Abstract
To test the hypothesis that aging has negative effects on stem-cell homing and engraftment, young or old C57BL/6 bone marrow (BM) cells were injected, using a limiting-dilution, competitive transplantation method, into old or young Ly5 congenic mice. Numbers of hematopoietic stem cells (HSCs) and progenitor cells (HPCs) recovered from BM or spleen were measured and compared with the numbers initially transplanted. Although the frequency of marrow competitive repopulation units (CRUs) increased approximately 2-fold from 2 months to 2 years of age, the BM homing efficiency of old CRUs was approximately 3-fold lower than that of young CRUs. Surprisingly, the overall size of individual stem-cell clones generated in recipients receiving a single CRU was not affected by donor age. However, the increased ages of HSC donors and HSC transplant recipients caused marked skewing of the pattern of engraftment toward the myeloid lineage, indicating that HSC-intrinsic and HSC-extrinsic (microenvironmental) age-related changes favor myelopoiesis. This correlated with changes after transplantation in the rate of recovery of circulating leukocytes, erythrocytes, and platelets. Recovery of the latter was especially blunted in aged recipients. Collectively, these findings may have implications for clinical HSC transplantation in which older persons increasingly serve as donors for elderly patients. (Blood. 2005; 106:1479-1487)
Introduction
Hematopoiesis is maintained throughout life by self-renewing stem cells with a high potential for proliferation and multilineage differentiation.1 Accumulating evidence indicates that as animals age, the number and the functional properties of hematopoietic stem cells (HSCs) become altered.2,3 However, these effects of aging on stem cells and their bone marrow (BM) microenvironment are not clearly defined. For example, marrow from old C57BL/6 mice contains more HSCs (measured by cobblestone area formation, primitive phenotype, and competitive repopulating ability) than BM from young mice.4-8 In contrast, the stem-cell pool from DBA/2 and all other mouse strains studied contracts during aging.4,9 There is compelling evidence that strain-specific variation in this and other stem/progenitor-cell parameters is regulated by cell-intrinsic mechanisms and is affected by several quantitative trait loci (QTL).7,10-15 In serial transplantation experiments, marrow cells from old animals was less able to engraft later passage recipients than young BM cells.16 Moreover, old HSCs exhibit a differentiation pattern skewed toward the myeloid lineage at the expense of lymphopoiesis.8,17 Further evidence of age-related changes in stem cells include the finding that a higher proportion of Thy-1loSca-1+Lin-Mac-1-CD4-c-kit+ cells from old mice are in S/G2/M phases of the cell cycle6 and the findings of Henckaerts et al,7 who showed that the proliferative response of Lin-Sca-1+c-kit+ marrow cells to the early-acting cytokines kit ligand (KL), FMS-like tyrosine kinase 3 ligand (Flt3L), and thrombopoietin (TPO) decreases dramatically with age.
In addition to such quantitative and functional changes of HSCs with aging, the density or activity of several cell-surface antigens and membrane transporters that facilitate their identification and isolation fluctuates during ontogeny and throughout adulthood.18-20 Therefore, functional measurements of HSC properties may actually reflect the effects of aging on this important population. The competitive repopulation assay is the most rigorous test for defining HSCs by their capacity for long-term reconstitution of the lymphohematopoietic system.21,22 When combined with a limiting-dilution design, this assay enables measurement of HSC numbers in vivo.22 In the present study, graded numbers of “test” BM cells were cotransplanted into myeloablated mice, together with competitor BM cells containing an allelic variant of the hematopoietic-cell-specific marker Ly-5 (also known as CD45 and Ptprc). The frequency of HSCs in the test population, measured as competitive repopulation units (CRUs), was then determined by applying maximum likelihood analysis and Poisson statistics.22 Because this method also identifies animals engrafted by a single HSC, it has the further advantage of determining the proliferation and differentiation status of HSCs at the individual cell level by permitting measurements of clone size and composition. Competitive repopulating advantages of young or old HSCs can then be ascribed to qualitative properties such as higher proliferative state or to quantitative variation in population size.23
In all experimental and clinical stem-cell transplantations, the critical first step leading to successful engraftment is homing of stem cells to the BM. Szilvassy et al23 previously showed that CRUs from fetal liver (FL) and young adult BM had roughly the same seeding efficiency to the marrow of lethally irradiated young recipients when measured 24 hours after intravenous transplantation. However, Morrison et al6 found that old stem cells have only approximately one fourth the competitive repopulating activity of young stem cells. As more older patients become candidates for transplantation in the treatment of hematologic malignancies and nonhematologic diseases, the effects of aging on the homing of stem/progenitor cells are clinically relevant yet remain largely unexplored.
Materials and methods
Animals
Female C57BL/6J (B6) mice (Ptprcb [Ly-5.2]) were used as BM donors, and congenic female B6.SJL(BoyJ) mice (Ptprca [Ly-5.1]) were used as recipients. Young mice (6-8 weeks of age) were purchased from Charles River Laboratories (Frederick, MD) through the National Cancer Institute Animal Program. Old mice (22-25 months of age) either were purchased from Harlan (Indianapolis, IN) through the National Institute on Aging Animal Program or were aged at our own facilities. Mice were maintained under specific pathogen-free conditions in the animal facility of the University of Kentucky Chandler Medical Center.
Homing assay for CRUs
Young or old B6.SJL mice were exposed to 9.0 Gy total body irradiation administered in 2 doses of 4.5 Gy approximately 3 hours apart. Later the same day, 6 irradiated mice (primary recipients) were injected intravenously with 3 × 106 B6 BM cells, and, 24 hours later, cells from pooled femora and tibiae were harvested into 1.5 mL medium. Assuming that the 4 long bones represented 25% of the total marrow mass of the mouse,24 this cell suspension thus contained 1.5 primary BM equivalents (3.0 equivalents analyzed in total for the 2 replicates of this experiment; performed as depicted in Tables 1 and 2) at a concentration of one “homed” BM equivalent per milliliter. To measure the B6 HSC content of this homed suspension, 4 groups of secondary, irradiated B6.SJL mice (6-8 weeks of age) were injected with graded numbers of primary BM cells (0.5%-12.5% homed BM equivalents per mouse), together with 2 × 105 B6.SJL competitor BM cells (6-8 weeks of age). The number of CRUs in the original B6 cell suspensions (fresh BM cells) was determined by limiting-dilution assays in separate sets of irradiated B6.SJL animals (6-8 weeks of age). Each of 4 groups of mice was injected with 2 × 103 to 6 × 104 BM cells admixed with 2 × 105 B6.SJL competitor BM cells, as previously described.23,25 Recipients of fresh or homed BM cells were bled from the retro-orbital sinus at 5, 10, 17, and 26 weeks after transplantation. Erythrocytes were depleted from each peripheral blood (PB) sample by hypotonic lysis, and the remaining leukocytes were then stained in triplicate with a donor (B6)-specific anti-Ly-5.2 monoclonal antibody (mAb) conjugated with fluorescein isothiocyanate (FITC) (clone ALI4A2; purified from hybridoma supernatant and conjugated in our laboratory) and phycoerythrin (PE)-conjugated mAbs (Becton Dickinson-Pharmingen, San Diego, CA) specific for B (anti-CD45R/B220; clone RA3-6B2) or T (anti-Thy-1.2; clone 30H12) lymphocytes or for granulocytes (anti-Ly6G/Gr-1; clone RB6-8C5) and macrophages (anti-CD11b/Mac-1; clone M1/70). Samples were analyzed using a FACScan instrument (Becton Dickinson Immunocytometry Systems, San Jose, CA). The frequencies of CRUs in the initial B6 BM suspensions, and in the BM of primary mice 24 hours after homing, were calculated from the proportions of negative recipients (in which less than 5% of the circulating B, T, and myeloid cells were regenerated by Ly-5.2+ stem cells) in each cell-dose group using L-Calc software (StemCell Technologies Inc, Vancouver, BC, Canada). The seeding efficiency of CRUs was then calculated by dividing the number of stem cells recovered in the BM of primary recipients by the number initially transplanted (×100%). Mice that received single HSCs were retrospectively identified by 2 criteria: they were determined by limiting-dilution analysis to have undergone transplantation with 0.3 CRU or less, and they contained more than 5% donor (B6)-derived PB cells detectable among B, T, and myeloid lineages.23 Although experiments to measure the homing of young CRUs to young BM were previously reported in part,23 these experiments were performed contemporaneously with the present studies using animals of varying ages.
Determination of the number of intravenously transplanted young CRUs that home to the BM of old irradiated mice
. | Negative mice* . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell type injected . | 5 wk . | 10 wk . | 17 wk . | 26 wk . | |||
| Fresh young BM¶ | |||||||
| 2 × 103 | 15 of 16 | 15 of 16 | 16 of 16 | 16 of 16 | |||
| 6 × 103 | 12 of 16 | 12 of 16 | 12 of 16 | 12 of 16 | |||
| 2 × 104 | 3 of 15 | 4 of 15 | 7 of 15 | 7 of 14 | |||
| 6 × 104 | 0 of 8 | 0 of 8 | 0 of 8 | 0 of 8 | |||
| 1/CRU frequency† (±SEM) | 15242 (12 124 ∼ 19 163) | 16 814 (13 350 ∼ 21 178) | 23 608 (18 571 ∼ 30 012) | 24 578 (19 232 ∼ 31 409) | |||
| No. CRUs injected‡ (±SEM) | 197 (157 ∼ 247) | 178 (142 ∼ 225) | 127 (100 ∼ 162) | 122 (96 ∼ 156) | |||
| Homed young BM¶ | |||||||
| 0.005 | 8 of 8 | 8 of 8 | 7 of 7 | 7 of 7 | |||
| 0.014 | 13 of 14 | 13 of 13 | 13 of 13 | 13 of 13 | |||
| 0.042 | 11 of 14 | 10 of 14 | 11 of 14 | 9 of 11 | |||
| 0.125 | 7 of 14 | 6 of 14 | 8 of 14 | 8 of 14 | |||
| CRU frequency, 1 per primary BM fraction (±SEM)§ | 0.182 (0.134 ∼ 0.247) | 0.159 (0.119 ∼ 0.213) | 0.231 (0.166 ∼ 0.32) | 0.247 (0.174 ∼ 0.352) | |||
| 1/CRU frequency, no. CRUs recovered per primary BM (±SEM)∥ | 5.5 (4 ∼ 7.5) | 6.3 (4.7 ∼ 8.4) | 4.3 (3.1 ∼ 6.0) | 4.0 (2.8 ∼ 5.7) | |||
. | Negative mice* . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell type injected . | 5 wk . | 10 wk . | 17 wk . | 26 wk . | |||
| Fresh young BM¶ | |||||||
| 2 × 103 | 15 of 16 | 15 of 16 | 16 of 16 | 16 of 16 | |||
| 6 × 103 | 12 of 16 | 12 of 16 | 12 of 16 | 12 of 16 | |||
| 2 × 104 | 3 of 15 | 4 of 15 | 7 of 15 | 7 of 14 | |||
| 6 × 104 | 0 of 8 | 0 of 8 | 0 of 8 | 0 of 8 | |||
| 1/CRU frequency† (±SEM) | 15242 (12 124 ∼ 19 163) | 16 814 (13 350 ∼ 21 178) | 23 608 (18 571 ∼ 30 012) | 24 578 (19 232 ∼ 31 409) | |||
| No. CRUs injected‡ (±SEM) | 197 (157 ∼ 247) | 178 (142 ∼ 225) | 127 (100 ∼ 162) | 122 (96 ∼ 156) | |||
| Homed young BM¶ | |||||||
| 0.005 | 8 of 8 | 8 of 8 | 7 of 7 | 7 of 7 | |||
| 0.014 | 13 of 14 | 13 of 13 | 13 of 13 | 13 of 13 | |||
| 0.042 | 11 of 14 | 10 of 14 | 11 of 14 | 9 of 11 | |||
| 0.125 | 7 of 14 | 6 of 14 | 8 of 14 | 8 of 14 | |||
| CRU frequency, 1 per primary BM fraction (±SEM)§ | 0.182 (0.134 ∼ 0.247) | 0.159 (0.119 ∼ 0.213) | 0.231 (0.166 ∼ 0.32) | 0.247 (0.174 ∼ 0.352) | |||
| 1/CRU frequency, no. CRUs recovered per primary BM (±SEM)∥ | 5.5 (4 ∼ 7.5) | 6.3 (4.7 ∼ 8.4) | 4.3 (3.1 ∼ 6.0) | 4.0 (2.8 ∼ 5.7) | |||
Lethally irradiated old (20-22 months of age) Ly-5.1 mice were injected with 3 × 106 BM cells from young (6-8 weeks of age) Ly-5.2 mice, and their marrow was harvested 24 hours later. Frequencies of CRUs in this primary BM and in the B6 suspension injected initially were determined by transplanting graded numbers of fresh or homed BM cells into separate sets of lethally irradiated Ly-5.1 recipients analyzed for donor (Ly-5.2+)-derived engraftment at the indicated times. Pooled data are from 2 independent experiments.
Negative mice are defined as animals in which less than 5% of the circulating B or T lymphocytes or myeloid cells, or any combination of them, were derived from donor (Ly-5.2+) stem cells at the time of analysis. Values for fresh young BM and homed young BM are proportions.
Shown are the number of BM cells containing one CRU. The range in CRU frequencies defined by ± 1 SEM is shown in parentheses.
Absolute values were determined by multiplying 1/CRU frequency (†) by 3 × 106 cells, the number of fresh cells injected into primary recipients.
Frequencies of homed CRUs are expressed as the proportion of primary BM cells that contain one CRU.
Absolute numbers of CRUs that homed to the total primary BM was determined by inverting the frequencies of homed CRUs (§).
No. fresh cells or fraction of homed primary BM injected per mouse.
Determination of the number of intravenously transplanted old CRUs that home to the BM of young irradiated mice
. | Negative mice . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell type injected . | 5 wk . | 10 wk . | 17 wk . | 26 wk . | |||
| Fresh old BM* | |||||||
| 2 × 103 | 12 of 15 | 12 of 15 | 11 of 14 | 11 of 14 | |||
| 6 × 103 | 5 of 14 | 5 of 14 | 8 of 14 | 8 of 14 | |||
| 2 × 104 | 1 of 14 | 1 of 14 | 2 of 14 | 3 of 14 | |||
| 6 × 104 | 0 of 8 | 0 of 8 | 0 of 8 | 0 of 8 | |||
| 1/CRU frequency (±SEM) | 7006 (5548 ∼ 8848) | 7006 (5548 ∼ 8848) | 10 019 (7923 ∼ 12 671) | 11 304 (8932 ∼ 14 306) | |||
| No. CRUs injected (±SEM) | 428 (339 ∼ 541) | 428 (339 ∼ 541) | 299 (237 ∼ 379) | 265 (210 ∼ 336) | |||
| Homed old BM* | |||||||
| 0.005 | 6 of 6 | 6 of 6 | 6 of 6 | 6 of 6 | |||
| 0.014 | 10 of 12 | 8 of 9 | 7 of 8 | 4 of 5 | |||
| 0.042 | 9 of 14 | 8 of 13 | 8 of 13 | 9 of 13 | |||
| 0.125 | 4 of 10 | 4 of 10 | 4 of 10 | 4 of 10 | |||
| CRU frequency, 1 per primary BM fraction (±SEM) | 0.113 (0.085 ∼ 0.151) | 0.116 (0.086 ∼ 0.157) | 0.115 (0.085 ∼ 0.155) | 0.124 (0.091 ∼ 0.169) | |||
| 1/CRU frequency, no. CRUs recovered per primary BM (±SEM) | 8.8 (6.6 ∼ 11.8) | 8.6 (6.4 ∼ 11.6) | 8.7 (6.5 ∼ 11.8) | 8.1 (5.9 ∼ 11) | |||
. | Negative mice . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell type injected . | 5 wk . | 10 wk . | 17 wk . | 26 wk . | |||
| Fresh old BM* | |||||||
| 2 × 103 | 12 of 15 | 12 of 15 | 11 of 14 | 11 of 14 | |||
| 6 × 103 | 5 of 14 | 5 of 14 | 8 of 14 | 8 of 14 | |||
| 2 × 104 | 1 of 14 | 1 of 14 | 2 of 14 | 3 of 14 | |||
| 6 × 104 | 0 of 8 | 0 of 8 | 0 of 8 | 0 of 8 | |||
| 1/CRU frequency (±SEM) | 7006 (5548 ∼ 8848) | 7006 (5548 ∼ 8848) | 10 019 (7923 ∼ 12 671) | 11 304 (8932 ∼ 14 306) | |||
| No. CRUs injected (±SEM) | 428 (339 ∼ 541) | 428 (339 ∼ 541) | 299 (237 ∼ 379) | 265 (210 ∼ 336) | |||
| Homed old BM* | |||||||
| 0.005 | 6 of 6 | 6 of 6 | 6 of 6 | 6 of 6 | |||
| 0.014 | 10 of 12 | 8 of 9 | 7 of 8 | 4 of 5 | |||
| 0.042 | 9 of 14 | 8 of 13 | 8 of 13 | 9 of 13 | |||
| 0.125 | 4 of 10 | 4 of 10 | 4 of 10 | 4 of 10 | |||
| CRU frequency, 1 per primary BM fraction (±SEM) | 0.113 (0.085 ∼ 0.151) | 0.116 (0.086 ∼ 0.157) | 0.115 (0.085 ∼ 0.155) | 0.124 (0.091 ∼ 0.169) | |||
| 1/CRU frequency, no. CRUs recovered per primary BM (±SEM) | 8.8 (6.6 ∼ 11.8) | 8.6 (6.4 ∼ 11.6) | 8.7 (6.5 ∼ 11.8) | 8.1 (5.9 ∼ 11) | |||
Experimental design and determination of the frequencies and absolute numbers of CRUs transplanted and recovered after homing are the same as described in Table 1, except that donor cells were harvested initially from old (20-22 months of age) mice and that young (6-8 weeks of age) mice were used as recipients. For negative mice, values for fresh young BM and homed young BM are proportions.
No. fresh cells or fraction of homed primary BM cells injected per mouse.
Homing assay for HPCs
Young or old B6 BM cells were injected into lethally irradiated old or young B6.SJL recipients (1 × 107 cells/mouse). Three hours after homing, the number of clonogenic progenitors in these BM suspensions and in the BM and spleen of mice that had undergone transplantation was determined by plating cells in duplicate 35-mm culture dishes (3 × 104-1 × 105 cells/dish) containing Methocult medium (StemCell Technologies), as previously described.25 BM and spleen cells from mice that underwent irradiation but not transplantation served as negative controls and did not generate any colonies in this assay. The total number of hematopoietic progenitor cells (HPCs) that had homed to the BM and spleen was calculated by multiplying progenitor-cell frequencies by total organ cellularities (in the case of the marrow, assuming that 2 femurs and 2 tibiae represented 25% of the total marrow mass of the mouse).24 The seeding efficiency of HPCs was then calculated by dividing progenitor cells that were recovered in the BM or spleen of primary recipients by the total number initially transplanted (×100%).
Analysis of lineage-specific engraftment kinetics
Young or old B6.SJL(BoyJ) mice were irradiated, as described in “Homing assays for CRUs,” and injected intravenously with 2 × 106 old or 3 × 106 young nucleated BM cells, respectively. PB was collected from the retro-orbital sinus 6, 9, 12, 15, 18, 25, 32, 42, 56, and 120 days after transplantation. Until day 25, only half the mice in each cohort were analyzed alternately at each time point so that no individual animal was bled more frequently than every 7 days. Circulating leukocyte, erythrocyte, and platelet counts were measured by analysis of 40 μL blood using a System 9118+ Hematology Series Cell Counter (Biochem Immunosystems, Allentown, PA).
Statistical analysis
All values represent the mean ± SEM. Statistical differences between means were assessed using the 2-tailed t test assuming unequal variances and analysis of variance (ANOVA) with 2 factors.
Results
Homing efficiency of HPCs declines with donor and recipient age
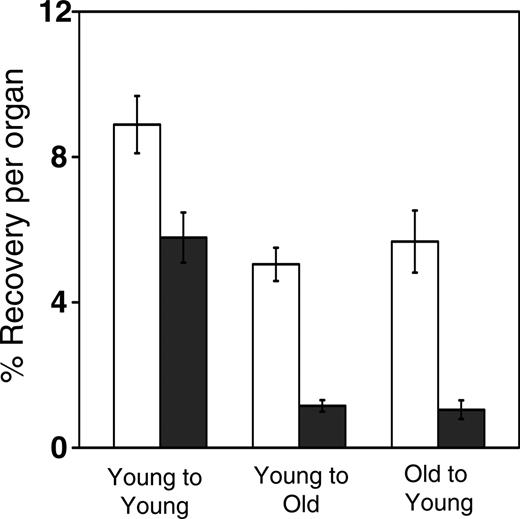
Compared with young adult BM progenitors, approximately 10-fold fewer fetal liver HPCs homed to the BM 3 hours after intravenous injection,25 and a similar trend toward reduced BM homing of FL HSCs has been reported.23 To determine whether aging of the BM also influenced homing, lethally irradiated young (6-8 weeks of age) or old (22-25 months of age) Ly-5.1 mice received transplanted BM cells from young or old Ly-5.2 mice, and 3 hours later recipient BM and spleen cells were assayed for HPCs. As shown in Figure 1, advanced donor or recipient age reduced the homing of HPCs to the BM by approximately one third (from approximately 9% to approximately 6%; P < .01). Age-related intrinsic (progenitor-dependent) and extrinsic (microenvironment-dependent) mechanisms contributing to decreased BM seeding efficiency were similar in magnitude. The effects of age on splenic homing of HPCs were even more dramatic. HPC recovery declined from approximately 6% for the young-into-young donor-host combination to approximately 1% for the young-into-old and old-into-young combinations (P < .001).
Homing efficiency of HSCs declines with donor and recipient age
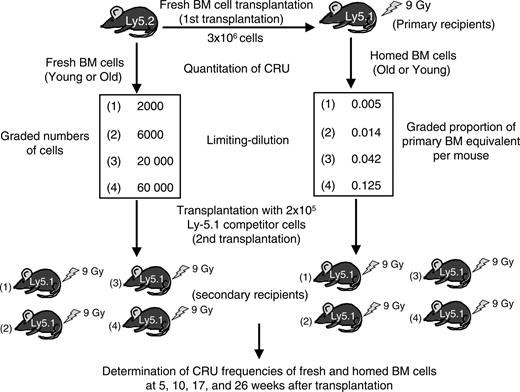
We hypothesized that aging had similar effects on the homing of more primitive HSCs that regenerate and maintain lymphohematopoiesis for up to 6 months after transplantation. To address this possibility, we used a limiting-dilution competitive repopulation assay to measure the 24-hour BM homing efficiencies of young adult BM cells transplanted into old recipients and old BM cells transplanted into young recipients. The design of this assay is depicted in Figure 2. BM cells isolated from young or old Ly-5.2 mice were injected into lethally irradiated, old or young Ly-5 congenic mice (3 × 106 cells/mouse). Twenty-four hours later, BM from these primary recipients was harvested and competitively transplanted into Ly-5.1 secondary recipients to measure the number of Ly-5.2+ CRU that had homed to this organ. Simultaneously, the number of CRUs present in the original young or old B6 BM-cell suspensions was measured by limiting-dilution competitive repopulation assays in a separate set of irradiated Ly-5.1 mice.
Reduced homing capacity of HPCs with donor and recipient age. Lethally irradiated young or old Ly-5.1 mice were injected with 2 × 106 old or young Ly-5.2 donor cells. Three hours later, HPCs that had homed to the BM (□) or spleen (▪) were measured by hematopoietic colony formation in vitro. Shown are the mean (± SEM) percentages of donor-derived HPCs recovered per organ, relative to numbers injected. Data are pooled from 3 independent experiments with 3 to 5 mice per group. Differences between young cells injected into young recipients and either young cells transplanted into old recipients or old cells transplanted into young recipients are significant (P < .05).
Reduced homing capacity of HPCs with donor and recipient age. Lethally irradiated young or old Ly-5.1 mice were injected with 2 × 106 old or young Ly-5.2 donor cells. Three hours later, HPCs that had homed to the BM (□) or spleen (▪) were measured by hematopoietic colony formation in vitro. Shown are the mean (± SEM) percentages of donor-derived HPCs recovered per organ, relative to numbers injected. Data are pooled from 3 independent experiments with 3 to 5 mice per group. Differences between young cells injected into young recipients and either young cells transplanted into old recipients or old cells transplanted into young recipients are significant (P < .05).
Results from the experiments performed with young BM cells transplanted into old recipients are given in Table 1, and results from the experiments performed with old BM cells transplanted into young recipients are given in Table 2. Comparison of the upper half of the 2 tables shows that the frequency of early (5- and 10-week time points) and long-term (17- and 26-week time-points) CRUs increased by approximately 2-fold from early adulthood to old age. For example, CRUs that contributed to hematopoiesis for at least 10 weeks after transplantation represented 1 per 20 000 young BM cells and 1 per 10 000 old BM cells. As shown previously, CRUs contributing to early engraftment were more prevalent (one 5-week CRU per approximately 15 000 young BM cells) than those detectable at later times (one 26-week CRU per 7000 old BM cells), consistent with the hierarchic organization of the stem-cell compartment.26,27 Furthermore, the HSC frequencies obtained in this study using a rigorous functional assay are similar to those determined previously using phenotypic or surrogate in vitro measures (such as cobblestone area formation) of stem cells and confirm that HSCs in B6 mice increase significantly with age.4,6,12,23
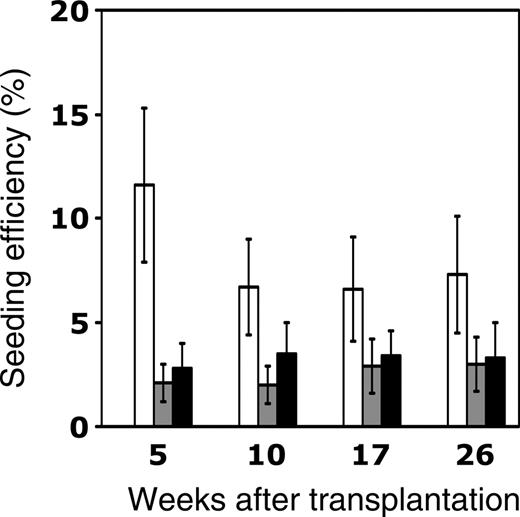
The seeding efficiency of young or old CRUs in old or young recipients, respectively, was determined by dividing the total number of CRUs recovered in the BM of primary recipients 24 hours after homing (as shown in the lower halves of Tables 1 and 2) by the absolute number of CRUs originally injected (calculated from the CRU frequencies in freshly isolated BM in the upper halves of Tables 1 and 2). These data are summarized in Figure 3 and are compared with previous studies begun contemporaneously with the present studies in which young BM CRUs were transplanted into young recipients.23 Twice as many young (6%-7%) as old (2%-3%) HSCs homed to the BM of young recipients the first day after transplantation. Moreover, CRUs that generated lymphoid and myeloid progeny at 5 weeks exhibited greater declines in homing as they aged than CRUs detectable at later times. Of equal importance, advanced recipient age also resulted in significantly reduced homing of HSCs (from 6%-7% to 2%-3%) compared with young CRUs transplanted into young hosts. Thus, the effects of donor or recipient aging are similar, each leading individually to a 50% to 60% decline in HSC homing compared with their young counterparts.
Experimental design for competitive repopulation studies. Lethally irradiated young or old Ly-5.1 mice were intravenously injected with 3 × 106 old or young Ly-5.2 BM cells. Twenty-four hours later, marrow from the primary recipients was harvested to assay the number of Ly-5.2+ stem cells that had homed there after transplantation. The frequencies of CRUs in the initial BM suspensions and in the BM of primary mice after homing were determined by competitive repopulation of 2 sets of Ly-5.1 mice that underwent transplantation with graded numbers of fresh or homed BM cells admixed with 2 × 105 Ly-5.1 competitor BM cells. Donor (Ly-5.2)-derived lymphoid and myeloid cells in PB were then measured at 5, 10, 17, and 26 weeks after transplantation. CRU frequencies were calculated from the proportions of negative mice in each cell-dose group, as described in “Materials and methods.”
Experimental design for competitive repopulation studies. Lethally irradiated young or old Ly-5.1 mice were intravenously injected with 3 × 106 old or young Ly-5.2 BM cells. Twenty-four hours later, marrow from the primary recipients was harvested to assay the number of Ly-5.2+ stem cells that had homed there after transplantation. The frequencies of CRUs in the initial BM suspensions and in the BM of primary mice after homing were determined by competitive repopulation of 2 sets of Ly-5.1 mice that underwent transplantation with graded numbers of fresh or homed BM cells admixed with 2 × 105 Ly-5.1 competitor BM cells. Donor (Ly-5.2)-derived lymphoid and myeloid cells in PB were then measured at 5, 10, 17, and 26 weeks after transplantation. CRU frequencies were calculated from the proportions of negative mice in each cell-dose group, as described in “Materials and methods.”
Effects of aging and previous transplantation on the proliferation potential of HSCs
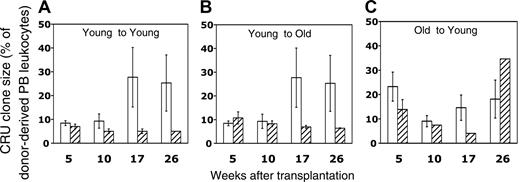
One advantage of the CRU assay is that it enables the retrospective identification of recipient mice repopulated by a single HSC. Circulating blood cells in such mice, identified using criteria described previously23 and in “Materials and methods,” were then analyzed in more detail to characterize the proliferation and differentiation potentials of individual CRUs in vivo. To assess the impact of aging on proliferation potential, we compared the sizes of clones generated by single young or old CRU (expressed as the percentage of donor-derived PB leukocytes) in recipients of varying ages. Average clone sizes of freshly isolated young CRUs in young recipients differed for stem cells whose progeny were detected early or late after transplantation (Figure 4A) (approximately 9% for 5- and 10-week CRUs compared with 25%-28% for 17- and 26-week CRUs; P < .05). This finding is consistent with the empirical use of longer assay times in repopulation assays to identify more primitive HSCs with a higher proliferation potential. Perhaps surprisingly, no difference was observed in the sizes of clones generated by young and old CRUs (Figure 4A, C). However, young and old long-term CRUs (17 and 26 weeks) selected by earlier homing to young or old BM generally produced smaller clones than those generated by freshly isolated HSCs. The sole exception was the larger clone generated by homed old CRUs at the 26-week time point; however, because this value was derived from a single clone in a single animal, we viewed it with caution. Interestingly, young CRUs that previously homed to young or old BM did not differ in their subsequent proliferation in young secondary recipients (Figure 4; compare panels A and B for homed HSCs). In aggregate, these data indicate that the proliferation potential of long-term repopulating HSCs is reduced significantly by previous transplantation.
Effects of aging and previous transplantation on the differentiation potential of HSCs
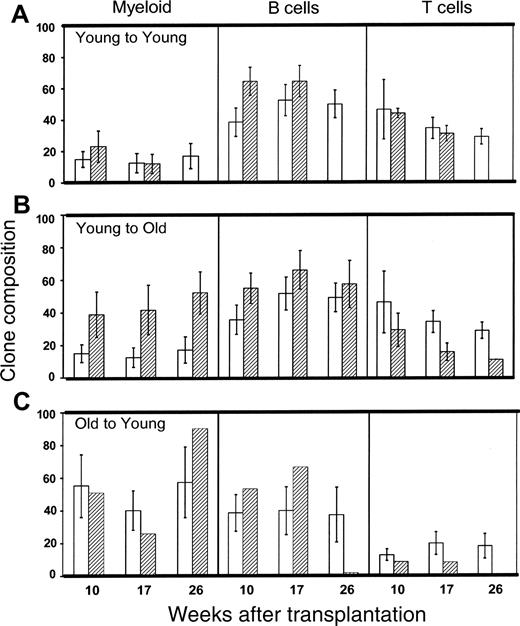
We next compared the proportions of B and T lymphocytes and of myeloid cells in the PB of young or old animals engrafted with single, freshly isolated, or homed CRUs that were isolated initially from young or old donor mice. As shown in Figure 5, clones generated by fresh old CRUs contained approximately 5-fold more myeloid cells (40%-60% of donor-derived cells) and approximately 2.5-fold fewer T cells (15%-20% of donor-derived cells) than those produced by young CRUs (Figure 5; compare panels A and C). This skewing of the differentiation potential of old HSCs toward myelopoiesis, at the expense of T-lymphocyte production, was also evident among old CRUs that had been subjected to earlier homing (Figure 4C). Myeloid skewing of older HSCs has been reported previously8,28 and suggests an age-related alteration of their proportional production of various blood cell lineages relative to that normally observed in early adulthood.
Age-related decline in the homing efficiency of murine HSCs. Lethally irradiated young or old Ly-5.1 mice were intravenously injected with 2-3 × 106 old or young Ly-5.2 donor cells, as depicted in Figure 2. The number of CRUs recovered from the BM 24 hours later was determined by limiting-dilution competitive repopulation assays conducted in young secondary recipients assessed for donor engraftment at 5, 10, 17, and 26 weeks after transplantation (Tables 1, 2). The homing efficiency of HSCs was calculated by dividing the number of CRUs recovered in the primary BM 24 hours after transplantation by the number of CRUs initially injected and then multiplying by 100%. Three different transplantation groups—young cells into young recipients (□), young cells into old recipients (▦), and old cells into young recipients (▪)—are depicted. Data for young-to-old and old-to-young groups are derived from the experiments described in Tables 1 and 2. Data for the young-to-young group, though collected contemporaneously with those for the other transplantation groups, were reported previously by Szilvassy et al23 and are shown for comparison. All values are mean ± SEM, and statistically significant differences are designated with an asterisk (P < .05).
Age-related decline in the homing efficiency of murine HSCs. Lethally irradiated young or old Ly-5.1 mice were intravenously injected with 2-3 × 106 old or young Ly-5.2 donor cells, as depicted in Figure 2. The number of CRUs recovered from the BM 24 hours later was determined by limiting-dilution competitive repopulation assays conducted in young secondary recipients assessed for donor engraftment at 5, 10, 17, and 26 weeks after transplantation (Tables 1, 2). The homing efficiency of HSCs was calculated by dividing the number of CRUs recovered in the primary BM 24 hours after transplantation by the number of CRUs initially injected and then multiplying by 100%. Three different transplantation groups—young cells into young recipients (□), young cells into old recipients (▦), and old cells into young recipients (▪)—are depicted. Data for young-to-old and old-to-young groups are derived from the experiments described in Tables 1 and 2. Data for the young-to-young group, though collected contemporaneously with those for the other transplantation groups, were reported previously by Szilvassy et al23 and are shown for comparison. All values are mean ± SEM, and statistically significant differences are designated with an asterisk (P < .05).
Aging of the recipient BM microenvironment had similar effects on HSC differentiation. Young CRUs homed in old recipients also produced approximately 3.5-fold more myeloid cells and approximately 2-fold fewer T cells than young CRUs homed to young BM or than freshly isolated CRUs (Figure 5; compare panels A and B; P < .05). There were no significant changes in the average proportional representation of B-lineage cells in the clones produced by freshly isolated or by homed HSCs in young or old recipients. These data suggest that HSC differentiation potential changes not only as a function of cell-autonomous, age-related mechanisms but also in response to extrinsic signals from the marrow microenvironment that are altered by aging.
Effects of aging and previous transplantation on the proliferation potential of CRUs. After determination of the CRU frequency in fresh and homed BM (Tables 1, 2), it was possible to retrospectively identify mice that had been injected with less than 0.3 CRU and in which the lymphoid and the myeloid compartments were subsequently repopulated with donor stem cells. On the basis of Poisson statistics, it is 95% probable that such mice were engrafted with a single HSC. Clone sizes generated by single, fresh (□) or homed (▨) CRUs as a function of donor and recipient age are represented as the mean ± SEM percentage of donor (Ly-5.2+)-derived PB leukocytes assessed 5 to 26 weeks after transplantation (4-8 mice per group from 2 pooled experiments). Note that data for the young-to-young group were collected contemporaneously with those for the other groups but were, in part, reported in Szilvassy et al.23
Effects of aging and previous transplantation on the proliferation potential of CRUs. After determination of the CRU frequency in fresh and homed BM (Tables 1, 2), it was possible to retrospectively identify mice that had been injected with less than 0.3 CRU and in which the lymphoid and the myeloid compartments were subsequently repopulated with donor stem cells. On the basis of Poisson statistics, it is 95% probable that such mice were engrafted with a single HSC. Clone sizes generated by single, fresh (□) or homed (▨) CRUs as a function of donor and recipient age are represented as the mean ± SEM percentage of donor (Ly-5.2+)-derived PB leukocytes assessed 5 to 26 weeks after transplantation (4-8 mice per group from 2 pooled experiments). Note that data for the young-to-young group were collected contemporaneously with those for the other groups but were, in part, reported in Szilvassy et al.23
Effect of aging and transplantation on the differentiation potential of CRUs. Mice that had been repopulated with a single, fresh (□) or homed (▨) CRU were identified as described in the Figure 4 legend and in “Materials and methods.” The lineage composition of clones generated by single, young, or old HSCs in old or young hosts was defined as the proportion of donor (Ly-5.2+)-derived leukocytes expressing markers for B (CD45R/B220+) or T (CD90/Thy-1.2+) lymphocytes or for myeloid (Gr-1/Ly6G+ and Mac-1/CD11b+) cells. Values shown for the 3 combinations of variably aged donors and recipients represent the mean ± SEM of 2 pooled experiments with 3 to 6 mice per group. Note that data for the young-to-young group were collected contemporaneously with those for other groups but were, in part, reported in Szilvassy et al.23
Effect of aging and transplantation on the differentiation potential of CRUs. Mice that had been repopulated with a single, fresh (□) or homed (▨) CRU were identified as described in the Figure 4 legend and in “Materials and methods.” The lineage composition of clones generated by single, young, or old HSCs in old or young hosts was defined as the proportion of donor (Ly-5.2+)-derived leukocytes expressing markers for B (CD45R/B220+) or T (CD90/Thy-1.2+) lymphocytes or for myeloid (Gr-1/Ly6G+ and Mac-1/CD11b+) cells. Values shown for the 3 combinations of variably aged donors and recipients represent the mean ± SEM of 2 pooled experiments with 3 to 6 mice per group. Note that data for the young-to-young group were collected contemporaneously with those for other groups but were, in part, reported in Szilvassy et al.23
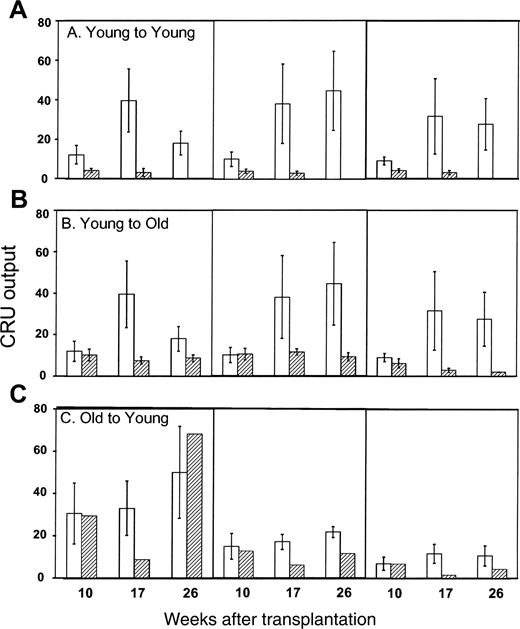
Finally, we compared the contributions individual CRUs made to all PB cells of the myeloid, B, or T lineages (Figure 6). In general, homed CRUs recovered from the BM of primary recipients 24 hours after transplantation made significantly smaller contributions to all 3 lineages in secondary mice than did fresh CRUs that were allowed to engraft primary hosts long term. Once again, donor aging resulted in increased myeloid contribution, and the total numbers of myeloid and B cells produced by young CRUs that had homed to old BM were 2- to 3-fold higher than those generated from young CRUs homed to young BM (Figure 6; compare panels A and B).
Differential contribution of single BM CRU to hematopoietic engraftment. Recipient mice that were repopulated with a single, fresh (□) or homed (▨) CRU were identified as described in the Figure 4 legend and in “Materials and methods.” The differential contribution of individual CRUs to engraftment was defined as the proportion of all circulating B (CD45R/B220+) or T (CD90/Thy-1.2+) lymphocytes or myeloid (Gr-1/Ly6G+ and Mac-1/CD11b+) cells that were Ly-5.2+. Values shown for the 3 combinations of variably aged donors and recipients represent the mean ± SEM of 2 pooled experiments with 3 to 6 mice per group. Note that data for the young-to-young group were collected contemporaneously with those for other groups but were, in part, reported in Szilvassy et al.23
Differential contribution of single BM CRU to hematopoietic engraftment. Recipient mice that were repopulated with a single, fresh (□) or homed (▨) CRU were identified as described in the Figure 4 legend and in “Materials and methods.” The differential contribution of individual CRUs to engraftment was defined as the proportion of all circulating B (CD45R/B220+) or T (CD90/Thy-1.2+) lymphocytes or myeloid (Gr-1/Ly6G+ and Mac-1/CD11b+) cells that were Ly-5.2+. Values shown for the 3 combinations of variably aged donors and recipients represent the mean ± SEM of 2 pooled experiments with 3 to 6 mice per group. Note that data for the young-to-young group were collected contemporaneously with those for other groups but were, in part, reported in Szilvassy et al.23
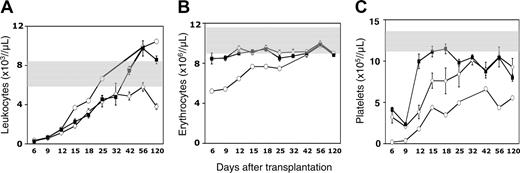
Differential engraftment kinetics of young and old BM cells in recipients of variable ages
We hypothesized that age-related variation in the homing efficiency of HSCs and HPCs would translate into differences in the subsequent rate of hematopoietic reconstitution. To address this possibility, young or old BM cells were injected into old or young Ly-5 congenic recipients, and the recovery of circulating blood cells was measured over 4 months after transplantation. Until day 12, the rate of leukocyte recovery was indistinguishable among the 3 groups (young into young, young into old, and old into young) (Figure 7A). Thereafter, the rate of leukocyte recovery in old mice that underwent transplantation with young BM cells began to surpass that of the other 2 groups. In young recipients engrafted with old BM, leukocyte production accelerated sharply after approximately 1 month and reached normal levels shortly after the young-to-old pairing. White blood cell recovery in young mice injected with young BM cells lagged behind that of the other 2 groups and failed to reach normal levels even after 4 months. This finding was consistent with the myeloid skewing of old CRUs, or as a result of recipient aging, that was observed in Figure 5. Young and old BM cells were similarly effective in preventing anemia after transplantation into young recipients (Figure 7B). In contrast, old mice that underwent transplantation with young BM cells became severely anemic early after transplantation, and erythrocytes recovered at a slow rate until they reached normal levels after 6 weeks.
Differential engraftment kinetics of young or old BM cells in old or young recipients. 2 × 106 young (⋄) or old (▪) Ly-5.2 BM cells were injected into lethally irradiated young mice, or 3 × 106 young Ly-5.2 BM cells were injected into lethally irradiated old Ly-5.1 mice (○). Shown are the mean ± SEM number of PB leukocytes (A), erythrocytes (B), and platelets (C) counted on the indicated days after transplantation (pooled data from 2 experiments with 10 mice per group). The absence of error bars for specific data points indicates that fewer than 3 animals were available for analysis. Note that the time after transplantation is not depicted on a linear scale. Shaded areas indicate normal ranges of blood cell counts in age-matched B6.SJL control mice. Data for the young-to-young group were collected contemporaneously with those of other groups but were, in part, reported in Szilvassy et al.23
Differential engraftment kinetics of young or old BM cells in old or young recipients. 2 × 106 young (⋄) or old (▪) Ly-5.2 BM cells were injected into lethally irradiated young mice, or 3 × 106 young Ly-5.2 BM cells were injected into lethally irradiated old Ly-5.1 mice (○). Shown are the mean ± SEM number of PB leukocytes (A), erythrocytes (B), and platelets (C) counted on the indicated days after transplantation (pooled data from 2 experiments with 10 mice per group). The absence of error bars for specific data points indicates that fewer than 3 animals were available for analysis. Note that the time after transplantation is not depicted on a linear scale. Shaded areas indicate normal ranges of blood cell counts in age-matched B6.SJL control mice. Data for the young-to-young group were collected contemporaneously with those of other groups but were, in part, reported in Szilvassy et al.23
Figure 7C depicts the dramatic age-related differences observed in platelet recovery kinetics. Young BM cells required nearly a month to regenerate near normal levels of platelets after transplantation into young recipients. In contrast, transplantation of old marrow into young mice led to normal platelet counts in approximately 15 days. Profound thrombocytopenia was still not resolved by 4 months after the transplantation of young marrow into old mice. These data illustrate the strong intrinsic bias of old BM cells toward platelet production and the failure of the old marrow microenvironment to foster platelet recovery when seeded with young marrow.
Discussion
Homing of primitive stem/progenitor cells to the BM represents the crucial first step to successful engraftment after transplantation. However, the use of the term homing in the literature has been confusing and duplicitous, referring to the initial events after hematopoietic-cell infusion, in which primitive cells lodge in supportive niches, and extending erroneously to the subsequent engraftment process, which is characterized by differentiated blood cell formation over many weeks and months. Experiments conducted in the 1960s to measure the 24-hour seeding efficiency of spleen colony-forming units (CFU-Ss), with “f” denoting factor,29 yielded estimates of 10% to 20%.29-31 Subsequent studies to determine the “f” of HSCs for BM seeding yielded estimates of approximately 1% per femur, or approximately 12% for the entire marrow mass of the mouse.32,33 As rigorous functional assays for HSCs have been refined, a similar value of approximately 10% was obtained for CRUs derived from murine BM and FL23 and for human CB- and FL-repopulating cells assessed in a xenotransplantation model.34
These findings, and those from clinical experience that demonstrate graft failure if too few stem cells are transplanted,35,36 beg reconciliation with experiments demonstrating engraftment with only a few HSCs or even one purified HSC.37-39 In many such studies claiming highly efficient seeding, it was long-term engraftment that was assessed, not homing of HSCs or HPCs within the first day of infusion. Small numbers of (or even single) HSCs will engraft if they fortuitously lodge in a receptive niche in the BM or the spleen. Cao et al40 used bioluminescence imaging to monitor engraftment derived from single luciferase-labeled HSCs. They showed that foci of hematopoiesis are detectable within the first few weeks after transplantation in the spleen and in widely dispersed BM sites throughout the skeleton, with no apparent preference for one site or another.40 Primitive hematopoietic cells emanating from the primary focus then initiated additional foci that nearly completely engrafted all hematopoietic sites in the following several weeks. These results are consistent with a dynamic series of events initiating widespread hematopoiesis involving multiple progeny of transplanted HSCs.
Despite increasing evidence that aging causes quantitative and qualitative changes directly in HSCs or indirectly through BM stromal cells,2 the effects of age on the homing of primitive hematopoietic cells remain poorly understood. In the present study, we provide the first measurement of the absolute homing efficiency of old HSCs to BM 24 hours after intravenous injection. Only 2% to 3% of transplanted CRUs were recovered at this time from the BM of young recipients. This value is 2- to 3-fold lower than that for young HSCs infused into young recipients (7%-10%), as shown here and in previous studies measuring homing of hematopoietic cells labeled with a vital fluorescent dye.41,42 This reduced seeding efficiency for old CRUs is consistent with results reported by Morrison et al,6 who found that a smaller proportion of young recipients retained engraftment long term when they underwent transplantation with limiting numbers of phenotypically identical old rather than young HSCs.
Several mechanisms may be involved in the intrinsic, age-associated decline of HSC homing. First, impaired homing may reflect changes in the levels or binding activity of receptors, extracellular matrix molecules, or adhesion molecules during aging. Indirect evidence indicates that CXCR4 expression on HSCs may change with age43 and may result in their altered ability to enter BM “niches.” Possible modulation of CD26—a peptidase that is expressed on engrafting hematopoietic cells and that negatively regulates homing and engraftment potential44 —during aging also deserves study. Second, changes in the cycling status of HSCs during aging may alter their homing. HSCs that are in the G0 phase of the cell cycle at the time of transplantation promote higher levels of engraftment than HSCs in the G1 phase45,46 and especially HSCs in the S phase.47,48 A greater proportion of HSCs in old mice are cycling,6 so this difference may underlie their diminished capability for homing. Third, it is possible there is an age-related change in radiosensitivity of HSCs manifested in the present experiments by differential ablation of host HSCs and thus a differential probability with which transplanted HSCs may find unoccupied niches. In addition, there may be an age-related change in the radiosensitivity of the BM microenvironment such that hematopoietic support for stem cells that manage to seed old BM is compromised. To our knowledge, the effect of age on the radiosensitivity of HSCs has not been tested experimentally, but this important area deserves further study.
In the present study, we have for the first time quantitatively determined that recipient age has a dramatic influence on HSC homing; the seeding efficiency of young HSCs in the BM of old mice is only one third to one half (2%-3%) that measured in young mice. This striking difference points to a decline in the capacity of the marrow stroma to capture or retain, or both, stem cells in old age. Stromal cells regulate HSCs by secreting various growth factors and providing appropriate cell contacts that define the BM niche. Aged stromal cells produce lower amounts of interleukin-7 (IL-7) and are less capable of supporting the proliferation of B lymphocytes in vitro.49 Although no studies have demonstrated unequivocally that IL-7 is involved in the homing of HSCs, IL-7 and other stromal-cell-derived chemokines, such as stromal-cell-derived factor-1 (SDF-1), may be down-regulated during the aging process, leading to defects in the ability of HSCs to maintain associations with the stroma.50
In assays to measure the homing of HPCs, we also observed a dramatic (approximately 80%) decline in homing to the spleen with donor and recipient age. Reduced splenic seeding was not caused by a decrease in organ size or cellularity with age (data not shown). In these studies, the homing of progenitors was determined after 3 hours rather than after the 24-hour period used for HSCs to preclude unpredictable population size changes (either losses or gains) in HPCs that resulted from proliferation or differentiation initiated 1 day after transplantation. Because HPCs mediate short-term engraftment and ameliorate myelosuppression immediately after ablative conditioning, the present findings may indicate that donor and recipient age might be important in the clinical setting.
Analysis of mice that underwent transplantation at limiting dilution with less than 0.3 CRU and that, after engraftment, were ascertained with 95% statistical confidence to have been repopulated by a single HSC revealed important age-related differences in stem-cell developmental potential. No difference was observed in the sizes of clones generated by young and old CRUs, indicating that HSC proliferation potential is not affected by donor age. In contrast, young CRUs recovered from the BM of primary mice 24 hours after homing exhibited diminished proliferation in secondary recipients, regardless of the age of the primary host. Donor and recipient aging was associated with enhanced myelopoiesis at the expense of lymphopoiesis and was further amplified in HSCs selected by earlier homing. Our findings corroborate and extend those of several previous reports of the link between aging, transplantation, and this characteristic pattern of lineage skewing.8,17,51 Interestingly, in contrast to these findings of diminished differentiation potential of previously homed BM, Lanzkron et al52 and Krause et al53 found that lineage-negative and quiescent BM cells reisolated from the BM 48 hours after transplantation not only contained long-term repopulating HSCs52 but were capable of differentiation into nonhematopoietic lineages.53 Clear methodologic differences between their findings and our study likely account for this discrepancy: we did not enrich stem cells from either the initial marrow source or the BM that was reisolated for secondary transplantation, and we recovered homed CRUs after 24 hours rather than after 48 hours. Nevertheless, our finding that age-related HSC changes were exacerbated by previous homing is consistent with the early finding of Harrison et al54 that transplantation itself induced decrements in HSC function.54 However, unlike Harrison et al,54 who attributed the erosion of HSC function to engraftment history rather than to aging, we found that donor and recipient age and previous homing are important.
Differences in the recovery rates of PB cells after BM transplantation (Figure 7) underscored the theme that old HSCs and an old marrow microenvironment both predispose HSC differentiation to myelopoiesis. Engraftment of young HSCs in old recipients dramatically enhanced leukocyte recovery at the particular expense of platelet recovery. Interestingly, anemia resulted in the transplantation of young marrow into old recipients but not in the transplantation of old marrow into young recipients. This suggests that the old microenvironment plays a more dominant role in determining the numbers of various lineages of terminally differentiated cells in the circulation early after transplantation than does the age of the HSC. This is not surprising given the important role the microenvironment plays in supporting hematopoiesis through cell-cell interactions and especially through cytokine production, the profile of which changes during aging (eg, IL-7 and B-cell production). Interestingly, the age of the transplanted HSC has meaningful effects on platelet formation during the first 2 weeks after transplantation and on leukocyte generation after the first month of engraftment. Old HSCs, in both cases, have enhanced generative capacity compared with young HSCs when engrafted into young recipients. Thus, diminishment in the homing ability of old stem cells may be more than offset by the increase with age in CRU numbers in C57BL6 mice, as shown here and as shown by the increased numbers of progenitors described previously.5 The enhancement of platelet recovery by old HSCs is noteworthy because prolonged thrombocytopenia is a consequence of clinical transplantation. Unfortunately, in the clinical setting, the recipient, not the donor, is more likely to be older, and, as can be seen in Figure 6, this age combination, at least in the present murine studies, results in a slow recovery pattern.
A final important result of this study is that decrements in the seeding efficiency of HSCs caused by procurement from old donors and by the marrow microenvironment of old recipients, individually and in concert, will affect calculations of stem frequency in experimental transplantation assays. For example, the measured frequency of BM CRUs from young C57BL/6 donors (1 in approximately 20 000) is approximately half that of HSCs taken from old mice (1 in approximately 10 000). However, because the marrow seeding efficiency (in young recipients) of young HSCs is 7% and that of old HSCs is 2% to 3%, the actual difference in stem-cell numbers may be twice again as large, or 4-fold overall between young and old C57BL/6 mice. It might be argued that a distinguishing characteristic of stem cells is their ability to home to hematopoietic sites, and the decline in seeding efficiency with HSC age may represent an age-related decline in function. If this argument were accepted, an adjustment in stem-cell frequency on the basis of a lower seeding efficiency would be unwarranted. However, Table 2 shows that the seeding efficiency in old recipients of young HSCs is 2-fold less than that for the same cells assayed in young recipients. In this case, because the alteration in seeding efficiency is extrinsic to the HSCs, an adjustment in measured stem-cell frequency would be justified.
Prepublished online as Blood First Edition Paper, April 12, 2005; DOI 10.1182/blood-2004-11-4282.
Supported by National Institutes of Health grants HL61392 to S.J.S., and grants AG24950, AG20917, and AG16653 to G.V.Z..
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Penny Ragland for technical assistance and Paula Thomason for editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal