The 1D11 antibody neutralizes all 3 isoforms of TGF-β.15 Antibodies were supplied by Genzyme (Boston, MA) and diluted in 0.9% saline before injection at 100 μg/dose. BMT recipients in the cGVHD model were treated in an identical schedule but received 20-μg doses because preliminary experiments confirmed this provided protection identical to that of 100-μg doses.
Assessment of GVHD
The degree of systemic GVHD was assessed by a scoring system that sums changes in 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (maximum index, 10).12,16,17 Animals with severe clinical GVHD (cGVHD) (score ≥ 6) were killed according to institutional animal ethics requirements, and the day of death was deemed to be the following day.
Fluorescence-activated cell sorter and engraftment analyses
Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) H-2b, H-2d, Ly-9.1, CD3, CD4, CD8, CD11b, CD11c, CD45.1, CD45.2, class 2, and Gr-1 and identical phycoerythrin (PE)- or biotin-conjugated antibodies were purchased from PharMingen (San Diego, CA). For engraftment studies in the aGVHD model, lethally irradiated B6D2F1 recipients (H2b/d) received whole spleen from C57Bl/6 donors (H2b). The percentage donor engraftment of peripheral-blood nucleated cells and splenocytes was defined as the percentage of H2bpos/H2dneg cells divided by the percentage of H2bpos/H2dneg + H2b/dpos cells. Donor cell engraftment in the B10.D2→Balb/c model of cGVHD was determined by examining the proportion of Ly-9.1-/(Ly-9.1- + Ly-9.1+) cells in peripheral blood after transplantation. Apoptotic lymphocytes were determined as AnnexinVpos/7-AADneg cells by labeling splenocytes with PE-conjugated Annexin V and 7-AAD (PharMingen), as previously described.18
Cell cultures
Mixed lymphocyte culture (MLC) and proliferation, as determined by the incorporation of [3H]-thymidine, were undertaken as previously described.11 To study the effect of TGF-β on cytotoxic T lymphocyte (CTL) induction in vitro, B6 (H-2b) T cells were enriched to greater than 85% purity by magnetic depletion of antibody-coated (CD19, B220, Gr-1, Ter-119, and CD11b) cells with Biomag beads (Qiagen, Hilden, Germany) and were stimulated with irradiated B6D2F1 (H-2b/d) splenocytes in the presence or absence of exogenous human TGF-β1 (R&D Systems, Minneapolis, MN). Seven days later, responding cells were washed, restimulated for another 3 days with irradiated splenocytes, and then used as effectors in cytotoxicity assays by standard chromium Cr 51 (51Cr) release assays, as previously described.11,13 In the 51Cr release assays ex vivo, 12 days after SCT, splenocytes from transplant recipients were pooled within the treatment group, and equal numbers of CD8+ effector cells (based on the CD8 staining of the input cells) were plated with 51Cr-labeled P815 (H2d) and EL4 (H2b) target cells.
CFSE labeling and assessment of in vivo cytotoxicity
Fluorescein labeling of T cells was performed as described.19 T cells were resuspended at a density of 3 × 107 cells/mL in phosphate-buffered saline (PBS), and carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) was added at a final concentration of 2 μM. Cells were analyzed using FACScan (BD Biosciences), and the data were processed with ModFit LT cell cycle analysis software (Verity Software House, Topsham, ME). The cytotoxic response to host antigens in vivo was studied in allogeneic B6D2F1 recipients of C57Bl/6 (H-2b, CD45.2+) splenocytes 12 days after SCT. Briefly, at day +12, recipients received equal numbers (20 × 106 cells/inoculum) of syngeneic B6 PTPRCA (H-2b, CD45.1+) unlabeled splenocytes and allogeneic B6D2F1 (H-2b/d, CD45.2+) CFSE-labeled splenocytes resuspended in L-15 medium by tail vein injection. Sixteen hours later, animals were bled, erythrocytes were lysed by hypotonic shock, and peripheral-blood leukocytes were stained with CD45.1 mAb. Percentages of remaining CD45.1+ and CFSE-labeled cells were determined by FACScan (BD Biosciences), and the index of in vivo cytotoxicity (measuring the in vivo cytotoxic response to allogeneic targets) was determined as the percentage of remaining syngeneic CD45.1+ cells divided by the percentage of remaining allogeneic CFSE+ cells.
Cytokine enzyme-linked immunosorbent assay and real-time PCR
Antibodies used in the interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) assays were purchased from PharMingen or Biolegend (San Diego, CA). All assays were performed according to the manufacturer's protocol. For real-time polymerase chain reaction (PCR) analysis of TGF-β, equivalent numbers of Mo-Flo-sorted cells were resuspended in Trizol (Gibco BRL, Carlsbad, CA) and snap frozen on dry ice, and RNA was extracted according to the manufacturer's protocol. cDNA was immediately reverse transcribed using AMVRT (Promega, Madison, WI) according to the manufacturer's protocol. cDNA was stored at -20°C until used. Real-time PCR was undertaken using Platinum SYBR Green qPCR SuperMix UDG (Invitrogen, Carlsbad, CA) carried out on a Rotor-Gene3000 (Corbett Research, Sydney, Australia), and data were analyzed using Rotor-Gene V-5.0 (Corbett Research). TGF-β cDNA copy numbers were then normalized for variations in the efficiency of RNA extraction and cDNA transcription against the β-2 microglobulin (B2M) housekeeping gene. Primers used for Tgfb reactions were GAG AGC GCT CAT CTC GAT TT (forward) and GGG TCT CCC AAG GAA AGG TA (reverse). Primers used for B2m reactions were TTT CTG GTG CTT GTC TCA CTG ACC G (forward) and GCA GTT CAG TAT GTT CGG CTT CCC A (reverse).
Histology/immunohistochemistry/preparation of skin cells for flow cytometry
Formalin-preserved skin, liver, and distal small bowel was embedded in paraffin, and 5-μm thick sections were stained with hematoxylin and eosin for histologic examination. Slides were coded and examined in a blinded fashion by one of the authors (A.D.C.) using a semiquantitative scoring system for abnormalities known to be associated with GVHD, as previously described.11,16,17,20 Scores were added to provide a total score of 24 for the skin, 28 for small bowel, and 40 for the liver. For immunohistochemistry, skin samples were embedded in optimal cutting temperature compound (ProSciTech, Queensland, Australia), snap frozen on dry ice, and stored at -70°C until analyzed. Frozen sections were stained using an avidin-biotin immunoperoxidase system (DAKO, Carpinteria, CA). Briefly, 5-μm-thick sections were fixed in acetone and rehydrated in PBS, and endogenous peroxidase was blocked with peroxidase blocking reagent (DAKO). Sections were then blocked for 15 minutes in 10% rat serum/10% fetal calf serum (FCS) in PBS followed by 60-minute incubation in biotinylated primary antibody. Staining was visualized with the streptavidin-biotin detection system (DAKO), as recommended by the manufacturer. Subsequently, the sections were counterstained with hematoxylin and imaged using an Olympus BX41 light microscope (Olympus, Hamburg, Germany) featuring its own camera and acquisition software, and equipped with a 100×/1.25 oil-immersion objective lens (Leica Microsystems, Wetzlar, Germany). Antibodies used were CD3 (KT31.1), CD11b (TIB128), and irrelevant isotype-matched antibodies. Cell suspensions from equivalently (1 cm2) sized skin samples were prepared for flow cytometry, as previously described.7
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. The Mann-Whitney U test was used for statistical analysis of the cytotoxicity data, cytokine data, and clinical scores. P less than .05 was considered statistically significant.
Results
Donor treatment with G-CSF induces TGF-β-dependent protection from aGVHD
Donor treatment with G-CSF attenuates the ability of donor T cells to induce GVHD after allogeneic SCT, but the mechanisms underlying this protective effect are complex; recent data suggest a process of active regulation.2 G-CSF is known to increase the production of TGF-β from CD4+ donor T cells.6 Therefore, we speculated that the production of TGF-β by donor cells may be an important mechanism controlling aGVHD after allogeneic SCT. We studied the effect of TGF-β inhibition on aGVHD mortality and morbidity in a well-established murine SCT model (B6→B6D2F1) that induces GVHD to major and minor histocompatibility antigens. Allogeneic B6 and syngeneic B6D2F1 donors received human G-CSF, and recipients received anti-TGF-β or control antibody, as described in “Materials and methods.” As shown in Table 1, 100% of non-GVHD controls that underwent transplantation with syngeneic splenocytes and received anti-TGF-β survived to day 35, confirming that this splenocyte dose contained sufficient stem cells to rescue lethally irradiated recipients and that anti-TGF-β treatment on its own did not induce non-GVHD-related mortality. Survival after allogeneic SCT is TGF-β dependent because TGF-β neutralization enhanced mortality; only 17% of allogeneic recipients treated with anti-TGF-β antibody survived the period of observation compared with 83% receiving control antibody (Table 1; P < .01). Furthermore, clinical GVHD, assessed by clinical scores in surviving animals at days 7, 14, and 21, demonstrated that anti-TGF-β-treated allogeneic recipients had more severe GVHD than the control antibody-treated group (Table 1).
TGF-β attenuates aGVHD after allogeneic SCT
. | G-CSF allogeneic anti-TGF-β . | G-CSF allogeneic control antibody . | G-CSF syngeneic anti-TGF-β . |
|---|---|---|---|
| Day 35 survival, % | 17* | 83 | 100 |
| Clinical score, mean ± SE | |||
| Day 7 | 4.6 ± 0.1* | 3.3 ± 0.2 | 1.8 ± 0.6 |
| Day 14 | 5.0 ± 0.4* | 2.8 ± 0.3 | 0 ± 0 |
| Day 21 | 5.3 ± 0.9† | 3.2 ± 0.2 | 0 ± 0 |
. | G-CSF allogeneic anti-TGF-β . | G-CSF allogeneic control antibody . | G-CSF syngeneic anti-TGF-β . |
|---|---|---|---|
| Day 35 survival, % | 17* | 83 | 100 |
| Clinical score, mean ± SE | |||
| Day 7 | 4.6 ± 0.1* | 3.3 ± 0.2 | 1.8 ± 0.6 |
| Day 14 | 5.0 ± 0.4* | 2.8 ± 0.3 | 0 ± 0 |
| Day 21 | 5.3 ± 0.9† | 3.2 ± 0.2 | 0 ± 0 |
Lethally irradiated B6D2F1 recipients underwent transplantation with splenocytes from G-CSF-treated allogeneic B6 (n = 6 per group) or syngeneic B6D2F1 (n = 4) donors, as described in “Materials and methods.” Recipients received anti-TGF-β or control antibody (100 μg/dose intraperitoneally) at day 0 and then 3 times weekly until day 35. Transplant recipients were monitored for survival and clinical score, as described in “Materials and methods.”
*P < 0.1 and †P < .05 compared with G-CSF control antibody and syngeneic groups. Results represent 1 of 3 similar experiments.
Neutralization of TGF-β promotes expansion and proliferation of donor T cells
To characterize the effects of TGF-β neutralization on donor T-cell function, we studied the proliferative response of donor T cells to alloantigen 14 days after SCT. As shown in Figure 1A, TGF-β neutralization augmented donor splenocyte proliferation to alloantigen relative to those from allogeneic SCT recipients treated with control antibody. Furthermore, the neutralization of TGF-β enhanced the expansion of all donor cell populations after transplantation (Figure 1B). Although IFN-γ levels in the sera of recipients (Figure 1C) were significantly higher in allogeneic SCT recipients treated with anti-TGF-β than in the control group (P < .05), this was not associated with an increase in systemic TNF-α production or GI tract abnormality.
To analyze whether the observed increase in absolute numbers of donor T cells after TGF-β neutralization reflected early effects on T-cell activation and expansion or later effects during differentiation, we undertook CFSE studies of T-cell proliferation in vivo. The proliferation index (fold expansion of input donor cells) of donor CD4+ T cells was increased relative to donor CD8+ T cells in allogeneic recipients, consistent with the CD4-dependent nature of the GVHD model (Figure 2). However, neutralization of TGF-β did not alter the proliferation of CD8+ donor T cells in allogeneic or syngeneic SCT recipients and had only a minor effect on the proliferation of CD4+ donor T cells in allogeneic SCT recipients. Neutralization of TGF-β increased the proportion of CD4 and CD8 cells undergoing apoptosis (annexin Vpos7-AADneg) 5 days after transplantation 2.3-fold and 1.7-fold, respectively; however, the proportions of cells undergoing apoptosis thereafter were equivalent (data not shown). Thus, differential effects of TGF-β neutralization on apoptosis in CD4 compared with CD8 populations did not appear responsible for the differences in CD8 expansion seen late after SCT (Figure 1B), suggesting a differential effect of TGF-β neutralization on the proliferation of CD8 compared with CD4 T cells in the latter phase of their expansion. Thus, TGF-β neutralization did not mediate effects on the proinflammatory axis of GVHD but instead modified donor CD4+ T-cell expansion early after SCT and subsequent donor CD8+ T-cell expansion late after SCT.
TGF-β and IL-10 from donor cells provide additive protection from aGVHD after allogeneic SCT
We have previously shown that protection from aGVHD afforded by donor treatment with pegylated G-CSF is mediated in part through IL-10 production from the donor T cell.2 Because TGF-β and IL-10 have similar and overlapping roles in regulation by the adaptive immune system, we next determined whether the protective effect of TGF-β on aGVHD occurred in association with IL-10. We compared survival and clinical scores of aGVHD in SCT recipients in which TGF-β was neutralized in the presence or absence of IL-10 production. As shown in Figure 3A, the survival rate at day 40 was 100% in recipients of syngeneic unfractionated splenocytes or IL-10-/- allogeneic TCD splenocytes treated with anti-TGF-β, confirming that adequate numbers of stem cells were transferred to allow hemopoietic reconstitution and that the inhibition of anti-TGF-β and IL-10 did not induce death in the absence of alloreactivity. Consistent with the data presented in Table 1, aGVHD was exacerbated after TGF-β neutralization. In addition, allogeneic SCT recipients of IL-10-/- splenocytes treated with control antibody survived for a median of 31 days (data not shown), which was significantly inferior to the median survival of allogeneic recipients of wild-type splenocytes treated with control antibody (median survival, more than 35 days; P < .05). Moreover, mortality in recipients of IL-10-/- splenocytes treated with anti-TGF-β was accelerated further (median survival, 17 days; P < .01 relative to recipients of wild-type splenocytes and anti-TGF-β antibody; P < .0001 relative to recipients of wild-type splenocytes and control antibody). cGVHD, assessed by clinical scores in surviving animals, was more severe in SCT recipients treated with anti-TGF-β antibody on days 14, 21, and 28 (P < .05) compared with recipients treated with control antibody (Figure 3B). There was a further aggravation of cGVHD in recipients of IL-10-/- splenocytes and anti-TGF-β antibody compared with recipients of wild-type splenocytes and anti-TGF-β antibody (Figure 3B) and with recipients of IL-10-/- splenocytes plus control antibody (data not shown). These data suggest GVHD that develops after allogeneic SCT is attenuated by the additive effects of donor-derived TGF-β and IL-10.
TGF-β neutralization augments donor T-cell proliferation and expansion to host antigens late after SCT. (A) Lethally irradiated B6D2F1 recipients underwent transplantation with G-CSF-treated B6 splenocytes and received anti-TGF-β (n = 5) or control antibody (n = 5) on day 0 and then 3 times weekly. On day+14, splenocytes from transplant recipients were pooled within the treatment group (numbers of CD3+ cells were equilibrated between the groups based on the CD3 staining of the input cells) and were stimulated with irradiated B6D2F1 peritoneal macrophages. Proliferation was measured at 48 hours by standard [3H]-thymidine incorporation assay, as described in “Materials and methods.” □ indicates allogeneic plus anti-TGF-β antibody; ○, allogeneic plus control antibody. Proliferation of nonstimulated splenocytes was 1157 ± 43.32 cpm and 1197 ± 90.39 cpm (allo plus anti-TGF-β antibody and allo plus control antibody, respectively). Results from one of 2 identical experiments. (B) Recipients received anti-TGF-β (□, n = 5-9) or control antibody (▪, n = 5-10), as in panel A. The absolute numbers of lineage and donor (H2bpos/H2dneg) cells were determined per spleen (× 106) 14 days after SCT. *P < .05; anti-TGF-β compared with control antibody. Pooled data from 2 experiments, expressed as mean ± SE. (C) B6D2F1 recipients underwent transplantation with G-CSF-treated donor B6 or B6D2F1 splenocytes and received anti-TGF-β (□, n = 5) or control antibody (▪, n = 5), as described in “Materials and methods.” Syngeneic recipients ( , n = 4) received control antibody. IFN-γ and TNF-α were determined in the sera of animals 5 and 14 days, respectively, after SCT by enzyme-linked immunosorbent assay (ELISA). GI tract abnormalities were determined 14 days after transplantation by semiquantitative histologic examination, as described in “Materials and methods.” Data are presented as mean ± SE.
, n = 4) received control antibody. IFN-γ and TNF-α were determined in the sera of animals 5 and 14 days, respectively, after SCT by enzyme-linked immunosorbent assay (ELISA). GI tract abnormalities were determined 14 days after transplantation by semiquantitative histologic examination, as described in “Materials and methods.” Data are presented as mean ± SE.
TGF-β neutralization augments donor T-cell proliferation and expansion to host antigens late after SCT. (A) Lethally irradiated B6D2F1 recipients underwent transplantation with G-CSF-treated B6 splenocytes and received anti-TGF-β (n = 5) or control antibody (n = 5) on day 0 and then 3 times weekly. On day+14, splenocytes from transplant recipients were pooled within the treatment group (numbers of CD3+ cells were equilibrated between the groups based on the CD3 staining of the input cells) and were stimulated with irradiated B6D2F1 peritoneal macrophages. Proliferation was measured at 48 hours by standard [3H]-thymidine incorporation assay, as described in “Materials and methods.” □ indicates allogeneic plus anti-TGF-β antibody; ○, allogeneic plus control antibody. Proliferation of nonstimulated splenocytes was 1157 ± 43.32 cpm and 1197 ± 90.39 cpm (allo plus anti-TGF-β antibody and allo plus control antibody, respectively). Results from one of 2 identical experiments. (B) Recipients received anti-TGF-β (□, n = 5-9) or control antibody (▪, n = 5-10), as in panel A. The absolute numbers of lineage and donor (H2bpos/H2dneg) cells were determined per spleen (× 106) 14 days after SCT. *P < .05; anti-TGF-β compared with control antibody. Pooled data from 2 experiments, expressed as mean ± SE. (C) B6D2F1 recipients underwent transplantation with G-CSF-treated donor B6 or B6D2F1 splenocytes and received anti-TGF-β (□, n = 5) or control antibody (▪, n = 5), as described in “Materials and methods.” Syngeneic recipients ( , n = 4) received control antibody. IFN-γ and TNF-α were determined in the sera of animals 5 and 14 days, respectively, after SCT by enzyme-linked immunosorbent assay (ELISA). GI tract abnormalities were determined 14 days after transplantation by semiquantitative histologic examination, as described in “Materials and methods.” Data are presented as mean ± SE.
, n = 4) received control antibody. IFN-γ and TNF-α were determined in the sera of animals 5 and 14 days, respectively, after SCT by enzyme-linked immunosorbent assay (ELISA). GI tract abnormalities were determined 14 days after transplantation by semiquantitative histologic examination, as described in “Materials and methods.” Data are presented as mean ± SE.
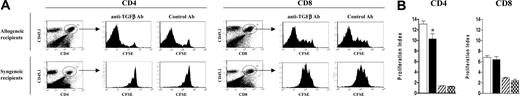
Effect of TGF-β neutralization on the expansion and proliferation of donor T cells early after SCT. B6 PTPRCA donors were treated with G-CSF, and purified T cells were labeled with CFSE before transplantation into lethally irradiated allogeneic B6D2F1 or syngeneic C57Bl/6 recipients. (A) Anti-TGF-β or control antibody was injected on days 0 and +1 after transplantation to allogeneic or syngeneic recipients (n = 3 per group), and CFSE intensity was determined in donor (CD45.1+) CD4+ or CD8+ cells 3 days after SCT. (B) The proliferation index (fold expansion of cell population over baseline) of donor CD4+ and CD8+ T cells was determined by Modfit analysis of CFSE intensity 3 days after SCT. Data are expressed as mean ± SE. *P < .05; anti-TGF-β compared with control antibody. □ indicates allogeneic anti-TGFβ Ab; ▪, allogeneic control Ab; ▨, syngeneic anti-TGF-β Ab; and  , syngeneic control Ab.
, syngeneic control Ab.
Effect of TGF-β neutralization on the expansion and proliferation of donor T cells early after SCT. B6 PTPRCA donors were treated with G-CSF, and purified T cells were labeled with CFSE before transplantation into lethally irradiated allogeneic B6D2F1 or syngeneic C57Bl/6 recipients. (A) Anti-TGF-β or control antibody was injected on days 0 and +1 after transplantation to allogeneic or syngeneic recipients (n = 3 per group), and CFSE intensity was determined in donor (CD45.1+) CD4+ or CD8+ cells 3 days after SCT. (B) The proliferation index (fold expansion of cell population over baseline) of donor CD4+ and CD8+ T cells was determined by Modfit analysis of CFSE intensity 3 days after SCT. Data are expressed as mean ± SE. *P < .05; anti-TGF-β compared with control antibody. □ indicates allogeneic anti-TGFβ Ab; ▪, allogeneic control Ab; ▨, syngeneic anti-TGF-β Ab; and  , syngeneic control Ab.
, syngeneic control Ab.
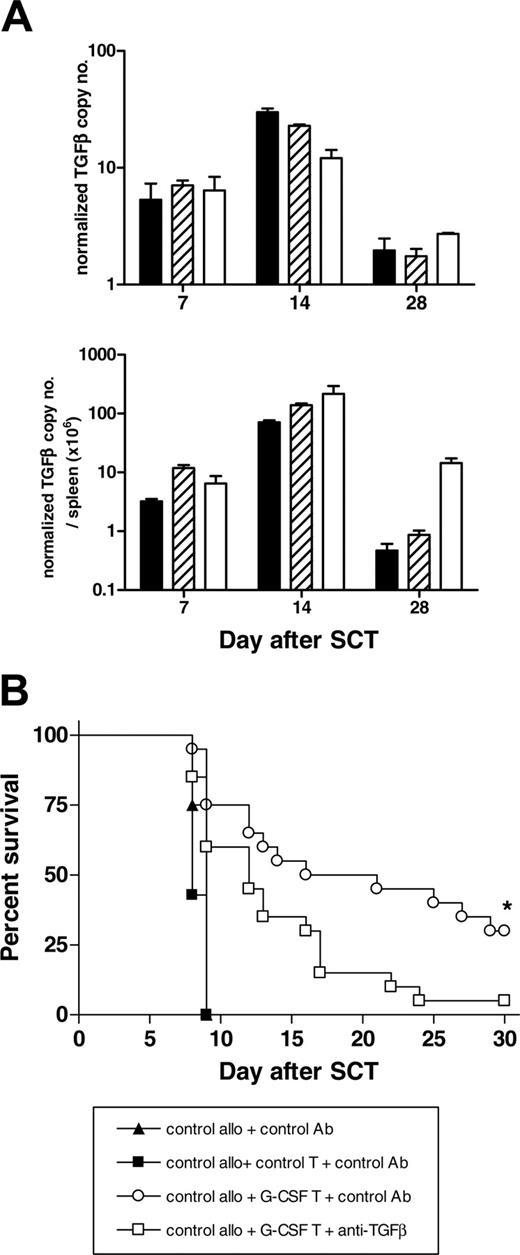
To characterize the source of TGF-β production during aGVHD, donor cells were purified from the spleens of SCT recipients 7, 14, and 28 days after SCT, and levels of TGF-β RNA expression were determined by real-time PCR. As shown in Figure 4A, TGF-β was derived predominantly from donor T cells in the first 2 weeks after SCT, with both the CD4+ and the CD8+ compartment contributing equally. At later times, non-T cells (predominantly CD11b+) become the dominant source of TGF-β. To establish that donor T cells from G-CSF-treated donors were able to regulate aGVHD, T cells from G-CSF- or control antibody-treated donors were added to splenocytes from control antibody-treated animals and were transplanted into lethally irradiated B6D2F1 recipients with subsequent control or anti-TGF-β antibody administration. As shown in Figure 4B, T cells from G-CSF-treated donors were able to attenuate lethal aGVHD induced by control T cells, and this effect was significantly impaired by the neutralization of TGF-β. Thus, the production of TGF-β by donor T cells is one of the regulatory mechanisms protecting against aGVHD early after allogeneic SCT.
TGF-β neutralization impairs cytotoxicity and GVL effects
Given that TGF-β appeared to predominantly modify donor T-cell responses after allogeneic SCT, we next studied the cytotoxic activity of T cells from anti-TGF-β and control antibody-treated recipients in vitro and in vivo. Surprisingly, cytotoxic activity in chromium release assays against host-type P815 targets was significantly impaired in splenocytes from anti-TGF-β-treated recipients relative to those from control antibody-treated recipients (Figure 5A). To confirm this was also the case in vivo, we developed a cytotoxicity assay that reflected the killing of CD45 disparate donor versus CFSE-labeled host splenocytes after SCT in vivo. Cytotoxicity in these assays was absolutely dependent on CD8+ donor T-cell effector function, though help from donor CD4+ T cells was required because the cytotoxicity index was reduced by 39% in the absence of CD4+ T cells (data not shown). As shown in Figure 5B, this assay also demonstrated a marked inhibition of cytotoxicity against host antigens when TGF-β was neutralized after allogeneic SCT, whereas there was no effect after syngeneic SCT. To further study the paradoxical effect of TGF-β neutralization on cytotoxicity seen in vivo, T cells were stimulated with alloantigen in the presence of exogenous TGF-β1 for 7 days in vitro. Responding T cells were then restimulated without further TGF-β1, and cytotoxicity was determined by chromium release assays 3 days later. As demonstrated in Figure 5C-D, exogenous TGF-β1 at a concentration of 2.5 ng/mL inhibited the expansion of CD8+ T cells to alloantigen but paradoxically enhanced their cytotoxicity, consistent with the effect of TGF-β neutralization seen in vivo. This effect was dose specific because high doses (10 ng/mL) of exogenous TGF-β1 inhibited cytotoxicity, whereas lower doses (1 ng/mL) had no effect (data not shown).
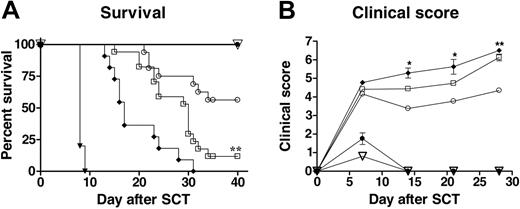
Donor treatment with G-CSF prevents aGVHD in a TGF-β- and IL-10-dependent manner. (A) Survival curves by Kaplan-Meier analysis, pooled from 3 experiments. B6 wild-type (wt) or B6 IL-10-/- donors and B6D2F1 donors were treated for 6 days with human G-CSF or control diluent, and splenocytes were transplanted into lethally irradiated B6D2F1 mice. Recipients received anti-TGF-β or control antibody at day 0 and then 3 times weekly. **P < .01; allogeneic recipients of G-CSF-pretreated wild-type splenocytes plus anti-TGF-β antibody (n = 17) compared with control antibody (n = 16) and IL-10-/- splenocytes treated with anti-TGF-β (n = 11). All allogeneic recipients of control pretreated B6 spleens treated after transplantation with control antibody died by day 14 (n = 5). All control allogeneic recipients of TCD IL-10-/- splenocytes (n = 5) and control syngeneic recipients of B6D2F1 splenocytes (n = 4) treated with anti-TGF-β antibody survived the period of observation. (B) GVHD clinical scores were determined as a measure of GVHD severity in animals, as described in “Materials and methods.” *P < .05 and **P < .01; allogeneic G-CSF wt and IL-10-/- recipients plus anti-TGF-β compared with control antibody. ▾ indicates control wt allo plus control Ab; ○, G-CSF wt allo plus control Ab; ♦, G-CSF IL-10-/- allo plus anti-TGFβ; □, G-CSF wt allo plus anti-TGFβ; •, G-CSF allo TCD plus anti-TGF-β; and ▿, control syn wt plus anti-TGFβ.
Donor treatment with G-CSF prevents aGVHD in a TGF-β- and IL-10-dependent manner. (A) Survival curves by Kaplan-Meier analysis, pooled from 3 experiments. B6 wild-type (wt) or B6 IL-10-/- donors and B6D2F1 donors were treated for 6 days with human G-CSF or control diluent, and splenocytes were transplanted into lethally irradiated B6D2F1 mice. Recipients received anti-TGF-β or control antibody at day 0 and then 3 times weekly. **P < .01; allogeneic recipients of G-CSF-pretreated wild-type splenocytes plus anti-TGF-β antibody (n = 17) compared with control antibody (n = 16) and IL-10-/- splenocytes treated with anti-TGF-β (n = 11). All allogeneic recipients of control pretreated B6 spleens treated after transplantation with control antibody died by day 14 (n = 5). All control allogeneic recipients of TCD IL-10-/- splenocytes (n = 5) and control syngeneic recipients of B6D2F1 splenocytes (n = 4) treated with anti-TGF-β antibody survived the period of observation. (B) GVHD clinical scores were determined as a measure of GVHD severity in animals, as described in “Materials and methods.” *P < .05 and **P < .01; allogeneic G-CSF wt and IL-10-/- recipients plus anti-TGF-β compared with control antibody. ▾ indicates control wt allo plus control Ab; ○, G-CSF wt allo plus control Ab; ♦, G-CSF IL-10-/- allo plus anti-TGFβ; □, G-CSF wt allo plus anti-TGFβ; •, G-CSF allo TCD plus anti-TGF-β; and ▿, control syn wt plus anti-TGFβ.
T cells from G-CSF-treated donors regulate aGVHD in a TGF-β-dependent fashion. (A) TGF-β was determined 7, 14, and 21 days after SCT from respective FACS-sorted CD4 (▪, n = 3), CD8 (▨, n = 3), and non-T-cell (□, n = 3) donor populations by real-time PCR, as described in “Materials and methods.” (B) Lethally irradiated B6D2F1 recipients received splenocytes from control treated B6 donors and control antibody with or without additional purified T cells from control B6 donors (control allo plus control T plus control antibody, n = 4; control allo plus control antibody, n = 4). To study the ability of T cells from G-CSF-treated B6 donors to modulate GVHD, purified T cells from G-CSF-treated donors were added to control splenocytes in the presence of anti-TGF-β or control antibody, as described in “Materials and methods” (control allo plus G-CSF T cells plus control antibody, n = 20; control allo plus G-CSF T cells plus anti-TGF-β antibody, n = 20). Survival curves by Kaplan-Meier analysis. *P < .02; anti-TGF-β compared with control antibody. ▴ indicates control allo plus control Ab; ▪, control allo plus control T plus control Ab; ○, control allo plus G-CSF T plus control Ab; and □, control allo plus G-CSF T plus anti-TGF-β.
T cells from G-CSF-treated donors regulate aGVHD in a TGF-β-dependent fashion. (A) TGF-β was determined 7, 14, and 21 days after SCT from respective FACS-sorted CD4 (▪, n = 3), CD8 (▨, n = 3), and non-T-cell (□, n = 3) donor populations by real-time PCR, as described in “Materials and methods.” (B) Lethally irradiated B6D2F1 recipients received splenocytes from control treated B6 donors and control antibody with or without additional purified T cells from control B6 donors (control allo plus control T plus control antibody, n = 4; control allo plus control antibody, n = 4). To study the ability of T cells from G-CSF-treated B6 donors to modulate GVHD, purified T cells from G-CSF-treated donors were added to control splenocytes in the presence of anti-TGF-β or control antibody, as described in “Materials and methods” (control allo plus G-CSF T cells plus control antibody, n = 20; control allo plus G-CSF T cells plus anti-TGF-β antibody, n = 20). Survival curves by Kaplan-Meier analysis. *P < .02; anti-TGF-β compared with control antibody. ▴ indicates control allo plus control Ab; ▪, control allo plus control T plus control Ab; ○, control allo plus G-CSF T plus control Ab; and □, control allo plus G-CSF T plus anti-TGF-β.
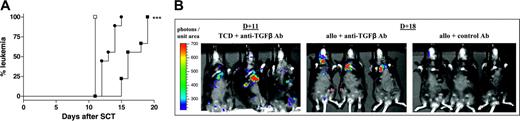
We next sought to study the relevance of this to GVL effects after transplantation. Allogeneic SCT was performed in the presence or absence of TGF-β neutralization with the addition of numbers of host-type P815 (50 000 per animal) to provide informative analysis of GVL effects before death that would otherwise occur from aGVHD. As shown in Figure 6A, the clearance of host-type P815 leukemia was significantly delayed by TGF-β neutralization in recipients after allogeneic SCT (P < .0001). Furthermore, biophotonic imaging of animals after transplantation with a lower dose of luciferase-transfected P815 confirmed this impairment of GVL. Allogeneic SCT recipients in which TGF-β was neutralized had evidence of leukemia by day 18, as demonstrated by high photon counts predominantly over the liver and spleen, whereas none was seen in the allogeneic SCT recipients receiving control antibody (Figure 6B). In contrast, recipients of TCD allogeneic splenocytes died of leukemia at day 11, regardless of whether they received control or anti-TGF-β antibody.
Delayed neutralization of TGF-β after SCT attenuates chronic GVHD
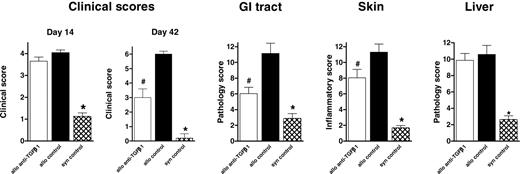
Our results confirm a beneficial role for TGF-β in protecting recipients from aGVHD after allogeneic SCT. Because TGF-β has been suggested as a major effector molecule in the fibrosis of chronic GVHD (cGVHD),7 we next sought to determine whether neutralization of TGF-β would also affect the severity of cGVHD. We used a well-established murine model of sclerodermatous cGVHD directed against isolated minor histocompatibility antigens, as recently described.4 Recipients of G-CSF-mobilized splenocytes received anti-TGF-β or control antibody starting at day 0 or at day 14 and then 3 times weekly until day 42. Donor CD4 and CD8 engraftment was assessed in the Ly-9.1-negative population and was equivalent in anti-TGF-β- and control antibody-treated recipients (92.49% ± 1.73% and 88.97% ± 2.16%, respectively; P = .4). Neutralization of TGF-β from day 0 to day 42 failed to attenuate cGVHD because histopathologic changes in the GI tract, skin, and liver were similar in anti-TGF-β- and control antibody-treated recipients (data not shown). TGF-β appeared to have a protective effect on GVHD early after SCT. Therefore, we investigated whether delayed neutralization of TGF-β (starting from day 14) could influence cGVHD severity. At day 14 after SCT, when TGF-β neutralization commenced, there was no difference in clinical scores between anti-TGF-β-treated and control antibody-treated recipients (Figure 7). However, subsequent neutralization of TGF-β resulted in significantly less cGVHD, as determined by clinical scores 42 days after transplantation (P < .05). Furthermore, histopathologic examination of GVHD target organs demonstrated that this treatment reduced GVHD of the GI tract and skin (P < .05) but that it did not prevent abnormalities of the liver (Figure 7). The parameters most discriminated after TGF-β neutralization were intestinal blunting and crypt regeneration in the gut and epidermal inflammation, dermal fibrosis, and subcutaneous fibrosis in the skin. In contrast to the recipients of splenocytes from G-CSF-treated donors, most (66%) recipients of equivalent numbers of splenocytes from control antibody-treated donors died of GVHD before day 42 with features of aGVHD (weight loss and diarrhea). We addressed the effect of TGF-β neutralization on GVHD histopathology in recipients of control grafts by transplanting a lower dose (2.5 × 107) of splenocytes that did not result in significant lethality, as previously described.4 Anti-TGF-β or control antibody was administered from day 14, as described in Figure 7. In the absence of donor treatment with G-CSF, the neutralization of TGF-β resulted in increased clinical scores at day 42 (3.9 ± 0.3 vs 1.4 ± 0.4; P < .01) in association with increased cutaneous histopathologic abnormalities (10.3 ± 2.0 vs 4.7 ± 1.4; P < .03) because of the promotion of an acute pathologic condition characterized by inflammatory cell infiltrates rather than fibrosis. Furthermore, GVHD in the gut (13.0 ± 1.2 vs 12.8 ± 0.6; P = .84) and liver (4.9 ± 0.9 vs 6.8 ± 0.8; P = .18) was unchanged by TGF-β neutralization. Thus, TGF-β is not pathogenic in this model unless chronic GVHD is induced by treating donors with G-CSF.
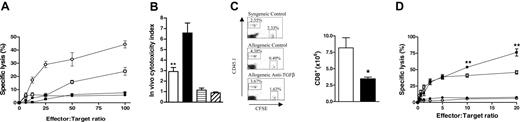
Neutralization of TGF-β attenuates antihost cytotoxicity and GVL. (A) Splenocytes from transplant recipients containing equal numbers of CD8+ T cells (based on the CD8 staining of the input cells) were used as effector cells against chromium-labeled P815 (host) and EL4 (donor) target cells. Percentage of specific lysis was determined in standard chromium-release assays, as described in “Materials and methods” against host-type P815 targets (open symbols) or donor-type EL4 targets (filled symbols) in allogeneic recipients of anti-TGF-β antibody (squares) or control antibody (circles). Data presented as mean ± SE of triplicate wells from 1 of 2 identical experiments. (B) At day +12 after transplantation, B6D2F1 recipients of G-CSF-mobilized allogeneic B6 (CD45.2) splenocytes that received anti-TGF-β or control antibody were injected intravenously with congenic B6 PTPRCA (CD45.1) and CFSE-labeled B6D2F1 (CD45.2) splenocytes. Sixteen hours later, CD45.1+ and CFSE+ peripheral-blood mononuclear cells from transplant recipients were quantified by FACS. (Left) In vivo cytotoxicity index was determined in allogeneic recipients as the ratio between remaining syngeneic (CD45.1+) and allogeneic (CFSE+) cells, as described in “Materials and methods” (allogeneic plus anti-TGF-β, □, n = 10; allogeneic plus control antibody, ▪, n = 12). Cytotoxicity was negligible (ratio of approximately 1.0) in B6D2F1 recipients of syngeneic B6D2F1 splenocytes, irrespective of the antibody treatment (syngeneic plus anti-TGF-β antibody, ▤, n = 3; syngeneic plus control antibody, ▨, n = 3). Data presented as mean ± SE. **P < .005, allogeneic plus anti-TGF-β antibody compared with allogeneic plus control antibody. (Right) FACS plots from representative syngeneic recipient (top) and allogeneic recipients of control antibody (middle) and anti-TGF-β antibody (bottom), illustrating the in vivo cytotoxicity index. (C) Purified B6 T cells were stimulated with irradiated B6D2F1 splenocytes in the absence (control) or presence of TGF-β1 (2.5 ng/mL). Seven days later equivalent numbers of T cells were restimulated with irradiated B6D2F1 splenocytes for an additional 3 days in the absence of exogenous TGF-β. Absolute numbers of CD8+ cells in MLC after secondary stimulation (control, □; TGF-β, ▪). Data represent mean ± SE from 3 identical experiments. *P = .05; TGF-β compared with control. (D) T cells were stimulated in MLC, as in panel C, and, after secondary stimulation, equal numbers of CD8+ cells were used as effectors in standard chromium release assays. Percentage of specific lysis against allogenic P815 (control, □; TGF-β, ▪) and syngeneic EL4 targets (control, ○; TGF-β, •). Data represent mean ± SE from quadruplicate wells combined from 3 identical experiments. **P < .01; TGF-β compared with control.
Neutralization of TGF-β attenuates antihost cytotoxicity and GVL. (A) Splenocytes from transplant recipients containing equal numbers of CD8+ T cells (based on the CD8 staining of the input cells) were used as effector cells against chromium-labeled P815 (host) and EL4 (donor) target cells. Percentage of specific lysis was determined in standard chromium-release assays, as described in “Materials and methods” against host-type P815 targets (open symbols) or donor-type EL4 targets (filled symbols) in allogeneic recipients of anti-TGF-β antibody (squares) or control antibody (circles). Data presented as mean ± SE of triplicate wells from 1 of 2 identical experiments. (B) At day +12 after transplantation, B6D2F1 recipients of G-CSF-mobilized allogeneic B6 (CD45.2) splenocytes that received anti-TGF-β or control antibody were injected intravenously with congenic B6 PTPRCA (CD45.1) and CFSE-labeled B6D2F1 (CD45.2) splenocytes. Sixteen hours later, CD45.1+ and CFSE+ peripheral-blood mononuclear cells from transplant recipients were quantified by FACS. (Left) In vivo cytotoxicity index was determined in allogeneic recipients as the ratio between remaining syngeneic (CD45.1+) and allogeneic (CFSE+) cells, as described in “Materials and methods” (allogeneic plus anti-TGF-β, □, n = 10; allogeneic plus control antibody, ▪, n = 12). Cytotoxicity was negligible (ratio of approximately 1.0) in B6D2F1 recipients of syngeneic B6D2F1 splenocytes, irrespective of the antibody treatment (syngeneic plus anti-TGF-β antibody, ▤, n = 3; syngeneic plus control antibody, ▨, n = 3). Data presented as mean ± SE. **P < .005, allogeneic plus anti-TGF-β antibody compared with allogeneic plus control antibody. (Right) FACS plots from representative syngeneic recipient (top) and allogeneic recipients of control antibody (middle) and anti-TGF-β antibody (bottom), illustrating the in vivo cytotoxicity index. (C) Purified B6 T cells were stimulated with irradiated B6D2F1 splenocytes in the absence (control) or presence of TGF-β1 (2.5 ng/mL). Seven days later equivalent numbers of T cells were restimulated with irradiated B6D2F1 splenocytes for an additional 3 days in the absence of exogenous TGF-β. Absolute numbers of CD8+ cells in MLC after secondary stimulation (control, □; TGF-β, ▪). Data represent mean ± SE from 3 identical experiments. *P = .05; TGF-β compared with control. (D) T cells were stimulated in MLC, as in panel C, and, after secondary stimulation, equal numbers of CD8+ cells were used as effectors in standard chromium release assays. Percentage of specific lysis against allogenic P815 (control, □; TGF-β, ▪) and syngeneic EL4 targets (control, ○; TGF-β, •). Data represent mean ± SE from quadruplicate wells combined from 3 identical experiments. **P < .01; TGF-β compared with control.
Neutralization of TGF-β attenuates GVL. (A) Leukemia development in a GVL model whereby recipients underwent transplantation with allogeneic (n = 10 per group) or allogeneic TCD (n = 3) splenocytes in conjunction with host-type P815 and control antibody or anti-TGF-β antibody, as described in “Materials and methods.” ***P < .001; all groups. (B) Xenogen biophotonic imaging of leukemia development in allogeneic (T-cell replete) and TCD recipients after cotransplantation of a lower dose of P815 transfected with a luciferase reporter gene, as described in “Materials and methods.” All TCD recipients developed leukemia on day 11 and required humane killing regardless of whether they received anti-TGF-β or control antibody. Allogeneic recipients receiving anti-TGF-β had evidence of leukemia by day 18, as demonstrated by biophotonic imaging. No leukemia was visualized in allogeneic recipients receiving control antibody. □ indicates TCD plus control Ab; ▪, allo plus control Ab; and •, allo plus anti-TGF-β Ab.
Neutralization of TGF-β attenuates GVL. (A) Leukemia development in a GVL model whereby recipients underwent transplantation with allogeneic (n = 10 per group) or allogeneic TCD (n = 3) splenocytes in conjunction with host-type P815 and control antibody or anti-TGF-β antibody, as described in “Materials and methods.” ***P < .001; all groups. (B) Xenogen biophotonic imaging of leukemia development in allogeneic (T-cell replete) and TCD recipients after cotransplantation of a lower dose of P815 transfected with a luciferase reporter gene, as described in “Materials and methods.” All TCD recipients developed leukemia on day 11 and required humane killing regardless of whether they received anti-TGF-β or control antibody. Allogeneic recipients receiving anti-TGF-β had evidence of leukemia by day 18, as demonstrated by biophotonic imaging. No leukemia was visualized in allogeneic recipients receiving control antibody. □ indicates TCD plus control Ab; ▪, allo plus control Ab; and •, allo plus anti-TGF-β Ab.
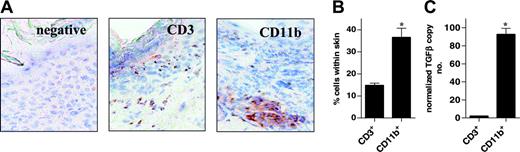
In this model, scleroderma resulting from the transplantation of G-CSF-mobilized grafts is determined by the non-T-cell myeloid component of the graft. This is demonstrated in mixing experiments whereby the transplantation of T-cell-depleted grafts from G-CSF-treated donors and purified control T cells induced cutaneous fibrosis, whereas T-cell-depleted grafts from control antibody-treated donors did not.4 The dependence on the myeloid component of the graft for the induction of chronic GVHD in this model is also consistent with clinical SCT.21 Because G-CSF predominantly expands cells of the myelomonocytic lineage, we studied the nature of the respective T-cell and non-T-cell infiltrate in these animals to determine the likely source of pathogenic TGF-β. As shown in Figure 8, though the cutaneous infiltrate included CD3+ T cells and CD11b+ mononuclear cells, the latter population predominated. Furthermore, when these populations were subjected to fluorescence-activated cell sorter (FACS) analysis and were assayed for TGF-β by real-time PCR, CD11b+ mononuclear cells produced 50-fold more TGF-β than did CD3+ T cells. Thus, the severity of scleroderma is dictated by the myeloid component of the graft; most TGF-β production in sclerodermatous skin is from the (non-T cell) CD11b+ fraction of cells, and neutralization of TGF-β attenuates the severity of scleroderma. It is, therefore, likely that TGF-β from CD11b+ cells contributes to the development of G-CSF-driven chronic GVHD.
Neutralization of TGF-β late after SCT attenuates chronic GVHD. Balb/c recipients underwent transplantation with 12.5 × 107 splenocytes from G-CSF-treated donors, as described in “Materials and methods.” Allogeneic recipients received anti-TGF-β (□, n = 13) or control antibody (▪, n = 13) at day 14 and then 3 times per week until day 42. Control syngeneic recipients ( , n = 8) were treated with control antibody. GVHD clinical scores were determined at days 14 and 42 after SCT as a measure of GVHD severity in animals, as described in “Materials and methods.” The extent of gastrointestinal, cutaneous, and hepatic GVHD was assessed by semiquantitative histopathologic examination, as described in “Materials and methods.” Data are pooled from 2 experiments and are expressed as mean ± SE. *P < .05, syngeneic compared with both allogeneic groups. #P < .01, allo plus anti-TGF-β compared with allo plus control antibody.
, n = 8) were treated with control antibody. GVHD clinical scores were determined at days 14 and 42 after SCT as a measure of GVHD severity in animals, as described in “Materials and methods.” The extent of gastrointestinal, cutaneous, and hepatic GVHD was assessed by semiquantitative histopathologic examination, as described in “Materials and methods.” Data are pooled from 2 experiments and are expressed as mean ± SE. *P < .05, syngeneic compared with both allogeneic groups. #P < .01, allo plus anti-TGF-β compared with allo plus control antibody.
Neutralization of TGF-β late after SCT attenuates chronic GVHD. Balb/c recipients underwent transplantation with 12.5 × 107 splenocytes from G-CSF-treated donors, as described in “Materials and methods.” Allogeneic recipients received anti-TGF-β (□, n = 13) or control antibody (▪, n = 13) at day 14 and then 3 times per week until day 42. Control syngeneic recipients ( , n = 8) were treated with control antibody. GVHD clinical scores were determined at days 14 and 42 after SCT as a measure of GVHD severity in animals, as described in “Materials and methods.” The extent of gastrointestinal, cutaneous, and hepatic GVHD was assessed by semiquantitative histopathologic examination, as described in “Materials and methods.” Data are pooled from 2 experiments and are expressed as mean ± SE. *P < .05, syngeneic compared with both allogeneic groups. #P < .01, allo plus anti-TGF-β compared with allo plus control antibody.
, n = 8) were treated with control antibody. GVHD clinical scores were determined at days 14 and 42 after SCT as a measure of GVHD severity in animals, as described in “Materials and methods.” The extent of gastrointestinal, cutaneous, and hepatic GVHD was assessed by semiquantitative histopathologic examination, as described in “Materials and methods.” Data are pooled from 2 experiments and are expressed as mean ± SE. *P < .05, syngeneic compared with both allogeneic groups. #P < .01, allo plus anti-TGF-β compared with allo plus control antibody.
Characterization of TGF-β production from the cellular infiltrate in sclerodermatous skin. (A) Balb/c recipient mice underwent transplantation with 12.5 × 107 splenocytes from G-CSF-treated B10.D2 donors, as described in “Materials and methods.” Frozen sections of skin were obtained 6 weeks after SCT and were stained by immunohistochemistry with CD3 and CD11b antibodies, as outlined in “Materials and methods.” (B) Splenocytes from G-CSF-treated B10.D2 donors were transplanted into Balb/c recipients (n = 6), as described in panel A. Six weeks after SCT, animals were humanely killed and dorsal skin was prepared for FACS analysis, as described in “Materials and methods.” Percentages of CD3+ and CD11b+ cells were assessed in the CD45.2+ population. Data are expressed as mean ± SE (*P < .05). (C) CD3+ and CD11b+ cells obtained by skin digestion were FACS sorted and assayed for TGF-β by real-time PCR, as described in “Materials and methods.” Data are expressed as mean ± SE from triplicate samples (*P < .05).
Characterization of TGF-β production from the cellular infiltrate in sclerodermatous skin. (A) Balb/c recipient mice underwent transplantation with 12.5 × 107 splenocytes from G-CSF-treated B10.D2 donors, as described in “Materials and methods.” Frozen sections of skin were obtained 6 weeks after SCT and were stained by immunohistochemistry with CD3 and CD11b antibodies, as outlined in “Materials and methods.” (B) Splenocytes from G-CSF-treated B10.D2 donors were transplanted into Balb/c recipients (n = 6), as described in panel A. Six weeks after SCT, animals were humanely killed and dorsal skin was prepared for FACS analysis, as described in “Materials and methods.” Percentages of CD3+ and CD11b+ cells were assessed in the CD45.2+ population. Data are expressed as mean ± SE (*P < .05). (C) CD3+ and CD11b+ cells obtained by skin digestion were FACS sorted and assayed for TGF-β by real-time PCR, as described in “Materials and methods.” Data are expressed as mean ± SE from triplicate samples (*P < .05).
Discussion
We have demonstrated that TGF-β separates aGVHD and GVL after allogeneic SCT. The protection from aGVHD afforded by the generation of TGF-β occurred in association with quantitative reductions in the expansion, proliferation, and IFN-γ generation of donor cells after SCT. The inhibition of GVHD was, at least in part, caused by TGF-β production from donor T cells because the ability of donor T cells from G-CSF-treated donors to regulate GVHD was impaired in the presence of TGF-β neutralization. In addition, TGF-β and IL-10 from G-CSF-treated donor grafts acted in an additive fashion to prevent aGVHD. In contrast to the protective effects in aGVHD, TGF-β contributed to the severity of cGVHD after stem-cell transplantation, confirming a differential role for TGF-β in aGVHD and cGVHD pathophysiology after allogeneic SCT.
Donor T cells from mice treated with G-CSF have a reduced capacity to induce GVHD on a per cell basis relative to those from control antibody-treated donors.10,11 In clinical studies, despite an approximate 20-fold increase in the T-cell content of G-CSF-mobilized leukapheresis products compared with unstimulated bone marrow harvests,10,22 there is no increase in the incidence of aGVHD.1,23 The qualitative changes in T-cell differentiation induced by G-CSF and the alteration in cytokine generation is critical in GVHD24 and has been widely proposed as an explanation for the equivalent rates of GVHD despite the very high numbers of T cells transplanted. We have recently demonstrated that donor pretreatment with G-CSF augments the protection from aGVHD in allogeneic SCT recipients because of the generation of IL-10-producing regulatory T cells.2 However, the regulatory mechanisms invoked were only partially IL-10 dependent, and we speculated that additional effector molecules such as TGF-β may provide supplementary regulatory activity after donor treatment with G-CSF. Recent studies have proposed a role for TGF-β in controlling T-cell alloreactivity25,26 and have confirmed that G-CSF administration to human stem-cell donors results in an increase in TGF-β from CD4+ T cells.6 This is also consistent with the clinical association of reduced serum TGF-β levels after engraftment and severe aGVHD.27 In the current study, neutralization of TGF-β after allogeneic SCT with IL-10-/- grafts further enhanced aGVHD, providing evidence that TGF-β and IL-10 act additively in preventing aGVHD after allogeneic SCT. This may in part explain the residual regulatory T-cell function in recipients of pegylated G-CSF-mobilized IL-10-/- SCT allografts.2 The nature of the TGF-β-producing regulatory T cell produced after G-CSF-based stem-cell mobilization remains to be determined, but the cytokine profile and CD4+ dependency of the model13 suggest it is likely to be CD4+ in nature. It is important to consider that other regulatory pathways may be invoked by stem-cell mobilization with G-CSF in addition to IL-10 and TGF-β.
The mechanism by which G-CSF-based growth factors augment regulatory T-cell activity is unclear but may be the result of “priming” by increased levels of IL-10 and TGF-β during stem-cell mobilization. Consistent with this, we have recently demonstrated that donor pretreatment with the G-CSF receptor and the Flt-3 receptor agonist progenipoetin-1 (ProGP-1) results in the production of large amounts of IL-10 and TGF-β from stem-cell grafts and—consistent with the in vitro effects of these cytokines—in the induction of donor T cells with impaired capacity to induce GVHD.11 Whether regulatory T cells generated in this fashion require one or both cytokines in the mobilization phase has not been determined, but the current study suggests that TGF-β is important in the effector phase, characterized by regulatory function. Additional studies are required to examine the role of IL-10 in both phases because our studies used IL-10-/- mice during stem-cell mobilization; hence, the subsequent reduction in regulatory function after SCT may reflect the loss of IL-10 activity during stem-cell mobilization, the posttransplantation effector phase, or both.
TGF-β can inhibit TH1 and TH2 differentiation and the acquisition of most, if not all, effector functions by naive T cells.28 During GVHD, systemic levels of IFN-γ were increased in the presence of TGF-β neutralization, suggesting the augmentation of type 1 differentiation. Acute GVHD has been established as a T-cell-dependent and a TH1-dominant disease,29 and the enhancement of T-cell proliferation or TH1 differentiation because of a reduction in regulation appears the predominant mechanism by which the neutralization of TGF-β augments the severity of aGVHD. We have demonstrated that the cytotoxic response of donor T cells to host-type allogeneic targets was impaired after the neutralization of TGF-β, resulting in significantly lower overall levels of cytotoxicity and associated impairment of GVL effects. This inhibition of donor T-cell cytotoxic function in animals in which TGF-β was neutralized is intriguing and is in contrast to findings from studies demonstrating potent inhibition of cytotoxicity by TGF-β itself when added to cultures during CTL expansion.28 However, TGF-β is known to play an important role in the growth and maturation of CD8+ cells.30 In our studies, TGF-β demonstrated a biphasic effect on T-cell cytotoxicity with enhancement of function at low doses and attenuation at high doses. Of interest, donor and host CD4+CD25+ regulatory T cells inhibit GVHD and are known to use TGF-β as an effector of suppression in a number of models.31,32 Despite this, CD4+CD25+ regulatory T cells do not appear to inhibit CD8+-mediated GVL.14,33 Thus, it appears that TGF-β may differentially influence donor CD4+ and CD8+ effector T-cell responses during allogeneic SCT and, in turn, separate GVHD and GVL. It is likely that the immunosuppression associated with severe acute GVHD34,35 itself inhibits cellular cytotoxicity after allogeneic SCT, and the prevention of GVHD by TGF-β produced from regulatory T cells may allow persistence of effective cytotoxicity in this setting.
Clinical and experimental data have demonstrated that donor treatment with G-CSF augments the incidence and severity of cGVHD.4,36 We have shown that the latter results from a high T-cell dose in conjunction with a putatively expanded myeloid lineage within the non-T-cell compartment.4 The treatment of stem-cell donors with G-CSF expands cells of the myelomonocytic lineage that have previously been suggested as effector cells of cutaneous fibrosis during cGVHD.7 In our murine model of cGVHD, cells of the monocyte-macrophage lineage producing large amounts of TGF-β were increased in the skin of SCT recipients. TGF-β is a potent fibrogenic cytokine known to stimulate collagen synthesis by fibroblasts, and TGF-β produced by monocytes has been described as an important mediator of fibrosis in experimental models of scleroderma.37 In these studies, the early neutralization of TGF-β attenuated cutaneous and pulmonary fibrosis. However, in the current model of G-CSF-dependent cGVHD after allogeneic SCT, only delayed (rather than immediate) neutralization of TGF-β attenuated the severity of cutaneous and gastrointestinal cGVHD, whereas hepatic GVHD was unchanged. This finding suggests that donor T-cell-derived TGF-β plays an important early regulatory role in preventing aGVHD, but subsequent pathologic TGF-β production (from an expanded mononuclear population) is partly responsible for the augmented manifestations of cGVHD after SCT. Clearly, this effect is only partial, and other, presumably T-cell-mediated, effects are responsible for the residual cGVHD after TGF-β neutralization. The neutralization of TGF-β in clinical SCT during the early posttransplantation period, when patients are at risk for acute GVHD, is likely to have a negative impact on both GVHD and GVL. Therefore, we propose that the potential beneficial effects of TGF-β neutralization on chronic GVHD are likely to be seen only when initiated beyond the first 100 days of transplantation, perhaps as immunosuppressive therapy is withdrawn.
Our results confirm a differential role of TGF-β in aGVHD and cGVHD after G-CSF-mobilized SCT. Early after SCT, TGF-β is an important regulator of donor engraftment and alloreactive T-cell function, and its neutralization leads to the expansion and activation of donor T cells, alteration of their cytokine profile, and increase in severity of aGVHD. In the pathogenesis of cGVHD, TGF-β regulates the expansion of myelomonocytic effector cells early after SCT, whereas later in the course of the disease, TGF-β production by these cells will have a profound pathogenic effect and will lead to extensive fibrosis of target organs. Thus, the therapeutic neutralization of TGF-β late after allogeneic SCT may attenuate the severity of cGVHD after transplantation of G-CSF-mobilized stem-cell allografts while maintaining the beneficial early regulatory effects of TGF-β on aGVHD and GVL.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2005-01-0062.
Supported in part by grants from the National Health and Medical Research Council (NHMRC) and Queensland Cancer Fund. G.R.H. is a Wellcome Trust Senior Overseas Research Fellow.
S.L. is employed by a company or a competitor of a company (Genzyme) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. TGF-β neutralization augments donor T-cell proliferation and expansion to host antigens late after SCT. (A) Lethally irradiated B6D2F1 recipients underwent transplantation with G-CSF-treated B6 splenocytes and received anti-TGF-β (n = 5) or control antibody (n = 5) on day 0 and then 3 times weekly. On day+14, splenocytes from transplant recipients were pooled within the treatment group (numbers of CD3+ cells were equilibrated between the groups based on the CD3 staining of the input cells) and were stimulated with irradiated B6D2F1 peritoneal macrophages. Proliferation was measured at 48 hours by standard [3H]-thymidine incorporation assay, as described in “Materials and methods.” □ indicates allogeneic plus anti-TGF-β antibody; ○, allogeneic plus control antibody. Proliferation of nonstimulated splenocytes was 1157 ± 43.32 cpm and 1197 ± 90.39 cpm (allo plus anti-TGF-β antibody and allo plus control antibody, respectively). Results from one of 2 identical experiments. (B) Recipients received anti-TGF-β (□, n = 5-9) or control antibody (▪, n = 5-10), as in panel A. The absolute numbers of lineage and donor (H2bpos/H2dneg) cells were determined per spleen (× 106) 14 days after SCT. *P < .05; anti-TGF-β compared with control antibody. Pooled data from 2 experiments, expressed as mean ± SE. (C) B6D2F1 recipients underwent transplantation with G-CSF-treated donor B6 or B6D2F1 splenocytes and received anti-TGF-β (□, n = 5) or control antibody (▪, n = 5), as described in “Materials and methods.” Syngeneic recipients (, n = 4) received control antibody. IFN-γ and TNF-α were determined in the sera of animals 5 and 14 days, respectively, after SCT by enzyme-linked immunosorbent assay (ELISA). GI tract abnormalities were determined 14 days after transplantation by semiquantitative histologic examination, as described in “Materials and methods.” Data are presented as mean ± SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2005-01-0062/6/m_zh80180583920001.jpeg?Expires=1769124193&Signature=g0gPTdHObllB9nISNB0SiPUXV1GG~0mSOQiXeEXaFuyrvHvPMxuJ1Qzw6IENE3zhfIUZvMRT1u1ZuHJUHPngRZQzl8oTXW9tjwpIuSr0~Bl2-3N7rsg5fOMxeUkI4TgwRhkxMiMhtaD8Bhgt3STL2xQuHJzJaxY65vBUECjsfK4XXpypq3dr4qqDX7Hn4s~g6dWxcpvZTGAhEcuAnznTg4ENoTFNjpoPudETRMMTbf76YhzhdH3wgNdHcFn0ZjfqtQHaIrlg2ZUnUyrWzV6O~6nmkuda0nqdKhWZ8dH~nw8kckzqAVAldJIdgsy8pr6n3Igx2ug~n9LfeF3de51PXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal