Abstract
Increasing evidence suggests that leukemias are sustained by leukemic stem cells. However, the molecular pathways underlying the transformation of normal cells into leukemic stem cells are still poorly understood. The involvement of a small group of key transcription factors into this process was suggested by their frequent mutation or down-regulation in patients with acute myeloid leukemia (AML). Recent findings in mice with hypomorphic transcription-factor genes demonstrated that leukemic stem-cell formation in AML could directly be caused by reduced transcription-factor activity beyond a critical threshold. Most interestingly, those experimental models and the paucity of biallelic null mutations or deletions in transcription-factor genes in patients suggest that AML is generally associated with graded down-regulation rather than complete disruption of transcription factors. Here, we discuss the effects of transcription-factor concentrations on hematopoiesis and leukemia, with a focus on the regulation of transcription-factor gene expression as a major mechanism that alters critical threshold levels during blood development and cancer.
The leukemic stem-cell model
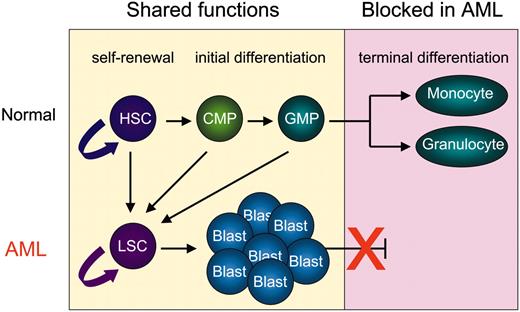
At least 2 principally different models have been proposed to explain the persistent production of neoplastic daughter cells during cancer development. One model assumes that all cells of a neoplasm are malignant in that every cell is able to give rise to cancerous daughter cells. The second model is based on growing evidence that only a minor subpopulation of cells are maintaining the uncontrolled formation of tumor cells.1-3 According to this model, a relatively small number of those tumor stem cells give rise to the bulk of tumor cells such as leukemic blasts. Leukemic stem cells (LSCs), similar to tissue stem cells under normal conditions, seem to be the cellular source of a hierarchy of daughter cells, which greatly differ in their capability of unlimited proliferation and maintaining the tumor.4,5 LSCs apparently share important stem-cell functions with normal hematopoietic stem cells (HSCs) such as self-renewal, initial differentiation, and survival (Figure 1). It is therefore believed that LSCs and HSCs are both controlled by a similar set of critical genes, which include the Wingless-type (Wnt) pathway,5 the ubiquitous transcription-factor junB,8 and the Polycomb family member Bmi-1.9 Depending on the transforming event, LSCs are thought to arise either from normal HSCs, whereby the activity of critical “stemness” genes is preserved during transformation, or from committed progenitors, which regain stem-cell functions.5-7 However, the overall molecular events that underlie the formation of LSCs remain poorly understood.
Acute myeloid leukemia (AML) is characterized by a terminal differentiation block of hematopoietic cells of the myeloid lineage, while self-renewal and proliferation is preserved.10 Thus, it is reasonable to assume that the molecular events underlying LSC development in AML must be severe enough to block terminal differentiation, but at the same time still allow basic stem-cell functions. In normal HSCs, lineage-specific transcription factors have been identified as potent regulators of these functional programs.
Transcription factor concentrations direct HSC activity
A small group of transcription factors with mostly lineage-restricted expression patterns was demonstrated to play a crucial role in controlling normal hematopoiesis.11 Among the best-studied examples are PU.1, CCAAT/enhancer-binding protein α (C/EBPα), AML1, GATA-1, c-myb, and SCL/Tal-1. Knockout of the genes encoding these factors in mice displayed profound hematopoietic defects.12 Moreover, those transcription factors were shown to regulate broad ranges of important target genes, hereby directly programming hematopoietic precursors to differentiate along a complex developmental pathway.13
A number of these transcription factors have been linked to HSC functions. AML1 and SCL/Tal-1 have been demonstrated to be indispensable for the specification of fetal liver HSCs.14,15 However, more recent studies using conditional knockout mice showed that AML1 and SCL/Tal-1 are less critical for the maintenance of HSCs in the adult organism,16,18 indicating that these nuclear factors may be required for the generation of HSCs from more pluripotent cells in the embryo but not for HSC self-renewal.
Several reports clearly link PU.1 to HSC function. Expression of PU.1 is detected in HSCs and increases progressively during differentiation into myeloid cells.19,20 Previous studies showed that, upon transplantation into congenic mice, PU.1–/– fetal liver HSCs poorly engrafted the bone marrow and were incapable of long-term reconstitution.21 Furthermore, although PU.1–/–-derived cells contributed to erythrocyte development in chimeric mouse studies, this effect was only transient, strongly suggesting a defect at the level of the HSCs.21 More recent approaches using both conventional and conditional PU.1 knockout mice suggest that PU.1–/– HSCs possess impaired self-renewal capacity and lack the ability to differentiate into the earliest lymphoid and myeloid progenitor stages, the common lymphoid progenitor (CLP) and the common myeloid progenitor (CMP) respectively.22-24
Important stem-cell functions are shared by HSCs and LSCs. Both normal HSCs and neoplastic LSCs have the ability to self-renew and initially differentiate into less pluripotent daughter cells. However, HSCs produce short-lived progenitors, such as common myeloid progenitors (CMPs) and granulocyte monocyte progenitors (GMPs), which terminally differentiate into mature monocytes and granulocytes. In contrast, LSCs give rise to leukemic blasts, which harbor a block in their terminal differentiation. Recent experiments using murine transplantation models suggest that both HSCs and committed myeloid progenitors can transform into an LSC.5-7
Important stem-cell functions are shared by HSCs and LSCs. Both normal HSCs and neoplastic LSCs have the ability to self-renew and initially differentiate into less pluripotent daughter cells. However, HSCs produce short-lived progenitors, such as common myeloid progenitors (CMPs) and granulocyte monocyte progenitors (GMPs), which terminally differentiate into mature monocytes and granulocytes. In contrast, LSCs give rise to leukemic blasts, which harbor a block in their terminal differentiation. Recent experiments using murine transplantation models suggest that both HSCs and committed myeloid progenitors can transform into an LSC.5-7
C/EBPα, a crucial regulator of granulopoiesis, has also been implicated in the control of HSCs. C/EBPα-deficient HSCs display enhanced competitive repopulation activity in murine transplantation models, possibly by a mechanism that includes altered unsymmetric cell divisions through increased Bmi-1 expression.25,26 Like PU.1, C/EBPα is expressed at lower levels in HSCs and is strongly up-regulated during myeloid differentiation.25
The pattern of lower level expression in HSCs and lineage-restricted up- or down-regulation in more mature cells is shared by a number of hematopoietic transcription factors, including PU.1, C/EBPα, GATA-1, and c-myb.19 This hallmark expression pattern suggests a stage-specific difference in the requirement for those proteins during differentiation and emphasizes a need for basic transcription-factor functions at the HSC level. Cytokine receptor genes are among the most important targets of lineage-specific transcription factors,13 and one crucial role of low-level transcription-factor expression in HSCs might be the induction of low-level cytokine receptor expression to enable their survival and expansion. Subsequent up-regulation of transcription-factor activity increases the expression of lineage-restricted cytokine receptor subsets, thereby inducing differentiation and loss of stem-cell potential.
PU.1 was the first hematopoietic transcription factor for which a causal association of different expression levels and differentiation fates has been shown. A model was developed by which different cellular concentrations of PU.1 direct distinct cell fates, with the highest levels required for macrophage development and lower levels for granulocytic and B-cell lineage adoption.27,28 Another transcription-factor with clear dosage sensitivity in hematopoiesis is c-myb. Whereas the null mutation of c-myb completely prevents progenitor cell development, low c-myb levels (5%-10% of wild type) do allow initial HSC differentiation into progenitors, but they are insufficient for further lineage commitment and terminal differentiation.29 Finally, GATA-1 appears to regulate erythroid, megakaryocytic, mast cell, and eosinophilic cell fates in a concentration-dependent manner.30-32 How is the pool of active transcription-factor molecules regulated in the cell? One possibility is through the protein stoichiometry of directly interacting transcription factors, as has been shown by the opposing interplay between PU.1 and GATA-1 in myeloid versus erythroid differentiation.33-36 Another mechanism is the regulation of transcription-factor gene expression.
Regulation of transcription-factor genes
The importance of transcription-factor gene regulation has strikingly been illustrated by the use of knock-in mice, knocking one factor into the genomic locus of another one. For instance, Spi-B, which is expressed exclusively in lymphocytes but not in myeloid cells, can functionally replace PU.1 during myeloid development when knocked into the PU.1 gene locus.37 Thus, Spi-B and PU.1 are largely interchangeable, and the unique role of PU.1 is due mainly to its unique expression in this lineage. In another study, C/EBPβ showed redundancy with C/EBPα after being knocked into the C/EBPα locus.38 From such experiments it became clear that temporal and tissue-specific expression of transcription factors is a critical determinant for their unique functions in hematopoiesis.
Transcription factor genes appear to be controlled by a complex pattern of both lineage- and stage-specific cis-regulatory DNA elements. In case of the PU.1 gene, it was shown that the proximal promoter region alone is unable to direct reporter gene expression in transgenic mice, suggesting that additional cis-regulatory elements are required for PU.1 gene expression.39 Further evidence for this hypothesis came from DNaseI hypersensitive studies that identified several potential PU.1 gene regulatory elements.39 One of these elements, which locates 14 kilobases (kb) upstream of the transcription start site (designated URE for upstream regulatory element) was found to have great implications for PU.1 expression. First, a combination of the URE and the PU.1 promoter was sufficient to drive reporter gene expression in stable cell lines and transgenic mice in a pattern resembling endogenous PU.1.39,40 Second, deletion of the URE in mice by homologous recombination led to an approximate 5-fold decrease in PU.1 expression in the bone marrow, resulting in markedly lower numbers of macrophages and B-cells41 (F.R. and D.G.T., unpublished observations, June 2004).
Transcription of the murine Gata1 gene also appears to be directed by several cis-regulatory elements. One distal element contains a potent enhancer core, which is able to confer reporter expression to erythroid and megakaryocytic lineages in transgenic mice.42 Targeted deletion of this element in mice ablated expression of GATA-1 in megakaryocytes, but left its expression in erythrocytes unchanged, perhaps because of cis elements elsewhere in the Gata1 gene.30 A GATA site positioned 500 base pairs (bp) upstream of the transcription start appears to be another crucial element for GATA-1 expression, because mice in which this GATA-binding site was deleted specifically lacked eosinophils, indicating a lineage-specific function of this site.32
SCL/Tal-1 is a third example of a systematically characterized transcription-factor gene locus. A total of 6 independent enhancers, each of which directs expression to a particular cellular subset, has been identified in the murine SCL/Tal-1 locus.43 The most intensively characterized enhancer is located 3′ of the SCL/Tal-1 coding region at +18/19 kb. This 3′ enhancer was shown to be active in endothelial cells and hematopoietic progenitors44,45 and was able to direct expression to long-term repopulating HSCs in frog embryos. However, in situ deletion of the 3′ enhancer in mice has not proven its essential role for SCL/Tal-1 expression, suggesting compensatory effects by other cis elements.43
Such detailed dissection of regulatory cis elements of important transcription-factor genes is necessary not only to understand the generation of distinct transcription-factor concentrations during normal differentiation but also to reveal the mechanisms of their dysregulation in cancer.
Transcription factors play a central role in human AML
The hallmark of acute leukemias such as AML is a severe block in the differentiation of early progenitors, leading to the accumulation of immature stages in the bone marrow and blood. Transcription factors are among the most frequently mutated or dysregulated genes in patients with AML, in their entirety similar to mutations in genes implicated in signal transduction, such as p21ras (ras) and Fms-like tyrosine kinase 3 (Flt3).46 The involvement of transcription factors in acute leukemias was first suggested by common somatically acquired chromosomal translocations.47 In patients with AML, the most frequently found translocation products involving transcription factors are AML1/ETO (eight twenty-one) [t(8;21)], core-binding factor β/myosin, heavy polypeptide 11 (CBFβ/MYH11) [inv16], mixed-lineage leukemia (MLL) gene-fusions [t11q23], and promyelocytic leukemia/retinoic acid receptor α (PML/RARα) [t(15;17)].46
More recently, smaller mutations in the coding regions of several transcription-factor genes have been identified in patients with AML. C/EBPα is among the most frequently mutated transcription-factor, especially in the French-American-British (FAB) M2 subtype.48 Approximately 58% of patients with C/EBPα mutations were found to carry N-terminal mutations that prevent the translation of the longer 42-kDa isoform but preserve the expression of a shorter 30-kDa variant. Mechanistically, such mutations result in reduced C/EBPα protein function through a dominant-negative effect of the transcriptionally inactive 30-kDa isoform on the active 42-kDa protein. In addition, many AML cases were identified to harbor C/EBPα mutations in the C-terminal DNA-binding domain.49-51 No C/EBPα mutations were found in patients with t(8;21) (AML1/ETO) or inv(16) translocations. However, blast cells from these patients showed decreased C/EBPα mRNA expression, suggesting that the block of granulocytic differentiation in these AML subtypes may be closely associated with C/EBPα down-regulation by oncogenic fusion products.52,53 In addition, Flt3–internal tandem duplication (ITD) mutants have been reported to lead to decreased C/EBPα expression.54,55 It should be emphasized that all of these mutagenesis studies demonstrated a conspicuous lack of patients with AML with null alleles, suggesting that cryptic or reduced C/EBPα function rather than a complete lack of function contributes to leukemogenesis.56
Although inherited mutations in the N-terminal zinc finger domain of GATA-1 are associated with congenital dyserythropoietic anemia and thrombocytopenia,57 somatically acquired GATA-1 mutations were exclusively found in patients with trisomy 21 (Down syndrome) who developed concomitant acute megakaryoblastic leukemia (AMKL) or transient myeloproliferative disorder (TMD).58 In such Down syndrome–associated leukemias, the majority of reported GATA-1 mutations involved small deletions or insertions in exon 2, which resulted in the disruption of the normal reading frame and introduced a premature stop codon.59,60 Similar to C/EBPα mutations, expression of full-length 47-kDa GATA-1 is prevented, while expression of a short truncated 40-kDa isoform (termed GATA-1s) is preserved.58 GATA-1s lacks the N-terminal activation domain but retains both zinc fingers and the entire C-terminus, and thus leads to a hypomorphic protein that is still able to bind to DNA and to friend-of-GATA (FOG), an important cofactor of GATA-1 protein function, but has reduced transactivation potential.59,61
A cooperative dosage effect of GATA-1 with a gene situated on the additional chromosome 21, potentially the AML1 gene, is suspected in patients with AMKL. The AML1 gene itself is mutated in 9% of all AML cases.62 In the FAB M0 subtype, many of these mutations were biallelic, which suggested that AML1 function may be completely abrogated in these patients.63 However, it is not yet fully understood whether these mutations in fact represent true null alleles and thus lead to complete loss of AML1 function or rather retain residual AML1 activity similar to what has been described for C/EBPα, GATA-1, and PU.1. Furthermore, it is very possible that functional redundancy among AML family members might compensate for a completely disrupted AML1 in FAB M0, thus providing the leukemic stem-cell compartment with residual AML activity required to induce the expression of critical target genes.
Mutations in the PU.1 gene were identified in one study in 7% of patients with AML.64 These mutations largely resulted in decreased ability of PU.1 to synergize with interacting proteins such as AML1 or c-Jun in the activation of target genes. In contrast, other studies found either no PU.1 mutations or that they occurred less frequently,65,66 and the exact reason for this discrepancy has yet to be revealed. However, PU.1 expression and/or function appears to be down-regulated by several important oncogenic products, such as AML1/ETO,67 FLT3-ITD,54,55 and PML-RARα (B.U. Mueller and D.G.T., unpublished observation, January 2005). Consequently, and similar to the absence of C/EBPα mutations in t(8;21) leukemia, this observation might explain the lack of frequent PU.1 mutations in human AML.
Together, these findings indicate that diminished function of a small number of key transcription factors might be a hallmark of acute leukemias. The block of normal differentiation upon transcription-factor reduction might result in the accumulation of a progenitor pool from which LSCs can arise. Reduction of transcription-factor activity below a crucial threshold appears therefore as a major molecular requirement of LSC development in AML.
Transcription factor concentrations in LSC development
Although transcription factors are frequently mutated in human AML, it is striking that such alterations generally do not lead to biallelic null mutations, which would result in a complete loss of transcription-factor function. Interestingly, those mutations rather produce hypomorphic situations, where residual transcription-factor expression and function are still preserved.48,58,62,64 In addition, the inhibitory effect of oncogenic products such as PML/RARα, AML1/ETO, or FLT3-ITD leads to decreased transcription-factor expression or function but not to a complete shutdown.52,55,67 Collectively, data gathered from patients with leukemia overwhelmingly support an idea in which transformation into LSCs is most efficiently achieved by graded decrease of transcription-factor activity to a critical threshold level, rather than by complete abrogation.
Corroborating evidence for this concept came recently from a number of animal models harboring hypomorphic transcriptionfactor function. First of all, PU.1 knockdown mice provided first definitive proof that graded down-regulation of a single transcription-factor to a level above nullizygosity is sufficient to induce myeloid transformation.41 In this study, PU.1 knockdown was engineered by deletion of the –14-kb PU.1 URE, thus allowing 20% residual PU.1 expression in HSCs and myeloid progenitors. These animals had normal numbers of HSCs, but an increased myeloid progenitor compartment, and after a short preleukemic phase they frequently developed an aggressive AML (Figure 2).
Although it has been reported that null alleles of PU.1 can also lead to leukemia,24 clear experimental evidence for the advantage of hypomorphic PU.1 function over its complete disruption in provoking AML came from the analysis of γ-irradiated mice.68 Cook et al68 showed that radiation-induced myeloid leukemias regularly acquired a combination of a deletion on one copy of chromosome 2, which included the PU.1 gene locus, and a recurring single point mutation in the ETS domain of the remaining PU.1 allele, which impaired DNA binding. Most strikingly, Cook et al could neither find any tumors which had both PU.1 alleles deleted nor detect any cases where the remaining PU.1 allele suffered a null mutation, suggesting a specific selection during transformation for those clones with preserved minimal PU.1 activity over those that suffered a complete loss of function. A very similar observation was very recently reported by another group in an independent study.69
C/EBPα is a second transcription-factor for which dosage sensitivity in leukemia can be suggested. First, although C/EBPα mutations occur frequently in patients with AML, no biallelic null mutations could be identified.56 Second, whereas neither conventional nor conditional C/EBPα-knockout mice developed AML,25 mice carrying engineered C/EBPα alleles that specifically disrupted the 42-kDa protein isoform and allow only expression of the 30-kDa isoform, regularly developed an AML-like disease.70 Moreover, knock-in mice with a targeted mutation in the C/EBPα basic region that specifically inhibits C/EBPα-E2F interaction (BRM2 mutation) also developed AML symptoms, suggesting that, although disruption of C/EBPα's cell-cycle control is important for AML induction, other C/EBPα functions must be preserved (C. Nerlov, unpublished data, February 2005).
Model of LSC development by a malignant transcription-factor threshold illustrated on the PU.1 gene. A range of PU.1 expression between normal (100%) and haploinsufficient (50%) levels supports normal HSC differentiation into myeloid progenitors and subsequent mature granulocytes and macrophages. Graded reduction of PU.1 activity below 20% of normal meets a critical threshold level leading to poor differentiation and subsequent accumulation of an abnormal progenitor pool which is reminiscent of a preleukemic phase. Additional secondary mutations, such as c-myc overexpression because of genomic instability, complete the transformation of those cells into LSCs, which give rise to a bulk of AML blasts (leukemic phase). PU.1 function has been shown to be repressed by a number of different mechanisms in human or murine leukemia, which include down-regulation by oncogenic products as well as mutations in the PU.1 coding sequence (CDS) and deletion of the URE.
Model of LSC development by a malignant transcription-factor threshold illustrated on the PU.1 gene. A range of PU.1 expression between normal (100%) and haploinsufficient (50%) levels supports normal HSC differentiation into myeloid progenitors and subsequent mature granulocytes and macrophages. Graded reduction of PU.1 activity below 20% of normal meets a critical threshold level leading to poor differentiation and subsequent accumulation of an abnormal progenitor pool which is reminiscent of a preleukemic phase. Additional secondary mutations, such as c-myc overexpression because of genomic instability, complete the transformation of those cells into LSCs, which give rise to a bulk of AML blasts (leukemic phase). PU.1 function has been shown to be repressed by a number of different mechanisms in human or murine leukemia, which include down-regulation by oncogenic products as well as mutations in the PU.1 coding sequence (CDS) and deletion of the URE.
This novel principle of a strong association between leukemo-genesis and hypomorphic instead of absent transcription-factor function appears to apply also to GATA-1. As in the case of C/EBPα, the analysis of Down syndrome–associated leukemias did reveal a conspicuous lack of patients with null alleles and suggested cryptic or reduced GATA-1 function as the mechanism for leukemogenesis.59,60 This hypothesis has recently been supported by the observation that GATA-1 knockdown mice developed leukemia whereas mice completely lacking GATA-1 did not. By comparing female GATA-1.05/X mice, which carry a promoter-interfered knockdown allele allowing for residual 5% GATA-1 expression,71 with female GATA-1-null/X mice, Shimizu et al72 observed clear differences in the sensitivity of both mutant lines to leukemogenesis. From their study, the researchers concluded that residual low-level GATA-1 expression is required for leukemia development because it is sufficient to support survival and proliferation but not differentiation, leading to the accumulation of progenitors that provide the pool for LSC formation.
Dose dependency has also been shown for fusion oncogenes in leukemia as well. A salient example is the PML/RARα fusion transcription factor. Transgenic PML/RARα mice had a markedly lower induction rate of acute promyelocytic leukemia (APL) than PML/RARα knock-in mice, which expressed only 3% of the PML/RARα transcripts of the amount measured in the transgenic model.73 This suggests that very low levels of PML/RARα expression in early myeloid cells may be optimal for the development of APL in mice. Similar effects have been shown for breakpoint cluster region/Abelson murine leukemia (BCR/ABL),74 and might also be suspected for AML1-ETO.75,76 The fact that fusion oncogenes can affect the expression and function of transcription factors such as C/EBPα and PU.1 raises the possibility that part of the dosage effects of these oncogenes is mediated through their effects on transcription-factor function.
Collectively, both observations on patient samples and analysis of experimentally generated myeloid leukemia in animal models support a concept in which LSC activity is generally associated with hypomorphic or cryptic activity rather than with complete abrogation of key transcription factors. Their quantitative reduction to a critical functional dosage might meet a highly malignant threshold level which is dominant over the effect of true null alleles in inducing cancer. The involvement of most leukemia-affiliated transcription factors in the regulation of basic stem-cell functions of normal HSCs, such as self-renewal and initial differentiation, could explain the general requirement for such residual activity to meet key LSC functions, which might not be as efficiently satisfied by completely abolished transcription-factor activity.
Although the lack of transformation in most leukemia-affiliated transcription-factor knockout mouse lines is likely due to the absence of the requisite stem or progenitor compartment from which LSCs develop (eg, C/EBPα–/– mice lack granulocyte-monocyte progenitors), hypomorphic animals such as the PU.1 enhancer mutant mice or female GATA-1.05/X mice have an expanded pool of such progenitors, placing them at higher risk of additional mutations. This situation may be somewhat different from human cancer, where a leukemic stem-cell arises from a single mutated cell of an otherwise normal precursor pool. However, analogous to genetically engineered mice, a mutated precursor might also be developmentally blocked and is thus placed at a higher risk to acquire additional events to complete transformation.
Transcription factors and the future: what are the challenges?
Although intensive research has provided ample evidence for the dominant roles of transcription factors in both normal and malignant stem-cell activity, there are still numerous open questions, in particular concerning the functional mechanisms. Most major insights into the biologic roles of transcription factors were derived from studying knockout mice, emphasizing the strength of those genetic models. However, such gene disruption usually leads to the complete abrogation of expression, and thus creates an extreme experimental situation. In real nature, it appears that genes are not simply expressed in a turn on or off mode but are rather expressed in tightly controlled graded levels. It is likely that for most genes small changes in their expression strength meet very specific thresholds, which trigger distinct biologic functions. Complete disruption may therefore reveal only a fraction of the possible functions of a gene and may miss those effects that reflect fine-tuned expression.
A major challenge for the future is therefore to consider the impact of small gradations in the dosages as well as cryptic functions of transcription factors on both normal and neoplastic development. It is essential for our understanding of malignant events that we obtain a detailed view of the mechanisms that adjust the concentrations of especially those transcription factors with a role in human cancer. We need to identify and characterize critical upstream and downstream pathways that control transcription factor-gene expression and function, and to learn how we could exploit them to improve cancer treatment. Such efforts require the close cooperation of basic and clinical research to best evaluate the experimentally gained data in human disease.
Prepublished online as Blood First Edition Paper, May 24, 2005; DOI 10.1182/blood-2005-02-0717.
Supported by DFG (German Research Foundation) research fellowship RO 2295/1-1 (F.R.) and research fellowship KO 2155/1-1 (S.K.), Dr Mildred Scheel Foundation for Cancer Research Fellowship D/03/41221 (U.S.), and the National Institutes of Health (NIH) grants R01 CA88046 and R01 CA72009 (D.G.T.).
We thank John Crispino, D. Gary Gilliland, and Bronwyn Owens for stimulating discussions and valuable comments on the manuscript and Claus Nerlov for sharing unpublished observations. We apologize to all investigators whose work could not be cited due to space limitations.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal