CD34 is a transmembrane protein expressed by vascular endothelial cells and hematopoietic stem/progenitor cells (HSCs),1 but its function has remained obscure. Recently, we observed that in mice, CD34 is highly expressed by committed mast-cell progenitors and their terminally differentiated progeny in vitro and in vivo.2 This was true of both mucosal- and connective-tissue-type mast cells. Although this observation highlights the problems associated with separating mast-lineage cells from stem cells (both of which express CD34, stem cell antigen 1 [Sca-1], c-kit, and interleukin-3 receptor [IL-3R], and lack lineage markers2 ), it also provides an additional avenue to explore CD34 function. We performed a detailed analysis of mast cells from wild-type (wt) and CD34-deficient mice and found that CD34 plays an important functional role in blocking adhesion.3 Specifically, we found that loss of CD34 leads to enhanced mast-cell aggregation in vitro and that loss of both CD34 and the distantly related sialomucin, CD43, impairs the ability of mast cells to repopulate their peripheral niches in vivo.3 Thus, we would argue on the basis of our data that CD34 and CD43 play an important role in enhancing the fidelity of hematopoietic-cell migration by decreasing their adhesion.
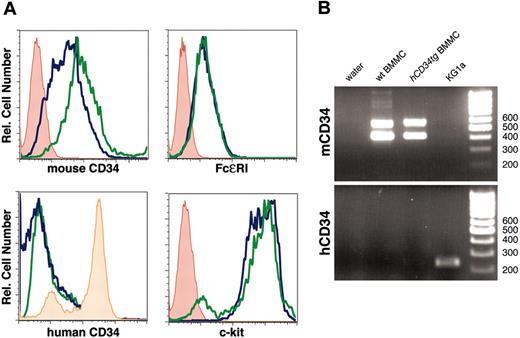
Mature mouse mast cells express mouse CD34 but not transgenic human CD34. (A) BMMC cultures were established from wild-type (wt) and hCD34tg bone marrow and assessed for expression of mouse and human CD34 and for c-kit and FcϵRI as markers of mast-lineage cells by flow cytometry. Blue lines indicate wild-type BMMCs; green lines, hCD34tg BMMCs; red lines, secondary antibody alone; and orange lines, human CD34 staining of KG1a cells. (B) Reverse-transcription-polymerase chain reaction (RT-PCR) analysis of BMMCs and KG1a cells for expression of mouse and human CD34 mRNA. Two bands in mouse CD34 lanes correspond to the expected size of the 2 naturally occurring splice variants of mouse CD34 detected by the murine primers.
Mature mouse mast cells express mouse CD34 but not transgenic human CD34. (A) BMMC cultures were established from wild-type (wt) and hCD34tg bone marrow and assessed for expression of mouse and human CD34 and for c-kit and FcϵRI as markers of mast-lineage cells by flow cytometry. Blue lines indicate wild-type BMMCs; green lines, hCD34tg BMMCs; red lines, secondary antibody alone; and orange lines, human CD34 staining of KG1a cells. (B) Reverse-transcription-polymerase chain reaction (RT-PCR) analysis of BMMCs and KG1a cells for expression of mouse and human CD34 mRNA. Two bands in mouse CD34 lanes correspond to the expected size of the 2 naturally occurring splice variants of mouse CD34 detected by the murine primers.
A caveat to these observations is the fact that, in humans, CD34 expression is lost upon mast-cell differentiation.4-6 To gain further insights into this discrepancy between mouse and human CD34 (hCD34) regulation, we examined the expression of the human CD34 gene in murine mast cells derived from transgenic mice harboring a 160-kilobase (kb) genomic DNA fragment spanning the hCD34 coding exons, promoter elements, 110 kb of 5′ upstream sequence and 30 kb of 3′ downstream flanking (hCD34tg).7 These mice have proved extremely useful for studying hCD34 gene regulation, since they faithfully recapitulate the normal pattern of hCD34 mRNA expression by vascular endothelia and bone marrow progenitor cells in the murine tissues.7-9
Bone marrow cells from hCD34tg and wild-type mice were cultured in IL-3 to obtain bone marrow-derived mast cells (BMMCs), and these were assessed for expression of human and mouse CD34 and for markers of mast cell differentiation (Figure 1). Consistent with our previous observations,2 murine CD34 (mCD34), Fcϵ receptor I (FcϵRI), and c-kit were all expressed at high levels by both wild-type and hCD34tg BMMCs. In sharp contrast to the expression of mCD34 on hCD34tg BMMCs, hCD34 was not detected on these cells (Figure 1). The human hCD34 antibody we used for flow cytometry (8G12) is reported to detect all glycoforms of hCD3410 ; however, we used RT-PCR to confirm the absence of hCD34 in transgenic BMMCs. Although we found high levels of endogenous mCD34 mRNA in both wild-type and transgenic mast cells, only trace amounts of hCD34 mRNA were found in hCD34tg mast-cell cultures. As a positive control, we found that the human KG1a cell line consistently showed high levels of hCD34 expression.
In summary, our results show that despite the ability of the human 160-kb CD34 transgene to drive hCD34 expression in mouse hematopoietic progenitors and vasculature,7-9 it is insufficient to drive hCD34 expression in murine mast cells. Furthermore, it supports the observation that in mature mast cells, CD34 is differentially regulated in mice and humans,4-6 and suggests that the hCD34 locus lacks key control elements that are present in the murine gene and are required for maintenance of CD34 expression in mature cells of this lineage.
Since we have shown that CD34 is important for blocking adhesion and aiding the appropriate homing of mast-lineage cells in mice, this raises an important question: why have mature human mast cells lost the expression of this molecule? An intriguing possibility is that this may reflect subtle differences in the way mast cells and their precursors populate tissues in mice and humans. In both species, mast cells leave the bone marrow as undifferentiated precursors, enter the circulation, and home to the appropriate target tissue where they undergo further local maturation into functional, granule-positive mast cells. Once these committed precursor cells reach their target tissue, the requirement for continued expression of this antigen by the mast-cell lineage may, therefore, no longer be necessary. Because mice continue to express CD34 on their mast cells long after they have matured in their peripheral niches, it is possible that this prolonged expression endows them with the capacity for further migration in response to pathogens or allergens. Conversely, loss of CD34 on human mast cells upon maturation may suggest that, once they have populated a tissue, they lose the capacity for future migration. Crossing hCD34tg mice into a mouse CD34 knock-out (KO) background may offer a suitable model to test this interesting hypothesis.
Supported by an operating grant from the Canadian Institutes of Health Research (CIHR). K.M.M. is a CIHR and a Michael Smith Foundation for Health Research Scholar. E.D. is supported by a Michael Smith Foundation for Health Research Trainee Scholarship and a Heart and Stroke Foundation Doctoral Scholarship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal