Abstract
Hepatic peptide hormone hepcidin is the key regulator of iron metabolism and the mediator of anemia of inflammation. Previous studies indicated that interleukin-6 (IL-6) mediates hepcidin increase and consequent hypoferremia during inflammation. Here we used an in vivo human endotoxemia model to analyze the effects of lipopolysaccharide (LPS) as a more upstream inflammation activator. The temporal associations between plasma cytokines, hepcidin levels, and serum iron parameters were studied in 10 healthy individuals after LPS injection. IL-6 was dramatically induced within 3 hours after injection, and urinary hepcidin peaked within 6 hours, followed by a significant decrease in serum iron. Serum prohepcidin showed no significant change within a 22-hour time frame. These in vivo human results confirm the importance of the IL-6-hepcidin axis in the development of hypoferremia in inflammation and highlight the rapid responsiveness of this iron regulatory system. (Blood. 2005;106: 1864-1866)
Introduction
Anemia of chronic disease occurs in patients with acute and chronic immune activation and represents an important clinical problem. It is a condition that has also been termed “anemia of inflammation” and that is thought to be mediated by hepcidin,1 a small, cysteine-rich cationic peptide produced by hepatocytes.2-4 Furthermore, hepcidin is proposed to be the key regulator of iron metabolism. Hepcidin overexpression in patients with hepatic adenomas5 or in transgenic mice6 resulted in severe iron-refractory microcytic anemia. Conversely, hepcidin deficiency in humans7 or mice8 has been associated with severe iron overload. Hepatic hepcidin expression is suppressed by hypoxia and anemia9 and induced by iron stores and inflammation.4
The induction of hypoferremia by inflammation is commonly seen in many infectious diseases. However, the mechanism remained unknown until the involvement of hepcidin was demonstrated in mice injected with bacterial lipopolysaccharide (LPS) or turpentine oil.4,9 Importantly, hepcidin-deficient mice did not develop hypoferremia after turpentine injection.9 In humans, increased urinary hepcidin levels were detected in patients with chronic infections or severe inflammatory diseases.10 In human hepatocyte cultures, hepcidin expression was induced after direct exposure to LPS or medium from LPS-activated human monocytes, and this response could be ablated by the addition of anti-interleukin-6 (anti-IL-6) antibodies. Furthermore, IL-6 infusion in human volunteers rapidly induced hepcidin and hypoferremia,11 whereas IL-6 knock-out mice injected with turpentine failed to increase hepcidin and develop hypoferremia.
In the present study, we used an in vivo human endotoxemia model to study the temporal associations between different plasma cytokines, urinary hepcidin, and serum iron. We demonstrate the existence of a highly responsive LPS-IL-6-hepcidin axis linking innate immunity and iron metabolism.
Study design
Research subjects
After approval from the local ethics committee was received, 10 healthy individuals (4 men, 6 women; mean age, 21; range, 18-24 years) gave written informed consent to participate in this study. Individuals who were taking prescription drugs (except oral contraceptives) or aspirin or other nonsteroid anti-inflammatory drugs were excluded. All research subjects were HIV- and hepatitis B-negative and had not had any febrile illness in the 2 weeks preceding the study. For 10 hours prior to the experiment, research subjects refrained from caffeine, alcohol, and food. Approval for these studies was obtained from the Radboud University Nijmegen Medical Centre's institutional review board.
Study protocol
Research subjects were intravenously injected with a bolus of 2 ng/kg body weight Escherichia coli O:113 LPS (United States Pharmacopeial Convention, Rockville, MD) between 8 and 9 am. Blood and urine samples were taken just before LPS injection and serially thereafter at regular time intervals up to 22 hours. In the hour prior to the LPS administration, research subjects were prehydrated with 1.5 L glucose (glc) 2.5% NaCl 0.45%. During the experiment, research subjects received 150 mL/h glc 2.5% NaCl 0.45%. Serum iron parameters, ferritin, C-reactive protein (CRP), prohepcidin, urinary creatinine, plasma cytokines, and routine hematology parameters were determined at the Radboud University Nijmegen Medical Centre, The Netherlands. Urine samples were preserved with 0.05% sodium azide and shipped frozen to UCLA, Los Angeles, CA, for urinary hepcidin measurement.
Laboratory measurements
Total serum iron and latent iron binding capacity (LIBC) were measured using the ascorbate/FerroZine colorimetric method, and urine creatinine was measured by colorimetric detection with picric acid (Roche Diagnostics, Mannheim, Germany). CRP was measured using immunologic agglutination detection with latex-coupled polyclonal anti-CRP antibodies (Abbott Laboratories, Abbott Park, IL), all measured by Aeroset (Abbott Laboratories). The serum ferritin was measured by a solid-phase, 2-site chemiluminescent immunometric assay (Immulite 2000; Diagnostic Products, Los Angeles, CA).
Routine hematology parameters were determined using flow cytometry (Sysmex XE-2100; Goffin Meyvis, Etten-Leur, The Netherlands).
Tumor necrosis factor-α (TNF-α), IL-6, IL-1β, IL-12, IL-10, and interferon-γ (IFN-γ) were measured in one batch using a multiplex Luminex Assay12 (Luminex, Austin, TX).
Serum prohepcidin concentration was measured by enzyme-linked immunoassay using a commercially available kit (DRG Diagnostics, Marburg, Germany).
Urinary hepcidin assay was performed as previously described.11 Cationic peptides were extracted from urine using CM-Macroprep (Bio-Rad Laboratories, Hercules, CA). Hepcidin concentrations were determined by an immunodot assay. Urine extracts equivalent to 0.1 to 0.5 mg of creatinine were dotted on Immobilon-P membrane (Millipore, Bedford, MA) along with a range of synthetic hepcidin standards (0 to 80 ng). Hepcidin was detected using rabbit anti-human hepcidin10 antibody with goat anti-rabbit horseradish peroxidase (HRP) as a secondary antibody. Dot blots were developed by the chemiluminescent detection method (SuperSignal West Pico Chemiluminescent Substrate; Pierce Chemical Rockford, IL) and quantified with the Chemidoc cooled camera running Quantity One software (Bio-Rad Laboratories). Hepcidin quantity in each sample was normalized using urinary creatinine, and urinary hepcidin levels were expressed as nanograms of hepcidin per millimole of creatinine.
Statistical analysis
Statistical analyses were performed with GraphPad Prism software (version 4.0). Differences were tested for statistical significance by 1-way repeated measurements analysis of variance (ANOVA) or 1-way ANOVA.
Results and discussion
Injection of 2 ng/kg LPS induced a hypoferremic effect (Figure 1A) already detectable 6 hours after injection and reaching a 57% fall in serum iron after 22 hours. Importantly, the changes in urinary hepcidin preceded serum iron decrease (Figure 1B). Maximal hepcidin excretion was detected at 6 hours after injection, after which the levels started declining but were still higher than preinjection levels at 12 to 22 hours. This time course of hepcidin induction and serum iron decrease was similar to the one observed in volunteers infused with IL-6.11 Hepcidin was recently shown to regulate cellular iron efflux in vitro by binding to the iron efflux channel ferroportin and inducing its internalization and degradation,13 and this mechanism could explain the rapid development of hypoferremia observed in research subjects who had been injected with LPS. Daily, macrophages export around 20 mg of iron through ferroportin, and the iron is taken up largely by the developing erythrocytes in the bone marrow. However, the plasma transferrin compartment contains only 2 to 4 mg of iron, which therefore must turn over every few hours. Accordingly, blocking macrophage iron efflux would be expected to decrease plasma iron concentration within hours.

Laboratory measurements. Serum iron (A), urinary hepcidin (B), serum prohepcidin (C), and CRP (D) were measured in 10 healthy volunteers more than 22 hours after LPS injection. Each point represents the mean ± SEM. Significant differences are indicated (*1-way repeated measurements ANOVA; **1-way ANOVA).

Laboratory measurements. Serum iron (A), urinary hepcidin (B), serum prohepcidin (C), and CRP (D) were measured in 10 healthy volunteers more than 22 hours after LPS injection. Each point represents the mean ± SEM. Significant differences are indicated (*1-way repeated measurements ANOVA; **1-way ANOVA).
Hepcidin is also known as a type II acute-phase protein10 and, in chronic inflammatory conditions, increased hepcidin levels correlate with increased ferritin levels. We measured acute-phase reactants CRP and ferritin in research subjects injected with LPS. CRP increased after LPS injection, but the time course lagged after hepcidin, with maximum levels detected 22 hours after injection (Figure 1D). Serum ferritin levels increased only slightly after 6 hours (results not shown) and stayed within normal reference intervals (10 to 150 μg/L) up to 22 hours, indicating that ferritin acute-phase response is probably delayed in comparison with hepcidin. The rapidity of the hepcidin response could be related to its proposed role as an inducer of hypoferremia that would restrict the flow of essential iron to infecting microbes and slow their multiplication in tissues. This host response could be particularly valuable during the earliest phases of infection, before other components of the innate and adaptive immunity are fully mobilized.14
LPS injection in human volunteers induced a cytokine response characteristic of inflammation.15 After an early and transient induction of the proinflammatory cytokines TNF-α and IFN-γ, the acute-phase response was boosted by a dramatic increase in IL-6, which peaked at 3 to 4 hours after LPS injection (Figure 2A-C). IL-1β expression, on the other hand, showed no significant changes within 4 hours, and IL-12 was undetectable in all research subjects (results not shown). Literature shows that anti-inflammatory cytokines, like IL-10, are able to counteract the proinflammatory IL-1β and IL-12 production.15 In this study we observed a transient increase in IL-10 that peaked at 2 to 3 hours after injection (Figure 2D), which might explain the mentioned cytokine suppression. The time course of IL-6 increase in relation to hepcidin induction and serum iron decrease coincides with that observed in research subjects injected directly with IL-6.11
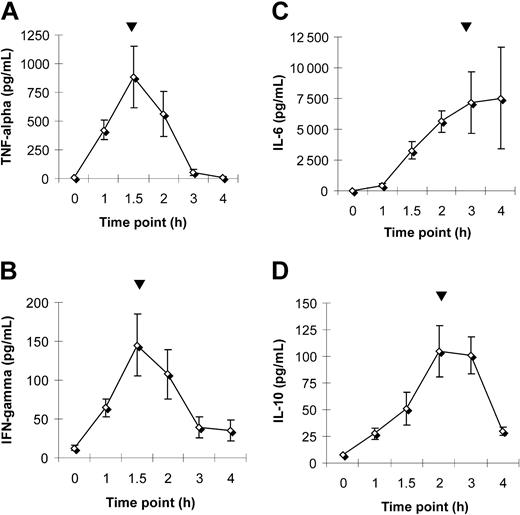
Plasma cytokine levels. Plasma levels of TNF-α (A), IFN-γ (B), IL-6 (C), and IL-10 (D) were measured in 10 healthy volunteers more than 4 hours after LPS injection. Each point represents the mean ± SEM. Peak values are indicated (▾).
Serum prohepcidin levels showed no significant change within the 22-hour period (Figure 1C). Previous reports on serum prohepcidin measurements also showed lack of correlation with other iron parameters and only minor concentration differences between various patient populations with disturbed iron metabolism.16 It remains to be determined whether the lack of correlation between the urinary hepcidin and serum prohepcidin measurements is due to technical limitations of serum assays or if serum prohepcidin concentration does not reflect inflammation or iron metabolism changes.
In conclusion, our in vivo human endotoxemia model highlights the role of hepcidin at the interface between host defense and iron regulation and further supports the importance of the IL-6-hepcidin axis in the development of hypoferremia and anemia of inflammation.
Prepublished online as Blood First Edition Paper, May 10, 2005; DOI 10.1182/blood-2005-03-1159.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mirrin Dorresteijn for her contribution to the managing and blood sampling of the volunteers.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal