Abstract
Heme oxygenase-1 (HO-1) is an intracellular enzyme that degrades heme and inhibits immune responses and inflammation in vivo. In most cell types, HO-1 is inducible by inflammatory stimuli and oxidative stress. Here we demonstrate that human monocyte-derived immature dendritic cells (iDCs) and several but not all freshly isolated rat splenic DC subsets and rat bone marrow-derived iDCs, spontaneously express HO-1. HO-1 expression drastically decreases during human and rat DC maturation induced in vitro. In human tissues, iDCs also express HO-1, whereas mature DCs do not. Induction of HO-1 expression with cobalt protoporphyrin (CoPP) in human and rat DCs inhibits lipopolysaccharide (LPS)-induced phenotypic maturation and secretion of proinflammatory cytokines, resulting in the inhibition of alloreactive T-cell proliferation. CoPP-treated DCs, however, retain the ability to produce the anti-inflammatory cytokine interleukin 10 (IL-10). Reactive oxygen species induced by LPS in DCs were inhibited by induction of HO-1. In conclusion, we identify, for the first time, the capacity of HO-1 to block maturation of DCs and to inhibit proinflammatory and allogeneic immune responses while preserving IL-10 production. This novel immune function for HO-1 may be of interest for the inhibition of immune responses in autoimmune diseases, transplantation, and other conditions involving activation of the immune system. (Blood. 2005;106:1694-1702)
Introduction
Heme oxygenases (HOs) are the rate-limiting intracellular enzymes that degrade heme to biliverdin, free divalent iron, and CO (for a review, see Otterbein and Choi1 ). Three distinct HO enzymes have been identified: HO-1, HO-2, and HO-3.1 HO-1 is a stress responsive gene whose expression is induced by a variety of stimuli including heme, heavy metals, inflammatory cytokines, and nitric oxide.1 HO-1 is known for its cytoprotective effect against oxidative injuries and inflammation.1 Induction of HO-1 expression by pharmacologic activators or gene transfer has had therapeutic effects in a variety of conditions or disorders involving the immune system, including transplantation and inflammatory disorders.2-8 Biliverdin and its metabolite, bilirubin, are known for their antioxidant9 and immunosuppressive effects.10 HO-1 and CO have been shown to inhibit lipopolysaccharide (LPS)-induced expression of proinflammatory cytokines and to increase LPS-induced expression of interleukin 10 (IL-10) in macrophages.11,12 Moreover, IL-10 induces HO-1 expression in macrophages.13-15 We previously reported that overexpression of HO-1, obtained with an HO-1-encoding adenovirus in rats having heart transplants, results in long-term allograft survival associated with an inhibition of cellular allogeneic immune responses, which could be mediated by adenoviral transduction of dendritic cells (DCs).6
DCs play a central role in the induction of immunity and tolerance (for a review, see Steinman et al16 ). In the absence of inflammation, immature DCs (iDCs) located in peripheral tissues specialize in taking up innocuous and cell-associated self antigens. They continuously capture antigens and migrate to draining lymph nodes where they can induce tolerance.16 In the presence of danger signals, DCs undergo maturation, a process involving up-regulation of surface major histocompatibility complex (MHC) class II and costimulatory molecules, secretion of proinflammatory and anti-inflammatory cytokines, and the acquired ability to stimulate differentiation of naive T cells into effector cells.
Our working hypothesis was that DCs can express HO-1, which can regulate DC functions. In this study, we demonstrate that human and rat iDCs express HO-1 and that HO-1 expression is down-regulated by maturation stimuli. Our results also demonstrate that induction of HO-1 expression renders DCs refractory to LPS-induced maturation, but preserves IL-10 secretion, suggesting that HO-1 may be used to regulate DC functions.
Materials and methods
Animals
Six- to 10-week-old Lewis.1A and Lewis.1W rats (Centre Janvier, Le Genest Saint-Isles, France) were used.
Cell preparation, culture, and treatments
Rat DCs. Rat splenic OX62+ DCs were purified as previously described.17 Briefly, spleen fragments were digested with collagenase D (Roche, Meylan, France). Low-density cells were isolated by centrifugation on a Nycodenz density gradient (Nycomed, Oslo, Norway). Cells were positively selected (> 90% purity)17 using anti-rat OX62-magnetic-activated cell sorting (MACS) microbeads and MiniMACS selection columns (Miltenyi Biotec, Paris, France).
Rat plasmacytoid DCs (pDCs) were purified by spleen digestion in collagenase D, followed by centrifugation over Ficoll-Hypaque (Amersham, Les Ulis, France). T-cell and partial B-cell depletion was then performed by incubating cells with the anti-T-cell receptor αβ (anti-TCRαβ) and γδ monoclonal antibodies (mAbs) R7.3 and V65 followed by incubation with a mixture of anti-mouse and anti-rat IgG-coated magnetic beads (Dynal Biotech, Oslo, Norway). Rat CD4+CD45RB+ pDCs (> 98% purity) were then sorted using the CD45RB-fluorescein isothiocyanate (FITC) and CD4-phycoerythrin (PE) mAbs (BD Pharmingen, San Diego, CA) with a fluorescence-activated cell sorting (FACS) Aria cell sorter (BD Biosciences; donated by the Crédit Agricole).
Rat bone marrow-derived DCs (BMDCs) were obtained as previously described.18 Briefly, bone marrow cells were cultured in medium supplemented with supernatant from COS cells transfected with rat IL-4 cDNA or murine granulocyte-macrophage colony-stimulating factor (GM-CSF) cDNA (final concentration of 4 ng/mL and 1.5 ng/mL of each cytokine, respectively). Cultures were fed with GM-CSF and IL-4 on days 3 and 6. At day 8, adherent immature bone marrow DCs (iBMDCs; < 1% T cells, 1% B cells, 1% macrophages) were used. BMDC maturation was induced by a 48-hour treatment with 500 U/mL human tumor necrosis factor α (TNF-α; BASF/Knoll, Ludwigshafen Germany), 25 μg/mL polyinosinic-polycytidylic acid (poly(I:C)), 0.5 μg/mL LPS (both from Sigma, St Louis, MO), 10 μM cytosine phosphate guanine (CpG; motif 2006, Sigma-Genosys), or 0.5 μg/mL CD40L (Alexis Biochemicals, San Diego, CA) in the presence of 1 μg/mL enhancer anti-Flag mAb (Sigma). Nonadherent mature DCs were collected for analysis.
Human monocyte-derived DCs. Human iDCs were generated as previously described.19 Briefly, monocytes were enriched by elutriation (> 85% CD14+) and cultured 6 days in medium supplemented with IL-4 (40 ng/mL; AbCys, Paris, France), and GM-CSF (500 IU/mL; AbCys). Next, iDCs were harvested and cultured (106 cells/mL) in plates coated with poly(2-hydroxyethyl methacrylate) (Sigma) to prevent cells from adhering. Maturation was induced by a 48-hour incubation using 20 ng/mL TNF-α (AbCys), 50 μg/mL poly(I:C) (Sigma), or 0.5 μg/mL CD40L (Alexis) in the presence of 1 μg/mL enhancer anti-Flag mAb (Sigma) or 1 μg/mL LPS (Sigma).
T cells. Rat lymph node T cells were prepared using nylon wool columns followed by negative selection of cells expressing CD45RA (clone OX33), CD45RB (clone His 24), CD11b/c (clone OX42), and CD161 (clone 3.2.3), with specific mAbs (all from BD PharMingen), followed by anti-mouse IgG-coated magnetic beads (Dynal Biotech). Purity was routinely 98% or higher.
Treatment of DCs with metalloprotoporphyrins or IL-10 and LPS
Rat or human iDCs were pulsed for 2 hours with 50 μM cobalt protoporphyrin (CoPP) or tin protoporphyrin (SnPP; Porphyrin Products, Logan, UT), an inducer and an inhibitor of HO-1, respectively.20-22 The cells were then washed twice and cultured for 16 hours. Protoporphyrins were protected from light at all times. This same protocol has already been shown to be effective in inducing and inhibiting HO-1 activity.23 Human iDCs were cultured for 18 hours with 20 ng/mL recombinant human IL-10 (AbCys). DCs treated with metalloprotoporphyrin or IL-10 were then cultured for a further 24 hours with LPS (1 μg/mL for human DCs [Escherichia coli 026:B6; Sigma] and 0.5 μg/mL for rat DCs [E coli 011:B4; InvivoGen, San Diego, CA]. Supernatants and cells were subsequently harvested for cytokine and phenotypic analysis, respectively. DC viability was analyzed by flow cytometry by staining with TO-PRO-3 iodide (Molecular Probes, Eugene, OR).
Mixed leukocyte reaction
Increasing numbers of treated DCs were cultured in triplicate in round-bottom 96-well plates with 105 allogeneic rat T lymphocytes or human peripheral blood lymphocytes (PBLs; < 1% CD14+ cells purified by elutriation). Proliferation was determined 4 days later by uptake of 3H-thymidine (Amersham, Orsay, France; 0.5 μCi/well [0.0185 MBq]) during the last 8 hours of culture.
Immunohistology and confocal image analysis
Human chronically infected tonsils and healthy, normal skin (kindly provided by Dr K. Renaudeau and Dr B. Dreno, CHU de Nantes) were embedded in cutting compound (Tissue-Tek, Elkhart, IN), frozen in isopentane and cryosectioned (5 μm). Tissue sections and DC cytospins were fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), pH 7.4. Slides were incubated with anti-MHC class II, anti-SIRP-1 (OX41), anti-CD5 (OX 19) (all from the European Collection of Cell Culture, Salisbury, United Kingdom), anti-CD4, anti-CD45RB (both from BD PharMingen), anti-DC-SIGN (R&D Systems, Minneapolis, MN), or PE-coupled anti-DC-LAMP (Immunotech, Marseille, France) Abs. Slides were next incubated with a biotinylated anti-mouse Ab and a streptavidin-Alexa 568 conjugate (except for anti-DC-LAMP). Tissue sections incubated with anti-DC-SIGN and biotinylated anti-mouse Abs were first incubated with 10% mouse serum. Negative controls included anti-human CD16 mAb (IgG1) and a mouse IgG1 isotype control (BD PharMingen) for rat cells and human cells/tissues, respectively. Cells or tissues were postfixed with 4% PFA, permeabilized with 0.5% saponin, incubated with a rabbit anti-rat HO-1 or mouse anti-human HO-1 Ab (Stressgen, Victoria, BC, Canada), followed by incubation with secondary FITC-labeled Abs. Cell nuclei were counterstained with TO-PRO-3 iodide (Molecular Probes) and slides were mounted in ProLong AntiFade reagent (Molecular Probes). Slides were analyzed with a Leica confocal microscope (Heidelburg, Germany) and the Leica TCS NT software. Positive cells (a minimum of 400 cells) were counted manually and coexpression of the different cell surface markers was assessed in each of these cells.
The antibilirubin mAb24 (kindly provided by M. Suematsu, Keio University, Tokyo, Japan) was used to analyze HO-1 enzymatic activity in 0.1% Triton-permeabilized DCs. Staining was performed as described.
Cell extracts and Western blot analysis
Western blot analysis was performed as previously described.5 Briefly, cell protein extracts were boiled, electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel and blotted. Membranes were blocked and incubated with either a rabbit anti-rat, a mouse anti-human HO-1 (Stressgen) Ab or a mouse anti-β tubulin mAb (Calbiochem, San Diego, CA). Membranes were then incubated with horseradish peroxidase-labeled secondary Abs (Jackson ImmunoResearch, West Grove, PA) and detection performed by enhanced chemoluminescence (Amersham).
Quantitative real-time RT-PCR
Total RNA was isolated using TRIzol (Invitrogen, Cergy Pontoise, France). RNA was treated with DNAse and reverse transcribed. Reverse transcription (RT) quantitative polymerase chain reaction (PCR) was performed with a GenAmp 7700 sequence detection system (Applied Biosystems, Foster City, CA) using SYBR Green PCR core reagents (Applied Biosystems). Rat and human primer sequences were used to target HO-1 and hypoxanthine-guanine phosphoribosyltransferase (HPRT).6,25 The PCR method and the 2-ΔΔCt quantification method, after normalization to HPRT values, have been previously described.6 The transcript accumulation index (TAI) is defined as the fold change in mRNA levels in a given sample (Q) relative to levels in a calibrator (CB)—in this case iDCs. The calibrator is the 1× expression of each gene. The TAI is calculated as follows: TAI = 2-ΔΔCt, where ΔΔCt = (CtTarget-CtHPRT)Q-(CtTarget-CtHPRT)CB. Specific amplifications were checked by amplicon melting curves.
Flow cytometry
Rat DCs were stained with the following PE- or FITC-conjugated mAbs: anti-MHC class II, anti-CD80, anti-CD86 (all from BD PharMingen) and anti-CD54 (Serotec, Oxford, United Kingdom). Human DCs were stained with the following PE- or FITC-conjugated mAbs: anti-CD80, anti-CD83, anti-CD86, and anti-HLA-ABC (all from Immunotech). Staining was assessed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Pont de Claix, France). PE- or FITC-labeled mouse anti-IgG1 Abs (Immunotech) were used as negative controls.
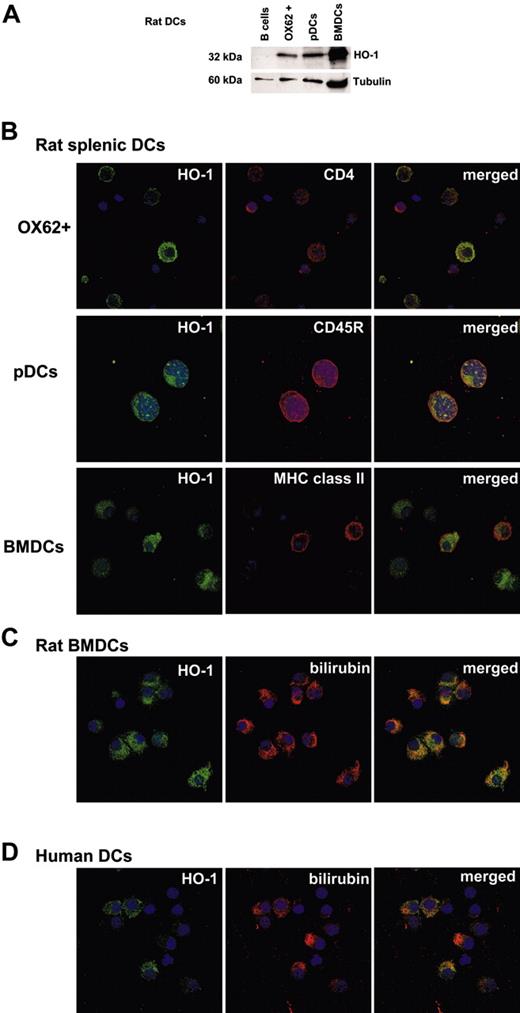
Rat and human DCs express functional HO-1. (A) Western blot analysis of HO-1 expression in rat freshly isolated splenic OX62+ DCs, freshly isolated plasmacytoid dendritic cells (pDCs), and BMDCs cultured for 8 days was performed using an anti-HO-1 antibody. Purified splenic B cells were used as a negative control. An anti-β tubulin antibody was used as a loading control. (B) Confocal micrographs show rat HO-1+ cells (green fluorescence); CD4+, CD45RB+, or MHC class II+ cells (red fluorescence); and merged images with dual labeling. Cell nuclei were counterstained with TO-PRO-3 (blue; objective, × 63/1.4). (C) Rat immature bone marrow-derived DCs express functional HO-1 as shown by the presence of HO-1+ cells (green fluorescence) also reactive with an antibilirubin mAb (red fluorescence). Merged images display dual labeling. Cell nuclei were counterstained with TO-PRO-3 (blue; objective, × 63/1.4). (D) Human immature monocyte-derived DCs express functional HO-1 as shown by the presence of HO-1+ cells (green fluorescence) also reactive with an antibilirubin mAb (red fluorescence). Merged images display dual labeling. Cell nuclei were counterstained with TO-PRO-3 (blue; objective, × 63/1.4). Similar results were obtained for each DC cell type in 2 to 5 independent experiments.
Rat and human DCs express functional HO-1. (A) Western blot analysis of HO-1 expression in rat freshly isolated splenic OX62+ DCs, freshly isolated plasmacytoid dendritic cells (pDCs), and BMDCs cultured for 8 days was performed using an anti-HO-1 antibody. Purified splenic B cells were used as a negative control. An anti-β tubulin antibody was used as a loading control. (B) Confocal micrographs show rat HO-1+ cells (green fluorescence); CD4+, CD45RB+, or MHC class II+ cells (red fluorescence); and merged images with dual labeling. Cell nuclei were counterstained with TO-PRO-3 (blue; objective, × 63/1.4). (C) Rat immature bone marrow-derived DCs express functional HO-1 as shown by the presence of HO-1+ cells (green fluorescence) also reactive with an antibilirubin mAb (red fluorescence). Merged images display dual labeling. Cell nuclei were counterstained with TO-PRO-3 (blue; objective, × 63/1.4). (D) Human immature monocyte-derived DCs express functional HO-1 as shown by the presence of HO-1+ cells (green fluorescence) also reactive with an antibilirubin mAb (red fluorescence). Merged images display dual labeling. Cell nuclei were counterstained with TO-PRO-3 (blue; objective, × 63/1.4). Similar results were obtained for each DC cell type in 2 to 5 independent experiments.
Cytokine measurement
Rat and human DCs were cultured for 2 days with various stimuli. Supernatants were serially diluted, and cytokine concentration assessed in duplicate by enzyme-linked immunosorbent assay (ELISA). Rat ELISA kits for IL-6, IL-10, TNF-α (OptEIA set; BD PharMingen) and IL-12 p40 (Biosource, Camarillo, CA) as well as human IL-12p70 and IL-10 ELISA kits (BD PharMingen) were used.
Measurement of reactive oxygen species
Rat iBMDCs or human iDCs were treated with CoPP or SnPP, cultured for 48 hours, and incubated or not with LPS for 15 minutes. DCs were incubated with 10 mM N-acetyl-l-cysteine (NAC) for 45 minutes prior to incubation with LPS. DCs were then incubated with 5 μM of the oxidative sensitive dye CM-H2DCFDA (Molecular Probes) for 15 minutes. Samples were then washed and analyzed by cytofluorimetry.
Statistical analysis
Statistical significance was assessed using the nonparametric one-way ANOVA test with a posttest. Differences were considered significant when P < .05.
Results
Expression of HO-1 in rat and human iDCs
To investigate the capacity of DCs to express HO-1, we analyzed several previously described rat splenic DC subpopulations17,26 and BMDCs,18 as well as human monocyte-derived DCs. Western blot analysis showed that freshly isolated and purified rat splenic OX62+ DCs and pDCs (OX62-), and rat iBMDCs expressed HO-1 (Figure 1A). Splenic B cells were used as negative controls (Figure 1A). Purified rat OX62+ DCs include 2 distinct subsets.17 A subpopulation of CD4-CD11b+SIRP-1-CD5- DCs, which are effective at presenting antigens to CD4+ but not to CD8+ T cells, produce large amounts of IL-12 but not interferon α (IFN-α), and induce Th1 responses.17 The second subpopulation of OX62+ DCs are CD4+CD11b+ SIRP-1+CD5+ cells that are effective at presenting antigens to CD4+ and CD8+ T cells, produce low amounts of IL-12 and IFN-α, and induce Th2/Th0 responses.17 Intracellular staining with an anti-HO-1 Ab revealed that the CD4- DC population did not express HO-1 and that HO-1 expression was restricted to the CD4+ DC population (32% ± 12% expressed HO-1, n = 3; Figure 1B). HO-1-expressing cells also expressed MHC class II antigens and were only found among SIRP-1+ and CD5+ cells (Figure S1; see the Supplemental Materials link at the top of the online article, at the Blood website). HO-1 expression was detected in 74% ± 12% (n = 2) of freshly isolated CD45RB+ pDCs (CD4+CD11b-26; Figure 1B). HO-1 expression was also detected in 80% ± 20% (n = 5) of iBMDCs (Figure 1B). All rat iBMDCs that expressed HO-1 also expressed bilirubin, which can only derive from heme degradation (Figure 1C). Bilirubin was undetectable in other cells lacking HO-1, such as 293 cells (data not shown).
HO-1 expression was also detected in most immature human monocyte-derived DCs as well as intracellular staining of bilirubin, demonstrating HO activity (Figure 1D).
In conclusion, these data establish that certain but not all rat iDC subsets and human monocyte-derived iDCs express functional HO-1.
HO-1 expression decreases during rat and human DC maturation
HO-1 expression was analyzed after maturation of rat and human iDCs induced by poly(I:C), LPS, or CpG, as well as by stimulation with inflammatory and lymphocyte stimuli, such as TNF-α or CD40L, respectively. DC maturation was confirmed in each experiment by an enhanced expression of MHC II, CD80, and CD86, and by secretion of the cytokines IL-12, IL-6, TNF-α, and IL-10. HO-1 expression by rat BMDCs was significantly decreased compared to control untreated DCs after maturation induced by all of the stimuli tested (mean decrease % ± SD): TNF-α, 83% ± 1%; poly(I:C), 82% ± 7.4%; CpG, 69% ± 2%; CD40L, 81% ± 2.5%; and LPS, 73 ± 15% (n = 3 for each, P < .05; Figure 2A). Inhibition of HO-1 expression on DC maturation was also observed in rat splenic OX62+ DCs and pDCs (data not shown).
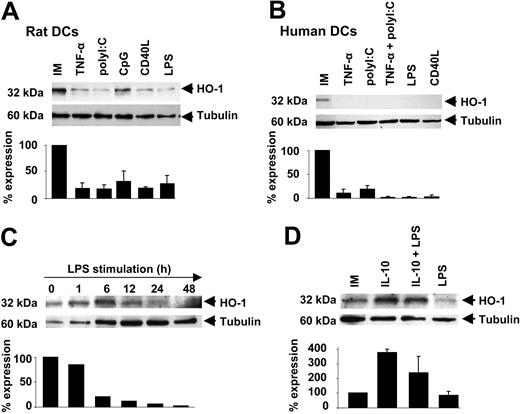
HO-1 expression decreases during rat and human DC maturation. (A) Western blot analysis of HO-1 expression in rat immature (IM) BMDCs and following maturation with TNF-α, poly(I:C), CpG, CD40L, and LPS was carried out using an anti-HO-1 antibody. Anti-β tubulin was used as a loading control. Bar graph shows densitometry analysis ± SD of HO-1 signals after normalization with β-tubulin from 3 different experiments. (B) Western blot analysis of HO-1 expression in human immature (IM) monocyte-derived DCs and human DCs following maturation induction with TNF-α, poly(I:C), CD40L, and LPS, performed using an anti-HO-1 antibody. Anti-β tubulin was used as a loading control. Bar graph shows densitometry analysis ± SD of HO-1 signals, after normalization with β-tubulin, from 2 different experiments. (C) Time-course Western blot analysis of HO-1 expression in human immature (IM) monocyte-derived DCs and human DCs following maturation induction with LPS, performed with an anti-HO-1 antibody. Anti-β tubulin was used as a loading control. Bar graph shows densitometry analysis of HO-1 signals after normalization with β-tubulin. (D) Human immature (IM) monocyte-derived DCs were cultured with or without 20 ng/mL of IL-10 for 18 hours and then with or without 1 μg/mL of LPS for 24 hours. Western blot was performed with an anti-HO-1. Anti-β tubulin was used as a loading control. Histograms show densitometry analysis ± SD of HO-1 signals after normalization with β tubulin, from 2 different experiments.
HO-1 expression decreases during rat and human DC maturation. (A) Western blot analysis of HO-1 expression in rat immature (IM) BMDCs and following maturation with TNF-α, poly(I:C), CpG, CD40L, and LPS was carried out using an anti-HO-1 antibody. Anti-β tubulin was used as a loading control. Bar graph shows densitometry analysis ± SD of HO-1 signals after normalization with β-tubulin from 3 different experiments. (B) Western blot analysis of HO-1 expression in human immature (IM) monocyte-derived DCs and human DCs following maturation induction with TNF-α, poly(I:C), CD40L, and LPS, performed using an anti-HO-1 antibody. Anti-β tubulin was used as a loading control. Bar graph shows densitometry analysis ± SD of HO-1 signals, after normalization with β-tubulin, from 2 different experiments. (C) Time-course Western blot analysis of HO-1 expression in human immature (IM) monocyte-derived DCs and human DCs following maturation induction with LPS, performed with an anti-HO-1 antibody. Anti-β tubulin was used as a loading control. Bar graph shows densitometry analysis of HO-1 signals after normalization with β-tubulin. (D) Human immature (IM) monocyte-derived DCs were cultured with or without 20 ng/mL of IL-10 for 18 hours and then with or without 1 μg/mL of LPS for 24 hours. Western blot was performed with an anti-HO-1. Anti-β tubulin was used as a loading control. Histograms show densitometry analysis ± SD of HO-1 signals after normalization with β tubulin, from 2 different experiments.
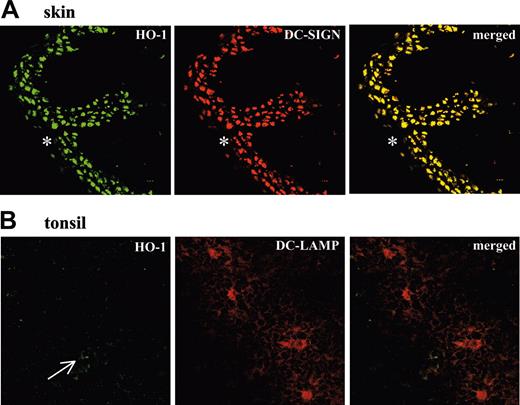
Detection of HO-1+ and HO-1- DCs in human tissues. (A) Confocal micrographs of cryostat sections from human skin show HO-1+ cells (green fluorescence), DC-SIGN+ cells (red fluorescence), and merged images with dual labeling. Dermal iDCs show complete overlap of HO-1 and DC-SIGN expression. The epidermis (*) shows very low or absent expression of both HO-1 and DC-SIGN. (objective, × 40/1.0). (B) Confocal micrographs of cryostat sections from a chronically infected human tonsil show few weakly HO-1+ cells (green fluorescence, arrow), DC-LAMP+ cells (red fluorescence), and merged images with dual labeling. Tonsil mature DCs expressing DC-LAMP do not express HO-1 (objective, × 63/1.4). Results are representative of 3 different skin and tonsils analyzed in the same way.
Detection of HO-1+ and HO-1- DCs in human tissues. (A) Confocal micrographs of cryostat sections from human skin show HO-1+ cells (green fluorescence), DC-SIGN+ cells (red fluorescence), and merged images with dual labeling. Dermal iDCs show complete overlap of HO-1 and DC-SIGN expression. The epidermis (*) shows very low or absent expression of both HO-1 and DC-SIGN. (objective, × 40/1.0). (B) Confocal micrographs of cryostat sections from a chronically infected human tonsil show few weakly HO-1+ cells (green fluorescence, arrow), DC-LAMP+ cells (red fluorescence), and merged images with dual labeling. Tonsil mature DCs expressing DC-LAMP do not express HO-1 (objective, × 63/1.4). Results are representative of 3 different skin and tonsils analyzed in the same way.
As observed in rat BMDCs, HO-1 expression in human monocyte-derived DCs was strongly decreased (mean decrease % ± SD) during DC maturation in response to TNF-α (90% ± 8.5%), poly(I:C) (81% ± 7%), TNF-α plus poly(I:C) (98.5% ± 4%), LPS (98.5% ± 2%), and CD40L (97 ± 3%; n = 2 for each; Figure 2B).
Kinetic analysis of HO-1 levels after LPS stimulation of human DCs showed levels comparable to those of untreated DCs at 1 hour and drastically reduced levels at 6, 12, 24, and 48 hours of stimulation (20%, 11%, 6%, and 2% versus untreated cells; Figure 2C).
Moreover, expression of HO-1 in rat and human iDCs and its strong decrease following maturation (> 85%, P < .05, n = 3) was confirmed by measuring HO-1 mRNA by quantitative real-time RT-PCR (Figure S2).
These data demonstrate that HO-1 expression in rat and human DCs is inhibited at the transcriptional or mRNA (or both) levels by Toll-like receptor (TLR), inflammatory, and lymphocyte stimuli.
HO-1 expression is increased by IL-10
Because IL-10 is potent at maintaining DCs in an immature state,16,27 we analyzed whether HO-1 expression could be increased by IL-10. As compared to LPS-treated DCs or untreated iDCs (100%; n = 2), pretreatment with IL-10 increased HO-1 expression levels (mean % ± SD) in iDCs (378% ± 23%) and in IL-10 plus LPS-treated DCs (240% ± 113%; Figure 2D). The inhibitory effect of IL-10 on LPS-induced DC phenotypic maturation was confirmed by FACS (Figure S3).
These data demonstrate that inhibition of DC maturation by IL-10 is associated with an increase in HO-1 expression.
HO-1 is expressed by immature DC-SIGN+ but not by mature DC-LAMP+ DCs in human tissues
We next investigated whether this dichotomy in HO-1 expression between iDCs and mature DCs also exists in vivo using chronically infected tonsils and healthy skin. Two specific DC markers were used to identify DCs in human tissue sections: DC-SIGN, a C-type lectin expressed in interstitial iDCs but not Langerhan cells,28,29 and DC-LAMP, a marker of mature DCs.30 As previously described,28-30 human skin displayed many DC-SIGN+ cells in the dermis but not in the epidermis (Figure 3) and DC-LAMP+ cells were absent (data not shown). Many HO-1+ cells were observed in the dermis and an almost complete overlap was observed between HO-1+ and DC-SIGN+ cells (Figure 3).
In contrast, and as previously described for human chronically infected tonsils,28-30 DC-LAMP was expressed by numerous cells (Figure 3), whereas DC-SIGN was nearly absent (data not shown). Only a few weakly HO-1+ cells were found in this tissue (Figure 3). Double staining showed that DC-LAMP+ cells were HO-1- (Figure 3).
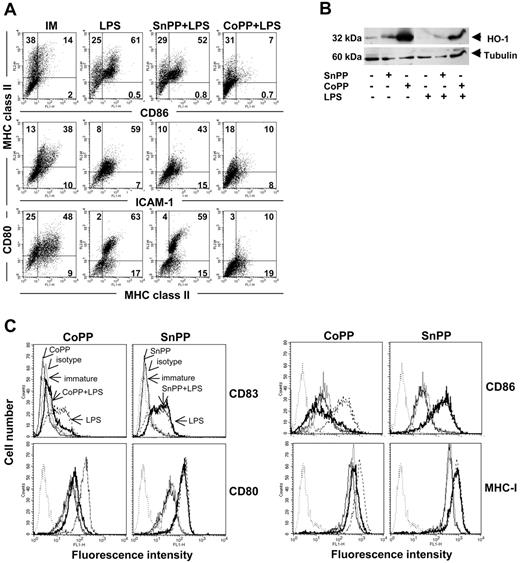
Induction of HO-1 renders DCs refractory to LPS-induced phenotypic maturation. (A) Western blot analysis of HO-1 levels in human iDCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours in the presence or absence of LPS. Anti-β tubulin was used as a loading control. (B) Flow cytometry analysis showing the phenotype of immature (IM) rat BMDCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours with or without LPS for 24 hours. Expression of MHC class II, CD80, CD86, and intercellular adhesion molecule 1 (ICAM-1) was assessed. Similar results were obtained in 5 independent experiments. Numbers within graph quadrants are percentage of positive cells. (C) Flow cytometry analysis showing the phenotype of immature human DCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then stimulated or not with LPS for 24 hours. Thin gray line indicates untreated immature DCs; dashed line, untreated DCs stimulated with LPS; thin black line, treatment with CoPP or SnPP; bold line, treatment with CoPP or SnPP and stimulated with LPS; and dotted line, isotype. Expression of CD83, CD80, CD86, and MHC class I was assessed. Similar results were obtained in 3 independent experiments.
Induction of HO-1 renders DCs refractory to LPS-induced phenotypic maturation. (A) Western blot analysis of HO-1 levels in human iDCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours in the presence or absence of LPS. Anti-β tubulin was used as a loading control. (B) Flow cytometry analysis showing the phenotype of immature (IM) rat BMDCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours with or without LPS for 24 hours. Expression of MHC class II, CD80, CD86, and intercellular adhesion molecule 1 (ICAM-1) was assessed. Similar results were obtained in 5 independent experiments. Numbers within graph quadrants are percentage of positive cells. (C) Flow cytometry analysis showing the phenotype of immature human DCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then stimulated or not with LPS for 24 hours. Thin gray line indicates untreated immature DCs; dashed line, untreated DCs stimulated with LPS; thin black line, treatment with CoPP or SnPP; bold line, treatment with CoPP or SnPP and stimulated with LPS; and dotted line, isotype. Expression of CD83, CD80, CD86, and MHC class I was assessed. Similar results were obtained in 3 independent experiments.
An irrelevant isotype-matched mAb did not show any reactivity with either tissue (Figure S4).
Thus, analysis of human tissues shows that iDCs express HO-1 in vivo, whereas mature DCs do not.
HO-1 induction renders rat and human DCs refractory to LPS-induced maturation
Next, we investigated whether HO-1 activity during maturation could play a functional role in the regulation of DC responses to pathogens.
To examine the effect of HO-1 activity on LPS-induced DC maturation, we pulsed-treated human iDCs and rat iBMDCs with CoPP or SnPP. CoPP is a strong inducer of HO-1 gene transcription,20-22 whereas SnPP irreversibly binds and inactivates HO-1 enzymatic activity.22
CoPP and SnPP 48-hour pulse treatments were not toxic to DCs, as determined by flow cytometric analysis of physical parameters and TO-PRO-3 staining (Figure S5). Porphyrin-pulsed DCs were cultured for 16 hours in the absence or presence of LPS. Expression of HO-1 in human (Figure 4A) and rat (data not shown) DCs was determined by Western blot analysis 24 hours later. HO-1 expression was strongly increased following treatment with CoPP and treatment with LPS partially abrogated this increase. SnPP slightly increased HO-1 levels, likely through a feedback mechanism due to inhibition of HO-1 activity, as previously described in other cells.31
Analysis of mRNA levels by quantitative RT-PCR showed that CoPP alone induced a 70- to 124-fold increase in HO-1 transcript levels versus those in iDCs, and a 29- to 69-fold increase when associated with LPS in rat DCs. A strong increase in HO-1 mRNA was also detected in CoPP-treated human DCs. Transcript levels increased 18- to 99-fold with CoPP alone and 29- to 67-fold when associated with LPS.
CoPP-pulsed human and rat DCs were refractory to LPS-induced maturation, as assessed by phenotypic analysis. A statistically significant inhibition of cell surface marker expression was observed in CoPP-pretreated rat and human iDCs compared to untreated and SnPP-pretreated cells incubated with LPS (Figure 4B-C; Table S1).
SnPP inhibition of HO-1 activity had no effect on DC maturation, suggesting that inhibition of HO-1 activity does not act as an inducer of DC maturation in the absence of specific DC maturation stimuli. The absence of an effect of SnPP on LPS-induced DC maturation is probably due to the inhibition of HO-1 expression on LPS stimulation.
Thus, induction of HO-1 in DCs resulted in a blockade of DC phenotypic maturation induced by LPS.
HO-1 induction inhibits the secretion of proinflammatory cytokines and maintains IL-10 production by DCs exposed to an inflammatory signal
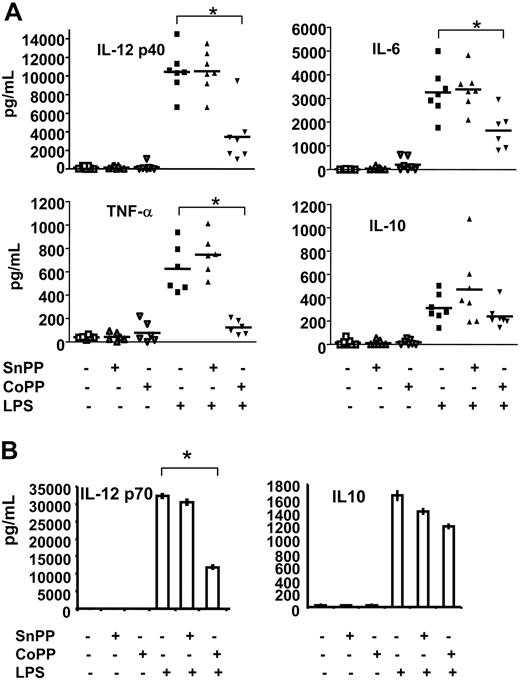
DC maturation results in secretion of diverse cytokines that mediate many of the functional effects of DCs on other cell populations. Production of the LPS-induced proinflammatory cytokines IL-12 p40, IL-6, and TNF-α significantly decreased when rat DCs were pulsed with the HO-1 inducer CoPP, whereas treatment with SnPP had no effect (Figure 5A). In contrast, HO-1 induction did not inhibit secretion of the LPS-induced anti-inflammatory cytokine IL-10 (Figure 5A).
Induction of HO-1 affects cytokine expression in rat and human DCs. (A) ELISA was used to assess the production of IL-12 p40, IL-6, TNF-α, and IL-10 in the supernatants of rat BMDCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours in the presence or absence of LPS. Induction of HO-1 inhibited expression of IL-12 p40, TNF-α, and IL-6 in DCs but did not alter IL-10 secretion. Horizontal bars represent the mean of individual values. Statistical significance compared to LPS-treated cells is indicated as *P < .001. (B) ELISA was used to assess the production of IL-12 p70 and IL-10 in the supernatants of human DCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours in the presence or absence of LPS. Induction of HO-1 inhibited expression of IL-12 p70 but did not alter IL-10 secretion. Data presented are mean ± SD of triplicate analysis of one experiment and are representative of 3 independent experiments. Statistical significance compared to LPS-treated cells is indicated as *P < .01.
Induction of HO-1 affects cytokine expression in rat and human DCs. (A) ELISA was used to assess the production of IL-12 p40, IL-6, TNF-α, and IL-10 in the supernatants of rat BMDCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours in the presence or absence of LPS. Induction of HO-1 inhibited expression of IL-12 p40, TNF-α, and IL-6 in DCs but did not alter IL-10 secretion. Horizontal bars represent the mean of individual values. Statistical significance compared to LPS-treated cells is indicated as *P < .001. (B) ELISA was used to assess the production of IL-12 p70 and IL-10 in the supernatants of human DCs treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours in the presence or absence of LPS. Induction of HO-1 inhibited expression of IL-12 p70 but did not alter IL-10 secretion. Data presented are mean ± SD of triplicate analysis of one experiment and are representative of 3 independent experiments. Statistical significance compared to LPS-treated cells is indicated as *P < .01.
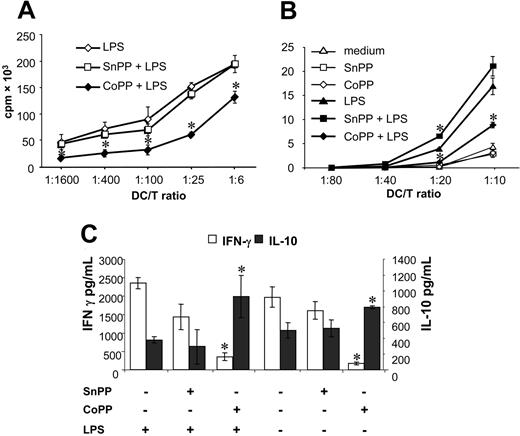
Induction of HO-1 inhibits the allogeneic stimulatory capacity of rat and human DCs. (A) Rat BMDCs and (B) human DCs were treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours in presence or absence of LPS. Thereafter, DCs were cultured with allogeneic T cells at different ratios for 4 days and proliferating T cells were labeled with 3H-thymidine for 8 hours before being harvested and counted. Results are shown as means ± SD of triplicate values after subtraction of spontaneous 3H-thymidine. Statistical significance compared with LPS-treated DCs is indicated as *P < .05. Similar results were obtained in 6 independent experiments for rat DCs and in 2 for human DCs. cpm indicates counts per minute. (C) In parallel, supernatants were collected after 48 hours from rat MLRs and analyzed by ELISA to determine levels of IFN-γ and IL-10. Data presented are mean ± SD of triplicate analysis of one experiment and are representative of 5 independent experiments. Statistical significance compared with LPS-treated DCs is indicated as *P < .01.
Induction of HO-1 inhibits the allogeneic stimulatory capacity of rat and human DCs. (A) Rat BMDCs and (B) human DCs were treated or not with SnPP or CoPP for 2 hours, cultured for 16 hours, and then for a further 24 hours in presence or absence of LPS. Thereafter, DCs were cultured with allogeneic T cells at different ratios for 4 days and proliferating T cells were labeled with 3H-thymidine for 8 hours before being harvested and counted. Results are shown as means ± SD of triplicate values after subtraction of spontaneous 3H-thymidine. Statistical significance compared with LPS-treated DCs is indicated as *P < .05. Similar results were obtained in 6 independent experiments for rat DCs and in 2 for human DCs. cpm indicates counts per minute. (C) In parallel, supernatants were collected after 48 hours from rat MLRs and analyzed by ELISA to determine levels of IFN-γ and IL-10. Data presented are mean ± SD of triplicate analysis of one experiment and are representative of 5 independent experiments. Statistical significance compared with LPS-treated DCs is indicated as *P < .01.
Similarly to rat DCs, a strong decrease in LPS-induced IL-12 p70 production associated with a preservation of IL-10 was also observed following CoPP treatment of human DCs (Figure 5B).
Treatment of immature rat and human DCs with SnPP or CoPP alone (no LPS) did not induce iDCs to secrete cytokines (Figure 5A-B).
These results show that HO-1 induction modulates LPS-induced DC maturation by decreasing proinflammatory cytokines production and preserving IL-10 secretion.
HO-1 induction decreases the capacity of rat and human DCs to stimulate MLRs
To further assess the effect of HO-1 on DC function, we determined whether HO-1 induction modified the T-cell allostimulatory capacity of rat and human DCs. Rat BMDCs that had been treated with LPS after induction of HO-1 by CoPP were poor stimulators in mixed lymphocyte reactions (MLRs) compared with control LPS-treated DCs, whereas HO-1 inhibition by SnPP treatment had no effect on rat allogeneic T-cell proliferation (Figure 6A).
Human DCs pretreated with CoPP and then with LPS were also poor stimulators in MLRs compared to LPS-treated DCs, and SnPP-pretreated DCs only increased proliferation at one T/DC (Figure 6B). MLR proliferation of rat (data not shown) or human (Figure 6B) DCs not matured with LPS was unmodified by pretreatment with SnPP or CoPP.
We also investigated cytokine production in supernatants from rat MLRs (Figure 6C). When DCs were treated with CoPP, IFN-γ production was significantly reduced and IL-10 significantly increased compared with control LPS-treated DCs. When DCs were treated with SnPP, IFN-γ and IL-10 production were unmodified compared to control DCs (Figure 6C). In MLRs using DCs not exposed to LPS but pretreated with CoPP, a reduction in IFN-γ production was observed, whereas IL-10 was increased and SnPP, on the other hand, had no effect (Figure 6C).
These results demonstrate that HO-1 induction not only inhibits the allostimulatory capacity of DCs, but also changes the cytokines produced during DC/T-cell interactions.
HO-1 induction decreases ROS levels in DCs
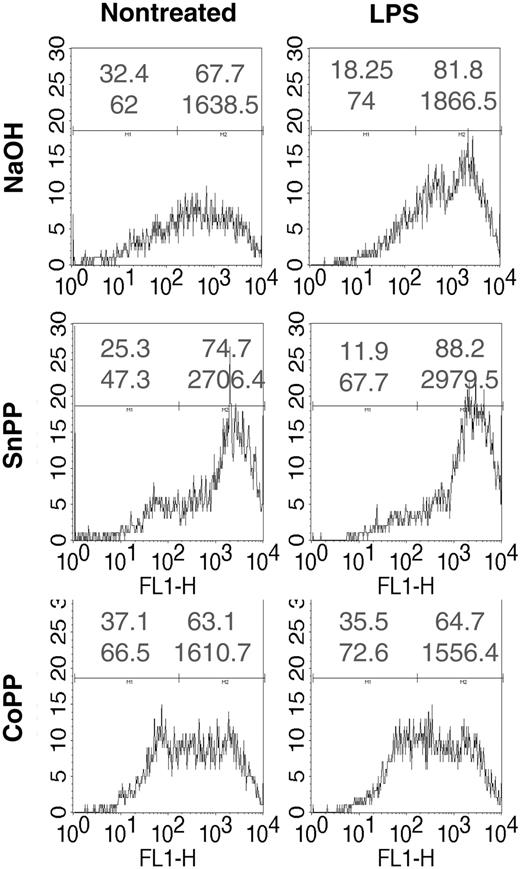
Increased levels of reactive oxygen species (ROS) have been shown to be involved in activation of DCs by different stimuli, and antioxidants inhibit DC activation.32 To assess potential intracellular mechanisms for HO-1 inhibition of DC maturation, we analyzed ROS levels in DCs pretreated with CoPP or SnPP, and matured with LPS, a stimulus known to increase ROS in DCs32 (Figure 7). ROS levels were increased following treatment with LPS. SnPP pretreatment increased ROS levels further in LPS-stimulated DCs but also in nonstimulated DCs. Conversely, treatment with CoPP decreased ROS levels in LPS but also in untreated DCs. Treatment with NAC reduced basal and LPS-induced ROS levels (Figure S6). Identical results were observed with human DCs (data not shown).
Induction of HO-1 inhibits the generation of ROS by LPS. Rat iBMDCs were treated with CoPP or SnPP, cultured, and incubated or not with LPS for 15 minutes. DCs were then incubated for 15 minutes with the oxidative sensitive dye CM-H2DCFDA and analyzed by cytofluorimetry. FL1-H is the log scale of fluorescence intensity. In each histogram upper numbers represent the percentage of negative (left) or positive (right) cells and the lower numbers the corresponding mean fluorescence intensity of cells. Data are representative of 3 independent experiments.
Induction of HO-1 inhibits the generation of ROS by LPS. Rat iBMDCs were treated with CoPP or SnPP, cultured, and incubated or not with LPS for 15 minutes. DCs were then incubated for 15 minutes with the oxidative sensitive dye CM-H2DCFDA and analyzed by cytofluorimetry. FL1-H is the log scale of fluorescence intensity. In each histogram upper numbers represent the percentage of negative (left) or positive (right) cells and the lower numbers the corresponding mean fluorescence intensity of cells. Data are representative of 3 independent experiments.
Thus, inhibition or induction of HO-1, respectively, increased or inhibited ROS levels in DCs.
Discussion
The present study demonstrates that rat and human DCs represent a previously unknown source of functionally active HO-1. Importantly, HO-1 expression is a specific feature of iDCs because fully mature DCs almost lack the capacity to express HO-1.
DCs constitute a heterogeneous cell population. Not only do these cells have distinct cell surface phenotype, localization, and differentiation status, but they also have different functions in the immune system, including the induction of effector immune responses and the establishment of central and peripheral self tolerance (for a review, see Shortman and Liu33 ). HO-1 is expressed in freshly isolated rat CD4+CD11b+ SIRP-1+CD5+ DCs, pDCs and BMDCs as well as in human monocyte-derived iDCs, but not in rat CD4-CD11b+SIRP-1-CD5- DCs.
Endothelial cells,34 artery smooth muscle cells,35 and glial cells36 show low or no expression of HO-1 in their resting state and increased HO-1 expression on LPS stimulation. The data concerning the effect of LPS on HO-1 expression by macrophages are contradictory. Whereas LPS has been shown not to increase HO-1 expression in the J774 mouse cell line,13 mouse primary macrophages,13 or human primary macrophages,14 it has been also shown to increase HO-1 expression in the J774 and RAW264.7 mouse cell lines14,37 as well as in mouse primary macrophages.14 Analysis of DCs stimulated with LPS using DNA microarrays also failed to detect an increase in HO-1 mRNA levels.27 Our results show that iDCs expressed HO-1 and that this expression was decreased by DC inflammatory stimuli. This suggests that HO-1 may exert anti-inflammatory and tolerogenic effects in iDCs and that inflammatory stimuli such as LPS should switch off its expression to fully initiate effector immune responses. In contrast, in parenchymal cells, the antiapoptotic and antioxidative effects of HO-1 may be needed to protect against inflammation once initiated and its expression is therefore increased by inflammatory stimuli. The decrease in HO-1 expression on DC maturation was observed at the protein and mRNA levels, not only for TLR-mediated activation signals (poly(I:C) and LPS) but also in response to a T cell-dependent signal (CD40L) or an inflammatory stimulus (TNF-α). CpG inhibited HO-1 expression less profoundly and this could be explained by a lower expression of TLR-9 in DCs other than pDCs.26,38 We showed that DC-SIGN+ iDCs but not DC-LAMP+ mature DCs expressed HO-1. Therefore, in vivo maturation of DCs is also accompanied by the inhibition of HO-1 expression.
Several immunomodulatory agents, including glucocorticoids,39 the antioxidant NAC,32 IL-10,40 transforming growth factor β1 (TGF-β1),41 and vitamin D3,42 have been shown to exert inhibitory effects on DC maturation. Here we demonstrate that treatment of DCs with IL-10 induces HO-1. Whether the effects of IL-10 on DCs are mediated by HO-1 is beyond the scope of this report. As is the case for all the compounds mentioned, induction of HO-1 in DCs interfered with the LPS-mediated maturation process in vitro, resulting in the down-regulation of the phenotypic maturation markers, as well as in modulation of cytokine production.
The present study demonstrated that HO-1 also modulates the secretion of cytokines critical for DC maturation and for efficient effector T-cell responses. Thus, HO-1 induction in DCs abrogated IL-12 secretion, whereas that of IL-10 was preserved. IL-10 is a potent inducer of tolerance and is the most efficient down-regulator of proinflammatory cytokine secretion in DCs.40 A decrease in IL-12 production and concomitant secretion of IL-10 have been shown to be involved in the induction of anergy and tolerance in T cells.40,43,44 Increased expression of HO-1 by mouse macrophages or treatment of these cells with CO has been shown to inhibit the production of proinflammatory cytokines induced by LPS stimulation, while preserving IL-10 secretion,11,12 although IL-10 did not mediate the anti-inflammatory effects of HO-1.11 Importantly, IL-10 has been shown to induce HO-1 expression in macrophages,13-15 although some controversy exists over whether13 or not14 HO-1 mediates the anti-inflammatory effect of IL-10 on these cells. We demonstrate that HO-1 promotes IL-10 expression in DCs, but whether the effect of HO-1 is mediated by IL-10 remains to be determined.
DCs exhibit the unique property of priming T cells through up-regulation of costimulatory molecules and secretion of IL-12.45-47 Disruption of the costimulatory pathways has been shown to be effective in inhibiting autoimmune diseases and allograft rejection.46,47 Here, we show that HO-1 overexpression induced the down-regulation of costimulatory molecules on DCs, which could have been responsible for the observed inhibition of alloreactive T-cell proliferation. The fact that HO-1 blocks phenotypic and functional maturation was further substantiated by its ability to decrease T-cell proliferation and production of IFN-γ in MLRs, whereas expression of IL-10 was increased. IL-10 has been shown to block MLRs and is considered as the main cytokine for the induction and maintenance of anergy or tolerance.40,48,49 Moreover, IL-10 expression in MLRs is linked to the generation of Tr1 cells.50 Further work is in progress to determine whether IL-10 acts downstream of HO-1, mediating suppression of DC maturation and possibly induction of tolerance. Alternatively, the inhibition of MLRs could also be related to the heme degradation end product CO, because CO was shown to inhibit T-lymphocyte proliferation.51 These results indicate that HO-1 is not only a marker of iDCs but could also affect DC functions by inhibiting Th1 responses and by inducing T-cell anergy. It is tempting to hypothesize that the beneficial effect of HO-1 overexpression in autoimmune disease,52 allotransplantation,6,53 and graft-versus-host disease54 (GVHD) may be associated with the ability of HO-1 to block DC maturation or inhibit Th1 responses and induce T-cell anergy.
In agreement with the known antioxidant properties of HO-1 (for a review, see Otterbein and Choi1 ), induction of HO-1 resulted in decreased ROS levels following LPS stimulation of DCs. Because ROS levels are inversely correlated to the degree of DC maturation55 and because antioxidants block DC maturation,32 the decrease in ROS levels by HO-1 could be involved in the inhibition of DC maturation. There are multiple downstream targets of ROS that will need to be analyzed to explain the inhibitory action of HO-1 on DC maturation. Although SnPP further increased ROS levels in LPS-treated cells, it is possible that the DC maturation process is fully activated by the levels reached with LPS alone.
In conclusion, we demonstrate, for the first time, that human and rat iDCs express HO-1 and that HO-1 expression is strongly down-regulated during DC maturation, thus suggesting a potential role for HO-1 in DC biology. We present evidence that induction of HO-1 expression not only suppressed the expression of cell surface molecules and proinflammatory cytokines but, importantly, maintained the expression of IL-10. This implies that HO-1 could selectively induce tolerogenic DCs.
The physiologic relevance of the present in vitro findings is strongly supported by the fact that mice lacking functional HO-156 and one human who had HO-1 deficiency57,58 have been associated with an inflammatory phenotype, and by results showing that overexpression of HO-1 inhibits allogeneic immune responses.5-7
Prepublished online as Blood First Edition Paper, May 26, 2005; DOI 10.1182/blood-2005-02-0494.
Supported by the Fondation Progreffe, EU grant QLK3-CT-2001-00422 (I.A.) and Agence pour la Recherche contre le Cancer (ARC, nationale; M.G.). S.R. and P.J.R. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to all of the researchers who kindly contributed with reagents or tissues.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal