Abstract
Megakaryocyte (MK)–specific transgene expression has proved valuable in studying thrombotic and hemostatic processes. Constitutive expression of genes, however, could result in altered phenotypes due to compensatory mechanisms or lethality. To circumvent these limitations, we used the tetracycline/doxycycline (Tet)–off system to conditionally over-express genes in megakaryocytes and platelets in vivo. We generated 3 transactivator transgenic lines expressing the Tet transactivator element (tTA), under the control of the MK-specific platelet factor 4 promoter (PF4-tTA-VP16). Responder lines were simultaneously generated, each with a bidirectional minimal cytomegalovirus (CMV)–tTA responsive promoter driving prokaryotic β-galactosidase gene, as a cellular reporter, and a gene of interest (in this case, the mitotic regulator Aurora-B). A transactivator founder line that strongly expressed PF4-driven tTA–viral protein 16 (VP16) was crossbred to a responder line. The homozygous double-transgenic mouse line exhibited doxycycline-dependent transgene overexpression in MKs and platelets. Using this line, platelets were conveniently indicated at sites of induced stress by β-galactosidase staining. In addition, we confirmed our earlier report on effects of constitutive expression of Aurora-B, indicating a tight regulation at protein level and a modest effect on MK ploidy. Hence, we generated a new line, PF4-tTA-VP16, that is available for conditionally overexpressing genes of interest in the MK/platelet lineage in vivo.
Introduction
Megakaryocytes (MKs) progress through distinct developmental stages, including lineage commitment, expansion, polyploidization, maturation, and fragmentation into platelets.1,2 To investigate mechanisms that control megakaryocytic development, gene targeting is required to manipulate levels of a potential regulatory protein of interest. More than a decade ago, the rat platelet factor 4 (PF4) gene promoter3 was used as a tool to specifically overexpress genes in early committed megakaryocytes in vivo. Since then, PF4 and other related promoters have been used to examine effects of deregulating the expression of cell-cycle genes, antiapoptotic proteins, receptors, thrombosis-modulating proteins, and others on megakaryocyte/platelet development and/or activity.4-14 PF4-driven constitutive expression is limited, depending on the transgene used, due to occasional lethality during development or adverse effects during adulthood. For instance, Kufrin et al demonstrated that PF4-driven expression of urokinase-type plasminogen activator in mice exhibited a bleeding diathesis, which may have limited the potential number of founder lines that were available for analysis.10 In addition, if a given transgene is not expressed due to regulatory elements within the site of genomic integration, increased numbers of founders need to be generated.13 Tetracycline-inducible promoters and transactivating mouse lines have been used throughout the literature to circumvent such limitations. A successful application of this system was reported for targeted expression in myeloid cells and hematopoietic progenitors in vivo,15-18 but no such study is available for cells in the megakaryocyte/platelet lineage.
As a model system for conditional and reversible overexpression of transgenes in megakaryocytes/platelets in vivo, we placed Aurora-B as well as prokaryotic β-galactosidase (β-gal; as a reporter gene) under a bidirectional minimal promoter (creating transresponder mouse lines). The minimal promoter can be activated by tetracycline/doxycycline (DOX)–mediated removal of an enhancing protein (Tet transactivator element [tTA]–VP16), as originally described by Gossen and Bujard.19 The latter is generated in a different line of mice under the control of the PF4 promoter (the transactivating line). In our study, one transactivating line, which was confirmed to express the enhancer protein, was selected for crossbreeding with several transresponder lines, yielding a transgenic line, which expressed the transgene dependent upon doxycycline. β-gal expression was easily detected in megakaryocytes and platelets of these double-transgenic lines, only upon withdrawal of doxycycline.
In summary, we demonstrated that the tetracycline/doxycycline system in conjunction with the PF4 promoter yields conditional overexpression of genes in vivo, which should be useful for targeted expression of lethality-inducing factors. The reversibility of gene expression might be an important trait when investigating effects of transformation- or thrombosis-promoting genes. The transactivator line produced here needs no further development, minimizing variation due to different integration sites in potentially new lines. A designated and well-examined transactivator line is now available for crossbreeding with any transresponder line of interest. It can also serve as a source of bone marrow (BM) cells for conditional overexpression of retroviral-mediated infection of cultured cells with the CMV-tTA–responsive promoter driving a gene of interest. Using this system, we were able to confirm an earlier report on constitutive overexpression of Aurora-B in megakaryocytes in vivo, indicating a tight regulation of this kinase at the protein level and a mild shift in megakaryocyte ploidy level.4 Moreover, using this mouse line, platelets were conveniently indicated at sites of induced stress by β-gal staining.
Materials and methods
Generation of transgenic constructs and mice
PF4-tTA-VP16 transgenic mice. A vector containing the rat 1.1-kb platelet factor 4 (PF4)4 promoter was linearized with SacI and NheI sites and cloned into pTet-off (catalog no. K1620-A; Clontech, Palo Alto, CA) to replace the existing cytomegalovirus (CMV) promoter at compatible multiple cloning sites. In the commercial construct, CMV drives the expression of the transactivator tTA fused to VP16. The existing simian virus 40 (SV40) polyadenylated RNA (poly A) in the pTet-off vector was also replaced by the 1.7 kb of the 3′ end of the human β-globin gene (including the 3′ intron and a poly A signal; base pair [bp] 71 022 to 72 711 from translation start of the clone described in GenBank accession number NT_000 007) to permit a stable mRNA in transgenic mice.4 The translated protein will not include β-globin because the transgene contains a stop codon. To prepare for injection, the construct was sequentially digested with BsmB1 and BsrB1 and the transgene was purified by electroelution.
CMV–Aurora-B/β-gal transgenic mice. The rat Aurora-B transgene, a 1.2-kb cDNA (GenBank accession no. D89731) construct, with N-terminal FLAG–tag sequence (DYKDDDDK) and 3′ linked β-globin gene (including the 3′ intron and a poly A signal) were isolated from a previously described transgenic construct.4 This transgene was then subcloned into pBI-G vector (7.7 kb, catalog no. 6150-1; Clontech) at sites Sal1 and Not1, yielding a 10.6-kb construct. This vector was linearized with Ase1 and Pst1 to exclude the vector sequence, and the resulting 8-kb fragment was purified by electroelution prior to injection. Furthermore, the FLAG tag at the 5′ end did not affect Aurora-B protein stability, as the half-lives of the endogenous or transfected tagged protein were similar (H.G.N., unpublished data, May 2003). Half-life determination was pursued in Y10/L8057 and HeLa cells as previously described.4
Transgenic production. The purified transgenes were injected into the pronucleus of mouse fertilized eggs (FVB strain; Jackson Laboratories, Bar Harbor, ME). Positive transgenic founders were identified by Southern blot analysis on mouse-tail genomic DNA, using a radiolabeled Aurora-B probe, or tTA-VP16 probe as described previously.8 In all analyses, age-matched (6-10 weeks old) and sex-matched F2 and higher generation mice were used. Transgenic mice were also identified by polymerase chain reaction (PCR). The primers for genotyping PF4-tTA transgenic mice were as follows: sense, 5′ gcttaatgaggtcggaatcg 3′ and antisense, 5′gcgacttgatgctcttgatc 3′, amplifying a 400-bp tTA-VP16 cDNA. For genotyping minimal CMV–Aurora-B/β-gal transgenic mice, primer sets included the following: sense, 5′ctcagaaagagaacgtctacc 3′ and antisense, 5′ cgatatgtctcactgtggcta 3′, amplifying 1.1-kb Aurora-B cDNA. To obtain double-transgenic mice, a highly expressing PF4-tTA-VP16 line (no. 42) was bred to homozygosity (confirmed by breeding to wild type) and then crossbred with 2 different homozygote CMV–Aurora-B/β-gal transgenic mice (also confirmed by breeding to wild type). The resulting offspring were designated as double-transgenic mice PF4-tTA-VP16/CMV–Aurora-B/β-gal after confirmation of the double transgene using the above method of detection involving repeated PCR of genomic tail DNA. Figure 1 displays a detailed scheme.
Doxycycline administration to transgenic mice
To inactivate the transactivator tTA, doxycycline (DOX; Sigma, St Louis, MO), which is more stable than tetracycline, was administered through drinking water placed in bottles wrapped with aluminum foil. The water was supplemented with 0.5 mg/mL DOX and was changed every 3 to 4 days to assure the activity of DOX. To induce transgene expression, DOX was removed from the drinking water 4 weeks prior to analysis. Analyses were also attempted at 2 weeks after DOX withdrawal, and transgene expression (by the transactivator line; Figure 1) was detected, albeit weaker than after 4 weeks. Typically, 10- to 14-week-old mice were analyzed.
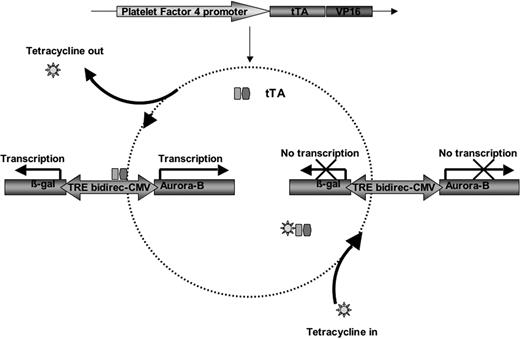
Engineering an in vivo system for conditional overexpression of genes in the megakaryocyte/platelet lineage. The scheme shown illustrates the Tet-on/off inducible gene expression system, involving the interaction between 2 transgenes of interest. The tissue-specific PF4 promoter drives the expression of the transactivator tTA (fused to the enhancing factor VP16). This line is crossbred to a tetracycline responsive element (TRE) bidirectional CMV line. The use of the TRE bidirectional CMV promoter allows the simultaneous regulation of both Aurora-B and β-gal by one central TRE. The TRE is a stretch of 7 copies of the 42-bp Tet operator sequences that bind to tTA-VP16 fusion protein in the absence of tetracycline/doxycycline. To achieve inducible transcription of 2 transgenes, the TRE is sandwiched between 2 minimal CMV promoters (directing the transcription of Aurora-B and β-gal in opposite directions, presumably in the absence of tetracycline).
Engineering an in vivo system for conditional overexpression of genes in the megakaryocyte/platelet lineage. The scheme shown illustrates the Tet-on/off inducible gene expression system, involving the interaction between 2 transgenes of interest. The tissue-specific PF4 promoter drives the expression of the transactivator tTA (fused to the enhancing factor VP16). This line is crossbred to a tetracycline responsive element (TRE) bidirectional CMV line. The use of the TRE bidirectional CMV promoter allows the simultaneous regulation of both Aurora-B and β-gal by one central TRE. The TRE is a stretch of 7 copies of the 42-bp Tet operator sequences that bind to tTA-VP16 fusion protein in the absence of tetracycline/doxycycline. To achieve inducible transcription of 2 transgenes, the TRE is sandwiched between 2 minimal CMV promoters (directing the transcription of Aurora-B and β-gal in opposite directions, presumably in the absence of tetracycline).
Transgenic mRNA expression
Total RNA was harvested from mouse bone marrow cells (using Trizol reagent, according to the manufacturer's instructions; Invitrogen, Carlsbad, CA). To detect transcripts of interest in rare megakaryocytes, reverse-transcriptase (RT)–PCR was pursued using Moloney murine leukemia virus (M-MLV) reverse transcriptase, 1 × First strain buffer, 0.5 mM deoxynucleoside triphosphate (dNTP), 5 mM dithiothreitol (DTT), 0.5 U/μL RNase inhibitor, 5 μM random primers (all purchased from Invitrogen), and primer sets designed to amplify both the FLAG tag and 1.1 kb of the Aurora-B gene (PCR primer sets: sense, 5′ gactacaaggacgatgacgacaag 3′ and antisense, 5′cgatatgtctcactgtggcta 3′). To differentiate between endogenous and exogenous expression of Aurora-B, one primer was specific to the FLAG sequence. The number of PCR cycles in our system was 20 for optimal transgene detection. Glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA expression was also apparent at 20 and 22 cycles and used for normalization.
Fluorescent immunohistochemical detection of transgenic proteins
Bone marrow (BM) cells were isolated from the femurs of transgenic and control mice as previously described.8 BM cells suspended in phosphate-buffered saline (PBS) were spun down (1 × 105 cells/slide) on Colofrost Plus microscope slides (Fisher Scientific, Sunwannee, GA) by a Cytospin3 (Shandon, Pittsburgh, PA) for 5 minutes at 120g. Cells were air dried, and fixed in 1% formaldehyde with 0.1% Triton X-100 buffered in PBS for 10 minutes. Slides were washed 4 times with PBS and then blocked with 2% goat serum and 1% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature. Mouse anti–Aurora-B antibody (1 μg/mL; BD Transduction Laboratories, Lexington, KY), or anti–β-galactosidase (Escherichia coli) (rabbit) (1:200 dilution, catalog no. 200-4136; Rockland Immunochemical, Gilbertsville, PA) or anti-VP16 antibody (1:1000 fold dilution; BD Transduction Laboratories) was reacted with the cells for 1 hour at room temperature and slides were then washed 4 times with PBS. As specified, a solution of Rhodamine-conjugated goat anti–mouse immunoglobulin G (IgG; Molecular Probes, Eugene, OR) at 100-fold dilution in PBS or Alexa Fluor 488–conjugated goat anti–rabbit IgG (Molecular Probes) at 200-fold dilution in PBS was subsequently added to the slides for 1 hour at room temperature. Slides stained with Rhodamine were subsequently incubated with fluorescein-isothiocyanate (FITC)–conjugated rat anti–mouse CD41 (integrin αIIb, 10 μg/mL) for 1 hour. All slides were stained with 1 μM Hoechst (Sigma) for 10 minutes and subsequently washed 4 times with PBS. Slides were mounted with ProLong Antifade Kit (Molecular Probes) and observed under an Olympus ×70 inverted fluorescence microscope (Melville, NY) using a ×20/1 numerical aperture objective. Images were documented with Hamamatsu CCD camera C4742-95 (Hamamatsu Photonics, headquartered in Hamamatsu City, Japan) and analyzed with OpenLab software (Improvision, Lexington, MA).
Immunoblot detection of transgenic proteins
Western blot analysis was performed as previously described.20 Briefly, freshly prepared bone marrow cells were lysed on ice with radioimmunoprecipitation assay (RIPA) buffer (1 × PBS, 1% nonidet P-40 [NP-40], 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 10 mg/mL phenylmethylsulphonyl fluoride [PMSF], and aprotinin [2 μg/mL]) freshly supplemented with 1 × protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN). Lysates were vigorously vortexed at 4°C for 15 minutes and cleared by centrifugation at 800 g for 8 minutes. Total cellular protein (50 μg) was loaded on a 10% SDS–polyacrylamide gel electrophoresis (PAGE). Gels were blotted and reacted with mouse monoclonal anti-VP16 antibody (1:1000-fold dilution; BD Biosciences, San Jose, CA) and anti–β-galactosidase (E coli, rabbit, 1:200-dilution, catalog no. 200-4136; Rockland Immunochemical, Gilbertsville, PA). The enhanced chemiluminescence (ECL) system was used for further reaction as previously described.20
In situ determination of β-galactosidase activity
The following assay was applied to both bone marrow cells and platelets. Briefly, bone marrow cells were isolated from femurs (as described above), and platelets were isolated from plasma by a low-speed centrifugation (5 minutes at 800g) of the whole blood via heart puncture and by centrifugation of the plasma at 3500 rpm (Sorval Biofuge Pico; Thermo Electron, Ashville, NC). The pellets were resuspended in buffered fixative for 20 minutes (0.5% glutaraldehyde, 0.02% NP-40, in 1 × PBS without MgCl2) and washed by centrifugation twice with 1 × PBS. Cells were incubated with 0.2 mL X-gal staining solution for 16 to 20 hours at 37°C. The X-gal staining solution was freshly prepared with the following composition: 400 μg/mL X-gal substrate (5-bromo-4-chloro-3-indolyl-β-D-galactoside), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM magnesium chloride, all dissolved in 1 × PBS. Blue precipitate (β-galactosidase activities) can be visualized via light microscopy after mounting cells onto slides.
Platelet counts
Mouse blood was collected from the tail and was diluted in IsoFlow Sheath Fluid (Beckman Coulter, Miami, FL) supplemented with 4 mM EDTA (ethylenediaminetetraacetic acid, 2 μL blood in 4 mL). Blood was serially diluted 10 times and platelet numbers were counted on a Beckman Coulter Z2 counter. Alternatively, 0.5 mL of blood obtained via heart puncture was collected in EDTA ready tubes (BD Biosciences) and subjected to automated counting on the LH755 Beckman Coulter machine at the Hematology Lab at Boston University School of Medicine (BUSM).
Ploidy analysis of megakaryocytes
Ploidy profile analyses were performed as previously reported.4 Briefly, freshly derived BM cells were isolated and washed in CATCH buffer (1 × Hanks Balance Salt Solution [Invitrogen], 5% fetal bovine serum [FBS], 0.38% sodium citrate, 1 mM adenosine, 2 mM theophylline). Cells were fixed with 70% ice-cold ethanol for 1 hour, washed twice, and stained with anti–CD41-FITC–conjugated antibody (specific megakaryocyte marker) at 1:200-fold dilution (BD Biosciences) or normal control mouse IgG-FITC at a similar concentration. Cells were then washed twice with 1 × PBS prior to a 10-minute staining with a propidium iodide solution (PI; Sigma) (0.05 mg/mL in 0.1% Triton X-100, 4 mM sodium citrate, pH 7.4, and 100 μg/mL DNase-free RNase A). An equal volume of salt solution (0.05 mg/mL PI, 0.1% Triton X-100, and 400 mM NaCl) was added. Cells were filtered through a 250-μm mesh filter and subjected to flow cytometric analysis on a FACScan system (Becton Dickinson, San Jose, CA). Data were collected and analyzed with CellQuest software (Becton Dickinson).
LPS-induced acute inflammation and tissue sections
Double-transgenic mice were anaesthetized with isoflurane (40 μg/g body weight) and intraperitoneally injected with lipopolysaccharide (LPS) (E coli serotype 0127;B8; Sigma), at a dosage of 60 μg/g of body weight, or saline in a total volume of up to 100 μL (control), essentially as described in Chen et al.21 Mice were killed 2 hours after LPS or saline administration and perfused for 5 minutes with 1 × PBS, followed by 2% paraformaldeldyde for 15 minutes and, finally, 1 × PBS for 10 minutes. The livers and lungs were surgically removed immediately after fixation and subjected to staining for β-galactosidase as described above. The stained organs were subsequently embedded in paraffin, thinly sectioned (5 micrometers), and stained with hematoxylin and eosin, all as in Jones et al,22 to reveal cell types and structures.
Results
Generation of PF4-tTA-VP16, CMV–Aurora-B/β-gal single- and double-transgenic lines
We sought to develop a system that would allow tissue-specific conditional overexpression of transgenes in megakaryocytes and platelets in vivo. Figure 1 illustrates the Tet-on/off inducible gene expression system, involving the interaction between a tissue-specific driven transgene and a minimal promoter. The tissue-specific PF4 promoter drives the expression of the transactivator tTA (fused to the enhancing factor VP16). This line was crossbred to a line carrying the tetracycline responsive element (TRE) bidirectional CMV (minimal promoter). The use of the TRE bidirectional CMV promoter allowed the simultaneous regulation of 2 genes by one central TRE. The tTA-VP16 fusion protein binds to the TRE in the absence of tetracycline/doxycycline. As a model system, we chose a combination of 2 transgenes, including the reporter gene encoding prokaryotic β-galactosidase (β-gal) and the mitotic regulator, Aurora-B. The former was chosen for convenience of detection3 and the latter was selected to compare results with a previously generated mouse line with constitutive expression of Aurora-B in megakaryocytes.4
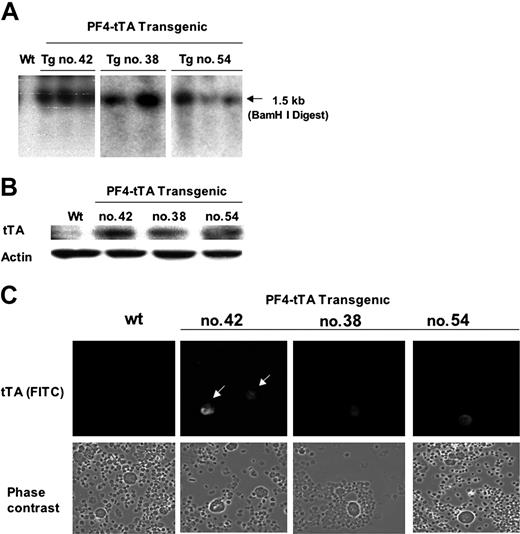
The first goal was to generate transgenic lines in which the transactivator is specifically expressed in megakaryocytes. Three such mouse lines were generated using the PF4 promoter (PF4-tTA-VP16 nos. 38, 42, 54), and this was confirmed by DNA integration into the genome (Figure 2A). Transgene expression was determined by Western blot analysis of bone marrow cells (Figure 2B) as well as by immunohistochemistry (Figure 2C). In all lines, tTA was specifically expressed in megakaryocytes (Figure 2C). PF4-tTA-VP16 line no. 42 was chosen for further breeding as indicated below.
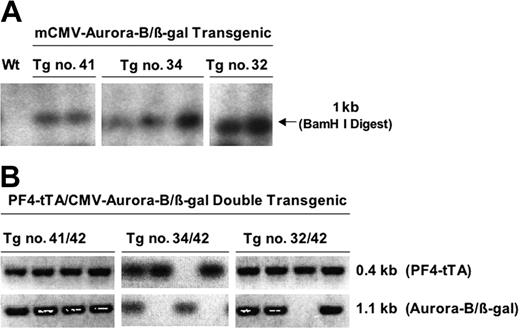
Several CMV–Aurora-B/β-gal transgenic lines (nos. 32, 34, 41) were also generated as confirmed by Southern blot analysis of tail genomic DNA (Figure 3A). As expected, these lines did not express the transgene (using methods of detection as below), since they are driven by a minimal promoter that requires tTA-VP16 for activation. Because it was not possible to determine which of these CMV–Aurora-B/β-gal lines has transgenes integrated in a chromosomal region that allows adequate expression, we used all these lines for crossbreeding with the PF4-tTA-VP16 no. 42 line and obtained double-transgenic offspring mainly with transgenic numbers 41/42 and 34/42 (Figure 3B). Line no. 32/42 was subsequently omitted due to leaky expression of the β-gal transgene under noninducing condition. Transgenic line no. 41/42 littermates were bred together to generate homozygous mice.
Generation of PF4-tTA-VP16 transgenic lines and confirmation of transgene expression. (A) Transgene integration. PCR of genomic DNA was used to screen for specific transgene integration (yielding a 460-bp product shown here) following protocols detailed in “Materials and methods.” Three founder lines, PF4-tTA-VP16 (referred to as PF4-tTA) nos. 38, 42, and 54, were identified, as confirmed by Southern blot analysis. (B-C) Transgene expression. After obtaining homozygous PF4-tTA-VP16 lines, we used Western blot analysis (B) to identify a line with the highest expression of the tetracycline responsive enhancer fusion protein (tTA-VP16). Bone marrow cells were subjected to Western blotting with anti-VP16 antibody to detect expression of tTA-VP16. Actin levels were determined to confirm equal loading of samples. Tissue-specific expression of tTA-VP16 was determined by immunohistochemical analysis of bone marrow cells (C) using anti-VP16 as a primary antibody and FITC-conjugated anti–mouse IgG as a secondary antibody. Since transgenic line no. 42 displays high expression of tTA-VP16 fusion protein in megakaryocytes (indicated by arrows), we decided to use this line for subsequent crossbreeding and experiments. Shown is a representative experiment in which the cells were viewed at a magnification of × 20. Images were captured using an Olympus ×70 microscope with Hamamatsu CCD camera C4742-95. Images were acquired with Openlab 3.1.2 software.
Generation of PF4-tTA-VP16 transgenic lines and confirmation of transgene expression. (A) Transgene integration. PCR of genomic DNA was used to screen for specific transgene integration (yielding a 460-bp product shown here) following protocols detailed in “Materials and methods.” Three founder lines, PF4-tTA-VP16 (referred to as PF4-tTA) nos. 38, 42, and 54, were identified, as confirmed by Southern blot analysis. (B-C) Transgene expression. After obtaining homozygous PF4-tTA-VP16 lines, we used Western blot analysis (B) to identify a line with the highest expression of the tetracycline responsive enhancer fusion protein (tTA-VP16). Bone marrow cells were subjected to Western blotting with anti-VP16 antibody to detect expression of tTA-VP16. Actin levels were determined to confirm equal loading of samples. Tissue-specific expression of tTA-VP16 was determined by immunohistochemical analysis of bone marrow cells (C) using anti-VP16 as a primary antibody and FITC-conjugated anti–mouse IgG as a secondary antibody. Since transgenic line no. 42 displays high expression of tTA-VP16 fusion protein in megakaryocytes (indicated by arrows), we decided to use this line for subsequent crossbreeding and experiments. Shown is a representative experiment in which the cells were viewed at a magnification of × 20. Images were captured using an Olympus ×70 microscope with Hamamatsu CCD camera C4742-95. Images were acquired with Openlab 3.1.2 software.
Generation of CMV–Aurora-B/β-gal transgenic lines and double-transgenic lines (PF4-tTA-VP16/CMV–Aurora-B/β-gal). (A) Generation of CMV–Aurora-B/β-gal transgenic lines. We generated 3 transresponder lines, nos. 32, 34, and 41, carrying the CMV–Aurora-B/β-gal transgene. Genomic integration was confirmed by Southern blot analysis and PCR of genomic tail DNA. Shown is a representative Southern blot analysis of DNA derived from F1 offspring of each of the lines and probed with a 1.1-kb DNA sequence encoding the rat Aurora-B gene. After obtaining homozygous lines by crossbreeding the confirmed F1 transgenic mice, we crossbred each of the 3 transresponder lines to the transactivator line no. 42 (Figure 2). (B) Confirmation of double-transgenic mice PF4-tTA-VP16/CMV–Aurora-B/β-gal. Shown is PCR screening for double-transgenic mice carrying PF4-tTA-VP16 and CMV–Aurora-B/β-gal transgenes, using primer sets that amplified a 460-bp and 1.1-kb DNA fragment coding for both transgenes, respectively. Lines that show both PCR products are double transgenic. More details are provided in “Materials and methods.”
Generation of CMV–Aurora-B/β-gal transgenic lines and double-transgenic lines (PF4-tTA-VP16/CMV–Aurora-B/β-gal). (A) Generation of CMV–Aurora-B/β-gal transgenic lines. We generated 3 transresponder lines, nos. 32, 34, and 41, carrying the CMV–Aurora-B/β-gal transgene. Genomic integration was confirmed by Southern blot analysis and PCR of genomic tail DNA. Shown is a representative Southern blot analysis of DNA derived from F1 offspring of each of the lines and probed with a 1.1-kb DNA sequence encoding the rat Aurora-B gene. After obtaining homozygous lines by crossbreeding the confirmed F1 transgenic mice, we crossbred each of the 3 transresponder lines to the transactivator line no. 42 (Figure 2). (B) Confirmation of double-transgenic mice PF4-tTA-VP16/CMV–Aurora-B/β-gal. Shown is PCR screening for double-transgenic mice carrying PF4-tTA-VP16 and CMV–Aurora-B/β-gal transgenes, using primer sets that amplified a 460-bp and 1.1-kb DNA fragment coding for both transgenes, respectively. Lines that show both PCR products are double transgenic. More details are provided in “Materials and methods.”
Inducible expression of β-galactosidase and generation of a mouse model for following platelet accumulation
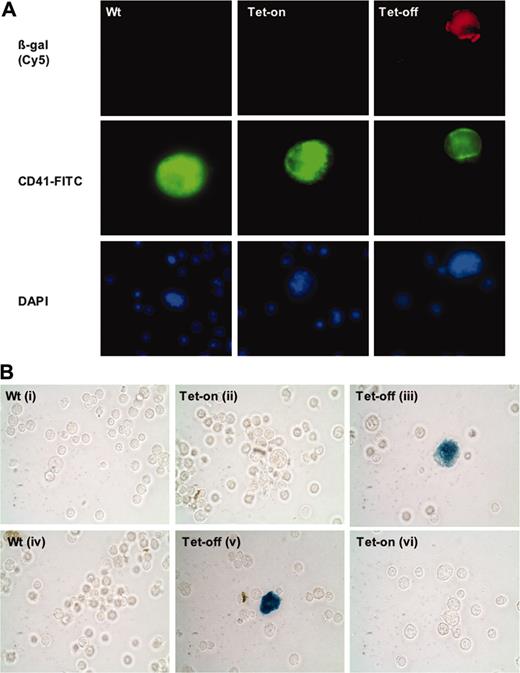
To confirm that transgene expression was inducible and reversible, we performed experiments with a double-transgenic line. Homozygosity at both transgenic loci was determined by genotyping and breeding. PF4-tTA-VP16/CMV–Aurora-B/β-gal (referred to as Tg no. 41/42), a result of crossbreeding PF4-tTA-VP16 no. 42 and CMV–Aurora-B/β-gal line no. 41, was used for analyses. As shown in Figure 4A, β-galactosidase was expressed only upon withdrawal of doxycycline from the drinking water, which induced expression of tTA-VP16 in megakaryocytes (Figure 3C) and, hence, activated the minimal CMV promoter (to which β-galactosidase was linked). Of interest, transgene expression was reversible, that is, it could be eliminated upon readdition of doxycycline to the drinking water (Figure 4C). This feature should be valuable in a variety of applications, including examination of overexpressed thrombotic agents on vascular/platelet function.
Conditional and reversible expression of transgenes in megakaryocytes. (A) Induced β-galactosidase expression. Shown are immunohistochemistry analyses of freshly isolated bone marrow cells from double-transgenic littermates PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42 shown in Figure 3B and age- and sex-matched wild-type mice. Tet-off mice were removed from doxycycline treatment (referred as Tet-off to keep with a terminology that refers to tetracycline inducible promoter) 4 weeks prior to analysis, whereas Tet-on mice were continuously supplemented with doxycycline. To detect β-galactosidase (β-gal)–induced expression, mouse monoclonal antibody against E coli β-gal was used (red). The same preparations were also stained for CD41 (specific marker for megakaryocytes) using anti–CD41-FITC (green). Images were captured with an Olympus microscope using a × 60 objective. DAPI indicates 4,6 diamidino-2-phenylindole. (B) Reversible transgene expression. To demonstrate that conditional gene expression was reversible, half of the Tet-off mouse population received doxycycline for 4 weeks (Bvi), while the other half of its littermates remained Tet-off (Bii). Similarly, half of the Tet-on transgenic mouse population (doxycycline supplement) was removed from doxycycline treatment for 4 weeks (Bv), while the other half remained on doxycycline supplement (Bvi). β-galactosidase was followed as in panel A (not shown) as well as by enzyme activity (shown here as a representative). Fresh bone marrow cells derived from the double-transgenic mice (PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42) and equivalent wild-type cells were stained for β-gal as outlined in “Materials and methods” (visualized via phase-contrast, × 40 original magnification). Megakaryocytes were clearly identified based on their size and morphology. Images were captured using an Olympus ×70 microscope with Hamamatsu CCD camera C4742-95. Images were acquired with Openlab 3.1.2 software.
Conditional and reversible expression of transgenes in megakaryocytes. (A) Induced β-galactosidase expression. Shown are immunohistochemistry analyses of freshly isolated bone marrow cells from double-transgenic littermates PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42 shown in Figure 3B and age- and sex-matched wild-type mice. Tet-off mice were removed from doxycycline treatment (referred as Tet-off to keep with a terminology that refers to tetracycline inducible promoter) 4 weeks prior to analysis, whereas Tet-on mice were continuously supplemented with doxycycline. To detect β-galactosidase (β-gal)–induced expression, mouse monoclonal antibody against E coli β-gal was used (red). The same preparations were also stained for CD41 (specific marker for megakaryocytes) using anti–CD41-FITC (green). Images were captured with an Olympus microscope using a × 60 objective. DAPI indicates 4,6 diamidino-2-phenylindole. (B) Reversible transgene expression. To demonstrate that conditional gene expression was reversible, half of the Tet-off mouse population received doxycycline for 4 weeks (Bvi), while the other half of its littermates remained Tet-off (Bii). Similarly, half of the Tet-on transgenic mouse population (doxycycline supplement) was removed from doxycycline treatment for 4 weeks (Bv), while the other half remained on doxycycline supplement (Bvi). β-galactosidase was followed as in panel A (not shown) as well as by enzyme activity (shown here as a representative). Fresh bone marrow cells derived from the double-transgenic mice (PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42) and equivalent wild-type cells were stained for β-gal as outlined in “Materials and methods” (visualized via phase-contrast, × 40 original magnification). Megakaryocytes were clearly identified based on their size and morphology. Images were captured using an Olympus ×70 microscope with Hamamatsu CCD camera C4742-95. Images were acquired with Openlab 3.1.2 software.
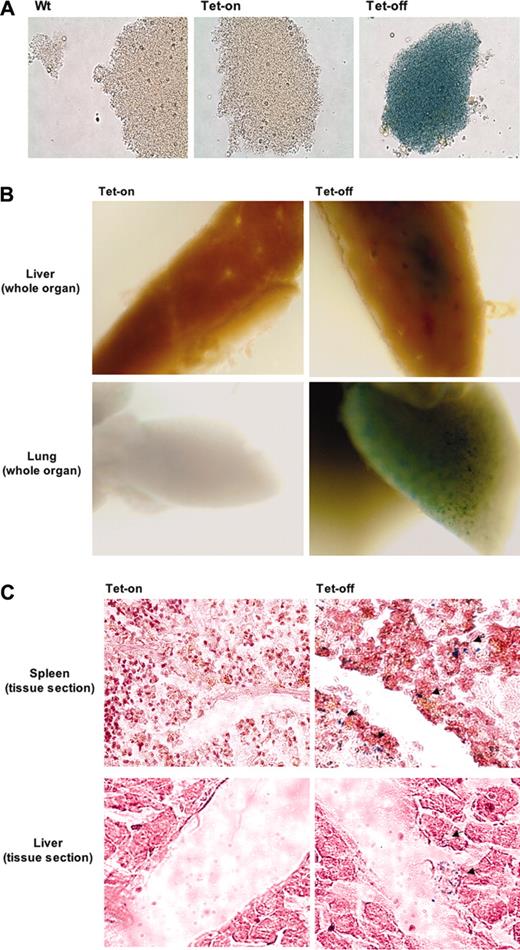
Conditional and reversible transgene overexpression in platelets was also confirmed (Figure 5). The clear detection of stained platelets indicated that this mouse model would be useful in studies that require a rapid and convenient identification of platelets in organs or sites of injury. To this end, LPS injection was used as a model system in Tg no. 41/42 mice under either Tet-on or Tet-off conditions. It has been recently reported that platelets accumulate in the livers and lungs of LPS-treated animals.21 In Figure 5B-C, platelet accumulation is clearly shown in β-gal stained livers, lungs, and spleens, but only in mice under Tet-off conditions. No significant platelet accumulation in the above tissues was observed in mice that were not treated with LPS compared with LPS-treated mice (data not shown). Although platelets may be detected by immunohistochemistry using fluorescent-labeled anti-CD41, it is technically difficult and time consuming to detect fluorescence in situ generated from platelet-sized cells. Furthermore, tissue staining with hematoxylin and eosin, which is needed to identify tissue morphology, increases background fluorescence. β-gal staining in the above mouse model provides an additional means to clearly detect platelets within tissues.
β-Gal activity is retained in platelets of the inducible Tet-on/off double-transgenic mouse model. (A) β-Galactosidase expression in platelets. To demonstrate the feasibility of retaining the expression of transgenes (β-gal in this case) after megakaryocytes fragmented into platelets, we isolated platelets from plasma of double-transgenic mice with Tet-on/off conditions along with age- and sex-matched wild-type mice. Shown are phase-contrast micrographs at × 60 objective of platelets stained for β-galactosidase activities. (B-C) Usage of the double-transgenic model (PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42) to trace platelets at sites of accumulation. Identifying and quantifying platelet accumulation at sites of injury or stress is a goal common to various experimental pursuits. As a model system, we used endotoxic shock using LPS injection as detailed in “Materials and methods.” Shown are livers, lungs, and spleen stained for β-gal activity. Whole tissues (B) as well as tissue sections (C) (spleen and liver shown as representatives) displayed accumulated platelets. Thin sections were stained only with eosin and visualized by light phase contrast; × 60 original magnification. Arrows indicate areas of platelet accumulation. Images were captured using an Olympus ×70 microscope with Hamamatsu CCD camera C4742-95. Images were acquired with Openlab 3.1.2 software.
β-Gal activity is retained in platelets of the inducible Tet-on/off double-transgenic mouse model. (A) β-Galactosidase expression in platelets. To demonstrate the feasibility of retaining the expression of transgenes (β-gal in this case) after megakaryocytes fragmented into platelets, we isolated platelets from plasma of double-transgenic mice with Tet-on/off conditions along with age- and sex-matched wild-type mice. Shown are phase-contrast micrographs at × 60 objective of platelets stained for β-galactosidase activities. (B-C) Usage of the double-transgenic model (PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42) to trace platelets at sites of accumulation. Identifying and quantifying platelet accumulation at sites of injury or stress is a goal common to various experimental pursuits. As a model system, we used endotoxic shock using LPS injection as detailed in “Materials and methods.” Shown are livers, lungs, and spleen stained for β-gal activity. Whole tissues (B) as well as tissue sections (C) (spleen and liver shown as representatives) displayed accumulated platelets. Thin sections were stained only with eosin and visualized by light phase contrast; × 60 original magnification. Arrows indicate areas of platelet accumulation. Images were captured using an Olympus ×70 microscope with Hamamatsu CCD camera C4742-95. Images were acquired with Openlab 3.1.2 software.
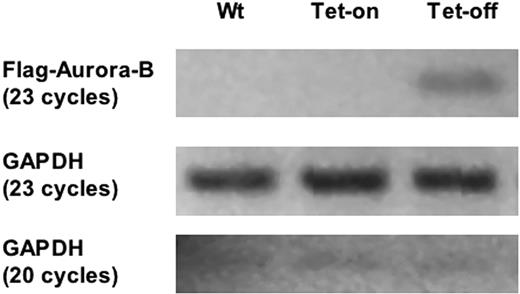
Inducible expressions of Aurora-B kinase in megakaryocytes. Primary bone marrow cell mRNA was isolated from double-transgenic mice (PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42) treated with and without doxycycline (Tet-on or Tet-off) and reverse transcribed to generate cDNA. PCR was subsequently conducted using primer sets specifically designed to amplify FLAG-tagged rat Aurora-B kinase. GAPDH was amplified to show that the RT-PCR was successfully conducted in all samples. GAPDH-specific primers and PCR cycles were performed, as indicated in “Materials and methods.”
Inducible expressions of Aurora-B kinase in megakaryocytes. Primary bone marrow cell mRNA was isolated from double-transgenic mice (PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42) treated with and without doxycycline (Tet-on or Tet-off) and reverse transcribed to generate cDNA. PCR was subsequently conducted using primer sets specifically designed to amplify FLAG-tagged rat Aurora-B kinase. GAPDH was amplified to show that the RT-PCR was successfully conducted in all samples. GAPDH-specific primers and PCR cycles were performed, as indicated in “Materials and methods.”
Inducible expression of Aurora-B and bone marrow analysis
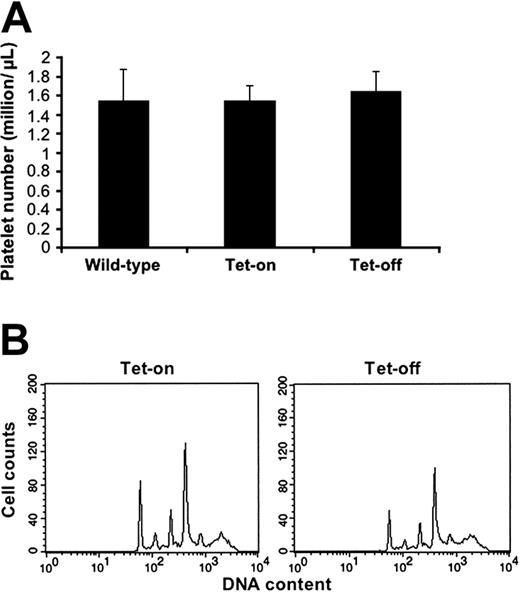
Bone marrow cells derived from Tg no. 41/42 mice treated with and without doxycycline were subjected to immunohistochemistry with anti–Aurora-B, as was pursued for β-galactosidase. In contrast to a high-level expression of the latter throughout the cell (Figure 4A), Aurora-B was not detected in large recognizable megakaryocytes (surveyed over 200 megakaryocytes; data not shown), although the transgenic mRNA was readily detectable (Figure 6). This phenotype correlates with the one reported earlier, involving constitutive expression of Aurora-B in megakaryocytes of transgenic mice,4 that is, Aurora-B typically peaks at mid mitosis, and this tightly regulated protein is not concomitantly elevated upon overexpression of its mRNA. In accordance, there was no change in platelet level in the induced mice compared with control (Figure 7). The change in ploidy level was small (Figure 7B), as also previously reported.4
Platelet and ploidy levels in the inducible double-transgenic mice (PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42). Double-transgenic mice and age- and sex-matched wild-type mice were killed after 4 weeks of treatment with and without doxycycline. Blood was withdrawn and subjected to platelet counts (A) as indicated in “Materials and methods.” Data are presented as platelet count averages ± SD for 10 mice in each group. To determine megakaryocyte ploidy status (B), primary bone marrow cells were isolated from the same population of mice, fixed, stained with anti-CD41 (FITC) antibody and propidium iodide, and subjected to flow cytometry analysis. A representative histogram is shown.
Platelet and ploidy levels in the inducible double-transgenic mice (PF4-tTA-VP16/CMV–Aurora-B/β-gal no. 41/42). Double-transgenic mice and age- and sex-matched wild-type mice were killed after 4 weeks of treatment with and without doxycycline. Blood was withdrawn and subjected to platelet counts (A) as indicated in “Materials and methods.” Data are presented as platelet count averages ± SD for 10 mice in each group. To determine megakaryocyte ploidy status (B), primary bone marrow cells were isolated from the same population of mice, fixed, stained with anti-CD41 (FITC) antibody and propidium iodide, and subjected to flow cytometry analysis. A representative histogram is shown.
Discussion
We have generated 2 systems that are valuable in studies involving gene manipulation in the megakaryocyte/platelet lineage. The already available PF4-tTA-VP16/CMV–Aurora-B/β-gal double-transgenic line might be used for following platelet accumulation at sites of induced injury. The transactivator line PF4-tTA-VP16 is available for crossbreeding with other newly generated or already available mouse lines in which the minimal CMV promoter is linked to genes of interest. Such available lines include the Id1 gene, involved in enhancing cellular proliferation and inhibiting cellular differentiation in a variety of cell types, TGFβ-1, transforming growth factor type β1, an important regulator of cell growth and differentiation, in addition to other successful related systems.23-30 Likewise, we created a transgenic line with rat Aurora-B linked to the minimal CMV promoter for further evaluation of the role of Aurora-B–induced overexpression in a wide range of tissues, including mammary (MMTV-tTA), skin (K5 promoter-tTA), liver (albumin promoter-tTA), and arterial (SM22α promoter-tTA) smooth muscle cells, via crossbreeding with lines that express the tTA under the control of the tissue-specific promoters indicated in the parentheses.23,28-30 Such new double-transgenic lines obtained will be useful for examination of conditional expression of transgenes, bypassing potential embryonic lethality. In addition, the reversibility of induced effects on the physiology might be examined.
Prepublished online as Blood First Edition Paper, May 12, 2005; DOI 10.1182/blood-2005-02-0638.
Supported by grant HL58547 from the National Heart, Lung, and Blood Institute (NHLBI) to K.R. K.R. is an established investigator with the American Heart Association.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to acknowledge Robin MacDonald of the BUSM Transgenic Facility and Paul Toselli for expertise in histology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal