Abstract
Hematopoietic progenitor cells (HPCs) traffic to and are retained in the marrow through the trophic effects of the chemokine stromal cell-derived factor-1α (SDF-1α) binding to its receptor, CXC chemokine receptor 4 (CXCR4). AMD3100 reversibly inhibits SDF-1α/CXCR4 binding, and AMD3100 administration mobilizes CD34+ cells into the circulation. We therefore tested the hypotheses that the combination of AMD3100 plus granulocyte colony-stimulating factor (G-CSF) (hereafter A + G) would be superior to G-CSF alone (hereafter G) in mobilizing hematopoietic progenitor cells (HPCs) and that A + G-mobilized cells would engraft as well as G-mobilized cells. The primary objective was to determine whether patients mobilized more progenitor cells per unit of blood volume of apheresis after A + G administration versus G alone. Secondary objectives were to determine whether patients mobilized with A + G compared with G alone required fewer apheresis procedures to reach the target level at least 5 × 106 CD34+ cells/kg for transplantation and to determine whether patients mobilized with A + G had at least a 90% success rate of autologous transplantation as assessed by neutrophil engraftment by day 21. Each patient served as his or her own control in a sequential mobilization design. All study objectives were met without significant toxicity. The results demonstrate that the combination of A + G is generally safe, effective, and superior to G alone for autologous HPC mobilization. (Blood. 2005;106:1867-1874)
Introduction
Autologous hematopoietic progenitor cell (HPC) transplantation (AHPCT) has emerged as a preferred strategy in the treatment of a variety of hematologic malignancies. The most common indications are for relapsed chemotherapy sensitive non-Hodgkin lymphoma (NHL) or for multiple myeloma (MM) in first or second remission.
The dose of HPCs infused, as quantitated by the number of CD34+ cells infused per kilogram of body weight, influences marrow recovery both qualitatively and quantitatively. Recovery of marrow function, particularly with regard to platelet production, is accelerated as HPC dose increases. Rapid platelet recovery is particularly reproducible at doses above 5 × 106 CD34+ cells/kg. At suboptimal HPC doses, hematopoietic recovery becomes unacceptably delayed or incomplete.1-14 As a consequence, many transplantation centers establish a minimal cell dose, often 1 to 2 × 106 CD34 cells/kg, as a limiting dose below which they will not proceed to transplantation.
One of the common approaches for HPC mobilization for collection by apheresis is to administer hematopoietic growth factors, most often granulocyte colony-stimulating factor (G-CSF). This cytokine produces a myeloid hyperplasia in the marrow, including expansion of both mature and immature myeloid cells. After 4 to 5 days, appreciable numbers of CD34+ cells are released into the circulation. Studies have suggested that mobilization is mediated, at least in part, through the release of neutrophil-derived proteases that cleave adhesion molecules as well as chemokines and their receptors.15-17
One of the predominant stimuli for the trafficking of HPCs to and their retention within the marrow occurs through the trophic influence of the chemokine stromal cell-derived factor-1α (SDF-1α) that binds to the CXC chemokine receptor 4 (CXCR4) receptor. Both SDF-1α and CXCR4 undergo degradation through the action of neutrophil-derived proteases released as a consequence of the neutrophilic hyperplasia seen after G-CSF administration, contributing to the HPC mobilization produced by that drug.15-17
AMD3100 is a bicyclam derivative that reversibly competes with and inhibits SDF-1α binding to CXCR4.18-20 This compound was originally tested clinically as an agent for treatment of human immunodeficiency virus (HIV).21 During those clinical trials, leukocytosis was noted. Further investigation demonstrated that CD34 cells were one component of this leukocytosis and that AMD3100 was, in fact, capable of mobilizing significant numbers of CD34 cells into the circulation.22 Mobilization of CD34 cells was also noted in patients with cancer.23 When AMD3100 was administered after 4 to 5 days of G-CSF, further significant sustained increases of circulating CD34 cells were observed in healthy subjects.24
On the basis of the above-mentioned observations, we hypothesized that the combination of AMD3100 plus G-CSF (hereafter A + G) would be superior to G-CSF alone (hereafter G). On the basis of successful engraftment of A + G-mobilized cells in SCID-Hu mice,25 we further hypothesized that A + G-mobilized cells would engraft at least as well as their G-mobilized counterparts. The current study represents the first clinical trial in patients with NHL and MM to begin to test these hypotheses. The primary objective of the study was to determine whether patients with MM and NHL mobilized more progenitor cells per unit blood volume of apheresis after treatment with A + G compared with G alone. The secondary objectives were to determine wither patients with MM and NHL mobilized with A + G compared with G alone required fewer apheresis procedures to reach the optimal target level of at least 5 × 106 cells/kg for transplantation and to determine whether patients with MM and NHL mobilized with A + G had at least a 90% success rate of autologous transplantation as measured by neutrophil engraftment by day 21. The trial has been completed, and the results are sufficiently mature such that all study endpoints can be addressed.
Patients, materials, and methods
Patient eligibility
Patients with multiple myeloma or non-Hodgkin lymphoma in first or second complete or partial remission were eligible for enrollment. Complete entry criteria are shown in Table 1. The study medical monitor was responsible for reviewing proposed study patients and approving any exceptions in entry criteria. In the latter portion of the study, some patients who had been treated with more than 3 prior chemotherapy regimens were allowed to enroll on study. Institutional review board approval for this study was obtained at Thomas Jefferson University, Philadelphia, PA; Washington University, St Louis, MO; University of Rochester, Rochester, NY; Virginia Commonwealth University, Richmond, VA; Hackensack University Medical Center, Hackensack, NJ; and Medical College of Wisconsin, Milwaukee, WI. Informed consent was obtained from all patients prior to their treatment on this study according to the Declaration of Helsinki.
Study entry criteria
Inclusion criteria |
| MM/NHL in 1st or 2nd CR or PR |
| Age, 18-70 y |
| ECOG performance status of 0 or 1 |
| Up to 3 prior chemotherapy regimens (thalidomide and dexamethasone were not considered chemotherapy for this purpose) |
| Resolution of all prior acute chemotherapy toxicities |
| WBC count greater than 3.0 × 109/L; ANC greater than 1.5 × 109/L; platelet count greater than 100 × 109/L |
| Cr no more than 195 μM |
| AST/ALT/bilirubin less than twice normal |
| LVEF greater than 45% |
| DLco greater than 45%, FEV1 greater than 50% predicted |
| HIV negative |
| Able to provide informed consent |
| For women, not being pregnant and being willing to use contraception |
| Exclusion criteria |
| High-risk comorbidities for acute treatment complications (eg, symptomatic coronary artery disease) |
| Residual acute conditions from prior chemotherapy |
| Brain metastases or carcinomatous meningitis |
| Acute infection or unexplained fever above 38°C |
| Hypercalcemia (0.25 mM above normal) |
| Weight greater than 150% of ideal weight |
| Experimental therapy within 4 wk |
| Recent cytokine administration (pegfilgrastim [Neulasta] within 21 d; other cytokines within 7 d) |
| For women, pregnancy or lactation |
| ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell; ANC, absolute neutrophil count; Cr, creatine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LVEF, alanine aminotransferase; DLco, diffusing lung capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second. |
Inclusion criteria |
| MM/NHL in 1st or 2nd CR or PR |
| Age, 18-70 y |
| ECOG performance status of 0 or 1 |
| Up to 3 prior chemotherapy regimens (thalidomide and dexamethasone were not considered chemotherapy for this purpose) |
| Resolution of all prior acute chemotherapy toxicities |
| WBC count greater than 3.0 × 109/L; ANC greater than 1.5 × 109/L; platelet count greater than 100 × 109/L |
| Cr no more than 195 μM |
| AST/ALT/bilirubin less than twice normal |
| LVEF greater than 45% |
| DLco greater than 45%, FEV1 greater than 50% predicted |
| HIV negative |
| Able to provide informed consent |
| For women, not being pregnant and being willing to use contraception |
| Exclusion criteria |
| High-risk comorbidities for acute treatment complications (eg, symptomatic coronary artery disease) |
| Residual acute conditions from prior chemotherapy |
| Brain metastases or carcinomatous meningitis |
| Acute infection or unexplained fever above 38°C |
| Hypercalcemia (0.25 mM above normal) |
| Weight greater than 150% of ideal weight |
| Experimental therapy within 4 wk |
| Recent cytokine administration (pegfilgrastim [Neulasta] within 21 d; other cytokines within 7 d) |
| For women, pregnancy or lactation |
| ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell; ANC, absolute neutrophil count; Cr, creatine; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LVEF, alanine aminotransferase; DLco, diffusing lung capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second. |
HPC mobilization
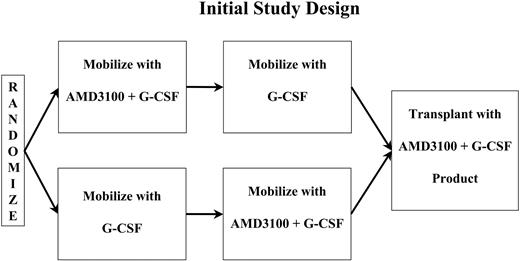
The initial study treatment schema is shown in Figure 1. Initially, patients were randomly assigned to receive either A + G or G alone as their initial mobilizing regimen, followed by a 2-week washout period and remobilization with the alternate regimen. Because of variability in the number of apheresis procedures required, the washout period ranged from 13 to 17 days from the time the first set of mobilizing cytokines was completed until the second set of mobilizing cytokines was initiated.
G mobilization consisted of the daily subcutaneous morning administration of 10 μg G-CSF/kg of actual body weight. Apheresis was initially begun on day 4 of G-CSF administration. Apheresis and G-CSF were continued daily for up to 4 consecutive daily collections through day 8 or until at least 5 × 106 CD34+ cells/kg had been collected, whichever occurred first. Patients could undergo apheresis for a maximum of 4 days. However, if the initial collections were very poor and, in the opinion of the treating physician, the patient was unlikely to collect 2 × 106 CD34+ cells/kg in 4 days, collections could be stopped after 2 days.
A + G mobilization consisted of administration of G-CSF as administered in the G regimen. Additionally, beginning on day 4, AMD3100 was administered subcutaneously followed 6 hours later by apheresis. AMD3100, G-CSF, and apheresis were continued daily thereafter through day 8 similar to the G portion of the study. Apheresis was limited to 3 blood volumes per day.
The first 8 patients received AMD3100 at a dose of 160 μg/kg. Subsequently, the trial was amended, and the AMD3100 dose was increased to 240 μg/kg when data from studies in healthy volunteers demonstrated better CD34+ cell mobilization at the higher dose.22 At the same time the initiation of AMD3100 and apheresis (in both treatment arms) was changed from day 4 to day 5 to match the schedule used in the studies of healthy volunteers. The study was amended again after treatment of the 12th patient, this time to discontinue randomization. Patients 13 through 25 all received G as the initial mobilizing regimen.
Patients subsequently received transplants following myeloablative conditioning. The choice of conditioning regimens was left to the discretion of the local principal investigator. Patients were to receive transplants with A + G-mobilized cells, if sufficient cells were mobilized, with G-mobilized cells retained as back up. If insufficient cells were mobilized using A + Go r at the local investigator's discretion, G cells or cells from both collections could be used for transplantation. Supportive care was left to the discretion of the local center. All centers participating in this study routinely administer growth factors after transplantation.
CD34 enumeration
CD34+ cells were enumerated using standard flow cytometric techniques both in the local transplantation laboratories and in a central reference laboratory (Esoterix, Memphis, TN). As prescribed in the protocol, the central counts were used for data analysis while physicians based their patient care decisions on local counts.
Definitions
For the purpose of this study, the minimal cell dose target for proceeding to transplantation was at least 2 × 106 CD34+ cells/kg. The ideal cell dose target was considered to be at least 5 × 106 CD34+ cells/kg. In comparing the number of CD34+ cells collected with the 2 regimens (either per day or in total), we considered a 50% increase in CD34+ cell number to represent an increment that would be considered clinically significant and useful by physicians caring for patients receiving autotransplants.
Engraftment
Neutrophil engraftment is defined as the first of 3 consecutive days in which the absolute neutrophil count exceeded 500 cells/μL. Platelet engraftment was defined as the first of 7 days in which the platelet count exceeded 20 000/μL without transfusion.
Statistical methods
An exact binomial test and calculation of the Clopper-Pearson 95% confidence interval were used to assess whether the number of CD34+ cells collected was skewed in favor of G or A + G.26 All data summaries and statistical tests were performed using SAS version 9 (SAS Institute, Cary, NC).
Results
Patient accrual
Twenty-five patients were enrolled on study (10 with MM and 15 with NHL) to meet the protocol goal of 24 patients who received transplants. One study patient reached the minimal HPC dose for transplantation (≥ 2 × 106 CD34+ cells/kg) based on counts obtained in the central laboratory but failed to do so in the local laboratory. For reasons unrelated to the study, the treating physician opted not to take this patient to transplantation. Characteristics of these 25 patients are provided in Table 2. There were 14 men and 11 women. Among patients with MM, there was a preponderance of male patients (7 of 10), whereas patients with NHL were almost equally distributed across both sexes. The majority of patients were white (19 patients; 76.0%) followed by 4 African American patients (16.0%), and 2 Hispanic/Latino patients (8.0%). Age ranged from 31 to 72 years (median, 60 years; mean, 58 years). Patients with MM had received a median of 1 treatment regimen (range, 1-4 regimens) for a median of 4 months/cycles (range, 3-15 months/cycles) of treatment prior to HPC collection. In contrast, patients with NHL had received a median of 2 treatment regimens (range, 1-5 regimens) for a median of 9 months/cycles (range 4-25 months/cycles) of treatment prior to HPC collection.
Patient characteristics
. | MM patient . | NHL patients . | All patients . |
|---|---|---|---|
| No. | 10 | 15 | 25 |
| Age, y | |||
| Mean/median | 61/63 | 56/59 | 58/60 |
| Minimum/maximum | 43/72 | 31/66 | 31/72 |
| Sex, no.(%) | |||
| Female | 3 (30.0) | 8 (53.3) | 11 (44.0) |
| Male | 7 (70.0) | 7 (46.7) | 14 (56.0) |
| Ethnic group, no. (%) | |||
| White | 5 (50.0) | 14 (93.3) | 19 (76.0) |
| African American | 4 (40.0) | 0 (0.0) | 4 (16.0) |
| Hispanic/Latino | 1 (10.0) | 1 (6.7) | 2 (8.0) |
| Prior treatment, median (range) | |||
| No. regimens | 1 (1-4) | 2 (1-5) | 2 (1-5) |
| Mo./no. cycles of treatment | 4 (3-15) | 9 (4-25) | 7 (3-25) |
. | MM patient . | NHL patients . | All patients . |
|---|---|---|---|
| No. | 10 | 15 | 25 |
| Age, y | |||
| Mean/median | 61/63 | 56/59 | 58/60 |
| Minimum/maximum | 43/72 | 31/66 | 31/72 |
| Sex, no.(%) | |||
| Female | 3 (30.0) | 8 (53.3) | 11 (44.0) |
| Male | 7 (70.0) | 7 (46.7) | 14 (56.0) |
| Ethnic group, no. (%) | |||
| White | 5 (50.0) | 14 (93.3) | 19 (76.0) |
| African American | 4 (40.0) | 0 (0.0) | 4 (16.0) |
| Hispanic/Latino | 1 (10.0) | 1 (6.7) | 2 (8.0) |
| Prior treatment, median (range) | |||
| No. regimens | 1 (1-4) | 2 (1-5) | 2 (1-5) |
| Mo./no. cycles of treatment | 4 (3-15) | 9 (4-25) | 7 (3-25) |
HPC mobilization
Consistent with findings in healthy adults, the number of CD34+ cells circulating in the blood increased a median of 2.9-fold (range, 1.1- to 13-fold) 6 hours after their first subcutaneous injection of AMD3100 (data not shown). Two patients, one with MM and one with NHL showed insignificant (1.1- and 1.1-fold) increases in CD34+ cells numbers during this 6-hour interval. These patients were among the better mobilizers with G-CSF alone. The remainder of the patients had at least a 1.9-fold or higher increase in the number of circulating CD34 cells over this interval.
To assess whether these increases in the number of circulating CD34+ cells translated into better collections from apheresis, the average number of CD34+ cells/kg patient body weight collected per day of apheresis and the total number of CD34+ cells collected per kg of patient body weight were analyzed. Table 3 demonstrates these results for all 25 patients studied. Patients with MM are grouped at the top of the table and patients with NHL at the bottom. The number of CD34+ cells collected per day with each regimen is illustrated in columns 4 and 5. In every case, more HPCs were collected per day of apheresis after A + G mobilization than after G alone, irrespective of which regimen was used first. This included 7 patients receiving A + G first and 18 receiving G first. The fold increase in CD34+ cells collected per day is shown in column 6. In 21 (84%) of 25 cases A + G mobilization produced a daily increase in the number of CD34+ cells collected of more than 50%. The 4 patients who did not mobilize 50% more cells/day with A + G included 3 patients with MM and 1 with NHL, one of whom was mobilized with A + G first and 3 of whom were mobilized with G first. Patients with MM mobilized a median of 3- to 3.5-fold (range, 1.3- to 10-fold) more CD34 cells per day of apheresis with A + G. Patients with NHL mobilized a median of 4.4-fold (range, 1.1- to 54.4-fold) more cells per day of apheresis with A+G.
Apheresis results after G and A + G priming
Disease and patient ID . | Initial treatment . | Mean CD34+ cells/kg/day . | . | Fold increase/day with A + G . | |
|---|---|---|---|---|---|
| . | . | G . | A + G . | . | |
| MM | |||||
| 01-102 | A + G | 1.6 × 106 | 2.2 × 106 | 1.4 | |
| 02-103 | A + G | 1.1 × 106 | 4.0 × 106 | 3.5 | |
| 01-301 | G alone | 2.8 × 106 | 4.2 × 106 | 1.5 | |
| 02-750 | G alone | 1.4 × 106 | 1.9 × 106 | 1.4 | |
| 05-751 | G alone | 1.1 × 106 | 4.9 × 106 | 4.6 | |
| 06-754 | G alone | 1.7 × 106 | 6.9 × 106 | 4.1 | |
| 01-756 | G alone | 8.6 × 106 | 2.5 × 107 | 3.0 | |
| 05-757 | G alone | 7.7 × 105 | 7.7 × 106 | 10.0 | |
| 03-760 | G alone | 5.3 × 106 | 7.1 × 106 | 1.3 | |
| 04-762 | G alone | 2.7 × 105 | 1.8 × 106 | 6.7 | |
| NHL | |||||
| 03-225 | A + G | 8.4 × 104 | 1.4 × 106 | 16.3 | |
| 03-227 | A + G | 1.8 × 105 | 2.5 × 106 | 14.1 | |
| 02-229 | A + G | 3.6 × 106 | 6.3 × 106 | 1.7 | |
| 03-304 | G alone | 3.0 × 106 | 5.1 × 106 | 1.7 | |
| 03-426 | G alone | 7.1 × 105 | 8.1 × 105 | 1.1 | |
| 03-549 | A + G | 1.8 × 104 | 1.0 × 106 | 54.3 | |
| 03-674 | A + G | 3.6 × 105 | 6.8 × 106 | 18.8 | |
| 03-873 | G alone | 1.5 × 106 | 2.5 × 106 | 1.6 | |
| 03-875 | G alone | 1.5 × 106 | 2.2 × 106 | 1.5 | |
| 03-878 | G alone | 4.0 × 104 | 6.8 × 105 | 17.2 | |
| 03-880 | G alone | 1.5 × 105 | 7.4 × 105 | 5.1 | |
| 03-881 | G alone | 3.7 × 105 | 1.6 × 106 | 4.4 | |
| 06-883 | G alone | 1.1 × 106 | 1.9 × 106 | 1.8 | |
| 03-886 | G alone | 1.3 × 106 | 5.6 × 106 | 4.4 | |
| 03-888 | G alone | 3.9 × 105 | 4.3 × 106 | 11.0 | |
Disease and patient ID . | Initial treatment . | Mean CD34+ cells/kg/day . | . | Fold increase/day with A + G . | |
|---|---|---|---|---|---|
| . | . | G . | A + G . | . | |
| MM | |||||
| 01-102 | A + G | 1.6 × 106 | 2.2 × 106 | 1.4 | |
| 02-103 | A + G | 1.1 × 106 | 4.0 × 106 | 3.5 | |
| 01-301 | G alone | 2.8 × 106 | 4.2 × 106 | 1.5 | |
| 02-750 | G alone | 1.4 × 106 | 1.9 × 106 | 1.4 | |
| 05-751 | G alone | 1.1 × 106 | 4.9 × 106 | 4.6 | |
| 06-754 | G alone | 1.7 × 106 | 6.9 × 106 | 4.1 | |
| 01-756 | G alone | 8.6 × 106 | 2.5 × 107 | 3.0 | |
| 05-757 | G alone | 7.7 × 105 | 7.7 × 106 | 10.0 | |
| 03-760 | G alone | 5.3 × 106 | 7.1 × 106 | 1.3 | |
| 04-762 | G alone | 2.7 × 105 | 1.8 × 106 | 6.7 | |
| NHL | |||||
| 03-225 | A + G | 8.4 × 104 | 1.4 × 106 | 16.3 | |
| 03-227 | A + G | 1.8 × 105 | 2.5 × 106 | 14.1 | |
| 02-229 | A + G | 3.6 × 106 | 6.3 × 106 | 1.7 | |
| 03-304 | G alone | 3.0 × 106 | 5.1 × 106 | 1.7 | |
| 03-426 | G alone | 7.1 × 105 | 8.1 × 105 | 1.1 | |
| 03-549 | A + G | 1.8 × 104 | 1.0 × 106 | 54.3 | |
| 03-674 | A + G | 3.6 × 105 | 6.8 × 106 | 18.8 | |
| 03-873 | G alone | 1.5 × 106 | 2.5 × 106 | 1.6 | |
| 03-875 | G alone | 1.5 × 106 | 2.2 × 106 | 1.5 | |
| 03-878 | G alone | 4.0 × 104 | 6.8 × 105 | 17.2 | |
| 03-880 | G alone | 1.5 × 105 | 7.4 × 105 | 5.1 | |
| 03-881 | G alone | 3.7 × 105 | 1.6 × 106 | 4.4 | |
| 06-883 | G alone | 1.1 × 106 | 1.9 × 106 | 1.8 | |
| 03-886 | G alone | 1.3 × 106 | 5.6 × 106 | 4.4 | |
| 03-888 | G alone | 3.9 × 105 | 4.3 × 106 | 11.0 | |
Italics indicate a significant increase, defined as a 50% increase in the number of CD34+ cells collected per day.
Successful HPC mobilization was defined as the a product of at least 2 × 106 CD34+ cells/kg, a value commonly used as the minimally acceptable cell dose for autotransplantation in the transplantation community. In 9 patients, it was not possible to collect this minimum cell dose during G-alone mobilization. In each of these 9 cases, A + G successfully mobilized the patient, while G alone failed. In no case was the reverse pattern seen. These 9 cases are further summarized in Table 4. The 4 patients at the bottom of the table were the first patients seen in the trial to successfully mobilize with one regimen but not the other. Because all 4 were mobilized successfully with A + G first and unsuccessfully with G alone thereafter, we became concerned that we were seeing a sequence effect such that A + G mobilization prevented successful G mobilization shortly thereafter. For this reason, randomization was discontinued, and all subsequent patients received G mobilization first. Subsequently, the remaining 5 patients in Table 4 were accrued to the study. In this case they initially failed to mobilize with G alone and then subsequently mobilized successfully with A + G. Clearly, a similar sequence effect could not be operative in these 5 cases. In retrospect, all 9 cases likely represent instances in which, in poorly mobilizing patients, A + G was a superior mobilizing regimen than G alone, and the fact that the first 4 instances were seen in patients mobilized with A + G first was the artifact of a small number of patients accrued at that time. A + G mobilized 5- to 102-fold (median, 21-fold) more cells in these 9 poor mobilizers. Eight of these 9 patients had NHL, while 1 had MM.
Total CD34+ cells (× 106 cells/kg) harvested in patients who poorly mobilized with G-CSF alone
First mobilizing regimen . | Collection after G-alone mobilization . | Collection after A + G mobilization . | Fold increase with A + G mobilization . |
|---|---|---|---|
| G alone | 0.08 | 2.8 | 35 |
| G alone | 0.29 | 2.9 | 10 |
| G alone | 0.73 | 3.7 | 5 |
| G alone | 1.6 | 8.5 | 6 |
| G alone | 0.55 | 5.2 | 9 |
| A + G | 0.16 | 5.3 | 33 |
| A + G | 0.36 | 7.6 | 21 |
| A + G | 0.04 | 4.1 | 102 |
| A + G | 0.63 | 13.6 | 22 |
First mobilizing regimen . | Collection after G-alone mobilization . | Collection after A + G mobilization . | Fold increase with A + G mobilization . |
|---|---|---|---|
| G alone | 0.08 | 2.8 | 35 |
| G alone | 0.29 | 2.9 | 10 |
| G alone | 0.73 | 3.7 | 5 |
| G alone | 1.6 | 8.5 | 6 |
| G alone | 0.55 | 5.2 | 9 |
| A + G | 0.16 | 5.3 | 33 |
| A + G | 0.36 | 7.6 | 21 |
| A + G | 0.04 | 4.1 | 102 |
| A + G | 0.63 | 13.6 | 22 |
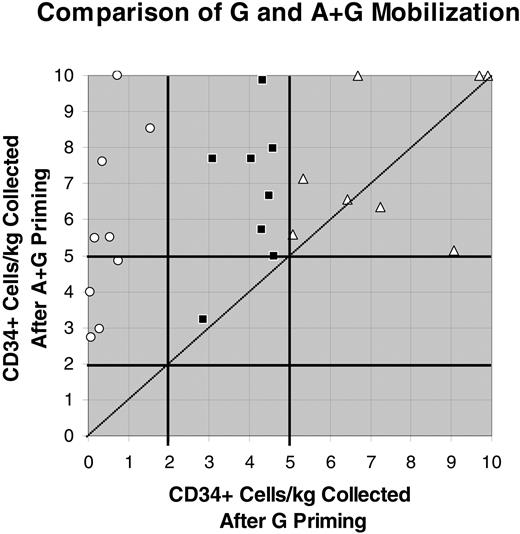
The mobilization of CD34+ cells/kg (× 10-6) after G or A + G mobilization. Values above 10 × 106 were reduced to 10 × 106 to reduce the scale of the figure and to allow the data points with lower values to be better separated and visualized. ○ indicates patients mobilizing less than 2 × 106 CD34+ cells/kg; ▪, those mobilizing at least 2 × 106 but less than 5 CD34+ cells/kg; and ▵ those mobilizing at least 5 × 106 CD34+ cells/kg. The line of identity (y = x) is shown as a dotted diagonal line.
The mobilization of CD34+ cells/kg (× 10-6) after G or A + G mobilization. Values above 10 × 106 were reduced to 10 × 106 to reduce the scale of the figure and to allow the data points with lower values to be better separated and visualized. ○ indicates patients mobilizing less than 2 × 106 CD34+ cells/kg; ▪, those mobilizing at least 2 × 106 but less than 5 CD34+ cells/kg; and ▵ those mobilizing at least 5 × 106 CD34+ cells/kg. The line of identity (y = x) is shown as a dotted diagonal line.
Optimal CD34+ cell mobilization was defined as a product exceeding 5 × 106 CD34+ cells/kg of patient body weight. At these doses, platelet engraftment, in particular, becomes more consistent and rapid. Of the 25 patients studied, 8 mobilized in excess of this value with G alone whereas, 20 mobilized in excess of this value with the combination of A + G. Figure 2 illustrates the mobilization of cells from each patient with G and A + G mobilization. In this figure, collections of more than 10 × 106 CD34+ cells/kg were graphed as 10 × 106 so as to reduce the scale of the figure and to allow the lower value data points to be more clearly seen. Of the 9 patients who mobilized less than 2 × 106 CD34+ cells/kg with G alone (○), 4 mobilized at least 2 × 106 but less than 5 × 106 CD34+ cells/kg, whereas 5 mobilized at least 5 × 106 CD34+ cells/kg with A + G. Eight patients mobilized at least 2 × 106 but less than 5 × 106 CD34+ cells/kg with G alone (▪). Seven of these patients mobilized at least 5 × 106 CD34+ cells/kg with A + G. Eight patients mobilized more than 5 × 106 CD34+ cells/kg with G alone (▵),and all mobilized more than 5 × 106 CD34+ cells/kg with A + G. Six of these patients mobilized more cells with A + G. Two patients had more cells collected after G-alone mobilization, but these 2 patients underwent more apheresis procedures after G mobilization than after A + G. Each of these patients collected more than 5 × 106 CD34+ cells/kg in a single apheresis procedure after A + G mobilization; therefore, apheresis was discontinued. In contrast, 2 or 3 apheresis procedures were required in these 2 patients after G mobilization. We formally assessed whether the data were skewed to one side of the line of identity (y = x) using an exact binomial test. If the data are not skewed to one side or the other, half the data points (0.50) should fall on each side of the line of identity. The data were found to be highly skewed toward improved collection with A + G (P ≤ .001). Moreover, the Clopper-Pearson 95% confidence interval was well removed from 0.50 (0.74-0.99).
Patients may benefit 3 ways from improved stem cell mobilization. Collection of more CD34+ cells may allow patients to proceed to transplantation who would not otherwise have had an adequate HPC product. Improved mobilization may allow the patient to complete collection with fewer apheresis procedures. Finally, even if the same number of apheresis procedures were required, improved mobilization may send patients to transplantation with a more robust HPC dose. We examined these 3 potential benefits from A + G mobilization in Table 5. As noted in column 2 of the table, 9 patients mobilized a transplantable cell dose with A + G but not with G alone. Columns 3 to 5 illustrate that 12 patients required fewer apheresis procedures with A + G mobilization to reach the ideal transplantation cell dose of at least 5 × 106 CD34+ cells/kg. Of these 12 patients, 6 required 1 fewer apheresis, 4 required 2 fewer apheresis procedures, and 2 required 3 fewer apheresis procedures. As illustrated in the table, despite requiring fewer days of apheresis to hit the target cell dose, 5 of these patients actually mobilized 50% more cells after A + G treatment than after G alone. Finally, 3 patients required the same number of apheresis procedures to reach target, but had 10% to 49% more cells collected. Note that one patient (02-750) is not included in Table 5. This patient had more cells and more apheresis procedures in the A + G treatment arm and, therefore, does not fall into any of the categories in the table. After 3 days of G mobilization, CD34 yields were decreasing and apheresis was stopped. After 3 days of A + G mobilization, essentially the same number of CD34+ cells had been collected. However, in contrast to the G-alone mobilization, after 3 days of A + G, CD34 yields were steadily increasing in this patient. Apheresis was thus continued, and more than half the total CD34+ cells collected were obtained on the fourth and final day of apheresis. Focusing primarily on those patients in the first 2 groups, where the clinical benefit was largest, 21 (84%) of the 25 patients enrolled on the study either became eligible for transplantation or would potentially have saved 1 or more days of apheresis using the A + G regimen.
Success of AMD3100 + G-CSF versus G-CSF alone for mobilization
. | No. patients successfully*mobilized when other treatment arm failed . | No. patients with fewer apheresis procedures (no. patients with ≥50% more cells) . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Regimen . | . | 1 less . | 2 less . | 3 less . | Same no. of apheresis procedures but more cells . | ||
| A + G | 9 | 6 (1) | 4 (3) | 2 (1) | 3† | ||
| G alone | 0 | 0 | 0 | 0 | 0 | ||
| Total | 9 | 6 | 4 | 2 | 3 | ||
. | No. patients successfully*mobilized when other treatment arm failed . | No. patients with fewer apheresis procedures (no. patients with ≥50% more cells) . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Regimen . | . | 1 less . | 2 less . | 3 less . | Same no. of apheresis procedures but more cells . | ||
| A + G | 9 | 6 (1) | 4 (3) | 2 (1) | 3† | ||
| G alone | 0 | 0 | 0 | 0 | 0 | ||
| Total | 9 | 6 | 4 | 2 | 3 | ||
The total number of patients is fewer than 25 because one patient (02-750) had more cells and more apheresis procedures in the A + G treatment arm, and therefore, does not fall into any of the categories in the table. In the same number of days of apheresis, this patient collected 105 more cells on the G-alone treatment arm.
Defined as collecting ≥2 × 106 cells/kg.
The 3 patients had 14% to 49% more cells.
A final means of comparing the 2 mobilization approaches is to ask how many patients could collect a minimum cell dose target or an ideal cell dose target in 1 or 2 apheresis procedures, allowing more extended days of apheresis to be avoided. These results are summarized in Table 6. In this study, daily apheresis procedures were limited to 3 blood volumes. Using G alone, only 5 (20%) of 25 patients reached the minimum cell dose after a single apheresis, whereas with A + G 14 (56%) of 25 did so. After 1 day, 2 (8%) of 25 patients on the G treatment arm mobilized an ideal product, whereas 9 (36%) of 25 on the A + G treatment arm did so. After 2 days of apheresis, all patients on the A + G treatment arm had reached the minimal cell dose, whereas 9 patients (36%) on the G treatment arm had not. Similarly, after 2 days, only 4 patients (16%) on the G treatment arm had reached the ideal cell dose versus 15 patients (60%) mobilized with A + G.
Mobilization of an adequate HPC dose after 1 or 2 days of apheresis
. | Cells collected on day 1, CD34+/kg . | . | . | Cells collected on days 1 and 2, CD34+/kg . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen . | Fewer than 2 × 106 . | Between 2 and 5 × 106 . | More than 5 × 106 . | Fewer than 2 × 106 . | Between 2 and 5 × 106 . | More than 5 × 106 . | ||||
| G alone | 20 | 3 | 2 | 9 | 12 | 4 | ||||
| A + G | 11 | 5 | 9 | 0 | 10 | 15 | ||||
. | Cells collected on day 1, CD34+/kg . | . | . | Cells collected on days 1 and 2, CD34+/kg . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen . | Fewer than 2 × 106 . | Between 2 and 5 × 106 . | More than 5 × 106 . | Fewer than 2 × 106 . | Between 2 and 5 × 106 . | More than 5 × 106 . | ||||
| G alone | 20 | 3 | 2 | 9 | 12 | 4 | ||||
| A + G | 11 | 5 | 9 | 0 | 10 | 15 | ||||
Engraftment
Twenty-four patients have received transplants to date and, of these, 19 received their A + G products only. Of these19 patients, 18 have demonstrated a consistent pattern of early engraftment with recovery of neutrophils between days 10 and 13 (median, day 10-11). One patient, who received a transplant with a cell dose of 2.3 × 106 CD34+ cells/kg (by local laboratory enumeraration), was an outlier regarding neutrophil engraftment, with recovery delayed until day 34. This patient did not mobilize successfully with G alone, and additional HPCs were thus unavailable. This patient had probable viral infection starting on day 4, documented Gramnegative sepsis on day 8, and fungal sepsis on day 9. The patient ultimately succumbed to sepsis and renal failure on day 35. In 5 cases, both A + G and G-only cells were administered. In 4 cases this was preplanned to administer a larger overall cell dose. In the fifth case, the A + G product was improperly thawed and had poor viability, and for this reason the G-only cells were thawed thereafter and administered. Because the contribution of the A + G cells to engraftment cannot be determined in these cases, they were not included in the analysis.
Platelet engraftment in patients who received transplants with A + G only occurred between 0 and 89 days (median, 16 days) after transplantation. Zero days until platelet engraftment refers to patients who never dropped their count below 20 000 and never received platelet transfusion. One patient, who received a CD34+ cell dose/kg of 3.96 × 106, was an outlier, because all other patients recovered their platelets by day 27. This patient failed to mobilize with G alone.
In this initial study, our focus was on neutrophil recovery. Daily platelet counts were not mandated after discharge; thus, many patients may have engrafted platelets earlier but without documentation. Additionally, clinical practice sometimes resulted in patients receiving transfusions prior to weekends so as to ensure counts did not drop too low before next checked. Because of these factors, the dates of platelet engraftment should be considered as a conservative or worst-case assessment only. It should be noted that engraftment was durable with no instances of late graft failure in patients who received transplants with A + G cells with a median follow-up of more than 575 days (range, 351-725 days).
Toxicity
Six patients experienced serious adverse events (SAEs) during the study. None occurred during A + G mobilization, and none were felt to be related to the study drug. Three SAEs (abdominal pain, jugular vein thrombosis, and hematuria) occurred during the mobilization phase of the study, but they occurred prior to the first administration of AMD3100, thus precluding any relationship to this agent. All resolved. The remaining SAEs occurred during the transplantation not the mobilization phase of the study and were felt to represent transplantation-conditioning regimen-related toxicities without relationship to the study drug. As noted under “Engraftment,” above, one patient died of sepsis and refractory hypotension. All other SAEs (neutropenic colitis, catheter infection, and gastroenteritis) resolved. No other transplant-related or other mortality has occurred in the study patients.
Milder adverse events (AEs) were seen during the study. The most frequently reported AEs were bone pain and tingling, and in almost all cases these AEs were considered unrelated to study drug. The most frequently reported AEs that were considered related to study drug were diarrhea, injection site redness (erythema), and nausea. These events were generally very mild and transient. No late sequelae of AMD3100 administration have been noted.
Discussion
This study is the first to test AMD3100, a reversible inhibitor of binding between the chemokine SDF-1α and CXCR4, its receptor, for the purposes of mobilizing human HPCs to be used for autologous transplantation. The results demonstrate both efficacy and safety of this agent when used along with G-CSF for mobilization of HPCs for autotransplantation for MM and NHL.
In this initial study, each patient was mobilized twice using G alone and A + G, thus allowing each patient to serve as his or her own control. This allows more conclusions of the relative efficacy of these 2 regimens to be drawn than would otherwise be possible from a study of this number of patients.
The combination of A + G proved superior to G alone in multiple ways. The higher numbers of CD34+ cells mobilized into the circulation translated into higher daily collections of CD34+ cells from apheresis in all patients. A + G was a superior mobilizing regimen than G alone, irrespective of which regimen was administered first. Pair-wise comparisons of CD34+ cells collected after A + G versus G alone were thus skewed significantly in favor of A + G. Clinically, this produced several potential benefits, including effective mobilization of patients who failed to mobilize with G-CSF alone, reducing the number of apheresis procedures required to reach a dose of 2 × 106 CD34+ cells/kg, and reducing the number of apheresis procedures required to reach a dose of 5 × 106 CD34+ cells/kg. Many of the patients also were able to collect appreciably more (50% or more increase) CD34+ cells, despite requiring fewer apheresis procedures to complete the collections. Twenty-one (84%) of 25 patients experienced such a potential clinical benefit. If a similar percentage is seen in follow-up studies, the clinical effect will be considerable.
The addition of AMD3100 to the mobilization regimen produced little in the way of toxicity beyond what is normally seen with G-CSF alone. The most common side effects attributed to AMD3100 in this study, mild gastrointestinal side effects and injection site erythema, are similar to the side effects observed in other studies using this drug in healthy subjects,22 patients with HIV,27 or patients with cancer.23 Higher doses of AMD3100 have been used in patients with HIV, and significant toxicities were infrequent even when using continuous infusions at 20-fold higher doses than used in this study. Thus, this drug appears to have an excellent track record of safety in more than 100 patients with cancer, 40 patients with HIV, and 60 healthy volunteers.
The difference in toxicity profile of AMD3100 versus G-CSF likely reflects their different effects on the marrow. G-CSF produces a substantial marrow hyperplasia that may be etiologically connected to its most common side effect, bone pain. Its effects in HPC mobilization are thought to be largely indirect, through cleavage of adhesion and trophic molecules as the result of enzymes released during the granulocytic marrow hyperplasia.15-17 In contrast, AMD3100 is thought to mediate HPC release more directly, by disrupting the SDF-1α/CXCR4 interaction that many currently consider as the most important physiologic signal for HPC migration to and retention in the marrow.15 Administration of AMD3100 in the absence of G-CSF does not produce bone pain, presumably because of the lack of the marrow hyperplasia.
The rapid increase in CD34 cells in the blood only 6 hours after AMD3100 administration, which is particularly pronounced in poor-mobilizing patients, cannot be readily explained through the proliferation of early hematopoietic progenitors. Neither SDF-1α nor AMD3100 is known to induce proliferation of HPCs. Moreover, the 6-hour interval is too short for this sort of expansion to occur even if HPC proliferation was stimulated. The results are more consistent with a model in which the CD34 cells were available in, but not released from, the marrow after G-CSF stimulation, with subsequent release into the circulation following AMD3100 administration. The specific mechanism of AMD3100-mediated release remains to be elucidated.
Although the numbers of patients in this study are too small for firm conclusions, the effects of AMD3100 seemed more pronounced in patients with NHL rather than with MM. The range of fold increase in CD34+ cell mobilization and collection was higher in patients with NHL. This likely reflects the fact that patients with NHL were more heavily pretreated and thus more skewed toward poor mobilization or nonmobilizers than their MM counterparts. This is reflected in the higher number of cytotoxic chemotherapy regimens and cycles of therapy that patients with NHL received prior to attempting HPC mobilization. Eight of the 9 patients who failed G-alone mobilization in this study had NHL.
In healthy volunteers, administration of a single dose of AMD3100 at a dose of 240 μg/kg results in mobilization of a comparable number of CD34+ cells into circulation as 4 days of G-CSF at 10 μg/kg/d, without the attendant bone pain. Theses data coupled with the current study data raise the question as to whether AMD3100 alone could be successfully used to mobilize MM patients for autotransplantation or healthy volunteers for allotransplantation. Trials in these clinical settings are ongoing.28 Additionally, the rapid and pain-free mobilization of HPCs after ADM3100 alone in chemotherapy-naive individuals raises questions as to the clinical utility of this agent in other clinical settings such as after revascularization of ischemic myocardium, after serious trauma, or in a variety of other clinical settings.
Other agents have been tested with and without G-CSF for HPC mobilization, including granulocyte-macrophage colony-stimulating factor (GM-CSF),29-32 interleukin 3 (IL-3),33,34 stem cell factor,35,36 flt-3 ligand,37 and others. None of these has generated widespread acceptance or approval by the Food and Drug Administration (FDA) in the United States either because of marginal efficacy or unacceptable toxicity, or both. G-CSF may be used at higher doses than the 10 μg/kg used in this study.38 Whether AMD3100 would boost stem cell mobilization if combined with higher doses of G-CSF and whether A + G used at the current doses and schedule will prove superior to higher doses of G-CSF will require further clinical trials. An alternate approach to HPC mobilization uses intermediate-dose chemotherapy combined with G-CSF with apheresis beginning during white blood cell recovery.39-42 Whether AMD3100 can be used to improve the yield of HPCs recovered after this sort of mobilization or alternatively to make the schedule of this type of mobilization more consistent will also require further trials, although preliminary data are encouraging.43
The current study administered AMD3100 around 8:00 am and began apheresis 6 hours later. This presents some additional logistical difficulties for processing laboratories. Recent data suggest that CD34+ cell mobilization actually peaks at 10 to 14 hours after administration and demonstrates a fairly prolonged plateau thereafter.22 For this reason, successor studies of A + G will administer AMD3100 the night prior to apheresis, both in an effort to further increase CD34+ cell mobilization and to simplify the logistics of collection.44
AMD3100 does not appear to have any untoward differentiating effects on HPCs or other effects that adversely affect engraftment. The median of about 10 days to neutrophil recovery is comparable to the fastest pace of ANC recovery seen clinically in the absence of this agent. The current study was less rigorous in requiring daily platelet counts after discharge or prescribing when platelet transfusions should be used. Thus, the data regarding platelet recovery must be viewed as a conservative estimate only. Future studies will be required to define the pace of platelet recovery more precisely. Nevertheless, these data do not suggest a significant delay in platelet recovery. Of the patients receiving A + G cells alone for rescue of hematopoiesis after transplantation, one patient showed delayed recover of neutrophils and platelets and another showed delayed recovery of platelets. Both patients failed to mobilize with G-CSF alone. The presence of small numbers of outliers in a sample of this size might be randomly observed clinically. This will be clarified as more patients are treated with AMD3100 alone or in combination with G-CSF.
In summary, this initial study suggests that the addition of AMD3100 to G-CSF may be clinically beneficial for autologous HPC mobilization. HPC mobilization is more consistent with the combination, particularly in poor mobilizers. Patients who could not produce an acceptable product otherwise may now generate clinically useful HPC products. The number of patients reaching optimal, rather than minimal, HPC targets also appears to be increased, and fewer apheresis procedures may be required to do so. The stem cells mobilized by A + G appear clinically efficacious, and few significant side effects are seen from the drug. Thus, the combination of AMD3100 and G-CSF appears to be generally safe and effective for human HPC mobilization and superior to G-CSF alone.
Prepublished online as Blood First Edition Paper, May 12, 2005; DOI 10.1182/blood-2005-02-0468.
Research support provided by AnorMED, Langley, BC, Canada.
K.B. and G.C. are employees of AnorMED, Inc., whose product was studied in the present work.
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 2003; and at the National Marrow Donor Program Council meeting, Minneapolis, MN October 29, 2004.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge the research assistance of Hanne Olsen, Brenda Baylis, and Christine Dehner, and helpful discussion and input from Gary Bridger.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal