Abstract
The immunosuppressive macrolide rapamycin and its derivative everolimus (SDZ RAD, RAD) inhibit the mammalian target of rapamycin (mTOR) signaling pathway. In this study, we provide evidence that RAD has profound antiproliferative activity in vitro and in NOD/SCID mice in vivo against Hodgkin lymphoma (HL) and anaplastic large cell lymphoma (ALCL) cells. Moreover, we identified 2 molecular mechanisms that showed how RAD exerts antiproliferative effects in HL and ALCL cells. RAD down-regulated the truncated isoform of the transcription factor CCAAT enhancer binding protein β (C/EBPβ), which is known to disrupt terminal differentiation and induce a transformed phenotype. Furthermore, RAD inhibited constitutive nuclear factor κB (NF-κB) activity, which is a critical survival factor of HL cells. Pharmacologic inhibition of the mTOR pathway by RAD therefore interferes with essential proliferation and survival pathways in HL and ALCL cells and might serve as a novel treatment option. (Blood. 2005;106: 1801-1807)
Introduction
Hodgkin lymphoma (HL) and anaplastic large cell lymphoma (ALCL) share morphologic and immunophenotypic markers in a subgroup of cases although they are biologically distinct entities.1 Therefore, pathologic diagnosis is sometimes difficult to achieve and these cases are classified as “gray-zone lymphomas.”2 Moreover, in both entities novel therapeutic options are needed, as curative therapy of HL is compromised by a high risk of long-term complications, and anaplastic lymphoma kinase (ALK)-negative ALCL still has a very unfavorable prognosis with current treatment strategies.3-5
The macrocyclic lactone everolimus (SDZ, RAD) RAD is a rapamycin derivative with potent immunosuppressive and antiproliferative properties.6-10 It is further known to inhibit growth factor-driven cell proliferation of hematopoietic and nonhematopoietic cells.6,10 In addition, RAD is a potent inhibitor of human Epstein-Barr virus (EBV)-transformed B lymphocytes in vitro and in vivo, arresting cell-cycle progression and increasing the apoptotic rate of EBV+ B cells.10 Therefore, it has been suggested that RAD might be effective in the prevention and treatment of human posttransplant lymphoproliferative disorders.10
Here, we investigated whether RAD inhibits tumor cell proliferation of HL and ALCL. We show that RAD significantly inhibits proliferation of HL and ALCL cells in vitro and arrests cell-cycle progression in G0/G1. Furthermore, we demonstrate that in vivo, RAD markedly suppresses tumor cell proliferation of HL and ALCL cells, xenotransplanted into NOD/SCID mice. Our data suggest that RAD might be used in combination chemotherapy for the treatment of HL and ALCL. Moreover, we studied the mechanisms of proliferation arrest mediated by the mammalian target of rapamycin (mTOR) inhibitor RAD to identify the molecular targets in HL and ALCL. The mTOR pathway controls the translation initiation machinery in response to nutrients and growth factors thereby coordinating cell growth with cell division.11 A transcription factor that is a critical target of mTOR is the CCAAT enhancer binding protein (C/EBP)β.11-13 C/EBPβ has previously been identified as an essential downstream target in tumors expressing activated cyclin D1.14 Our data demonstrate that ALCL and some HL cells express abundant amounts of C/EBPβ, in particular of its rapamycin-sensitive, truncated (Tr) isoform liver-enriched inhibitory protein (LIP) that emerges as a result of alternative translation initiation of the C/EBPβ mRNA. We further show that RAD treatment inhibits proliferation of HL and ALCL cells and down-regulates expression of the LIP C/EBPβ isoform. Ectopic expression of LIP in the ALCL cell line Karpas 299 abrogated the antiproliferative effect of RAD, suggesting that translationally deregulated expression of C/EBPβ plays an important role in ALCL.
Our further analysis of the molecular mechanisms of RAD effects also identified the nuclear factor κB (NF-κB) signaling pathway as a novel target. We had previously shown that NF-κBis constitutively activated and serves as a survival factor of HL cells.15-18 Here we show that RAD down-regulates constitutive NF-κB DNA binding activity in HL L540cy cells and ALCL Karpas 299 cells. Overexpression of NF-κB-p65 rescues L540cy cells from RAD-mediated proliferation arrest, indicating that RAD exerts its antiproliferative effects at least in part through inhibition of constitutive NF-κB. We conclude that pharmacologic inhibition of mTOR signaling by RAD affects at least 2 important proliferation and survival pathways in HL and ALCL and that RAD treatment might therefore serve as an alternative therapeutic approach.
Materials and methods
Cell culture
The following human cell lines were analyzed in this study: the HL cell lines L428, KM-H2, L1236, HD-LM2 (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ], Braunschweig, Germany), and L540cy, a subclone of the HL cell line L540 that grows in SCID mice (kindly provided by A. Engert, University Hospital Cologne, Germany); the ALCL cell lines Karpas 299, JB6, and SU-DHL1 carrying the t(2;5) chromosomal translocation and expressing nucleophosmin (NPM)-ALK, which involves the fusion of the NPM gene to an ALK (DSMZ) and the NPM-ALK-negative ALCL cell line FE-PD (kindly provided by Dr Michael Hummel, Berlin, Germany). Cell lines were maintained in RPMI 1640 (Biochrom, Berlin, Germany), 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, and 100 μg/mL streptomycin, 1 mM sodium pyruvate, and 2 mM glutamine (GIBCO, Karlsruhe, Germany). Cell lines were treated with RAD (Novartis Pharma, Basel, Switzerland) in various concentrations (0 to 50 nM) for 24, 48, and 96 hours as indicated. Electroporation of L540cy cells was performed using a Gene-Pulser II (Bio-Rad, München, Germany) with 960 microfarads (μF) and 0.18 kV. Transfection-associated cell death was negligible. Cells were transfected with 6 μg 6NF-κBtkluc,19 40 μg p65-green fluorescent protein (GFP) expression plasmid or 36 μg pcDNA3 along with 4 μg pEGFP-N3 plasmid. Luciferase assays were performed as previously described.20
DNA constructs
The following expression plasmids were used: p65-pEGFP (kindly provided by J. Schmid, Vienna, Austria), pcDNA3 (Invitrogen, Karlsruhe, Germany), and pEGFP-N3 (Clontech Laboratories, Heidelberg, Germany). The rC/EBPβ-Tr construct was cloned as previously described12 and was C-terminally tagged with an epitope and cloned in pMSCVpuro vector (Clontech). Fusion protein NPM-ALK was cloned in pcDNA3 (kindly provided by S.W. Morris, Memphis, TN).
Retroviral methods
The amphotropic-packaging cell line Phoenix A was transiently transfected with the calcium phosphate-DNA precipitation method, and infectious virus was harvested after 48 hours. Karpas 299 target cells (5 × 105) were infected as described21 and selected for puromycin (1.5 μg/mL) resistance.
Proliferation assay and cell-cycle analysis
Cell lines were cultured in triplicate at 1 × 105 cells per 6 wells for 96 hours in the presence of various concentrations of RAD and counted in Neubauer chambers. Cell counts were determined as cells per milliliter. For cell-cycle analysis, cell lines were cultured in 0 nM to 10 nM RAD for 48 and 96 hours. The cells were washed with phosphate-buffered saline (PBS) and fixed in 70% ice-cold ethanol (-20°C). After at least overnight at -20°C, cells were washed and stained with 4 mg/mL propidium iodide (Sigma, Deisenhofen, Germany) and incubated with 10 mg/mL RNase A (Roche, Basel, Switzerland) for 10 minutes at room temperature (dark). DNA content was then measured by flow cytometry analysis. Statistical analysis was performed via ModFit LT program.
NOD/SCID mice experiments
Immunodeficient 7- to 8-week-old NOD/SCID mice were kept under pathogen-free conditions in a laminar flow unit, and were supplied with sterile food and water. L540cy cells (1 × 107) and Karpas 299 cells (1 × 107) were inoculated into mice subcutaneously in 100 μL PBS together with 100 μL matrigel (Becton Dickinson, Heidelberg, Germany). Palpable subcutaneous tumors developed 1 to 2 weeks after cell injection. RAD treatment (5 mg/kg per day) was initiated after palpable tumor growth and was given once a day by gavage. Tumor volume (V) was calculated in all experiments according to V = ab2/2, where a and b designate long and short diameters of the tumor, respectively. The mice that underwent transplantation were monitored for tumor growth for a period of up to 3 to 4 weeks. Mice experiments were performed by EPO GmbH (Berlin, Germany) according to the German Animal Protection Law with permission of the responsible authorities. At the end of the study a complete autopsy was performed, including the tumors, all internal organs (liver, kidneys, heart, spleen, lung, small and large bowel), and lymph nodes. In the second experiment, 1 × 107 vector and C/EBPβ-Tr-transfected Karpas 299 cells were inoculated into mice. RAD treatment (5 mg/kg per day) was initiated one day after tumor cell inoculation and was given once a day by gavage. Statistical significance was determined by Mann and Whitney U test (*P < .05).
Immunoblotting
Cell extracts were prepared and quantitated as described.22 Proteins (30 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Protein load was normalized by Ponceau Red staining. Membranes were incubated with rabbit polyclonal anti-C/EBPβ, anti-p50, anti-p65, and anti-IκBα antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and with rabbit polyclonal anti-phospho S6 ribosomal protein (Ser235/236; New England Biolabs, Frankfurt, Germany), followed by goat anti-rabbit horseradish peroxidase (HRP)-conjugated antibodies (Dianova, Hamburg, Germany) and anti-Flag tag (Eastman Kodak, Rochester, NY) followed by goat anti-mouse HRP-conjugated antibodies (Pharmingen), and detected by enhanced chemiluminescence (Amersham Pharmacia, Freiburg, Germany).
Electrophoretic mobility shift assay (EMSA)
Whole cell extracts were prepared essentially as described previously.23 After washing the cells with PBS, lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.9], 350 mM NaCl, 0.5 mM EDTA [ethylenediaminetetraacetic acid], 0.1 mM EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], 1 mM MgCl2, 10% Glycerol, 1% Nonident P-40, 1 mg/mL Pefabloc (4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride), 1 mg/mL aprotinin, 1 mg/mL leupeptin, 1 mg/mL pepstatin A, 10 mM NaF, 8 mM β-glycerophosphate, and 1 mM dithiothreitol [DTT]) was added and after a 10-minute incubation at 4 °C the lysate was centrifuged for 10 minutes at 16 000g [14 000 rpm], 4°C. EMSA was performed as described previously.15
Northern blot analysis
Total RNA was prepared using the RNeasy kit (Qiagen, Hilden, Germany). Total RNA (10 μg) was subjected to gel electrophoresis on a 1.1% formaldehyde/1.2% agarose gel and transferred to a nylon membrane (Appligene, Heidelberg, Germany). After UV cross-linking, the membrane was prehybridized (ExpressHyb solution; Clontech) and thereafter hybridized with α-[32P]deoxycytidine triphosphate (α-[32P]dCTP)-labeled random prime-labeled DNA probes (IκBα) overnight at 68°C. Membranes were washed at room temperature in 2× SSC and 0.05% SDS and then in 0.1× SSC and 0.1% SDS.
Results
RAD inhibits tumor-cell proliferation of HL and ALCL in vitro
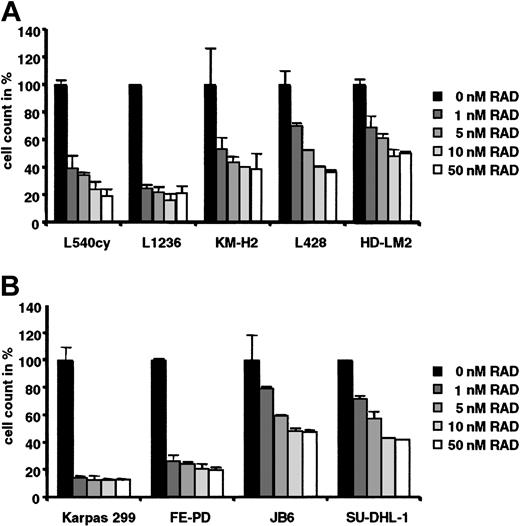
The indicated HL and ALCL cell lines (Figure 1) were grown for 96 hours in 0 nM to 50 nM RAD to determine its effect on cell proliferation. The HL cell lines L1236 and L540cy (a subclone of the HL cell line L540 that forms tumors in SCID mice) and the ALCL cell lines Karpas 299 (ALK+) and FE-PD (ALK-) were highly sensitive to RAD-mediated inhibition of proliferation (Figure 1A-B). In L540cy, L1236, and FE-PD cells treatment with 10 nM RAD resulted in 80% inhibition of proliferation. Proliferation of Karpas 299 cells was already inhibited to 90% by 1 nM RAD with no further increase after dose escalation. These results were comparable to the inhibition of EBV+ B-cell lines derived from patients with posttransplantation lymphoproliferative disorders.10 The HL cell lines KM-H2, L428, and HD-LM2 and the ALCL cell lines JB6 and SU-DHL-1 were less sensitive toward RAD treatment, excluding generalized toxicity of RAD. Even the maximally inhibited cultures (10%-20% of control) such as Karpas 299, FE-PD, L540cy, or L1236 had either increased or maintained cell numbers per well after RAD treatment compared with starting cultures. The degree of response was confirmed by determination of [3H] thymidine incorporation (data not shown).
RAD inhibits proliferation of HL and ALCL cells. (A) The HL cell lines (L540cy, L1236, KM-H2, L428, HD-LM2) and (B) the ALCL cell lines (Karpas 299, FE-PD, JB6, SU-DHL1) were incubated with 0 nM to 50 nM RAD for 96 hours. Cell counts are given as the mean of 3 independent experiments. Counts of RAD-treated lymphoma cells are given relative to counts of untreated cells, which were set arbitrarily at 100% for each cell line. Error bars indicate standard deviation (SD).
RAD inhibits proliferation of HL and ALCL cells. (A) The HL cell lines (L540cy, L1236, KM-H2, L428, HD-LM2) and (B) the ALCL cell lines (Karpas 299, FE-PD, JB6, SU-DHL1) were incubated with 0 nM to 50 nM RAD for 96 hours. Cell counts are given as the mean of 3 independent experiments. Counts of RAD-treated lymphoma cells are given relative to counts of untreated cells, which were set arbitrarily at 100% for each cell line. Error bars indicate standard deviation (SD).
RAD blocks cell-cycle progression in HL and ALCL
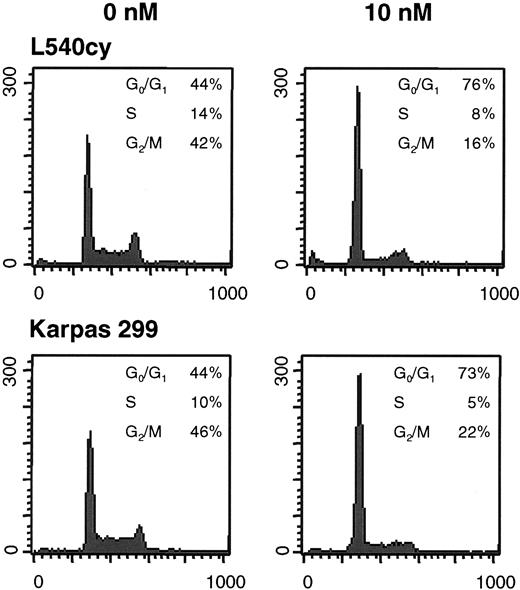
Flow cytometry analysis was performed after 48 hours (Figure 2, Table 1) and 96 hours (data not shown) to analyze whether RAD blocks cell-cycle progression in G0/G1 phase as previously reported.10 Increase of G0/G1 and concomitant decrease in G2/M cells was observed in all RAD-treated HL and ALCL cell lines. There was no increase in cells in sub-G1 phase and no enhanced expression of Annexin V (data not shown), indicating that RAD did not induce apoptosis leading to loss of DNA content. These data suggest that failure to expand in cell numbers is due to cell-cycle arrest in G0/G1 rather than apoptosis or necrosis.
Relative increases and decreases of RAD-treated cells in G0G1 and G2/M phases of the cell-cycle of HL and ALCL cells
. | Cell-cycle profiles . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | G0/G1, % . | . | S, % . | . | G2/M, % . | . | |||||
| Cell line . | 0 nM . | 10 nM . | 0 nM . | 10 nM . | 0 nM . | 10 nM . | |||||
| L540cy | 44 | 76 | 14 | 8 | 42 | 16 | |||||
| L1236 | 45 | 73 | 13 | 11 | 42 | 16 | |||||
| KM-H2 | 41 | 59 | 10 | 10 | 49 | 31 | |||||
| L428 | 37 | 62 | 16 | 8 | 47 | 30 | |||||
| HD-LM2 | 56 | 73 | 29 | 18 | 15 | 9 | |||||
| Karpas 299 | 44 | 73 | 10 | 5 | 46 | 22 | |||||
| FE-PD | 48 | 64 | 16 | 11 | 34 | 25 | |||||
| JB6 | 32 | 52 | 15 | 10 | 53 | 38 | |||||
| SU-DHL | 41 | 60 | 18 | 14 | 41 | 26 | |||||
. | Cell-cycle profiles . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | G0/G1, % . | . | S, % . | . | G2/M, % . | . | |||||
| Cell line . | 0 nM . | 10 nM . | 0 nM . | 10 nM . | 0 nM . | 10 nM . | |||||
| L540cy | 44 | 76 | 14 | 8 | 42 | 16 | |||||
| L1236 | 45 | 73 | 13 | 11 | 42 | 16 | |||||
| KM-H2 | 41 | 59 | 10 | 10 | 49 | 31 | |||||
| L428 | 37 | 62 | 16 | 8 | 47 | 30 | |||||
| HD-LM2 | 56 | 73 | 29 | 18 | 15 | 9 | |||||
| Karpas 299 | 44 | 73 | 10 | 5 | 46 | 22 | |||||
| FE-PD | 48 | 64 | 16 | 11 | 34 | 25 | |||||
| JB6 | 32 | 52 | 15 | 10 | 53 | 38 | |||||
| SU-DHL | 41 | 60 | 18 | 14 | 41 | 26 | |||||
Cell cycles of HL and ALCL cells were analyzed by propidium iodide staining and statistical analysis was performed via ModFit LT program. Untreated cells in G0/G1 and G2/M phase were set arbitrarily at 100% for each cell line.
RAD treatment of HL and ALCL cells in xenotransplant models
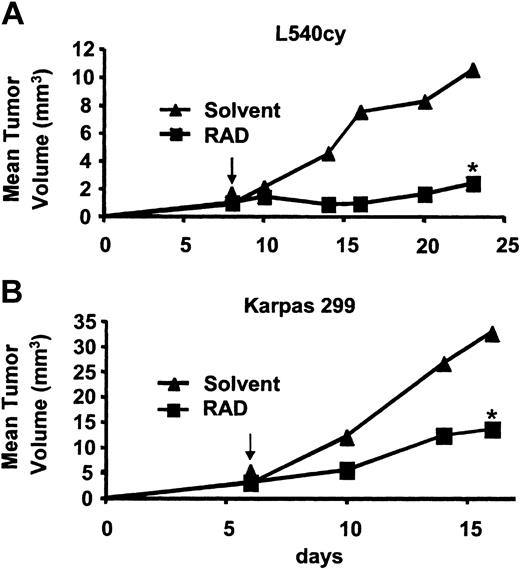
A xenotransplant model of the HL cell line L540cy and the ALCL cell line Karpas 299 in NOD/SCID mice was established to determine the effect of RAD in vivo (Figure 3). Mice were injected subcutaneously with 1 × 107 L540cy cells or Karpas 299 cells. Treatment with RAD started when tumors were palpable (day 6 or day 8). In the untreated L540cy control group, tumors grew rapidly and reached a mean tumor volume of 10.5 mm3 within 23 days. In the RAD-treated group, tumor growth was delayed and by day 23 tumors had a mean volume of about 2.4 mm3 (Figure 3A). Subcutaneously injected Karpas cells grew to a mean tumor volume of 32.7 mm3 after 16 days (Figure 3B). RAD treatment resulted in a delay of tumor development and a reduction of tumor size (mean volume: 13.7 mm3). Furthermore, in RAD-treated mice no lymph node metastasis of Karpas 299 cells was detected, whereas untreated animals showed invasion of axillary and inguinal lymph nodes. Untreated animals were killed at day 16 because of massive tumor size. Our data indicate that treatment of HL and ALCL cells with RAD significantly inhibits tumor cell proliferation and metastasis in xenotransplant models.
RAD mediates inhibition of cell-cycle progression in HL and ALCL cells. Cell lines were incubated with 0 nM to 10 nM RAD for 48 hours, labeled with propidium iodine, and analyzed by flow cytometry. Cell-cycle profiles of L540cy and Karpas 299 cells are shown as representative examples. X-axis shows DNA content; y-axis, cell numbers.
RAD mediates inhibition of cell-cycle progression in HL and ALCL cells. Cell lines were incubated with 0 nM to 10 nM RAD for 48 hours, labeled with propidium iodine, and analyzed by flow cytometry. Cell-cycle profiles of L540cy and Karpas 299 cells are shown as representative examples. X-axis shows DNA content; y-axis, cell numbers.
RAD controls tumor-cell proliferation through modulation of the C/EBPβ protein isoform ratio in HL and ALCL cells
As shown in Figure 4A (lower panels), C/EBPβ proteins were highly expressed in all HL and ALCL cell lines examined. Of particular interest was the observation that the truncated LIP isoform of C/EBPβ that is highly expressed in breast cancer cells24,25 was also highly expressed in Hodgkin L540cy and in ALCL Karpas 299, FE-PD, JB6, and SU-DHL-1 cells (Figure 4A) and moderately expressed in Hodgkin L1236 and L428 cells. We and others12,24,25 have recently shown that the truncated isoform promotes proliferation and may induce transformation and that rapamycin blocks expression of the truncated isoform through partial inhibition of the translation initiation factor elF4E.12 As shown in Figure 4A, treatment with 10 nM RAD for 96 hours down-regulated the activity of the mTOR signaling pathway as evidenced by strongly diminishing phosphorylation of the ribosomal S6 protein (Figure 4A, upper panels) and 4E-BP1 (data not shown). Concomitantly, the ratio of C/EBPβ isoform expression was modulated such that the truncated C/EBPβ isoform was specifically decreased in all cell lines examined (Figure 4A).
RAD-mediated inhibition of in vivo proliferation of HL and ALCL cells. NOD/SCID mice were inoculated subcutaneously with (A) L540cy and (B) Karpas 299 cells, and daily treatment with RAD (5 mg/kg) was started when tumors were palpable. Tumor volume (mean in mm3) was compared between RAD-treated versus control animals. Statistical significance was determined by Mann and Whitney U test (*P < .05). Arrows define the start of the treatment.
RAD-mediated inhibition of in vivo proliferation of HL and ALCL cells. NOD/SCID mice were inoculated subcutaneously with (A) L540cy and (B) Karpas 299 cells, and daily treatment with RAD (5 mg/kg) was started when tumors were palpable. Tumor volume (mean in mm3) was compared between RAD-treated versus control animals. Statistical significance was determined by Mann and Whitney U test (*P < .05). Arrows define the start of the treatment.
To determine whether abrogation of expression of the truncated LIP isoform of C/EBPβ contributes to the inhibition of proliferation of RAD-treated Karpas 299 cells, we stably introduced the truncated C/EBPβ isoform (fused to a Flag immuno-epitope) by retroviral gene transfer (Tr-Flag, Figure 4B). Ectopic expression of the Flag-tagged truncated C/EBPβ isoform alleviated RAD-induced proliferation arrest as shown in Figure 4C (RAD treatment for 48 hours). Thus, translational up-regulation of the truncated C/EBPβ isoform sustains proliferation and might thereby contribute to transformed phenotype in Karpas 299 cells. This finding suggests translational control of C/EBPβ isoform expression as a target of proliferation control and therapeutic intervention.
RAD down-regulates constitutive NF-κB activity in L540cy and Karpas 299 cells
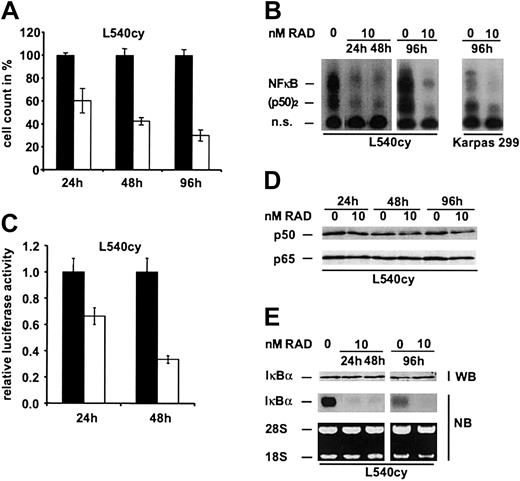
Activated NF-κB p50/p65 has been shown to be essentially required for HL tumor cell proliferation and survival.16 We therefore asked whether RAD also affects the function of NF-κBin HL cells. As shown in Figure 5B, 10 nM RAD down-regulated NF-κB DNA binding activity and inhibited proliferation of L540cy cells in a time-dependent fashion (Figure 5A). Similarly, RAD inhibited constitutive NF-κB activity in the ALCL cell line Karpas 299, whereas NF-κB activity did not change in the other RAD-treated HL and ALCL cell lines (Figure 5B and data not shown). To further determine RAD effects on transcriptional activity of NF-κB, we performed luciferase assays with a NF-κB dependent reporter construct (Figure 5C). Our data demonstrate that RAD treatment down-regulated transcriptional activity of NF-κB in L540cy cells.
Constitutive ectopic expression of truncated C/EBPβ renders ALCL cells resistent to RAD-mediated inhibition of proliferation. (A, left) Down-regulation of the mTOR target gene, ribosomal S6 protein, was demonstrated after treatment of cell lines with 0 nM to 10 nM RAD for 96 hours. (Right) Endogenous expression of C/EBPβ protein isoforms was determined in HL and ALCL cells, which were treated with 0 nM to 10 nM RAD for 96 hours. Fl indicates full-length isoform; Tr, truncated isoform. (B) Flag-tagged truncated C/EBPβ was ectopically expressed in Karpas 299 cells by retroviral transduction. Ectopic and endogenous expression of C/EBPβ protein isoforms was analyzed by immunoblotting with antibodies against C/EBPβ after treatment with 0 nM to 10 nM RAD for 96 hours. (C) Control and C/EBPβ-Tr-transfected Karpas 299 cells were incubated with 0 nM to 10 nM RAD for 48 hours. RAD-treated and untreated cells were counted and counts of untreated cells were set arbitrarily at 100%. Cell counts are given as the mean of 3 independent experiments. Error bars indicate SD.
Constitutive ectopic expression of truncated C/EBPβ renders ALCL cells resistent to RAD-mediated inhibition of proliferation. (A, left) Down-regulation of the mTOR target gene, ribosomal S6 protein, was demonstrated after treatment of cell lines with 0 nM to 10 nM RAD for 96 hours. (Right) Endogenous expression of C/EBPβ protein isoforms was determined in HL and ALCL cells, which were treated with 0 nM to 10 nM RAD for 96 hours. Fl indicates full-length isoform; Tr, truncated isoform. (B) Flag-tagged truncated C/EBPβ was ectopically expressed in Karpas 299 cells by retroviral transduction. Ectopic and endogenous expression of C/EBPβ protein isoforms was analyzed by immunoblotting with antibodies against C/EBPβ after treatment with 0 nM to 10 nM RAD for 96 hours. (C) Control and C/EBPβ-Tr-transfected Karpas 299 cells were incubated with 0 nM to 10 nM RAD for 48 hours. RAD-treated and untreated cells were counted and counts of untreated cells were set arbitrarily at 100%. Cell counts are given as the mean of 3 independent experiments. Error bars indicate SD.
To investigate whether reduced NF-κB p50 and p65 protein levels may account for the down-regulated NF-κB DNA binding activity in L540cy cells, we performed Western blot analysis. NF-κB p50 and p65 levels remained unaltered over time (Figure 5D), indicating that the loss of NF-κB activity could not be explained by down-regulated expression levels. We next analyzed inhibitor kappa B (IκB)α protein and mRNA expression in response to RAD (Figure 5E), because IκBα is the main inhibitor and an important target gene of NF-κB. As expected, IκBα mRNA was significantly reduced since NF-κB activity was down-regulated (Figure 5E; 24, 48, and 96 hours). However, expression of IκBα protein, which has a very short half life, remained unaltered in L540cy cells over 96 hours, in contrast to its mRNA expression, indicating a posttranslational stabilization of the protein (Figure 5E). We conclude that the loss of NF-κB activity is caused by the posttranslational stabilization of IκBα and results in down-regulation of mRNA expression of IκBα as its main target. Our results clearly show that RAD treatment can functionally interfere with the NF-κB signaling pathway.
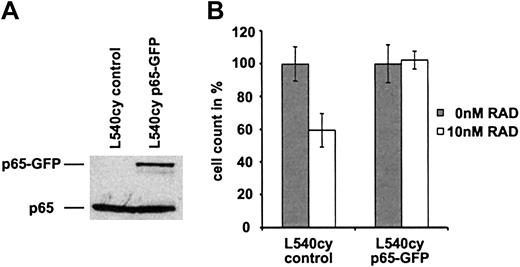
NF-κB-p65 overexpression rescues L540cy cells from RAD-mediated inhibition of proliferation
To examine a potential causal relationship between down-regulation of constitutive NF-κB and RAD-mediated inhibition of proliferation in L540cy cells, we transiently transfected L540cy cells with a NF-κB-p65-GFP expression plasmid (Figure 6A) and determined rescue from RAD-mediated proliferation arrest (Figure 6B). As shown in Figure 6B, treatment with 10 nM RAD of control-transfected L540cy cells for 24 hours resulted in approximately 40% inhibition of proliferation (cell count, see also Figure 5A). p65-GFP-transfected L540cy cells, however, became resistant to RAD treatment, indicating that overexpression of NF-κB-p65 rescues L540cy cells from RAD-mediated proliferation arrest. The p65-GFP-mediated activation of NF-κB was controlled by reporter assay and EMSA analysis.23 Furthermore, the cotransfection of IκBα reduced NF-κB activity of the construct, suggesting proper regulation of the fusion protein.23 Thus, our data show that in addition to regulation of C/EBPβ isoform expression, the NF-κB system is also involved and a target in RAD-mediated inhibition of proliferation in L540cy cells. We suggest that in L540cy cells, down-regulation of both the truncated LIP isoform and NF-κB activity contribute to the RAD-mediated effect but neither change is sufficient to the effect.
Down-regulation of NF-κB DNA binding activity by RAD in L540cy and Karpas 299 cells. (A) The HL cell line L540cy was incubated with 0 nM (▪) to 10 nM (□) RAD for 24, 48, and 96 hours. Cell counts are given as the mean of 3 independent experiments. Counts of RAD-treated lymphoma cells are given relative to counts of untreated cells, which were set arbitrarily at 100%. (B) L540cy and Karpas 299 cells were treated with 0 nM to 10 nM RAD for indicated times. Whole cell extracts were analyzed by EMSA for NF-κB DNA binding activity. Free DNA probe is not shown. n.s. indicates nonspecific. (C) L540cy cells were transfected with an NF-κB-dependent reporter construct (6NF-κBtkluc) and transcriptional activity of NF-κB was detected 24 and 48 hours after treatment with 0 nM (▪) to 10 nM (□) RAD. Luciferase counts are given relative to control-treated L540cy cells, which are set arbitrarily at 1. Luciferase activity was determined for triplicate experiments. (D) Protein expression levels p50 and p65 in response to RAD analyzed by Western blot analysis in L540cy cells. (E) Protein and total mRNA were prepared after indicated times and analyzed for expression of IκBα by Western and Northern blotting in L540cy cells. Error bars indicate SD.
Down-regulation of NF-κB DNA binding activity by RAD in L540cy and Karpas 299 cells. (A) The HL cell line L540cy was incubated with 0 nM (▪) to 10 nM (□) RAD for 24, 48, and 96 hours. Cell counts are given as the mean of 3 independent experiments. Counts of RAD-treated lymphoma cells are given relative to counts of untreated cells, which were set arbitrarily at 100%. (B) L540cy and Karpas 299 cells were treated with 0 nM to 10 nM RAD for indicated times. Whole cell extracts were analyzed by EMSA for NF-κB DNA binding activity. Free DNA probe is not shown. n.s. indicates nonspecific. (C) L540cy cells were transfected with an NF-κB-dependent reporter construct (6NF-κBtkluc) and transcriptional activity of NF-κB was detected 24 and 48 hours after treatment with 0 nM (▪) to 10 nM (□) RAD. Luciferase counts are given relative to control-treated L540cy cells, which are set arbitrarily at 1. Luciferase activity was determined for triplicate experiments. (D) Protein expression levels p50 and p65 in response to RAD analyzed by Western blot analysis in L540cy cells. (E) Protein and total mRNA were prepared after indicated times and analyzed for expression of IκBα by Western and Northern blotting in L540cy cells. Error bars indicate SD.
Overexpression of NF-κB-p65 protects L540cy cells from RAD-induced cell-cycle arrest in G0/G1. (A) L540cy cells were transfected with a p65-GFP expression construct or with control (pcDNA3) and EGFP-expressing (pEGFP-N3) constructs. At 24 hours after transfection, whole cell extracts were analyzed by Western blotting for the expression of p65-GFP and endogenous p65 using a p65-specific antibody. (B) L540cy cells were transfected with a p65-GFP expression construct or with pcDNA3 and pEGFP-N3 constructs. At 6 hours after transfection, cells were incubated with 0 nM and 10 nM RAD for 24 hours. RAD-treated and untreated cells were counted and counts of untreated cells were set arbitrarily at 100%. Cell counts are given as the mean of 3 independent experiments. Error bars indicate SD.
Overexpression of NF-κB-p65 protects L540cy cells from RAD-induced cell-cycle arrest in G0/G1. (A) L540cy cells were transfected with a p65-GFP expression construct or with control (pcDNA3) and EGFP-expressing (pEGFP-N3) constructs. At 24 hours after transfection, whole cell extracts were analyzed by Western blotting for the expression of p65-GFP and endogenous p65 using a p65-specific antibody. (B) L540cy cells were transfected with a p65-GFP expression construct or with pcDNA3 and pEGFP-N3 constructs. At 6 hours after transfection, cells were incubated with 0 nM and 10 nM RAD for 24 hours. RAD-treated and untreated cells were counted and counts of untreated cells were set arbitrarily at 100%. Cell counts are given as the mean of 3 independent experiments. Error bars indicate SD.
Discussion
Data presented here show that the macrolide fungicide RAD, a known immunosuppressant and antitumor agent for human posttransplantation lymphoproliferative disorders,10 inhibits tumor cell proliferation of HL and ALCL (Figure 1). We show that RAD potently suppresses cell-cycle progression in G0/G1 phase of HL and ALCL cells (Figure 2, Table 1) and significantly delays tumor development in NOD/SCID mice that had received xenotransplantations (Figure 3). Moreover, we identify 2 molecular targets that account for the RAD-mediated substantial antiproliferative effects through down-regulation of both the truncated isoform of the transcription factor C/EBPβ and NF-κB activity.
Tumor cell lines used in this study are derived from patients with advanced stages of HL and ALCL that were heavily pretreated. It is therefore conceivable that less malignant forms of HL and ALCL cases that would have to be obtained earlier in treatment are even more sensitive to RAD, when tested in future studies. In contrast to the apoptotic effect of RAD or rapamycin on EBV+ B cells and B-percursor leukemia cells,10,26 programmed cell death did not appear to play a major role in its antitumor activity toward HL and ALCL cells (data not shown). Majewski et al10 demonstrated that RAD induced apoptosis in EBV+ B-cell lines, in contrast to HTLV-I+ malignant T-cell lines, and Brown et al26 used a B-precursor acute lymphoblastic leukemia cell line to determine rapamycin-induced apoptosis. The ALCL cell lines and 2 of the HL cell lines (L540cy and HD-LM2) were of T-cell origin and might be relatively resistant to induction of apoptosis by RAD in contrast to the B-cell lines used in former studies. In addition, HL cell lines are known to be resistant toward induction of apoptosis by various drugs.16
Our data also suggest that RAD might not be used as a single therapeutic drug since it did not eradicate xenotransplanted tumor cells but only delayed tumor outgrowth. However, from 2 studies it is already known that rapamycin cannot only enhance apoptosis but also potentiate dexamethasone-induced apoptosis and increase sensitivity to cisplatin in lymphoblastoid cells.27,28 Therefore, in clinical settings of HL and ALCL, RAD might be used in combination therapy with conventional chemotherapeutic drugs.
Novel concepts for the treatment of HL and ALCL have to be based on insights of the molecular mechanisms that are responsible for deregulated proliferation and differentiation. Rapamycin inhibits the protein kinase activity of mTOR and thereby controls the translation of key mRNAs that encode proteins required for cell-cycle progression and differentiation.11 We recently demonstrated that inhibiting the mTOR signaling pathway by rapamycin controls the protein isoform expression of the transcription factor C/EBPβ through alteration of the activity of the eukaryotic translation initiation machinery.12 Different translationally initiated C/EBPβ proteins display isoform-specific biologic activities that adjust cell proliferation versus differentiation.12-14 The full-length C/EBPβ isoform functions as a transcriptional activator that induces differentiation,13,29-32 whereas a truncated, downstream-initiated isoform permits the cell cycle to proceed.32 Moreover, the truncated isoform can counteract the functions of the full-length isoform.13 Therefore, the biologic effects evoked by C/EBP proteins strongly depend on the ratio of C/EBP isoforms. We show that C/EBPβ and in particular its truncated, proliferation-supporting isoform is abundantly expressed in ALCL and some HL cells (Figure 4A). Similar to what has been shown in rapamycin-treated adipocytes,12 RAD strongly reduced expression of the truncated C/EBPβ isoform, whereas the full-length isoform remained relatively constant. Karpas 299 cells that were stably transfected with truncated C/EBPβ were less sensitive to RAD-mediated inhibition of proliferation in vitro, demonstrating a growth-supporting function for C/EBPβ in ALCL (Figure 4B-C). Taken together, our data suggest that modulation of the translational control of the C/EBPβ isoform expression by RAD essentially contributes to inhibition of proliferation in HL and ALCL cells.
Increased NF-κB activity is an essential factor for malignant transformation in HL.15-18 Our previous data showed that constitutive NF-κB activity is a survival factor for HL cells.15-18 Furthermore, pharmacologic inhibition of IKK/NF-κB activity by arsenic can also overcome drug resistance and therefore is a powerful treatment option for HL.23 Several reports suggested that rapamycin can prevent activation of NF-κB by stabilization of its major inhibitor IκBα.33-36 Here we demonstrate that RAD strongly inhibits NF-κB DNA-binding activity only in L540cy HL and Karpas 299 ALCL cells (Figure 5), whereas more conventional mTOR pathway targets such as ribosomal S6 protein were down-regulated in all cell lines (Figure 4A, upper panels). Thus, in L540cy cells the loss of NF-κB activity is caused by the posttranslational stabilization of its main inhibitor IκBα, which has been previously described in Jurkat T cells.31 We further show that overexpression of NF-κB-p65 rescues L540cy cells from RAD-mediated inhibition of proliferation, indicating that RAD also exerts its antiproliferative effects and at least in part through inhibition of constitutive NF-κB (Figure 6).
In conclusion, our data provide evidence for the inhibitory activity of RAD against HL and ALCL cell proliferation in tissue culture and in the animal. These results suggest that important targets of the antiproliferative activity of RAD include C/EBPβ isoform expression and constitutive NF-κB activity. Therefore, targeting mTOR-dependent signal transduction may represent a novel and an alternative strategy for potent lymphoma combination therapy.
This view is in agreement with conclusions drawn from classical tumor virology that suggested that inhibition of translation by rapamycin through mTOR and its downstream targets S6K and 4E-BPs effectively blocks oncogenic transformation induced by a subgroup of pathways such as activated P3K or Akt but not by other oncoproteins.37 Our study might therefore provide a basis for testing RAD in new clinical trials to overcome classical drug resistance and achieve improved outcome in incurable cases of HL and ALCL. Further studies are needed to determine its efficacy in combination with standard therapeutic drugs.
Prepublished online as Blood First Edition Paper, May 10, 2005; DOI 10.1182/blood-2004-11-4513.
Supported in part by the Berliner Krebsgesellschaft.
F.J. and N.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Walter Schuler (Novartis Pharma, Basel, Switzerland) for providing us with the SDZ RAD.
Christine Müller and Cornelis F. Calkhoven are currently affiliated with the Institute of Molecular Biotechnology, Jena, Germany.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal