In a recent paper published in Blood by Seré and coworkers,1 a number of misconceptions are generated relative to earlier work published by our laboratory dealing with the inhibition of prothrombin activation by protein S.
Our laboratory reported that protein S inhibits prothrombin activation by competition for phospholipid binding sites.2 The paper by Seré et al1 concludes that our hypothesis was based on experiments performed with protein S preparations that contained multimeric forms of protein S. This conclusion was based upon studies performed with their preparations of protein S, using which they observed that protein S was polymerized when analyzed in the absence of Ca2+.3 Their data, in fact, confirmed a previous publication from our laboratory4 that examined the polymerization of protein S and the Ca2+ dependence of this process. In the latter paper, we showed that protein S, both intact and thrombin cleaved, polymerizes in the absence of Ca2+ but is totally depolymerized by 0.8 mM calcium with a Ca2+ dissociation constant of 0.42 mM. Sedimentation equilibrium studies conducted in the presence of Ca2+ revealed that protein S was homogeneous with a molecular weight of 75 800 ± 4200 (S vedberg units = 4.2). In the presence of ethylenediaminetetraacetic acid (EDTA), polymerized forms of the protein were observed both by sedimentation equilibrium and sedimentation velocity (S vedberg units = 7.2). We cannot comment on the qualities of the protein S prepared by Seré and colleagues.
The experiments performed by our laboratory on prothrombin activation reported that protein S slowed the rate of prothrombin activation in experiments conducted using solutions equilibrated with Ca2+ (2 mM) prior to mixing in the reaction system. We also showed that phospholipids overcame the inhibition that was observed, consistent with a phospholipid-dependent process, and that the inhibition occurred in systems with platelets and factors II, VIIa, IX, X, VIII, and V at their mean physiologic concentration, with reactions initiated with tissue factor.
In the report by Seré et al,1 there are fundamental defects in the experimental protocol that overcome the ability to interpret the experimental results. Their experiments are conducted in diluted citrate plasma with initiation of the reactions by the addition of Ca2+. Thus, at the start of each experiment protein S is polymerized, and depolymerization is occurring during the course of the reaction itself. The investigators, in fact, make a point of this and indicate that the preaddition of CaCl2 to the experimental system caused a “decrease in lag time”1(p3626) consistent with the rate processes required for the conformational changes of procoagulant vitamin-K-dependent proteins as well as depolymerization of the inhibitor protein S. These conflicting processes render the experimental results uninterpretable. In addition, in the endogenous thrombin potential (ETP) measurements used by the investigators, a substrate for thrombin is included. This addition, however, confuses the results of ETP interpretations by providing a competitive substrate for thrombin, thus influencing thrombin concentration throughout the measurements.
To conclude, the authors state that they “quantified the APC-independent anticoagulant activity of protein S in its natural environment [emphasis added].”1(p3629) This is hardly the case. The natural environment for protein S is blood that, in addition to the components of plasma, contains cells and platelets, but does not include dilution, citrate, or a synthetic thrombin inhibitor.
Misconceptions about protein-S multimers
The critique by Dr Mann of our study1 enables us to dispel several misconceptions about the importance of in vitro-generated protein-S dimers2 and multimers.3 First, we would like to emphasize that there is a difference between the protein-S dimers (called polymers by Dr Mann) reported by Pauls et al2 and the protein-S multimers described by Seré et al.3 Pauls et al2 described quantitative dimerization of purified protein S at high concentrations (micromolar range) in ethylenediaminetetraacetic acid (EDTA) that was reversible upon addition of Ca2+ ions. We demonstrated that purified protein-S preparations contain a small percentage of higher-order multimers that do not dissociate in the presence of Ca2+ ions and that are stable at low protein-S concentrations (nanomolar range).3 The low-abundance multimers bind with a high affinity (kDa < 1 nM) to phospholipids and are very effective inhibitors of phospholipid-dependent reactions at low phospholipid concentrations.3 Concordantly, increasing the phospholipid concentration overcomes the inhibitory activity of the multimers.4
Protein-S multimers are absent in plasma.3 In theory, protein-S dimerization could occur in plasma as a result of chelation of calcium by sodium citrate used as an anticoagulant with blood collection. However, protein-S dimerization occurs at concentrations far above the plasma concentration: “at 17 μM, the associated product is principally a dimer.”2 Inspection of Figure 5 of the paper by Pauls et al2 indicates that, at the free protein-S concentration present in plasma (∼ 150 nM), protein S is principally a monomer.
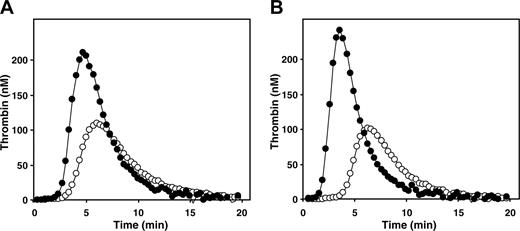
Anticoagulant activity of protein S in the absence of APC in recalcified normal pooled citrated plasma. (A) Normal pooled plasma was incubated with antibodies against protein C with (•) or without (○) antibodies against protein S. After a 15-minute incubation, thrombin generation was started with 3.5 pM tissue factor, 10 μM phospholipid vesicles 20/60/20 1,2-Dioleoyl-sn-glycero-3-phosphoserine (DOPS)/1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC)/1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and 16 mM CaCl2 (final concentrations). (B) A similar experiment in which thrombin generation was measured in plasma in which 16 mM CaCl2 was already present during the 15-minute preincubation with antibodies.
Anticoagulant activity of protein S in the absence of APC in recalcified normal pooled citrated plasma. (A) Normal pooled plasma was incubated with antibodies against protein C with (•) or without (○) antibodies against protein S. After a 15-minute incubation, thrombin generation was started with 3.5 pM tissue factor, 10 μM phospholipid vesicles 20/60/20 1,2-Dioleoyl-sn-glycero-3-phosphoserine (DOPS)/1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC)/1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and 16 mM CaCl2 (final concentrations). (B) A similar experiment in which thrombin generation was measured in plasma in which 16 mM CaCl2 was already present during the 15-minute preincubation with antibodies.
In spite of this, Dr Mann believes that inhibitory protein-S dimers are present at the start of our experiments, and that slow depolymerization of these dimers after recalcification of plasma invalidated our conclusions.1
We refuted this possibility by recalcifying the plasma 15 minutes before initiation of thrombin generation with tissue factor/phospholipid. This provided ample time for the Ca2+-induced conformational changes of vitamin-K-dependent proteins to occur, and for depolymerization of protein-S dimers if present. Figure 1 shows that after “pre”-recalcification of plasma the activated protein C (APC)-independent inhibitory effect of protein S on thrombin generation prevailed.
The endogenous thrombin potentials (ETPs) determined in the absence of functional protein S were similar without and with preincubation of plasma with CaCl2 (Figure 1). In the presence of protein S (Figure 1 open symbols), the ETP determined in plasma preincubated with CaCl2 (521 nM thrombin min) was even lower than the ETP of plasma that was not preincubated with CaCl2 (659 nM thrombin min). Protein S decreased the ETP in freshly recalcified citrated plasma by 30%, compared with 40% when plasma was preincubated with CaCl2, and thus became an even better inhibitor of thrombin generation. Moreover, if protein-S dimers in citrated plasma had caused the observed phenomena, the inhibitory effect of protein S on thrombin generation would have been abrogated at high phospholipid concentrations.2 However, increasing phospholipid concentrations did not affect the anticoagulant potential of protein S in plasma.1 This demonstrates that the inhibition of thrombin formation by protein S in plasma is not due to competition for phospholipid binding sites.
Dr Mann raised an important point regarding the effect of the thrombin substrate on thrombin generation. Indeed, it has been shown that the measured ETP increases with increasing substrate concentrations,5 likely due to the fact that the substrate protects thrombin against inhibition by antithrombins. However, it should be emphasized that only free thrombin (ie, thrombin that is not occupied by substrate) will be available for feedback reactions. Surprisingly, when the free-thrombin concentrations present in plasma were calculated using the Michaelis-Menten constant (Km) and substrate concentrations, superimposable thrombin generation curves (free thrombin versus time) were obtained.5 This indicates that the generation of thrombin hardly depends on the action of thrombin but that another component is rate limiting, namely factor Xa,6 and that the presence of the substrate has virtually no effect on the mechanism of prothrombin activation.5
We agree with Dr Mann that there are ample differences between our reaction conditions and the natural environment in which protein S acts. We have used the phrase “natural environment” to emphasize the significance of the natural form and integrity of protein S in plasma, in which it, in the absence of APC, could play an important role in the in vivo down-regulation of coagulation.
Correspondence: Tilman M. Hackeng, Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), University Maastricht, PO Box 616, 6200 MD Maastricht, the Netherlands; email: t.hackeng@bioch.unimaas.nl.
This research was supported by National Institutes of Health (NIH) grant NIH PPG HL46703.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal