Abstract
We report about new apoptotic and non-apoptotic death pathways in neutrophils that are initiated via the surface molecule sialic acid-binding immunoglobulin-like lectin (Siglec)-9. In normal neutrophils, Siglec-9 ligation induced apoptosis. Inflammatory neutrophils obtained from patients with acute septic shock or rheumatoid arthritis demonstrated increased Siglec-9, but normal Fas receptor-mediated cytotoxic responses when compared with normal blood neutrophils. The increased Siglec-9-mediated death was mimicked in vitro by short-term preincubation of normal neutrophils with proinflammatory cytokines, such as granulocyte/macrophage colony-stimulating factor (GM-CSF), interferon-α (IFN-α), and IFN-γ, and was demonstrated to be caspase independent. Experiments using scavengers of reactive oxygen species (ROS) or neutrophils unable to generate ROS indicated that both Siglec-9-mediated caspase-dependent and caspase-independent forms of neutrophil death depend on ROS. Interestingly, the caspase-independent form of neutrophil death was characterized by cytoplasmic vacuolization and several other nonapoptotic morphologic features, which were also seen in neutrophils present in joint fluids from rheumatoid arthritis patients. Taken together, these data suggest that apoptotic (ROS- and caspase-dependent) and nonapoptotic (ROS-dependent) death pathways are initiated in neutrophils via Siglec-9. The new insights have important implications for the pathogenesis, diagnosis, and treatment of inflammatory diseases such as sepsis and rheumatoid arthritis. (Blood. 2005;106:1423-1431)
Introduction
Neutrophils are important effector cells in inflammation. Delayed neutrophil apoptosis has been associated with several acute and chronic inflammatory diseases and appears to be mediated, at least partially, by the overexpression of neutrophil survival cytokines such as granulocyte-colony-stimulating factor (G-CSF),1-3 granulocyte/macrophage colony-stimulating factor (GM-CSF),1,2,4,5 and macrophage migration inhibitory factor (MIF).6 Any failure in the process of neutrophil apoptosis likely results in the initiation of an inflammatory response and/or in the maintenance of an already existing inflammation. Therefore, studying neutrophil apoptosis under normal and inflammatory conditions seems to be important.
The induction of neutrophil apoptosis during the resolution of a neutrophilic inflammatory response can be mimicked by culturing the cells with insufficient amounts of survival factors, a process, which is called spontaneous apoptosis. Spontaneous neutrophil apoptosis can be enhanced by Fas receptor stimulation.7 Similar observations have been made in eosinophil in vitro cultures. Eosinophils undergo spontaneous apoptosis, which can be inhibited by survival cytokines such as interleukin 5 (IL-5) or GM-CSF.8 Eosinophils also express functional Fas receptors that accelerate in vitro cell death.9,10
Sialic acid-binding immunoglobulin-like lectins (Siglecs) belong to the immunoglobulin (Ig) supergene family and are characterized by the presence of an N-terminal V-set domain that binds sialic acid.11 The expression of the Siglecs is at least partially restricted. For instance, Siglec-1 is a macrophage-restricted adhesion molecule12 and Siglec-2 is specifically expressed by B cells.13 Moreover, Siglec-8 has been shown to be expressed on eosinophils, basophils, and mast cells.14 In contrast, Siglec-9 is predominantly expressed on monocytes and neutrophils.15,16
Since no functional role for Siglec-9 has been described in primary cells, we investigated this molecule in human neutrophils. Similarly to CD95, Siglec-9 ligation resulted in the induction of neutrophil apoptosis. However, when the experiments were performed in the presence of some proinflammatory cytokines, we observed an unexpected additional cell death, which was characterized by a nonapoptotic morphology, caspase independence, as well as mitochondrial and reactive oxygen species (ROS) involvement. Moreover, we provide evidence that this form of cell death may play a role during inflammatory responses in vivo.
Materials and methods
Antibodies
Anti-Siglec-9 (both unlabeled and phycoerythrin [PE]-conjugated), anti-CD7, anti-CD36, anti-Src homology domain 2 (SH2)-containing tyrosine phosphatase-1 (SHP-1) and anti-caspase-3 monoclonal antibodies (mAbs), as well as allophycocyanin (APC)-conjugated anti-CD11b and PE-conjugated anti-CD16 mAbs were obtained from Becton Dickinson Biosciences (Basel, Switzerland). Anti-caspase-8 mAb was from Cell Signaling Technology (distributed by BioConcept, Allschwil, Switzerland). F(ab) and F(ab')2 fragments of the unlabeled anti-Siglec-9 mAb were produced by digestion and subsequent purification using a protein A-Sepharose column F(ab) and F(ab')2 preparation kit from Pierce Chemical (Rockford, IL). The purity of the F(ab) and F(ab')2 fragments was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis and their Siglec-9 binding activity tested by flow cytometric analysis. Goat anti-Bid Ab was purchased from R&D Systems (distributed by Bühlmann AG, Basel, Switzerland). Anti-Fas agonistic mAb (CH11) was obtained from LabForce AG (Nunningen, Switzerland). Antiphosphotyrosine (antiptry) mAb was from Upstate Biotechnology (clone 4G10; distributed by Lucerne Chem AG, Lucerne, Switzerland). Blocking death receptor Abs were anti-Fas mAb from MLB (clone ZB4; distributed by LabForce AG), anti-tumor necrosis factor-receptor 1 (TNF-R1) mAb from Alexis (clone H398; Lausen, Switzerland), and anti-TRAIL mAb from Becton Dickinson Biosciences (clone RIK-2). Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mAb was from Chemicon International (Temecula, CA). Horseradish peroxidase (HRP)-conjugated secondary Abs were obtained from Amersham Pharmacia Biotech (Dübendorf, Switzerland). Control immunoglobulin G1 (IgG1), control F(ab) and F(ab')2 fragments, as well as the F(ab')2 fragment of secondary goat antimouse (GaM) Ab were from Jackson ImmunoResearch Laboratories (Milan Analytica, La Roche, Switzerland). Anti-CD16 and anti-CD14 microbeads for eosinophil and monocyte isolation, respectively, were from Miltenyi Biotec (Bergisch-Gladbach, Germany).
Cells
Blood neutrophils were isolated from healthy individuals as well as patients suffering from rheumatoid arthritis (RA) associated with acute joint inflammation, acute septic shock, and chronic granulomatous disease (CGD). Blood eosinophils were purified from healthy control individuals. Briefly, peripheral blood mononuclear cells (PBMCs) were separated by centrifugation on Ficoll-Hypaque (Seromed-Fakola AG, Basel, Switzerland). The lower phase, mainly granulocytes and erythrocytes, was treated with erythrocyte lysis solution (155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA[ethylenediaminetetraacetic acid], pH 7.3). The resulting cell populations contained mostly neutrophils.17 Joint fluid neutrophils from patients with RA were isolated using the same protocol. Blood eosinophils were further isolated by negative selection using anti-CD16 mAb-labeled microbeads and a magnetic cell-separation system (MACS; Miltenyi Biotec) using a column type C attached to a 21-gauge needle in the field of a permanent magnet.17 Monocytes were positively selected from PBMCs using anti-CD14 mAb-labeled microbeads. Cell purity was assessed by staining with Diff-Quik (Baxter, Düdingen, Switzerland) and light microscopy analysis. The purity of the resulting populations was as follows: at least 95% for neutrophils, at least 98% for eosinophils, and at least 95% for monocytes.
Bone marrow neutrophils were isolated from normal bone marrow aspirates by centrifugation on a 2-step discontinuous percoll density gradient followed by a negative selection procedure using anti-CD7 and anti-CD36 mAbs to eliminate contaminating lymphoid and erythroid precursors.18 The resulting cell population contained more than 97% cells of the neutrophil lineage as determined by MPO staining, analysis of lineage-associated surface proteins, as well as by staining with Diff-Quik and light microscopy. In average, we counted 15% myeloblasts, 10% promyelocytes, 35% myelocytes, 30% metamyelocytes and band cells, and 10% mature neutrophils in these mixed neutrophil populations.18 All patients and control individuals donating blood or bone marrow aspirates signed a written informed consent agreement in accordance with the Declaration of Helsinki. The study was approved by the local medical ethics committee (the Kantonale Ethikkommission Bern).
Immunofluorescence
Siglec-9 surface expression was analyzed by flow cytometry following incubation of the cells with saturating concentrations of PE-conjugated anti-Siglec-9 and isotype-matched control mAbs. In some experiments, cells were pretreated with 0.1 U/mL Vibrio cholerae α2-3,6,8-neuraminidase (Calbiochem) at 37°C for 60 minutes before the staining procedure.19 Two-color immunofluorescence analysis using fluorescein isothiocyanate (FITC)-conjugated anti-CD16 mAb and anti-CD11b mAb, respectively, was carried out to define the maturation step, in which neutrophils gain Siglec-9 expression.
Cell cultures
Cells were cultured at 1 × 106/mL in the presence or absence of cytokines and/or Abs for the indicated times using complete culture medium (RPMI 1640 containing 10% fetal calf serum [FCS] and 200 IU/mL penicillin/100 μg/mL streptomycin, all from Life Technologies, Basel, Switzerland). If not indicated otherwise, cells were stimulated with 35 μg/mL anti-Siglec-9 mAb in combination with 20 μg/mL unlabeled F(ab')2 fragments of secondary GaM Ab. Blocking of Siglec-9 was achieved by preincubation with 50 μg/mL anti-Siglec-9 F(ab) fragments for 1 hour before stimulation with the complete anti-Siglec-9 mAb. In the plate-bound experiments, microtiter plates were directly coated with 35 μg/mL anti-Siglec-9 mAb and washed with RPMI 1640 before use. Death receptor blocking mAbs were added to the cell cultures at the following concentrations: anti-Fas mAb at 1 μg/mL, anti-TNF-R1 mAb at 10 μg/mL, and anti-TRAIL mAb at 200 ng/mL. MIF was produced as a maltose binding protein (MBP) fusion protein as previously described.6 GM-CSF (Novartis Pharma GmbH, Nürnberg, Germany) was used at 25 ng/mL, G-CSF (Aventis Pharma AG, Zurich, Switzerland) at 25 ng/mL, IL-1β (R&D Systems) at 25 ng/mL; interferon-α (IFN-α) (PBL Biomedical Laboratories, distributed by Alexis) at 500 U/mL, IFN-γ (R&D Systems) at 85 ng/mL, and anti-Fas mAb (CH11) at 1 μg/mL. The caspase inhibitors N-benzyloxycarbonyl (z)-Val-Ala-Asp (VAD)-fluormethylketone (fmk) and z-Ile-Glu-Thr-Asp (IETD)-fmk (both from Alexis) were used at 50 μM. N-Acetyl-L-cysteine (NAC; Sigma) was used at 10 μM. For nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-inhibition experiments, neutrophils were incubated with 20 μM diphenyleneiodonium (DPI) (Calbiochem, distributed by Juro Supply GmbH, Lucerne, Switzerland) at 37°C for 5 minutes, and then washed twice with RPMI 1640 before culture.
Determination of cell death and apoptosis
Cell death was assessed by uptake of 1 μM ethidium bromide and flow cytometric analysis (FACScan, Becton Dickinson).6,18 To determine the form of cell death, morphologic analysis,20 annexin V staining,6,18 and DNA-fragmentation6,18 were performed. For morphologic analysis, an Axiovert 35 microscope equipped with a 630 ×/1.4 oil objective lens was used (Carl Zeiss, Heidelberg, Germany). Images were processed with Adobe Photoshop 5.0 software (Adobe, San Jose, CA).
Transmission electron microscopy
Neutrophils (1 × 106) were resuspended in 1 mL of 2.5% phosphate-buffered glutaraldehyde at 4°C. They were then washed with PBS and postfixed with 1% OsO4 for 1.5 hours at room temperature. Cell blocks were then embedded in Spurr low viscosity resin (Electron Microscopy Sciences, Hatfield, PA). Grey thin sections were contrasted with uranylacetate and lead citrate. The neutrophils were observed with an electron microscope (Zeiss EM 10 C, Oberkochen, Germany), and 20 to 30 cells were photographed per sample.
Enzymatic caspase-3 assay
Neutrophils were cultured at the indicated conditions, washed with cold phosphate-buffered saline (PBS) and subsequently lysed in cell-lysis buffer (50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.4; 0.1% Chaps; 5 mM dithiothreitol [DTT]; 0.1 mM EDTA) using a Teflon glass homogenizer (VWR International, Ismaning, Germany) on ice for 10 minutes. Caspase-3-like activity was measured in 10 μl cell lysate as enzymatic conversion of the colorimetric substrate Ac-DEVD-pNA at 405 nm according to the manufacturer's instructions (QuantiZyme caspase-3 cellular activity assay kit; Biomol, Plymouth Meeting, PA).
Oxidative burst measurements
Following a 4-hour stimulation, neutrophils were incubated with 1 μM dihydrorhodamine 123 (DHR; Sigma) at 37°C for 30 minutes, placed on ice, and subsequently analyzed by flow cytometry.21
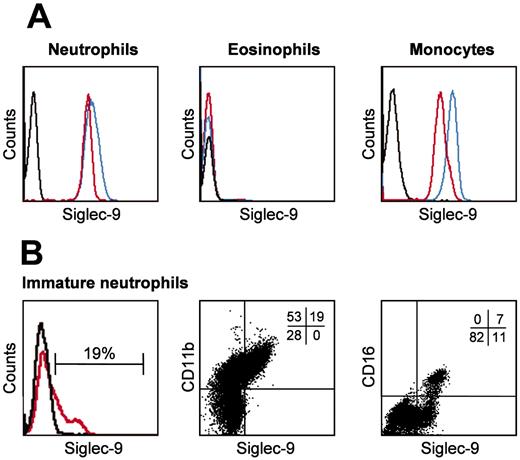
Neutrophils but not eosinophils express Siglec-9. Freshly isolated mature blood and immature bone marrow neutrophils, as well as blood monocytes and eosinophils, were analyzed by flow cytometry. (A) Neutrophils and monocytes, but not eosinophils, demonstrated evidence for Siglec-9 expression (red). A significant proportion of monocytic Siglec-9 was additionally detectable after sialidase treatment (blue). (B) A subpopulation of the bone marrow neutrophils was Siglec-9 positive (red). Two-color immunofluorescence analysis demonstrated a correlation between CD11b and Siglec-9 expression. CD11b is a differentiation marker associated with myelocytes, metamyelocytes, and mature neutrophils. Representative examples of 4 independent experiments are shown. Staining with isotype-matched control mAb is indicated by black lines in the histograms. In panel B, middle and right subpanels, the quantitative analysis is shown by percent at the top right corner of each dot plot.
Neutrophils but not eosinophils express Siglec-9. Freshly isolated mature blood and immature bone marrow neutrophils, as well as blood monocytes and eosinophils, were analyzed by flow cytometry. (A) Neutrophils and monocytes, but not eosinophils, demonstrated evidence for Siglec-9 expression (red). A significant proportion of monocytic Siglec-9 was additionally detectable after sialidase treatment (blue). (B) A subpopulation of the bone marrow neutrophils was Siglec-9 positive (red). Two-color immunofluorescence analysis demonstrated a correlation between CD11b and Siglec-9 expression. CD11b is a differentiation marker associated with myelocytes, metamyelocytes, and mature neutrophils. Representative examples of 4 independent experiments are shown. Staining with isotype-matched control mAb is indicated by black lines in the histograms. In panel B, middle and right subpanels, the quantitative analysis is shown by percent at the top right corner of each dot plot.
Immunoprecipitation and immunoblotting
Statistical analysis
Statistical analysis was performed by using the Student t test. If mean levels are presented, the standard error of the mean (SEM) and the number (n) of independent experiments are indicated in each case. A probability value of less than .05 was considered statistically significant.
Results
Siglec-9 is expressed by mature blood neutrophils but not eosinophils
In agreement with previously published work,15,16 we observed surface Siglec-9 expression on freshly purified human blood neutrophils and monocytes but not on eosinophils as assessed by flow cytometry (Figure 1A). The same results were observed when nonpurified neutrophils and monocytes were analyzed in whole blood samples (data not shown). Siglec receptors can be masked by binding in cis to sialic acids present as nonreducing ends of oligosaccharide chains attached to a wide variety of glycoproteins and glycolipids on the same plasma membrane.20 Therefore, Siglec-9 surface staining was also performed following sialidase (neuraminidase) treatment to remove sialic acids. Sialidase treatment of neutrophils had no effect on the staining properties of the anti-Siglec-9 mAb, and eosinophils remained Siglec-9 negative (Figure 1A). In contrast, we observed stronger fluorescent signals in monocytes, indicating the possibility that a proportion of Siglec-9 molecules might have been masked by endogenous cis-interactions on the cell surface. However, other mechanisms such as a decrease in net negative charge upon release of sialic acid, reduction in steric interactions, or alterations in Siglec-9 conformation cannot be excluded.
To investigate Siglec-9 expression during differentiation, we purified human bone marrow neutrophils. Siglec-9 expression was exclusively observed on CD11b+ neutrophils. Moreover, all CD16+ and some CD16- neutrophils were Siglec-9 positive, but the majority of CD16- neutrophils were Siglec-9 negative (Figure 1B). This suggested that immature neutrophils gain Siglec-9 expression not before the myelocyte stage but before CD16 expression.
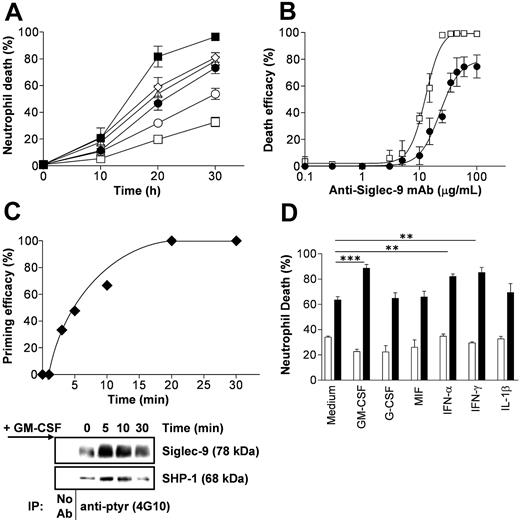
Siglec-9 cross-linking accelerates spontaneous neutrophil death, which is enhanced following GM-CSF priming. Cell death was assessed by ethidium bromide uptake and flow cytometric analysis. (A) Time-dependent acceleration of neutrophil death by Siglec-9 and Fas receptor cross-linking, respectively. GM-CSF-enhanced Siglec-9-mediated, but not Fas-triggered, cell death (n = 4). ▪ indicates GM-CSF + anti-Siglec-9; ⋄, anti-Fas; ▵, GM-CSF + anti-Fas; •, anti-Siglec-9 alone; ○, medium; and □, GM-CSF alone. (B) Concentration-effect curve of anti-Siglec mAb in 20-hour neutrophil cultures. Maximal death effects in the absence of cytokine priming were seen at 35 μg/mL. GM-CSF preincubation for 20 minutes and its presence in subsequent culture resulted in higher death efficacy and potency of the mAb (n = 3). Symbols are as in panel A. (C, top) Priming by GM-CSF was time dependent. Addition of GM-CSF after Siglec-9 cross-linking had no effect. Cells were cultured for 20 hours. (Bottom) Stimulation with GM-CSF resulted in rapid tyrosine phosphorylation of Siglec-9 and SHP-1. In these experiments, neutrophil lysates were immunoprecipitated (IP) with anti-ptyr mAb followed by immunoblotting with anti-Siglec-9 and anti-SHP-1 mAbs. One representative out of 4 independent experiments is shown. (D) GM-CSF, IFN-α, and IFN-γ, but not G-CSF, MIF, and IL-1β were able to enhance the Siglec-9-mediated neutrophil death. Results of 24-hour cultures are shown (n = 4). Cytokines were preincubated for 20 minutes before cross-linking. □ indicates control IgG1; and ▪, anti-Siglec-9. **P < .01; ***P < .001. In panels A, B, and D, data are expressed as means ± SEM.
Siglec-9 cross-linking accelerates spontaneous neutrophil death, which is enhanced following GM-CSF priming. Cell death was assessed by ethidium bromide uptake and flow cytometric analysis. (A) Time-dependent acceleration of neutrophil death by Siglec-9 and Fas receptor cross-linking, respectively. GM-CSF-enhanced Siglec-9-mediated, but not Fas-triggered, cell death (n = 4). ▪ indicates GM-CSF + anti-Siglec-9; ⋄, anti-Fas; ▵, GM-CSF + anti-Fas; •, anti-Siglec-9 alone; ○, medium; and □, GM-CSF alone. (B) Concentration-effect curve of anti-Siglec mAb in 20-hour neutrophil cultures. Maximal death effects in the absence of cytokine priming were seen at 35 μg/mL. GM-CSF preincubation for 20 minutes and its presence in subsequent culture resulted in higher death efficacy and potency of the mAb (n = 3). Symbols are as in panel A. (C, top) Priming by GM-CSF was time dependent. Addition of GM-CSF after Siglec-9 cross-linking had no effect. Cells were cultured for 20 hours. (Bottom) Stimulation with GM-CSF resulted in rapid tyrosine phosphorylation of Siglec-9 and SHP-1. In these experiments, neutrophil lysates were immunoprecipitated (IP) with anti-ptyr mAb followed by immunoblotting with anti-Siglec-9 and anti-SHP-1 mAbs. One representative out of 4 independent experiments is shown. (D) GM-CSF, IFN-α, and IFN-γ, but not G-CSF, MIF, and IL-1β were able to enhance the Siglec-9-mediated neutrophil death. Results of 24-hour cultures are shown (n = 4). Cytokines were preincubated for 20 minutes before cross-linking. □ indicates control IgG1; and ▪, anti-Siglec-9. **P < .01; ***P < .001. In panels A, B, and D, data are expressed as means ± SEM.
Siglec-9 ligation induces neutrophil death, which is accelerated by GM-CSF and interferons
Since Siglec-9 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM),15,16 which is usually able to bind and activate inhibitory phosphatases, we hypothesized that Siglec-9 ligation might have inhibitory effects on neutrophil viability. To activate Siglec-9, we used an anti-Siglec-9 mAb, which was used in conjunction with F(ab')2 fragments of a polyclonal antimouse Ab to enhance cross-linking. Cross-linking of Siglec-9 resulted in the induction of significant neutrophil death in a time- (Figure 2A) and concentration-dependent manner (Figure 2B). The use of an isotype-matched control mAb (IgG1) had no effect in this system (Figure 3A). The anti-Siglec-9-mediated death was slightly less efficient compared with the death induced by anti-Fas mAb treatment. However, whereas GM-CSF preincubation for 20 minutes had no significant effect on neutrophil viability in conjunction with anti-Fas mAb, it dramatically enhanced the death effect following Siglec-9 cross-linking (Figure 2A). GM-CSF not only increased the efficiency of Siglec-9-mediated neutrophil death, it also increased its potency, meaning that the concentration of anti-Siglec-9 mAb required to reach a certain cytotoxic effect was much less following cytokine priming of the cells (Figure 2B).
That GM-CSF promoted death of neutrophils in association with Siglec-9 cross-linking was a surprising finding, since GM-CSF has been considered as a survival factor for neutrophils.1,2,4,5 We were therefore interested in the exact conditions under which GM-CSF promoted neutrophil death. When GM-CSF was added at the same time or after addition of anti-Siglec-9 mAb, the cytokine had no effect. The Siglec-9-mediated death-enhancing capacity was only observed when neutrophils were preincubated with GM-CSF and subsequently Siglec-9 stimulated. The death priming effect mediated by GM-CSF was time dependent, being detectable already after 3 minutes, and reaching its maximum after 20 minutes (Figure 2C).
Interestingly, short-term stimulation with GM-CSF rapidly increased tyrosine phosphorylation of Siglec-9, peaking between 5 and 10 minutes (Figure 2C, right panel). SHP-1 was found in increased amounts associating with one or more tyrosine-phosphorylated protein(s). Since the anti-Siglec-9 mAb used in these experiments did not efficiently immunoprecipitate Siglec-9 (data not shown), we were not able to investigate potential direct interactions between Siglec-9 and SHP-1 in this study. No elevated surface expression of Siglec-9 following GM-CSF stimulation was observed in neutrophils (data not shown). In addition, blocking mAbs preventing Fas, TNF-α, and TRAIL receptor signaling had no effect on Siglec-9-mediated neutrophil death both in the presence and absence of GM-CSF (data not shown). Therefore, the cytokine priming effect might be related to increased tyrosine phosphorylation but not the result of increased expression of Siglec-9 or the initiation of death receptor signaling pathways. All following cytokine priming experiments reported in this manuscript were carried out using a preincubation time of 20 minutes.
We also tested additional cytokines for their capacity to enhance the Siglec-9-mediated cytotoxic activity in neutrophils. Similarly to GM-CSF, preincubation with IFN-α or IFN-γ increased neutrophil death induced by Siglec-9 cross-linking. In contrast, the proinflammatory cytokine IL-1β as well as the neutrophil survival factors G-CSF and MIF did not prime the Siglec-9-initiated death pathway (Figure 2D). Thus, the increased efficacy and potency of Siglec-9-mediated cytotoxicity is limited to a subset of cytokines and not linked to their capacity of increasing neutrophil survival.
Cross-linking with F(ab')2 fragments demonstrates specific Siglec-9-mediated effects
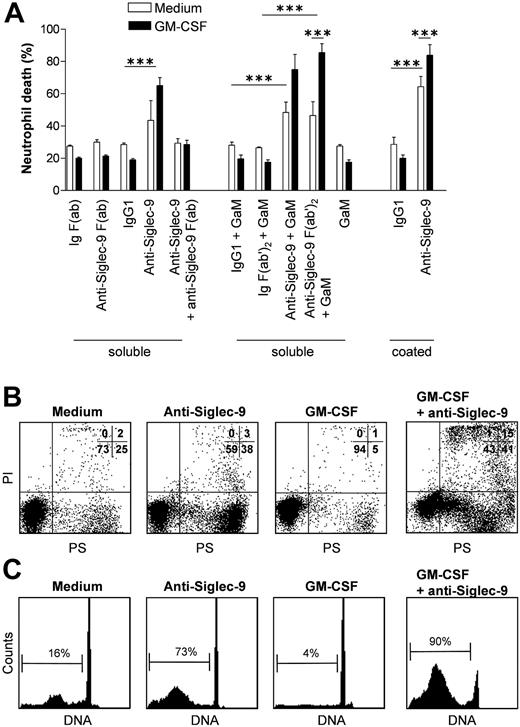
To exclude the possibility of Fc-receptor-mediated effects, several control experiments were performed (Figure 3A). First, cross-linking of an isotype-matched IgG1 mAb with F(ab')2 fragments of goat antimouse secondary Ab (GaM) had no effect both in the presence and absence of GM-CSF. Second, we generated F(ab′)2 fragments of the anti-Siglec-9 mAb to cross-link Siglec-9. In parallel experiments, F(ab')2 fragments of an IgG1 control mAb were used. Using this method of activating Siglec-9, we observed no differences compared with the experiments using intact Abs. Addition of the secondary Ab (GaM) alone had also no effect in this system. Third, Siglec-9-negative eosinophils that carry Fc-receptors,23 did not demonstrate accelerated cell death following anti-Siglec-9 mAb incubation both in the presence and absence of GM-CSF (data not shown). Moreover, we performed experiments using plate-bound anti-Siglec-9 mAb without the use of secondary Ab. Both induction of cell death in the absence of GM-CSF as well as enhanced Siglec-9-mediated neutrophil death in the presence of GM-CSF were confirmed under these conditions (Figure 3A, right panel). For practical reasons, however, we used the soluble stimulation system in all subsequent experiments.
Cross-linking of Siglec-9 using different techniques excludes the possibility of nonspecific death effects. (A) F(ab')2 fragments of the anti-Siglec-9 mAb and intact mAb revealed same results. Cross-linking by a plate-bound primary anti-Siglec-9 mAb also resulted in neutrophil death. GM-CSF was under these 3 different conditions always an efficient death sensitizer. F(ab) fragments of the anti-Siglec-9 mAb had no cytotoxic effect. Moreover, preincubation of F(ab) fragments of the anti-Siglec-9 mAb prevented neutrophil death induced by the intact mAb both in the presence and absence of GM-CSF. Results of 20-hour cultures are shown (n = 4). ***P < .001. Data in panel A are expressed as means ± SEM. (B,C) Anti-Siglec-9 mAb stimulation in the absence of GM-CSF-induced PS redistribution (9-hour cultures) and DNA fragmentation (13-hour cultures) indicative for the induction of neutrophil apoptosis. In presence of GM-CSF, Siglec-9 ligation resulted in even more DNA fragmentation, but an abnormal subpopulation of neutrophils with low annexin-binding capacity was observed. These panels show one representative experiment out of 5. In panel B, quantitative analysis is shown in percent at the top right corner of each dot plot. In panel C, the relative number of apoptotic cells is indicated by the bracket in each subpanel.
Cross-linking of Siglec-9 using different techniques excludes the possibility of nonspecific death effects. (A) F(ab')2 fragments of the anti-Siglec-9 mAb and intact mAb revealed same results. Cross-linking by a plate-bound primary anti-Siglec-9 mAb also resulted in neutrophil death. GM-CSF was under these 3 different conditions always an efficient death sensitizer. F(ab) fragments of the anti-Siglec-9 mAb had no cytotoxic effect. Moreover, preincubation of F(ab) fragments of the anti-Siglec-9 mAb prevented neutrophil death induced by the intact mAb both in the presence and absence of GM-CSF. Results of 20-hour cultures are shown (n = 4). ***P < .001. Data in panel A are expressed as means ± SEM. (B,C) Anti-Siglec-9 mAb stimulation in the absence of GM-CSF-induced PS redistribution (9-hour cultures) and DNA fragmentation (13-hour cultures) indicative for the induction of neutrophil apoptosis. In presence of GM-CSF, Siglec-9 ligation resulted in even more DNA fragmentation, but an abnormal subpopulation of neutrophils with low annexin-binding capacity was observed. These panels show one representative experiment out of 5. In panel B, quantitative analysis is shown in percent at the top right corner of each dot plot. In panel C, the relative number of apoptotic cells is indicated by the bracket in each subpanel.
To answer the question whether cross-linking of Siglec-9 is required for the death effect, we generated F(ab) fragments of the Siglec-9 mAb. Anti-Siglec-9 F(ab) fragments had no effect on neutrophil viability. Moreover, preincubation of neutrophils with anti-Siglec-9 F(ab) fragments completely blocked the death effect of intact anti-Siglec-9 mAb in the presence and absence of GM-CSF (Figure 3A, left panel). These data indicate that Siglec-9 cross-linking is required for the observed cytotoxic effects in neutrophils.
Siglec-9 activates caspase-dependent and -independent death pathways in neutrophils
We next investigated whether the neutrophil death induced following Siglec-9 cross-linking was due to induction of apoptosis. Anti-Siglec-9 mAb stimulation in the absence of GM-CSF increased redistribution of phosphatidyl serine (PS), a characteristic feature of apoptotic neutrophils (Figure 3B).6,18 Moreover, Siglec-9 ligation accelerated DNA fragmentation, which is associated with spontaneous apoptosis,6,24 as assessed by staining DNA with propidium iodide (PI) and flow cytometric analysis (Figure 3C). These data suggested that Siglec-9 cross-linking in the absence of costimulatory signals initiates a proapoptotic pathway in neutrophils in vitro. In the presence of GM-CSF, however, the picture appeared to be less clear following Siglec-9 cross-linking. Although the generation of hypoploid DNA correlated with the amount of cell death, annexin binding was somewhat low, at least in a subpopulation of the neutrophils undergoing death. A subpopulation of annexin low binders was also seen in the PI up-taking cells under these conditions (Figure 3B).
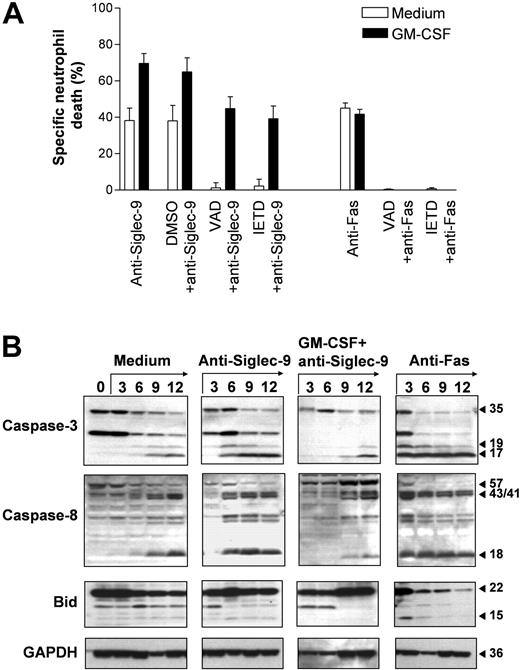
Caspases play a critical role in the execution phase of neutrophil apoptosis.25 Therefore, we investigated whether the death induced via Siglec-9 involves caspase activation. Both the caspase-8 inhibitor z-IETD-fmk and the pan-caspase inhibitor z-VAD-fmk completely blocked Siglec-9-mediated cell death in the absence of GM-CSF, confirming that Siglec-9 transduces proapoptotic signals under these conditions. Similarly, Fas receptor-mediated death was completely blocked by the 2 inhibitors, confirming previously published work.26 However, the enhanced Siglec-9-mediated cell death seen in the presence of GM-CSF was only partially blocked by both caspase inhibitors, suggesting the involvement of caspase-independent death mechanisms at least in a subpopulation of dying neutrophils (Figure 4A).
To more precisely investigate the involvement of caspases, we performed immunoblotting experiments. Caspase-3 plays a critical role in neutrophil apoptosis.26-28 Caspase-3 was present as an inactive pro-form in freshly purified blood neutrophils. The active 17-kDa fragment,24 however, was not detectable. Culturing the cells resulted in the appearance of the 17-kDa form, which was clearly detectable after a 9-hour culture period (Figure 4B). Cleavage of caspase-3 was accelerated in anti-Fas mAb stimulated neutrophils, in which the 17-kDa caspase-3 fragment was already detectable in 3 hours upon Fas receptor activation. Compared with Fas-stimulated cells, Siglec-9-mediated caspase-3 cleavage was delayed and the 17-kDa form was first detectable in 6-hour neutrophil cultures. Strikingly, in GM-CSF-primed and anti-Siglec-9 mAb-stimulated neutrophils, cleavage of caspase-3 appeared even later since the 17-kDa fragment was not seen in cells cultured less than 9 hours (Figure 4B). We also analyzed caspase-3-like DEVDase activity, which correlated with the data obtained by immunoblotting (OD405 in 9-hour cultures: medium, 0.35; GM-CSF, 0.14; anti-Fas mAb, 0.75; anti-Siglec-9 mAb, 0.55; GM-CSF + anti-Siglec-9 mAb, 0.29). Thus, although Siglec-9 ligation in GM-CSF-primed neutrophils caused massive cell death, caspase-3 activation appeared to be blocked and was not different compared with spontaneously dying neutrophils.
Similar results were obtained when caspase-8 cleavage was analyzed. The active 18-kDa fragment was first detectable in anti-Fas mAb (3 hours) and then in anti-Siglec-9 mAb (6 hours) stimulated neutrophils. In untreated neutrophils, this fragment was seen in 9-hour cultures. In GM-CSF-primed and anti-Siglec-9 mAb-stimulated neutrophils, cleavage of caspase-8 appeared to be delayed since the 18-kDa fragment also did not occur in cells cultured less than than 9 hours (Figure 4B). Since Bid is a target for caspase-8, we also analyzed Bid cleavage. The active 15-kDa Bid fragment was rapidly generated in anti-Fas mAb-treated neutrophils, but hardly detectable in Siglec-9-mediated cell deaths independent from the presence or absence of GM-CSF (Figure 4B). Taken together, these data suggest that Siglec-9 cross-linking causes caspase-dependent apoptosis in neutrophils as long as GM-CSF is not present. However, in GM-CSF-primed neutrophils, caspase-3 and caspase-8 activation is inhibited and ligation of Siglec-9 under these conditions results in cell death, which proceeds even in the presence of caspase inhibitors.
Evidence for caspase-dependent and -independent Siglec-9-mediated neutrophil deaths. (A) The caspase inhibitors z-VAD-fmk and z-IETD-fmk completely blocked Siglec-9- and Fas receptor-mediated neutrophil deaths. However, the enhanced Siglec-9-triggered death following GM-CSF priming was only partially blocked by the 2 caspase inhibitors. Results of 20-hour cultures are shown (n = 3). Data are expressed as means ± SEM. (B) Spontaneous neutrophil death was associated with the occurrence of the 17-kDa caspase-3 cleavage product and of the 18-kDa caspase-8 fragment (both detectable in 9-hour cultures). Cleavage of both caspases was accelerated in anti-Fas mAb-treated cultures (the active fragments of caspase-3 and caspase-8 are both detectable in 3-hour cultures). In addition, active 15-kDa Bid was seen in 3-hour anti-Fas mAb-treated neutrophil cultures. In anti-Siglec-9-treated neutrophils, the 2 active caspase products were detectable in 6-hour cultures. Strikingly, although GM-CSF enhanced Siglec-9-mediated neutrophil death, the active caspase cleavage products were not seen in neutrophils cultured less than 9 hours. Immunoblots are representative of 3 independent experiments.
Evidence for caspase-dependent and -independent Siglec-9-mediated neutrophil deaths. (A) The caspase inhibitors z-VAD-fmk and z-IETD-fmk completely blocked Siglec-9- and Fas receptor-mediated neutrophil deaths. However, the enhanced Siglec-9-triggered death following GM-CSF priming was only partially blocked by the 2 caspase inhibitors. Results of 20-hour cultures are shown (n = 3). Data are expressed as means ± SEM. (B) Spontaneous neutrophil death was associated with the occurrence of the 17-kDa caspase-3 cleavage product and of the 18-kDa caspase-8 fragment (both detectable in 9-hour cultures). Cleavage of both caspases was accelerated in anti-Fas mAb-treated cultures (the active fragments of caspase-3 and caspase-8 are both detectable in 3-hour cultures). In addition, active 15-kDa Bid was seen in 3-hour anti-Fas mAb-treated neutrophil cultures. In anti-Siglec-9-treated neutrophils, the 2 active caspase products were detectable in 6-hour cultures. Strikingly, although GM-CSF enhanced Siglec-9-mediated neutrophil death, the active caspase cleavage products were not seen in neutrophils cultured less than 9 hours. Immunoblots are representative of 3 independent experiments.
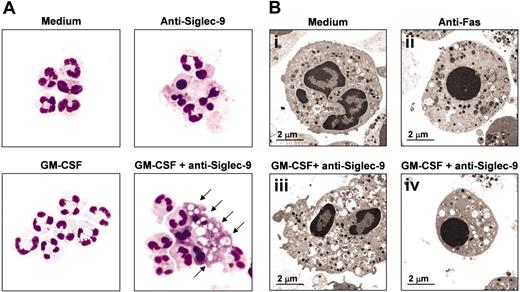
The Siglec-9-mediated death in GM-CSF-primed neutrophils is nonapoptotic and morphologically characterized by the formation of cytoplasmic vacuoles
Since we obtained evidence for a caspase-independent form of cell death in GM-CSF-primed and Siglec-9 cross-linked neutrophils, we thought to investigate its morphologic features. Whereas Siglec-9 ligation alone was associated with increased numbers of apoptotic neutrophils compared with untreated neutrophils (15-hour cultures), priming with GM-CSF and subsequent Siglec-9 cross-linking resulted in neutrophils with an aberrant morphology (Figure 5A). The most characteristic feature of these cells was a cytoplasmic vacuolization. In addition, the chromatin appeared to be more disintegrated and less condensed compared with classical apoptotic neutrophils, which were also present in GM-CSF-primed and anti-Siglec-9 mAb-stimulated cells. The proportion of the cells with aberrant morphology was at this time point approximately 65% (25% were apoptotic and 10% had a normal viable morphology).
We also examined the aberrant cells by transmission electron microscopy. In GM-CSF-primed and anti-Siglec-9 mAb-stimulated neutrophil populations, we found again, aside from apoptotic cells, neutrophils that did not appear to die via classical apoptosis. As previously described,20 apoptotic neutrophils were characterized by condensation and fragmentation of the nuclei, as well as shrinkage of the cells. In contrast, the aberrant cells had many vacuole-like double-membrane structures, their cytoplasm looked electron translucent and their mitochondria were at least partially swollen, characteristics that accompany necrotic cell death. However, the plasma membrane appeared to be intact and cell shrinkage was also observed, excluding primary cell lysis and therefore a classical necrotic cell death. Moreover, we observed nuclear condensation in approximately 25% of these cells (Figure 5B). It is possible that the described observations within the cytoplasm precede the nuclear morphologic changes in this aberrant neutrophil death. Taken together, GM-CSF-primed and anti-Siglec-9 mAb-stimulated neutrophils undergo a nonapoptotic form of cell death, which is characterized by vacuolization, mitochondrial swelling, nuclear condensation, and normal plasma membrane integrity.
Morphologic characterization of the Siglec-9-mediated caspase-independent neutrophil death. (A) Light microscopy: anti-Siglec-9 cross-linking alone induced neutrophil apoptosis (reduced cell volume and nuclear condensation). GM-CSF followed by Siglec-9 engagement resulted in both apoptotic and aberrant neutrophil death. The aberrant death was characterized by cytoplasmic vacuolization (arrows). Cells were stained with Giemsa-May-Grünwald (Diff-Quik). Original magnification, × 1000. Representative examples of 4 independent experiments are shown. (B) Transmission electron microscopy: panel i demonstrates a normal viable neutrophil; panel ii shows an apoptotic neutrophil with condensation of the nucleus and cell shrinkage. Panels iii and iv demonstrate the aberrant morphology of GM-CSF-primed and Siglec-9-stimulated neutrophils, which contained many vacuole-like double-membrane structures. Approximately 25% of these cells showed condensation of the nucleus and cell shrinkage. The mitochondria were partially swollen. The plasma membrane appeared to be intact. The cells in panels A and B were from 15-hour cultures.
Morphologic characterization of the Siglec-9-mediated caspase-independent neutrophil death. (A) Light microscopy: anti-Siglec-9 cross-linking alone induced neutrophil apoptosis (reduced cell volume and nuclear condensation). GM-CSF followed by Siglec-9 engagement resulted in both apoptotic and aberrant neutrophil death. The aberrant death was characterized by cytoplasmic vacuolization (arrows). Cells were stained with Giemsa-May-Grünwald (Diff-Quik). Original magnification, × 1000. Representative examples of 4 independent experiments are shown. (B) Transmission electron microscopy: panel i demonstrates a normal viable neutrophil; panel ii shows an apoptotic neutrophil with condensation of the nucleus and cell shrinkage. Panels iii and iv demonstrate the aberrant morphology of GM-CSF-primed and Siglec-9-stimulated neutrophils, which contained many vacuole-like double-membrane structures. Approximately 25% of these cells showed condensation of the nucleus and cell shrinkage. The mitochondria were partially swollen. The plasma membrane appeared to be intact. The cells in panels A and B were from 15-hour cultures.
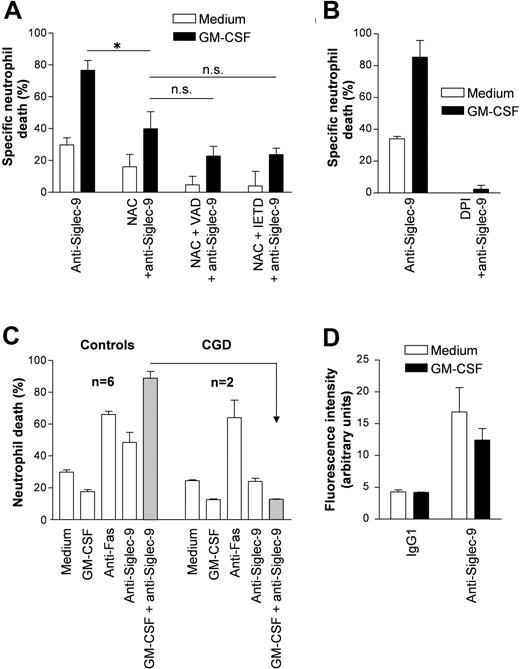
The Siglec-9-mediated neutrophil death is dependent on the formation of reactive oxygen species
Since we noticed mitochondrial swelling in neutrophils undergoing the largely caspase-independent and nonapoptotic cell death, we hypothesized that mitochondria-derived ROS mediate, at least partially, the cytotoxic effect. ROS have previously been described as regulators of both apoptosis and necrosis in several cellular systems.29 NAC, a well-characterized ROS scavenger, had some effects on Siglec-9-mediated apoptosis. However, inhibition of cell death by NAC was dramatically increased in GM-CSF-primed and anti-Siglec-9 mAb-stimulated neutrophils. The antideath activity of NAC was dose dependent, reaching its maximum at 10 μM (data not shown). Moreover, additional death inhibition was observed when the cells received combined NAC and caspase inhibitor treatment, although this increase did not reach statistical significance (Figure 6A).
The death mediated by Siglec-9 upon GM-CSF priming is ROS-dependent. (A) The ROS scavenger NAC partially blocked Siglec-9-mediated death in normal GM-CSF-primed neutrophils. NAC and caspase inhibitors had an additive blocking effect, although no complete inhibition was achieved. Results of 20-hour cultures are shown (n = 4). *P < .05. (B) The inhibitor of ROS production DPI completely blocked Siglec-9-mediated death both in the absence (□) and presence (▪) of GM-CSF. Results of 20-hour cultures are shown (n = 4). (C) Comparison of normal (n = 6) and CGD (n = 2) neutrophils. Although spontaneous death of CGD neutrophils appeared to be delayed, they demonstrated normal responses to GM-CSF, anti-Fas mAb. However, the death effect of anti-Siglec-9 mAb appeared to be reduced, and the death priming effect of GM-CSF was not at all detectable in CGD neutrophils. Results of 24-hour cultures are shown. (D) Anti-Siglec-9 mAb stimulation resulted in increased production of ROS no matter whether GM-CSF was present or not. Neutrophils were stimulated for 4 hours (n = 3). Data in all panels are expressed as means ± SEM.
The death mediated by Siglec-9 upon GM-CSF priming is ROS-dependent. (A) The ROS scavenger NAC partially blocked Siglec-9-mediated death in normal GM-CSF-primed neutrophils. NAC and caspase inhibitors had an additive blocking effect, although no complete inhibition was achieved. Results of 20-hour cultures are shown (n = 4). *P < .05. (B) The inhibitor of ROS production DPI completely blocked Siglec-9-mediated death both in the absence (□) and presence (▪) of GM-CSF. Results of 20-hour cultures are shown (n = 4). (C) Comparison of normal (n = 6) and CGD (n = 2) neutrophils. Although spontaneous death of CGD neutrophils appeared to be delayed, they demonstrated normal responses to GM-CSF, anti-Fas mAb. However, the death effect of anti-Siglec-9 mAb appeared to be reduced, and the death priming effect of GM-CSF was not at all detectable in CGD neutrophils. Results of 24-hour cultures are shown. (D) Anti-Siglec-9 mAb stimulation resulted in increased production of ROS no matter whether GM-CSF was present or not. Neutrophils were stimulated for 4 hours (n = 3). Data in all panels are expressed as means ± SEM.
In neutrophils, ROSs are mostly generated by the inducible nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system. To further investigate the potential role of ROS in Siglec-9-mediated cell death in neutrophils, we used the NADPH oxidase-inhibitor DPI. In contrast to the ROS scavenger studies with NAC, blocking of ROS synthesis by DPI was more efficient and completely abrogated Siglec-9-mediated death in both the presence and absence of GM-CSF (Figure 6B). To confirm the data using DPI, we investigated neutrophils from 2 patients with chronic granulomatous disease (CGD) that are unable to generate ROS based on a genetic defect in the NADPH oxidase. Due to the rarity of this disease and the low availability of untreated patients, this experiment was restricted to 2 patients only. Although the genetic defect was localized in different NADPH oxidase subunits (p22phox and gp91phox, respectively) in the 2 patients with CGD, their neutrophils exhibited very similar responses toward anti-Siglec-9 mAb stimulation both in the presence and absence of GM-CSF (Figure 6C). The death induced by anti-Fas mAb appeared to be as efficient as in normal neutrophils, but the cytotoxic effect of anti-Siglec-9 mAb was completely abolished both in the presence and absence of GM-CSF in CGD neutrophils. Moreover, morphologic analysis revealed no evidence for a nonapoptotic form of cell death in the GM-CSF-primed and anti-Siglec-9 mAb-stimulated CGD neutrophils (data not shown).
To investigate whether Siglec-9 stimulation would itself increase ROS production, we performed flow cytometric measurements using DHR.21 Upon 4-hour stimulation with anti-Siglec-9 mAb, we obtained clear evidence for increased production of ROS, and no significant difference was observed in the presence and absence of GM-CSF (Figure 6D). Taken together, these data suggest that ROSs largely contribute to both the Siglec-9-mediated apoptotic and nonapoptotic neutrophil deaths.
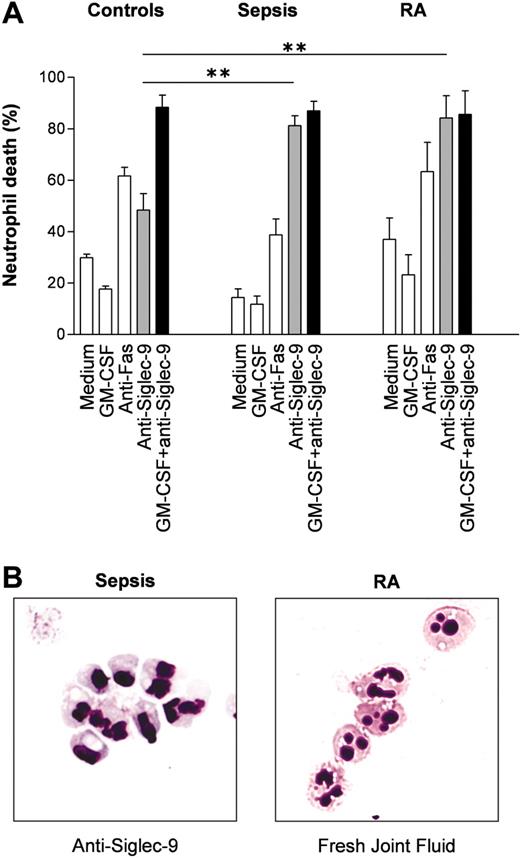
Siglec-9-mediated nonapoptotic neutrophil death in inflammation: ex vivo and in vivo studies suggest a potential role in sepsis and rheumatoid arthritis
Since neutrophils are exposed to proinflammatory cytokines during inflammatory responses, we hypothesized that inflammatory neutrophils derived from patients may respond with an enhanced death rate following Siglec-9 cross-linking compared with normal neutrophils. In sepsis, neutrophils are exposed to high levels of GM-CSF,30 giving rise to the possibility that these cells gain increased susceptibility toward Siglec-9-mediated cytotoxic effects in vivo. Indeed, compared with normal blood neutrophils, neutrophils from patients suffering from acute septic shock responded with an enhanced in vitro death rate following Siglec-9 cross-linking, and this effect could not be increased by GM-CSF preincubation (Figure 7A). We also performed experiments using joint fluid neutrophils from patients with RA, which had likely been exposed to IFN-γ and/or GM-CSF.31 These tissue neutrophils exhibited the same properties as the blood neutrophils from sepsis patients: enhanced anti-Siglec-9 cytotoxicity in the absence of in vitro priming and no effect of GM-CSF preincubation (Figure 7A). In contrast, both sepsis blood neutrophils and RA joint fluid neutrophils demonstrated normal death responses upon Fas receptor cross-linking. Surface expression of Siglec-9 assessed by flow cytometry was not significantly altered in both diseases compared with healthy controls (data not shown). However, morphologic analysis of the in vivo-primed neutrophils derived from septic shock patients revealed a large proportion of cells with visible vacuolization following Siglec-9 cross-linking (Figure 7B, left panel), suggesting that these cells underwent the same form of death as normal neutrophils following GM-CSF in vitro priming and subsequent anti-Siglec-9 mAb stimulation.
To obtain direct evidence that this morphologically distinct form of cell death indeed occurs under in vivo conditions, we prepared cytospins from cells found in freshly obtained joint fluids of patients with RA (n = 20) and analyzed the morphologic features of neutrophils. Aside from normal (mean: 74%) and apoptotic (mean: 16%) neutrophils, we observed a relative high proportion (mean: 10%) of neutrophils with cytoplasmic vacuoles (Figure 7B, right panel).
Discussion
We investigated functional consequences of Siglec-9 ligation in granulocytes under normal and several inflammatory conditions. The following new findings are reported. Neutrophils initiate the expression of Siglec-9 at the myelocyte stage during differentiation in the bone marrow. Siglec-9 transduces death signals into neutrophils but not eosinophils. Siglec-9 ligation results in neutrophil apoptosis under noninflammatory conditions. In inflammatory disease neutrophils, however, Siglec-9 cross-linking initiates both apoptotic and nonapoptotic pathways. GM-CSF, IFN-α, and IFN-γ sensitize neutrophils for the Siglec-9-mediated nonapoptotic pathway. GM-CSF increases tyrosine phosphorylation of Siglec-9 in neutrophils. Siglec-9 stimulates the production of ROS, which is required for both apoptotic and nonapoptotic pathways in neutrophils. The Siglec-9-mediated nonapoptotic death is characterized by cytoplasmic vacuolization. We provide evidence that the nonapoptotic death described in this report occurs under in vivo inflammatory conditions.
Neutrophils from patients with sepsis and patients with RA demonstrate increased Siglec-9-mediated cell death susceptibility. (A) Blood neutrophils from patients with sepsis and joint fluid neutrophils from patients with RA appeared to be primed in vivo. Additional in vitro priming with GM-CSF had no effect on these inflammatory neutrophils. Results of 20-hour cultures are shown (control individuals: n = 6; septic shock neutrophils: n = 5; joint fluid RA neutrophils: n = 4). **P < .01. (B) Cytoplasmic vacuolization associated with inflammation. (Left) Sepsis blood neutrophils following 15-hour anti-Siglec-9 mAb treatment alone in vitro. (Right) Neutrophils from fresh RA joint fluids. Neutrophils were stained with Giemsa-May-Grünwald (Diff-Quik). Original magnification, × 1000.
Neutrophils from patients with sepsis and patients with RA demonstrate increased Siglec-9-mediated cell death susceptibility. (A) Blood neutrophils from patients with sepsis and joint fluid neutrophils from patients with RA appeared to be primed in vivo. Additional in vitro priming with GM-CSF had no effect on these inflammatory neutrophils. Results of 20-hour cultures are shown (control individuals: n = 6; septic shock neutrophils: n = 5; joint fluid RA neutrophils: n = 4). **P < .01. (B) Cytoplasmic vacuolization associated with inflammation. (Left) Sepsis blood neutrophils following 15-hour anti-Siglec-9 mAb treatment alone in vitro. (Right) Neutrophils from fresh RA joint fluids. Neutrophils were stained with Giemsa-May-Grünwald (Diff-Quik). Original magnification, × 1000.
Siglecs exhibit a specific expression pattern among hematopoietic cells, which is indicative of specific functions.11-16 In contrast, there is only little evidence for the expression of Siglecs on nonhematopoietic cells. Although the cytoplasmic tails of Siglecs vary in sequence and length, almost all, including Siglec-9, share regions of sequence similarity surrounding 2 conserved tyrosine motifs that are implicated in signaling functions. The first motif, also present in Siglec-9, fulfils the classical criteria of an ITIM, which is able to bind and activate inhibitory tyrosine phosphatases such as SHP-1.11 Functional evidence that Siglecs can indeed mediate inhibitory signals has been obtained for CD33 (Siglec-3)32 and Siglec-7.33 Taken together, Siglecs are believed to participate in the process of negative regulation of hematopoietic cells by raising activation thresholds.
This is the third report that suggests the initiation of a proapoptotic pathway by a member of the Siglec family of proteins. Similarly to our observations concerning Siglec-9 and neutrophils, Siglec-8 cross-linking has been demonstrated to induce eosinophil death, and the cytokines IL-5 and GM-CSF enhanced this effect.34 Whereas the type of Siglec-8-mediated eosinophil death in the absence of cytokines was apoptosis, a nonapoptotic death effect in the presence of IL-5 or GM-CSF cannot be excluded from the published data. Similarly to our study, it is possible but remains to be shown that these cytokines also enhance the susceptibility of eosinophils under in vivo inflammatory conditions. Clearly, our studies encourage additional investigations on the type of eosinophil death mediated by Siglec-8 following IL-5 or GM-CSF priming. The second previously published study concerns CD33. Cross-linking of CD33 also resulted in the induction of apoptosis in acute myeloid leukemic cells.35 Based on these data, it is tempting to speculate that Siglecs represent a novel family of death receptors. However, we cannot exclude that the direct death-inducing effect mediated by Siglecs is limited to granulocytes and, perhaps, other myeloid cells.
We demonstrated accelerated caspase activation following Siglec-9 cross-linking in neutrophils. However, how caspases are activated remains unclear. The cytoplasmic tail of Siglec-9 does not contain a death domain as seen in the Fas receptor or related molecules. Therefore, caspase-8 activation is unlikely to occur via Fas-associated protein with death domain (FADD). One possibility is that SHP-1 is indeed activated following Siglec-9 ligation, in particular in the presence of GM-CSF, which increased tyrosine phosphorylation of Siglec-9 in neutrophils. SHP-1 may then inactivate phosphoinositide 3′-kinase (PI3K), perhaps by physical interaction with its p85 regulatory subunit.36 The PI3K pathway is an important antiapoptotic pathway in many cells, including neutrophils,37 and SHP-1 has been implicated in the regulation of neutrophil apoptosis.38 For instance, decreased enzymatic SHP-1 correlates with inhibition of neutrophil apoptosis, since formyl-methionyl-leucyl-phenylalanine (FMLP) mediates PKC-mediated SHP-1 inactivation39 as well as delayed apoptosis5 in these cells. Moreover, a role of SHP-1 in regulating neutrophil numbers was suggested by the observation that this phosphatase is overexpressed in patients with severe neutropenia.40 Increased enzymatic SHP-1 may block cytokine effects important for both antiapoptosis and differentiation,41 resulting in low neutrophil numbers. Taken together, reduction of the baseline PI3K activity by SHP-1 might be one possible mechanism of how spontaneous apoptosis is accelerated by Siglec-9.
Our data suggest that Siglec-9 ligation generates a respiratory burst, which results in increased cytotoxicity both in the presence and absence of GM-CSF. This conclusion is based on the observation that neutrophils, which cannot generate ROS (due to a genetic defect or pharmacologic inhibition of NADPH oxidase), demonstrated reduced or no Siglec-9-mediated death, depending on the conditions. Moreover, the use of a ROS scavenger in normal neutrophils reduced anti-Siglec-9-induced death in the absence of GM-CSF and also reversed the cytokine priming effect.
Interestingly, the Siglec-9-mediated death observed following cytokine priming was at least partially caspase-independent and nonapoptotic. This nonapoptotic death was characterized by cytoplasmic vacuolization and may represent an autophagosomal-lysosomal cell death.42 Interestingly, a similar caspase-independent death pathway has recently been described in human neutrophils stimulated with TNF-α in the presence of z-VAD-fmk.43 Moreover, earlier work has described similar morphologic features in cell lines following TNF-α- or Fas-mediated44,45 cytotoxicity in the presence of caspase inhibitors. Our immunoblotting experiments suggest that, in the presence of GM-CSF, caspase activation is inhibited. Thus, the possibility exists that caspase inhibition by GM-CSF is responsible for the switch from the apoptotic into the aberrant form of neutrophil death. In contrast, in the case of concurrent GM-CSF and Fas receptor activation, no caspase inhibition by GM-CSF is observed, since the antiapoptotic signal is disrupted already at the plasma membrane.22 Clearly, additional work is required to better define the intracellular requirements and mechanisms responsible for the nonapoptotic and caspase-independent form of cell death mediated by Siglec-9.
The fact that neutrophils with cytoplasmic vacuolization were also seen in joint fluids of patients with RA demonstrates that this type of cell death occurs in vivo. Moreover, we obtained evidence for increased Siglec-9-mediated cytotoxicity in freshly isolated neutrophils derived from patients suffering from sepsis and RA, suggesting that the neutrophils were exposed to GM-CSF and/or interferons under in vivo conditions. Interestingly, priming in RA was seen only in joint fluid but not in blood neutrophils (data not shown), suggesting that inflammation occurs primarily in the joint. In contrast, blood neutrophils from patients with sepsis demonstrated an enhanced Siglec-9-mediated cytotoxic effect, suggesting a strong systemic inflammatory response. The priming effect was restricted to the acute shock phase and was not seen in later stages of septic patients when expression levels of proinflammatory cytokines might have been declined (data not shown).
Siglec-9 recognizes both α2-3- and α2-6-linked sialic acids.16 One important limitation of our study is that Abs rather than a natural ligand were used to cross-link Siglec-9. However, since the exact physiologic ligands of virtually all Siglecs are unknown, all functional studies so far have been performed using agonistic Abs. By using F(ab')2 fragments, we excluded that the effects following Ab incubation were mediated via Fc-receptors. Moreover, cross-linking of Siglec-9 induced only death in Siglec-9-positive neutrophils, but not in Siglec-9-negative eosinophils, confirming that the observed effects were specific. Like anti-Siglec-9 Abs, the physiologic ligand of Siglec-9 must be able to form Siglec-9 clusters. Therefore, we speculate that the death-promoting activities initiated by Siglec-9 are most likely induced via trans-interactions with sialic acids on other cells.
Although a great deal is known about the molecular basis for sialic acid recognition by Siglecs, little is known about their functions. Therefore, our study provides significant progress in the field. Together with the information that Siglec-8 induces apoptosis in eosinophils,34 it can be concluded that Siglecs are surface molecules involved in the molecular control of granulocyte death, and, consequently, in the regulation of granulocyte numbers. These mechanisms may play an important role in the innate immune system both under physiologic and pathologic conditions. In addition, it is possible that molecular interactions between Siglec-9 and its ligand contribute to the phenomenon of “immune privilege” seen in some organs.46
Prepublished online as Blood First Edition Paper, April 12, 2005; DOI 10.1182/blood-2004-10-4112.
Supported by the Swiss National Science Foundation (grant no. 31-58 916.99); the Bonizzi Theler Foundation, Zurich; and the Kurt and Senta Hermann Foundation, Vaduz.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs D. Simon (Dept of Dermatology, University of Bern) and M.F. Fey (Dept of Medical Oncology, University of Bern) for the organization of blood samples and bone marrow aspirates, respectively, from control individuals. We also thank I. Schmid, E. Kozlowski, and V. Winkelmann, who provided excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal