Abstract
T-cell tolerance is mandatory for major histocompatibility complex (MHC)-mismatched stem-cell transplantation without cytoreduction. Here, we used a cytotoxicity assay based on the infusion of differentially carboxyfluorescein succinimidyl ester (CFSE)-labeled syngeneic and donor splenocytes to determine the survival of donor cells in vivo. In vivo cytotoxicity data showed that treatment with anti-CD40 ligand monoclonal antibody in combination with a low dose of MHC-mismatched bone marrow cells was sufficient to induce T-cell tolerance. However, CFSE-labeled donor cells were still eliminated. A similar elimination pattern was observed in T-cell and natural killer T-cell (NKT-cell)-deficient mice, suggesting the involvement of natural killer (NK) cells. Indeed, in vivo NK-cell depletion resulted in a prolonged survival of CFSE-labeled donor cells, confirming the role of NK cells in this process. Transplantation of a megadose of MHC-mismatched bone marrow cells was required for a complete survival of CFSE-labeled donor cells. This NK-cell tolerance was donor specific and was associated with mixed chimerism. Additional NK-cell depletion significantly enhanced engraftment and allowed long-term chimerism after transplantation of a relatively low dose of donor bone marrow cells. These data demonstrate the importance of NK cells in the rejection of MHC-mismatched hematopoietic cells and may explain the high numbers of bone marrow cells required for transplantation over MHC barriers. (Blood. 2005;106:2215-2220)
Introduction
Allogeneic stem-cell transplantation (SCT) is a potential treatment modality for different malignant and nonmalignant diseases.1 However, the application of this approach is hampered by the high conditioning-related toxicity, graft-versus-host disease, graft rejection, and the limited availability of major histocompatibility (MHC)-matched siblings.2 A reduction in morbidity and mortality would greatly facilitate a widespread application of hematopoietic SCT. To achieve successful acceptance of allogeneic stem cells in the absence of cytoreduction, the induction of donor-specific tolerance is mandatory. Because T cells play a key role in the rejection of allogeneic stem cells, most transplantation studies have focused on the induction of T-cell tolerance.
Natural killer (NK) cells represent another barrier that prevents engraftment following transplantation over MHC barriers. Because of their ability to kill virus-infected or malignant host cells without prior sensitization, NK cells are part of the first line of defense against pathogens. Their activation is coordinated by different inhibitory and activating receptors. Classic and nonclassic MHC class I molecules function as ligands for these receptors and a perturbation of the class I expression, due to virus-infection or tumorigenesis, can result in NK-cell activation.3-5 Differences in MHC class I expression on allogeneic hematopoietic cells can also result in NK-cell alloreactivity, making NK cells potential effector cells in the early rejection of hematopoietic stem cells.6 However, data concerning NK-cell elimination of allogeneic stem cells have been inconclusive. Some studies demonstrate an important role for NK cells in the elimination of allogeneic cells,7-9 whereas other studies indicate that allogeneic stem cells are relatively resistant toward NK-cell cytotoxicity.10,11
In this study, we used an in vivo cytotoxicity assay based on the infusion of differentially carboxyfluorescein succinimidyl ester (CFSE)-labeled syngeneic and allogeneic donor splenocytes to analyze the elimination kinetics of MHC-mismatched hematopoietic cells in an anti-CD40 ligand (anti-CD40L)-based BALB/c into C57BL/6 SCT model in the complete absence of cytoreduction. We demonstrate the importance of NK cells in the elimination of MHC-mismatched hematopoietic cells after the induction of T-cell tolerance and further show that transplantation of a high dose of stem cells is required to induce NK-cell tolerance. Finally, we show that additional NK-cell depletion allows engraftment and the induction of long-term chimerism after transplantation of a reduced number of stem cells.
Materials and methods
Mice
C57BL/6 (H-2b), BALB/c (H-2d), C3H/J (H-2k), and BALB/c nu/nu (H-2d) mice were purchased from Charles River Laboratories (Maastricht, The Netherlands), and C57BL/6 nu/nu (H-2b) mice were purchased from EDRIS A. Brunink (Valburg, The Netherlands). All mice were female, 8 to 12 weeks old at the start of each experiment, and maintained in the animal facilities of the Leiden University Medical Center in accordance with the national guidelines for animal care. All experiments were performed in accordance with the Guidelines for Animal Research and approved by the Ethics Committee on Animal Research of the Leiden University Medical Center (Leiden, The Netherlands).
Conditioning and bone marrow transplantation
Donor bone marrow was flushed from femurs, tibias, and humeri of BALB/c or BALB/c nu/nu mice using a 26-gauge needle and RPMI 1640 (BioWittaker, Verviers, Belgium) containing 2% fetal calf serum (FCS; BioWittaker) and heparin (24 IU/mL; pharmacy L.U.M.C., Leiden, The Netherlands). Recovered cells were filtered through a sterile nylon mesh (100 μm; BD Biosciences, Erembodegem, Belgium), washed with phosphate-buffered saline (PBS; pharmacy L.U.M.C.), and injected intravenously in a volume of 0.2 mL PBS. Anti-CD40L monoclonal antibody (mAb; MR1) was administered intraperitoneally at the day of transplantation in a dose of 0.5 mg in a volume of 0.5 mL PBS. Anti-NK1.1 mAb (PK136) and anti-CD8 mAb (2.43) was administered intraperitoneally in 2 doses of 0.25 mg in a volume of 0.5 mL PBS on day -5 and day -1 before the required depletion.
In vivo cytotoxicity assay
Splenocytes were isolated by gently mashing the spleen through a sterile nylon mesh (100 μm; BD Biosciences) using RPMI 1640 containing 2% FCS and separated by Ficoll-Hypaque (pharmacy L.U.M.C.) centrifugation. Target and internal control splenocytes (107/mL in PBS) were then incubated with 5.0 μM or 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes, Leiden, The Netherlands), respectively, at 37°C for 10 minutes. FCS (final concentration 10%) was added to stop the reaction. After washing, the cells were mixed in a 1:1 ratio and resuspended in PBS, and 107 cells were injected intravenously in 0.2 mL PBS. Peripheral blood was collected from individual mice at different time points. After lysis of red blood cells (incubation for 10 minutes in NH4Cl [8.4 g/L]/KHO3 [1 g/L] buffer at 4°C), peripheral-blood lymphocytes were analyzed for CFSE expression by fluorescence-activated cell sorting (FACS) using a FACSCalibur and CellQuest software (both from Becton Dickinson, Erembodegem, Belgium).
Peripheral-blood leukocyte staining
Flow cytometric analysis on heparinized peripheral-blood leukocytes was performed after lysis of red blood cells (incubation for 10 minutes in NH4Cl [8.4 g/L]/KHO3 [1 g/L] buffer at 4°C). Confirmation of NK1.1 depletion (> 99%) was carried out using allophycocyanin (APC)-conjugated anti-NK1.1 (clone PK136) and fluorescein isothiocyanate (FITC)-conjugated anti-Ly49D (clone 4E5). CD8 depletion was confirmed (> 99%) using APC-conjugated anti-CD3e (clone 145-2C11), FITC-conjugated anti-CD8a (clone 53-6.7), and phycoerythrin (PE)-conjugated anti-CD4 (clone RM4-5). Flow cytometric analysis of multilineage chimerism was performed using APC-conjugated anti-CD3e (clone 145-2C11), anti-B220 (clone RA3-6B2), and anti-GR1 (clone RB6-8C5), FITC-conjugated anti-H-2Kb (clone AF6-88.5), FITC-conjugated anti-H-2Dd (clone KH95), and PE-conjugated anti-H-2Kd (clone SF1-1.1). All antibodies were obtained from Pharmingen (Alphen aan de Rijn, The Netherlands), and all samples were analyzed using a FACSCalibur and CellQuest software.
Results
Anti-CD40L mAb treatment prevents the onset of a memory T-cell response
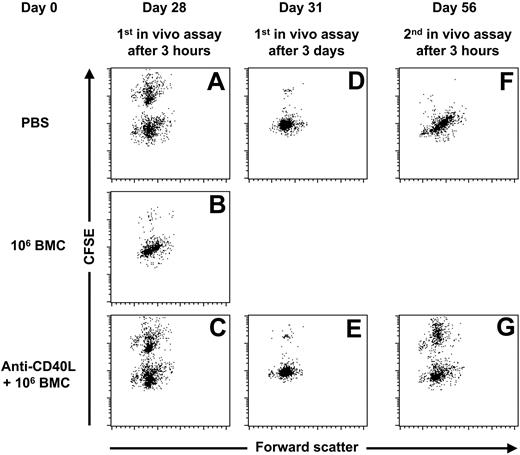
To determine the effect of anti-CD40L mAb treatment on the induction of a memory T-cell response, we treated 3 groups of C57BL/6 mice with PBS, 106 BALB/c bone marrow cells (BMCs), and 106 BALB/c BMCs in combination with anti-CD40L mAb. Four weeks later we performed an in vivo cytotoxicity assay. C57BL/6 mice initially treated with 106 BALB/c BMCs eliminated almost all CFSE-labeled BALB/c splenocytes within 3 hours (Figure 1B), indicating that the infusion of 106 BALB/c BMCs was sufficient to trigger a memory T-cell response. C57BL/6 mice treated with 106 BALB/c BMCs in combination with anti-CD40L mAb displayed similar rejection kinetics as the mice treated with PBS (Figure 1). These results indicate that anti-CD40L mAb treatment prevented the induction of a memory T-cell response. To confirm that anti-CD40L mAb treatment prevented the induction of a memory T-cell response, we subsequently performed a second in vivo cytotoxicity assay, 4 weeks after the first in vivo cytotoxicity assay. The C57BL/6 mice initially treated with PBS eliminated almost all CFSE-labeled BALB/c splenocytes within 3 hours (Figure 1F), indicating that the first in vivo cytotoxicity assay triggered a memory T-cell response. In contrast, the C57BL/6 mice initially treated with anti-CD40L mAb and 106 BALB/c still did not show an accelerated elimination of CFSE-labeled BALB/c cells (Figure 1G), indicating that a single injection of anti-CD40L mAb is sufficient to prevent the onset of a memory T-cell response, even after multiple infusions with donor antigens.
Anti-CD40L mAb treatment prevents the onset of a memory T-cell response. At day 0, 3 groups of C57BL/6 mice were given injections with PBS (n = 5), 106 BALB/c BMCs (n = 5), or 106 BALB/c BMCs in combination with anti-CD40L mAb (n = 5). At day 28, an in vivo cytotoxicity assay was performed and peripheral-blood samples were collected 3 hours and 3 days later. The C57BL/6 mice initially treated with PBS eliminated 32.3% ± 4.0% of the CFSE-labeled BALB/c splenocytes within 3 hours (A) and 96.4% ± 1.4% within 3 days after infusion (D). The C57BL/6 mice initially treated with 106 BALB/c BMCs eliminated 96.8% ± 3.2% of the CFSE-labeled BALB/c splenocytes within 3 hours after infusion (B), and the C57BL/6 mice initially treated with anti-CD40L mAb and 106 BALB/c BMCs eliminated 25.6% ± 3.7% of the CFSE-labeled BALB/c splenocytes within 3 hours (C) and 96.9% ± 1.5% within 3 days after infusion (E). At day 56, 4 weeks after the first in vivo cytotoxicity assay, a second in vivo cytotoxicity assay was performed. The C57BL/6 mice initially treated with PBS eliminated 99.2% ± 0.6% of the CFSE-labeled BALB/c splenocytes within 3 hours after infusion (F). The C57BL/6 mice initially treated with anti-CD40L mAb and 106 BALB/c BMCs eliminated 39.1% ± 5.5% of the CFSE-labeled BALB/c splenocytes within 3 hours (G). For each group a representative dot plot is displayed.
Anti-CD40L mAb treatment prevents the onset of a memory T-cell response. At day 0, 3 groups of C57BL/6 mice were given injections with PBS (n = 5), 106 BALB/c BMCs (n = 5), or 106 BALB/c BMCs in combination with anti-CD40L mAb (n = 5). At day 28, an in vivo cytotoxicity assay was performed and peripheral-blood samples were collected 3 hours and 3 days later. The C57BL/6 mice initially treated with PBS eliminated 32.3% ± 4.0% of the CFSE-labeled BALB/c splenocytes within 3 hours (A) and 96.4% ± 1.4% within 3 days after infusion (D). The C57BL/6 mice initially treated with 106 BALB/c BMCs eliminated 96.8% ± 3.2% of the CFSE-labeled BALB/c splenocytes within 3 hours after infusion (B), and the C57BL/6 mice initially treated with anti-CD40L mAb and 106 BALB/c BMCs eliminated 25.6% ± 3.7% of the CFSE-labeled BALB/c splenocytes within 3 hours (C) and 96.9% ± 1.5% within 3 days after infusion (E). At day 56, 4 weeks after the first in vivo cytotoxicity assay, a second in vivo cytotoxicity assay was performed. The C57BL/6 mice initially treated with PBS eliminated 99.2% ± 0.6% of the CFSE-labeled BALB/c splenocytes within 3 hours after infusion (F). The C57BL/6 mice initially treated with anti-CD40L mAb and 106 BALB/c BMCs eliminated 39.1% ± 5.5% of the CFSE-labeled BALB/c splenocytes within 3 hours (G). For each group a representative dot plot is displayed.
Elimination of MHC-mismatched hematopoietic cells by NK cells
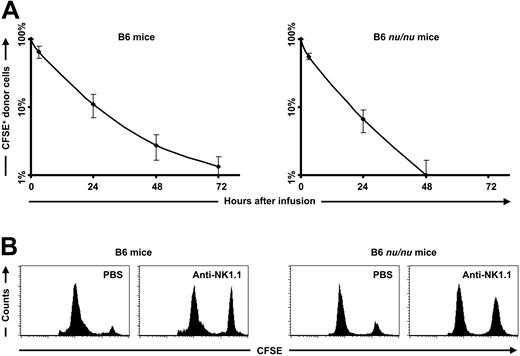
A single injection of anti-CD40L mAb prevented the induction of a memory T-cell response, but elimination of CFSE-labeled donor splenocytes was still observed (Figure 1E). Therefore, we hypothesized the involvement of NK cells in this process. A more detailed analysis of the elimination kinetics of the CFSE-labeled BALB/c splenocytes in untreated C57BL/6 mice showed a gradual elimination starting from the moment of infusion (Figure 2A). To exclude a role for T and NKT cells in this process, we repeated the experiment in T and natural killer T-cell (NKT-cell)-deficient nu/nu mice.12 Analysis of the elimination kinetics of CFSE-labeled BALB/c nu/nu splenocytes in untreated C57BL/6 nu/nu mice showed the same elimination kinetics as seen in normal C57BL/6 mice (Figure 2A). In accordance with the higher NK-cell activity in T and NKT-cell-deficient C57BL/6 nu/nu mice,13,14 the elimination rate in these mice was higher compared to the elimination rate of normal C57BL/6 mice. Treatment with anti-NK1.1 mAb resulted in a prolonged survival of CFSE-labeled BALB/c splenocytes in both C57BL/6 and C57BL/6 nu/nu mice (Figure 2B), confirming the role of NK cells in this process. These data indicate a pivotal role for NK cells in the rejection of MHC-mismatched hematopoietic cells.
NK cells mediate the early elimination of major mismatched hematopoietic cells. (A) Data from an in vivo cytotoxicity assay performed in untreated C57BL/6 mice (n = 5) showed that 35.1% ± 11.5% of the CFSE-labeled BALB/c splenocytes were eliminated within 3 hours, 88.8% ± 3.5% within 1 day, 97.2% ± 1.0% within 2 days, and 98.7% ± 0.5% within 3 days after infusion. Data from an in vivo cytotoxicity assay performed in untreated T and NKT-cell-deficient C57BL/6 nu/nu mice (n = 5) showed that 44.6% ± 6.1% of the CFSE-labeled BALB/c nu/nu splenocytes were eliminated within 3 hours, 93.4% ± 2.4% within 1 day, and 99.0% ± 0.7% within 2 days after infusion. (B) Normal C57BL/6 mice and T and NKT-cell-deficient C57BL/6 nu/nu mice were treated with PBS or anti-NK1.1 mAb at day -5 and -1. At day 0, a mixture of differentially CFSE-labeled donor and syngeneic splenocytes was injected; 1 or 2 days later, FACS analysis on peripheral-blood samples was performed. The PBS-treated C57BL/6 mice (n = 5) eliminated 88.5% ± 8.2% of the CFSE-labeled BALB/c splenocytes within 2 days, whereas the NK-cell-depleted C57BL/6 mice (n = 5) eliminated 46.0% ± 2.6% of the CFSE-labeled BALB/c splenocytes within 2 days. The PBS-treated C57BL/6 nu/nu mice (n = 5) eliminated 79.8% ± 9.9% of the CFSE-labeled BALB/c splenocytes within 1 day, whereas the NK-cell-depleted C57BL/6 nu/nu mice eliminated 25.8% ± 8.2% of the CFSE-labeled BALB/c splenocytes within 2 days. The displayed histograms are representative of each group.
NK cells mediate the early elimination of major mismatched hematopoietic cells. (A) Data from an in vivo cytotoxicity assay performed in untreated C57BL/6 mice (n = 5) showed that 35.1% ± 11.5% of the CFSE-labeled BALB/c splenocytes were eliminated within 3 hours, 88.8% ± 3.5% within 1 day, 97.2% ± 1.0% within 2 days, and 98.7% ± 0.5% within 3 days after infusion. Data from an in vivo cytotoxicity assay performed in untreated T and NKT-cell-deficient C57BL/6 nu/nu mice (n = 5) showed that 44.6% ± 6.1% of the CFSE-labeled BALB/c nu/nu splenocytes were eliminated within 3 hours, 93.4% ± 2.4% within 1 day, and 99.0% ± 0.7% within 2 days after infusion. (B) Normal C57BL/6 mice and T and NKT-cell-deficient C57BL/6 nu/nu mice were treated with PBS or anti-NK1.1 mAb at day -5 and -1. At day 0, a mixture of differentially CFSE-labeled donor and syngeneic splenocytes was injected; 1 or 2 days later, FACS analysis on peripheral-blood samples was performed. The PBS-treated C57BL/6 mice (n = 5) eliminated 88.5% ± 8.2% of the CFSE-labeled BALB/c splenocytes within 2 days, whereas the NK-cell-depleted C57BL/6 mice (n = 5) eliminated 46.0% ± 2.6% of the CFSE-labeled BALB/c splenocytes within 2 days. The PBS-treated C57BL/6 nu/nu mice (n = 5) eliminated 79.8% ± 9.9% of the CFSE-labeled BALB/c splenocytes within 1 day, whereas the NK-cell-depleted C57BL/6 nu/nu mice eliminated 25.8% ± 8.2% of the CFSE-labeled BALB/c splenocytes within 2 days. The displayed histograms are representative of each group.
NK cells mediate the elimination of donor cells after anti-CD40L mAb treatment
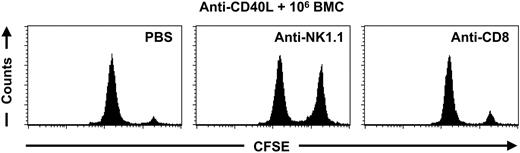
To determine the role of NK cells in the elimination of BALB/c splenocytes after treatment with anti-CD40L mAb, C57BL/6 mice were given injections with anti-CD40L mAb in combination with 106 BALB/c BMCs. Four weeks later we performed an in vivo cytotoxicity assay. At day 5 and day 1 before the in vivo cytotoxicity assay, 2 groups of mice were treated with anti-NK1.1 mAb or anti-CD8 mAb to determine whether NK cells or CD8+ cells were responsible for rejection of donor cells. Depletion of NK cells resulted in a prolonged survival of CFSE-labeled BALB/c splenocytes, whereas depletion of CD8+ cells did not result in a prolonged survival of CFSE-labeled BALB/c splenocytes (Figure 3), suggesting that NK cells, rather than cytotoxic T cells are responsible for the elimination of BALB/c splenocytes after the treatment with anti-CD40L mAb in combination with 106 BALB/c BMCs.
NK cells mediate the elimination of donor cells after anti-CD40L mAb treatment. C57BL/6 mice were treated with anti-CD40L mAb in combination with 106 BALB/c BMCs at day 0. At day 23 and day 27 the mice received an injection with PBS (n = 2), anti-NK1.1 mAb (n = 4), or anti-CD8 mAb (n = 4). At day 28 an in vivo cytotoxicity assay was performed and peripheral-blood samples were collected 2 days later for FACS analysis. The PBS-treated C57BL/6 mice eliminated 94.2% ± 0.2% of the CFSE-labeled BALB/c splenocytes within 2 days, whereas the NK1.1-depleted C57BL/6 mice eliminated 32.1% ± 10.9% of the CFSE-labeled BALB/c splenocytes within 2 days. The C57BL/6 mice depleted for CD8+ cells eliminated 89.6% ± 1.8% of the CFSE-labeled BALB/c splenocytes within 2 days. The displayed histograms are representative of each group.
NK cells mediate the elimination of donor cells after anti-CD40L mAb treatment. C57BL/6 mice were treated with anti-CD40L mAb in combination with 106 BALB/c BMCs at day 0. At day 23 and day 27 the mice received an injection with PBS (n = 2), anti-NK1.1 mAb (n = 4), or anti-CD8 mAb (n = 4). At day 28 an in vivo cytotoxicity assay was performed and peripheral-blood samples were collected 2 days later for FACS analysis. The PBS-treated C57BL/6 mice eliminated 94.2% ± 0.2% of the CFSE-labeled BALB/c splenocytes within 2 days, whereas the NK1.1-depleted C57BL/6 mice eliminated 32.1% ± 10.9% of the CFSE-labeled BALB/c splenocytes within 2 days. The C57BL/6 mice depleted for CD8+ cells eliminated 89.6% ± 1.8% of the CFSE-labeled BALB/c splenocytes within 2 days. The displayed histograms are representative of each group.
Suppression of NK-cell alloreactivity by increasing the dose of donor BMCs
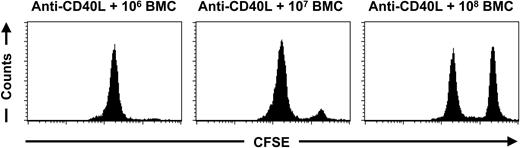
It is well known that high numbers of BMCs are required for the transplantation over MHC barriers.15 We therefore investigated the effect of increasing doses of donor BMCs on the survival of CFSE-labeled donor cells. C57BL/6 mice were treated with anti-CD40L mAb in combination with 106, 107, and 108 BALB/c BMCs. Four weeks later an in vivo cytotoxicity assay was performed. Treatment with anti-CD40L mAb followed by the infusion of increasing numbers of BALB/c BMCs resulted in a dose-dependent prolongation of the survival of BALB/c splenocytes (Figure 4). Treatment with anti-CD40L mAb and 108 BMCs even resulted in complete nonresponsiveness. This effect was donor-specific because CFSE-labeled MHC-mismatched third-party splenocytes, derived from C3H/J mice, were still eliminated (Figure 5). To exclude a role for T and NKT cells in this phenomenon, we infused 106, 107, and 108 T and NKT-cell-deficient BALB/c nu/nu BMCs into T and NKT-cell-deficient C57BL/6 nu/nu mice. Survival of CFSE-labeled BALB/c nu/nu splenocytes was tested 4 weeks later. Again, infusion of increasing numbers of MHC-mismatched BMCs correlated with a prolonged survival of donor splenocytes (Figure 6). These results indicate that infusion of increasing numbers of MHC-mismatched BMCs results in decreased NK-cell alloreactivity.
Suppression of alloreactivity by increasing the dose of donor BMCs. C57BL/6 mice were treated with anti-CD40L mAb in combination with 106 (n = 6), 107 (n = 6), or 108 (n = 6) BALB/c BMCs at day 0. At day 28 an in vivo cytotoxicity assay was performed and peripheral-blood samples were collected 3 days later for FACS analysis. The mice treated with anti-CD40L mAb and 106 BALB/c BMCs eliminated 98.1% ± 1.3% of the CFSE-labeled BALB/c cells within 3 days. The mice treated with anti-CD40L mAb and 107 BALB/c BMCs eliminated 92.5% ± 3.8% of the BALB/c cells within 3 days, whereas no significant elimination of CFSE-labeled donor cells was detected (0.0% ± 6.8% within 3 days) in the group treated with anti-CD40L mAb and 108 BALB/c BMCs. The displayed histograms are representative of each group.
Suppression of alloreactivity by increasing the dose of donor BMCs. C57BL/6 mice were treated with anti-CD40L mAb in combination with 106 (n = 6), 107 (n = 6), or 108 (n = 6) BALB/c BMCs at day 0. At day 28 an in vivo cytotoxicity assay was performed and peripheral-blood samples were collected 3 days later for FACS analysis. The mice treated with anti-CD40L mAb and 106 BALB/c BMCs eliminated 98.1% ± 1.3% of the CFSE-labeled BALB/c cells within 3 days. The mice treated with anti-CD40L mAb and 107 BALB/c BMCs eliminated 92.5% ± 3.8% of the BALB/c cells within 3 days, whereas no significant elimination of CFSE-labeled donor cells was detected (0.0% ± 6.8% within 3 days) in the group treated with anti-CD40L mAb and 108 BALB/c BMCs. The displayed histograms are representative of each group.
NK-cell depletion reduces the number of donor BMCs required for long-term donor chimerism
Our results indicate an important role for NK cells in the initial rejection of MHC-mismatched BMCs. A high dose of donor BMCs was required to overcome the NK-cell barrier, and this NK-cell nonresponsiveness was correlated with mixed chimerism (data not shown). Altogether, these results suggest that NK-cell depletion prior to anti-CD40L mAb treatment and transplantation should result in enhanced engraftment. To test this hypothesis, C57BL/6 mice were treated with PBS or anti-NK1.1 mAb at day 5 and day 1 before treatment with anti-CD40L mAb in combination with 108 BALB/c BMCs. Peripheral-blood samples were collected at different time points and analyzed for the presence of donor cells using FACS analysis. NK-cell depletion resulted in increased donor chimerism compared to the PBS-treated mice (Table 1). NK-cell depletion followed by anti-CD40L mAb treatment and the transplantation of 30 × 106 BALB/c BMCs showed a more pronounced difference when compared to undepleted C57BL/6 mice. All NK-cell-depleted C57BL/6 mice showed long-term multilineage chimerism, where after the treatment with anti-CD40L mAb and 30 × 106 BALB/c BMCs alone only 20% of the C57BL/6 control mice showed long-term multilineage chimerism (Table 1). These results indicate that NK-cell depletion prior to transplantation results in enhanced engraftment and also suggest that NK cells are capable of rejecting 30 × 106 MHC-mismatched BMCs.
Chimerism in C57BL/6 recipients of BALB/c BMCs
BM dose and anti-NIK1.1 mAb treatment . | Long-term donor chimerism, no./total (%) . | Donor chimerism in chimeric mice 24 wk after transplantation, % . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | GR-1+ . | B220+ . | CD3+ . | ||
| 100 × 106 cells | ||||||
| Without treatment | 5/5 (100) | 26.7 ± 9.5 | 5.3 ± 2.5 | 1.4 ± 0.9 | ||
| With treatment | 5/5 (100) | 37.3 ± 11.1 | 7.1 ± 3.9 | 1.8 ± 1.1 | ||
| 30 × 106 cells | ||||||
| Without treatment | 1/5 (20)* | 6.2 | 1.4 | 0.1 | ||
| With treatment | 5/5 (100) | 5.8 ± 3.3 | 1.6 ± 0.9 | 0.3 ± 0.2 | ||
BM dose and anti-NIK1.1 mAb treatment . | Long-term donor chimerism, no./total (%) . | Donor chimerism in chimeric mice 24 wk after transplantation, % . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | GR-1+ . | B220+ . | CD3+ . | ||
| 100 × 106 cells | ||||||
| Without treatment | 5/5 (100) | 26.7 ± 9.5 | 5.3 ± 2.5 | 1.4 ± 0.9 | ||
| With treatment | 5/5 (100) | 37.3 ± 11.1 | 7.1 ± 3.9 | 1.8 ± 1.1 | ||
| 30 × 106 cells | ||||||
| Without treatment | 1/5 (20)* | 6.2 | 1.4 | 0.1 | ||
| With treatment | 5/5 (100) | 5.8 ± 3.3 | 1.6 ± 0.9 | 0.3 ± 0.2 | ||
Two groups of C57BL/6 mice were treated with anti-CD40L mAb and 100 × 106 BALB/c BMCs at day 0. One group (n = 5) received 2 injections of anti-NK1.1 mAb at day 5 and day 1 before transplantation, and the other group (n = 5) did not have depletion of NK1.1+ cells. Two other groups of C57BL/6 mice were treated with anti-CD40L mAb and 30 × 106 BALB/c BMCs on day 0. Again, one group (n = 5) received 2 injections of anti-NK1.1 mAb at day 5 and day 1 before transplantation and the other group (n = 5) was not depleted for NK1.1+ cells.
Two mice in this group showed transient chimerism, but no detectable chimerism was observed 12 weeks after transplantation.
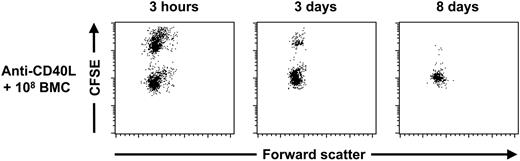
NK-cell tolerance after treatment with anti-CD40L mAb and 108 donor BMCs is donor-specific. C57BL/6 mice were treated with anti-CD40L mAb in combination with 108 BALB/c BMCs (n = 5) at day 0. At day 28 these mice were given injections of differentially CFSE-labeled MHC-mismatched, C3H/J-derived third-party splenocytes and BALB/c splenocytes; peripheral-blood samples were collected 3 hours, 3 days, and 8 days later for FACS analysis. Survival of the highly CFSE+ third-party cells was calculated using the intermediate CFSE+ donor cells as internal control cells. Three hours after the infusion of the CFSE-labeled splenocytes, no detectable elimination of the CFSE-labeled third-party cells was observed. Three days later 88.7% ± 6.5% of the third-party cells were eliminated and 8 days later 98.4% ± 1.4% of the cells were eliminated. These elimination kinetics were similar compared to anti-CD40L mAb-treated C57BL/6 mice (data not shown). The displayed dot plots are representative of each time point.
NK-cell tolerance after treatment with anti-CD40L mAb and 108 donor BMCs is donor-specific. C57BL/6 mice were treated with anti-CD40L mAb in combination with 108 BALB/c BMCs (n = 5) at day 0. At day 28 these mice were given injections of differentially CFSE-labeled MHC-mismatched, C3H/J-derived third-party splenocytes and BALB/c splenocytes; peripheral-blood samples were collected 3 hours, 3 days, and 8 days later for FACS analysis. Survival of the highly CFSE+ third-party cells was calculated using the intermediate CFSE+ donor cells as internal control cells. Three hours after the infusion of the CFSE-labeled splenocytes, no detectable elimination of the CFSE-labeled third-party cells was observed. Three days later 88.7% ± 6.5% of the third-party cells were eliminated and 8 days later 98.4% ± 1.4% of the cells were eliminated. These elimination kinetics were similar compared to anti-CD40L mAb-treated C57BL/6 mice (data not shown). The displayed dot plots are representative of each time point.
Suppression of NK-cell alloreactivity by increasing the dose of donor BMCs. T and NKT-cell-deficient C57BL/6 nu/nu mice were treated with an infusion of 106 (n = 5), 107 (n = 5), or 108 (n = 5) BALB/c nu/nu BMCs at day 0. At day 28 an in vivo cytotoxicity assay was performed, and peripheral-blood samples were collected 2 days later for FACS analysis. The mice treated with 106 BALB/c nu/nu BMCs eliminated 99.7% ± 0.3% of the CFSE-labeled BALB/c nu/nu cells within 2 days. The mice treated with 107 BALB/c nu/nu BMCs eliminated 96.8% ± 2.9% of the BALB/c nu/nu cells within 2 days, whereas the group given transplants with 108 BALB/c nu/nu BMCs eliminated 64.6% ± 32.8% of the BALB/c nu/nu cells within 2 days. The displayed histograms are representative of each group.
Suppression of NK-cell alloreactivity by increasing the dose of donor BMCs. T and NKT-cell-deficient C57BL/6 nu/nu mice were treated with an infusion of 106 (n = 5), 107 (n = 5), or 108 (n = 5) BALB/c nu/nu BMCs at day 0. At day 28 an in vivo cytotoxicity assay was performed, and peripheral-blood samples were collected 2 days later for FACS analysis. The mice treated with 106 BALB/c nu/nu BMCs eliminated 99.7% ± 0.3% of the CFSE-labeled BALB/c nu/nu cells within 2 days. The mice treated with 107 BALB/c nu/nu BMCs eliminated 96.8% ± 2.9% of the BALB/c nu/nu cells within 2 days, whereas the group given transplants with 108 BALB/c nu/nu BMCs eliminated 64.6% ± 32.8% of the BALB/c nu/nu cells within 2 days. The displayed histograms are representative of each group.
Discussion
The induction of mixed hematopoietic chimerism over MHC barriers using nonablative conditioning would allow a widespread use of allogeneic SCT. Recent studies have demonstrated that it is indeed possible to obtain long-term chimerism after SCT over MHC barriers without cytoreductive treatment.16-19 However, a better understanding of the conditions required for successful engraftment should result in more robust transplantation strategies. In this study, we demonstrated the impact of the different barriers in a nonablative MHC-mismatched SCT setting. By specifically targeting these barriers, we were able to induce long-term chimerism with a relative low number of MHC-mismatched BMCs.
We have used an SCT model in the absence of cytoreduction and first looked at the effect of anti-CD40L mAb treatment on the onset of a memory T-cell response. In vivo cytotoxicity data demonstrated that the accelerated elimination of CFSE-labeled donor splenocytes observed in C57BL/6 mice initially infused with 106 BALB/c BMCs was prevented following the infusion of 106 BALB/c BMCs in combination with anti-CD40L mAb treatment. In fact, even after repetitive infusions of donor cells, no signs of a memory T-cell response were observed, demonstrating that a single infusion of anti-CD40L mAb was sufficient to prevent the onset of a memory T-cell response. However, elimination of donor hematopoietic cells was still observed with approximately the same kinetics as in nonsensitized mice. Therefore, we hypothesized the involvement of NK cells in this process.
Detailed analysis of the elimination kinetics in wild-type C57BL/6 mice and T and NKT-cell-deficient C57BL/6 mice showed a gradual elimination of donor splenocytes and a prolonged survival of donor splenocytes after in vivo depletion of NK cells. In vivo depletion of NK cells in C57BL/6 mice initially treated with anti-CD40L mAb in combination with 106 BALB/c BMCs also resulted in a significant prolonged survival of donor splenocytes. In contrast, CD8 depletion did not affect the elimination of donor splenocytes. These data indicate that NK cells, rather than CD8+ cells, were responsible for the elimination of donor cells after the induction of T-cell tolerance. Dose escalation studies in both T and NKT-cell-deficient and wild-type C57BL/6 mice showed that the transplantation of increasing numbers of MHC-mismatched BMCs correlated with a prolonged survival of donor splenocytes. Transplantation of a megadose of MHC-mismatched BMCs even resulted in complete nonresponsiveness. This effect was associated with donor chimerism and was donor specific because CFSE-labeled MHC-mismatched third-party splenocytes were still eliminated.
These data suggested that additional NK-cell depletion prior to anti-CD40L mAb treatment and SCT might enhance engraftment. Indeed, NK-cell depletion prior to anti-CD40L mAb treatment and transplantation of 108 MHC-mismatched BMCs resulted in higher levels of donor chimerism. However, the most pronounced effect of NK-cell depletion was seen in mice treated with anti-CD40L mAb treatment in combination with the transplantation of 30 × 106 MHC-mismatched BMCs. Additional NK-cell depletion completely prevented graft rejection, whereas graft rejection was observed in 80% of the mice that did not receive additional NK-cell depletion. These data indicated that NK-cell depletion facilitates engraftment and allows long-term chimerism after transplantation of a reduced dose of MHC-mismatched stem cells.
A recent study, which also used anti-CD40L mAb treatment and MHC-mismatched SCT model in the complete absence of cytoreduction, showed that pretransplantation conditioning with a donor-specific transfusion significantly improved engraftment. A pretransplantation depletion of CD8+ cells did not exhibit an engraftment-enhancing effect, whereas a pretransplantation depletion of CD122+ cells did.18 Because anti-CD122 mAb treatment has been shown to delete NK-cell activity in vivo20-22 and the effect of pretransplantation depletion of CD8+ cells was minimal, this study also supports an important role for NK cells in the early rejection of MHC-mismatched stem cells.
In the absence of an immune response, the percentage of donor chimerism is determined by the ratio of donor and recipient stem cells. Estimates state that the bone marrow compartment of an adult mouse contains approximately 2 × 108 BMCs,23,24 explaining the relatively high dose of donor bone marrow required for detectable chimerism following transplantation over MHC barriers. Preliminary data indicate that the transplantation of 1 × 108 BMCs in a congenic setting resulted in approximately 35% donor chimerism within the granulocyte population (data not shown). This percentage corresponds with data from our MHC-mismatched transplantation experiments, suggesting that NK-cell depletion and treatment with anti-CD40L mAb result in engraftment with approximately the same efficiency as congenic stem cells. It is therefore conceivable that a reduction of the host cell content would allow engraftment following transplantation of a lower number of stem cells. Results from a congenic stem-cell transplantation study in rats demonstrated that by using an isoform-specific anti-CD45 mAb treatment, a significant depletion of host stem cells could indeed be obtained.25 However, further research is needed to address the efficiency of this approach.
NK-cell function is coordinated by complex interactions between different inhibitory and activating receptors,26,27 making it difficult to study the mechanism responsible for NK-cell tolerance. Preliminary data of Ly49A and Ly49D expression on host NK cells support the conclusion that the observed NK-cell nonresponsiveness was indeed the result of NK-cell tolerance. The Ly49A receptor is an inhibitory NK-cell receptor and the Ly49D receptor is an activating NK-cell receptor, both specific for the BALB/c MHC class I molecule H-2Dd.28-32 After treatment of C57BL/6 mice with anti-CD40L mAb in combination with the transplantation of 108 BALB/c BMCs, a slight increase in the percentage of host NK cells bearing the Ly49A receptor and a slight decrease in the percentage of host NK cells bearing the Ly49D receptor were observed (data not shown). This observation corresponds with data from Sykes and colleagues, who found an altered expression of Ly49 receptors in mixed allogeneic bone marrow chimeras, but no depletion of NK cells with a specific NK-cell receptor.33,34 Altogether, these data are in accordance with the hypothesis that the induction of mixed chimerism is associated with NK-cell tolerance, rather than a deletion of alloreactive NK cells.
Recent evidence suggests that tolerance induction is more difficult in mice that have experienced multiple infections, a phenomenon being referred to as heterologous immunity.35-37 We therefore hypothesize that that differences between our study and other studies with respect to the induction of chimerism may be related to differences in the microbiologic status of the mice. This issue will be further addressed in future studies.
In conclusion, our results show that transplantation of a high dose of BMCs allows engraftment and long-term chimerism over a MHC barrier after anti-CD40L mAb treatment in the absence of cytoreduction. This relatively large stem-cell dose is needed to induce NK-cell tolerance. Because NK cells are relatively radioresistant, these results may explain the requirement of the larger stem-cell dose in MHC-mismatched SCT in general. Furthermore, our results suggest that in vivo NK-cell depletion allows engraftment and long-term chimerism of a relatively low number of stem cells following MHC-mismatched SCT.
Prepublished online as Blood First Edition Paper, May 31, 2005; DOI 10.1182/blood-2005-04-1391.
Supported by grant no. RUL 2001-2494 from the Dutch Cancer Society.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Thorbald van Hall and Dr Jeroen van Bergen for support and helpful discussion.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal