Abstract
Resistance to cytotoxic drugs frequently emerges during treatment of leukemia with conventional chemotherapy. New classes of anticancer drugs, such as the farnesyltransferase inhibitors (FTIs), show therapeutic promise, but whether cells will easily develop resistance against them is not known. Here, we grew breakpoint cluster region/Abelson murine leukemia (Bcr/Abl) P190 lymphoblasts on stroma and made them resistant to the FTI SCH66336/lonafarnib to model emerging drug resistance in a patient. These cells exhibited greatly increased (> 100-fold) expression levels of a novel ATP (adenosine triphosphate)-binding cassette (ABC) transporter-homologous gene, ATP11A. We showed that overexpression of this gene provided protection against the effects of SCH66336, whereas knockdown of endogenous ATP11a using small interfering RNA (siRNA) made cells more sensitive to this drug. The lymphoblasts that were resistant to this FTI were also more resistant to FTI-276 and to GGTI-298, 2 other structurally similar inhibitors. Surprisingly, the cells were also able to survive higher concentrations of imatinib mesylate, the Bcr/Abl tyrosine kinase inhibitor. However, the cells remained sensitive to vincristine. Our results show that elevated levels of ATP11a can protect malignant lymphoblastic leukemia cells against several novel small molecule signal transduction inhibitors. A determination of the expression levels of this gene may have prognostic value when treatment with such classes of drugs is contemplated. (Blood. 2005;106: 1355-1361)
Introduction
Philadelphia (Ph)-positive leukemias, including chronic myelogenous leukemia (CML) and a subclass of acute lymphoblastic leukemia (ALL), are caused by the fusion of the BCR gene to the ABL proto-oncogene.1,2 The P190 type of breakpoint cluster region/Abelson murine leukemia (Bcr/Abl) protein is associated with Ph chromosome-positive ALL. Prognosis for this type of cancer is poor.3-5 Even though the tyrosine kinase inhibitor imatinib mesylate is effective in treatment of chronic-phase CML, Phpositive ALL responds only transiently to treatment with this drug.6,7 Also, responses of patients with CML in blast phase to the standard dose of imatinib mesylate may be transient, and resistance to this drug can develop.8-10 Other treatments are therefore clearly needed.
A class of molecules that interfere with the activity of the enzyme farnesyltransferase (FT) has emerged in preclinical models as a possible alternative treatment for this type of leukemia.11-13 Experiments in mouse models suggest the drug may be very effective; in addition, cells of patients that are resistant to imatinib mesylate appear to be sensitive to farnesyltransferase inhibitors (FTIs).14-17
Experience with most anticancer drugs, including imatinib mesylate,18,19 has shown that invariably, in some patients, resistance against such drugs arises. FTIs are currently in phase 1 and phase 2 clinical trials for a variety of cancers, including leukemias.20-23 However, whether resistance against such drugs can develop is unknown. In view of the potential use of FTIs in the treatment of Ph-positive ALL, we set out to explore possible mechanisms of drug resistance against FTIs. We subjected cells expressing Bcr/Abl P190 that were derived from a transgenic mouse model to increasing concentrations of the FTI SCH66336 (lonafarnib) and obtained a fully drug-resistant derivative. The current study describes a novel mechanism of resistance against the FTI SCH66336 and other small molecule signal transduction inhibitors.
Materials and methods
Isolation of B-1 cells, generation of B-1R cells, and drug treatment
Bin-1 (B-1) cells were grown in culture from the lymphoma of a 7-month-old male P190 BCR/ABL transgenic mouse from the 623 line.24,25 Briefly, the lymphoma was dissected into several small pieces, which were then pressed between the frosted parts of 2 microscope slides and washed with phosphate-buffered saline supplemented with 1 U/mL heparin. The cell suspension was filtered through a 35-μm nylon mesh. B-1 cells were grown in McCoy 5A Modified Medium (Invitrogen, Carlsbad, CA) with 15% fetal calf serum (heat-inactivated), 110 mg/mL sodium pyruvate, 2 mM l-glutamine, 100 U/mL penicillin/streptomycin, and freshly added 50 μM β-mercaptoethanol and 10 ng/mL murine interleukin 3 (IL-3). Cells were maintained on irradiated (100 Gy [10 000 rad]) primary mouse embryonic fibroblast feeder layers.
To isolate cells that were able to tolerate increased concentrations of FTI, the B-1 cells were grown on mitotically inactivated fibroblasts over a period of 3 months in the presence of dimethyl sulfoxide (DMSO) or DMSO with 0.05 to 16 μM SCH66336. The medium was refreshed every 2 to 3 days with inclusion of fresh SCH66336. Lymphoma cells were exposed to a starting concentration of 0.05 μM SCH66336, which killed about half of the cells. When the cells stopped dying and resumed proliferation, which took about 1 to 2 weeks, the doses of SCH66336 were doubled. After the final adaptation, to 16 μM SCH66336, all cells were harvested on the same day after an identical period after refreshing of the medium. RNA and protein was isolated.
Cells were exposed to FTI-276 (10 μM), GGTI-298 (20 μM) (both from Calbiochem, La Jolla, CA), STI571 (5 μM), or vincristine sulfate (0.5 μM) (from Faulding Pharmaceutical, Parasmus, NJ). After drug exposure, cells were collected and resuspended in culture medium containing 0.1% (wt/vol) trypan blue. Trypan blue-excluding and total cells were counted using a hemocytometer. All drug sensitivity assays were done in triplicate. Viability of the cells is expressed as the percentage of trypan blue-excluding cells of the total number of cells. Data points show the mean plus or minus SEM of triplicate samples.
Sequencing farnesyltransferase
We used the ELXR Exon locator and extractor26 (http://exon.swmed.edu/about.html) to identify primers suitable for sequencing of murine exons 1, 5, and 11 of the FTβ subunit. These were used to amplify the corresponding exons from the genomic DNA of B-1R using PfuI polymerase. Fragments were gel purified, and 150 ng product was sequenced. Primers used to amplify exon 1 were forward (Fw) 5′-atgtgtaggtgggaggagagatt-3′ and reverse (Rv) 5′-gtgcactacgggagttgtagttc-3′; for exon 5 Fw 5′-ccaggtctaccaaagatctcctc-3′ and Rv 5′-ggcagtagtcagtgccatacagt-3, for exon 11 Fw 5′-tcctctggattcctgtagtaggg-3′ and Rv 5′-gaataaggtcccagcatctctg-3′.
Preparation of RNA and Northern blotting
RNAs were extracted using Trizol according to the supplier's specifications (Invitrogen, Carlsbad, CA). Samples in loading buffer with ethidium bromide (15 μg total RNA) were run on a 1.0% agarose gel containing 20 mM guanidine thiocyanate27 in 1 × TBE (Tris [tris(hydroxymethyl)aminomethane]-borate-EDTA [ethylenediaminetetraacetic acid]) buffer. Gels were blotted in 3M NaCl, 0.2 MNaH2 PO4, 0.02 M EDTA, pH 7.4, to Hybond-N (Amersham, Arlington Heights, IL). The ATP11a probe for Northern and Southern blotting was generated using the primers described for reverse transcriptase-polymerase chain reaction (RT-PCR) using total mouse heart RNA. Hybridization was for 24 hours in 50% deionized formamide, 5 × standard saline citrate (SSC), 5 × Denhardt, 0.5% sodium dodecyl sulfate (SDS), 100 μg/mL herring sperm DNA, 10% dextran sulfate, at 42°C; the final posthybridization wash was at 0.01 × SSC at 65°C.
(Real-time) RT-PCR
Total RNAs isolated from B-1, B-1R, and B-1S were subjected to RT-PCR for ATP11a using as forward primer 5′-cgctcgtgcgcagatactg (presumably from exon 1a, nt 113-132 in NM_015804, version 1/7/02; the ATG codon is at nt 91) and reverse primer 5′-gacgaagaagagtgggagc (nt 399-381; presumed exon 3). Murine ATP (adenosine triphosphate)-binding cassette transporter A1 (ABCA1) forward and reverse primers28 were 5′-ggacatgcacaaggtcctga and 5′-cagaaaatcctggagcttcaaa. Annealing temperature was 55°C for both. As control, the same RNAs were subjected to RT-PCR for actin. Intactness of total RNA was assessed by electrophoresis on a non-denaturing agarose gel upon staining with ethidium bromide. For quantitative RT-PCR, RNAs were treated with DNAse I (amplification grade; Invitrogen). First-strand synthesis on 10 μg total, DNAse I-treated RNA was performed using SuperScript (Invitrogen) and random primers in a 50-μL volume. PCR reactions were on 1 μL cDNA. The ATP11a and ABCA1 primer combinations were used at annealing temperatures of 57°C. For ATP11a, the same forward primer but a different reverse primer (5′-ggaatgtaggcctctgcac, nt 215-197, presumed exon 2) was used, which gave a product of 101 base pairs (bp); for ABCA1 the same forward and reverse primers as for RT-PCR were used. To check whether the specific small interfering RNAs (siRNAs) silenced the expression of ATP11a, cells were harvested for real-time RT-PCR 36 hours after transfection with siRNAs. cDNA was synthesized using an EndoFree RT reverse transcriptase kit (Ambion, Austin, TX) according to the supplier's specifications.
ATP11a cDNAs
The murine Atp11a and human ATP11A genes (GeneCard identifier GC13P112526)29 appear to encode multiple spliced forms, presumed to result in proteins with different N- and C-terminal ends. These include for the N-terminus: MDCSLVRTLVHRY... cage (FB2776_E08 and XM_085028 version 2; 1134 amino acid residues, human), and the murine homolog MDCSLLRTLVRRY... cage (NM_015804, version 7/1/2, 1188 amino acid residues, mouse), with the residues in capital letters representing the alternative first amino acid residues, and those in small letters the common residues. An alternative N-terminal end for human is MGEKLSGKLSPCLRRGVAQQPR... cage (XM_085028, version 3, 1202 amino acid residues, human).
Alternative C-terminal ends include sqq(tlydtayltlynisftslpillyslmeqhvgidvlkrdptlyr)dvak.... (AL161996, human, residues missing via spliced out exon indicated between brackets; this would remove 1 putative transmembrane domain and 1 extracellular domain); TATERVQ... tksqclsveqstifmlsqtssslsf* (XM_085028 human version 2 01/08/02; this version is shared by at least 4 other human clones such as KIAA1021); TATERTQ... ngcaqprdrdseftplaslqspgyqstcpsaawysshsqqvtlaawkekvstepppilggshhhcssipshscprsrvgmlv* (XM_085028 human version 3 of 17/10/03) and TATERVQ... niqhqdsiseftplaslpswgaqgsrllaaqcsspsgrvvcsrweseecpvlplhpglphkarygccrsslempt* (NM_015804 mouse, gi 81334324).
We obtained a full-length human ATP11A cDNA clone FB2768 (XM_085028 version 2) from OriGene Technologies (Rockville, MD). This clone has an insert of around 5.5 kb, with the insert directionally cloned between the EcoRI and SalI sites of the pCMV-XL4 vector.
Transfections, cell-cycle analysis, and Western blots
To verify that the ATP11A cDNA clone FB2768 expressed a protein, we inserted it in frame with the Xpress tag of pcDNA and the presence of protein was verified after transfection and Western blotting.
To test for activity, we used the untagged ATP11A cDNA clone FB2768. 293A cells, which are sensitive to 20 μM SCH66336, were transfected with enhanced green fluorescent protein (EGFP) and either the ATP11A cDNA or pcDNA vector control and then treated with 20 μM SCH66336 24 hours after transfection. Twenty-four and 36 hours after the start of treatment, the cells were collected, stained with propidium iodide, and analyzed using fluorescence-activated cell sorting.
Predesigned siRNAs against mouse ATP11a (NM_015804) were obtained from Ambion. NIH3T3 cells were tested to be sensitive to 50 μM SCH66336. Cells were transfected with 2 different ATP11a siRNAs (ASU, no. 72127, nt 204-222 of NM_015804, exon 2; and ASV, no. 72223, nt 423-441 of NM_015804, exon 5) and a nonspecific control siRNA in Optimem (400 pmol siRNA per 10-cm dish) using Lipofectamine 2000 (Invitrogen). Twenty-four hours after transfection, the cells were exposed to 50 μM SCH66336. After 48 hours of drug treatment, all cells were collected, and total and viable cell counts were performed. Bright field images of the cells in PBS were taken using a Hamamatsu (Bridgewater, NJ) ORCA-ER digital CCD camera and Compix (Cranberry Township, PA) SimplePCI acquisition software. Images were processed using Powerpoint (Adobe Systems, San Jose, CA). Images were captured with a Leica Microsystems (Wetzlar, Germany) MZ FL III motorized fluorescence stereomicroscope with a 1× PlanApo lens and 10× zoom, at 100× magnification.
HDJ-2 (human DnaJ homologue 2) antibodies detect a 47-kDa chaper-one protein. The mobility shift and appearance of a slower migrating, unprocessed form of HDJ-2 on SDS-polyacrylamide gel electrophoresis (PAGE) is the result of FT inhibition. Monoclonal anti-HDJ-2 antibodies were obtained from NeoMarkers (Fremont, CA).
Results
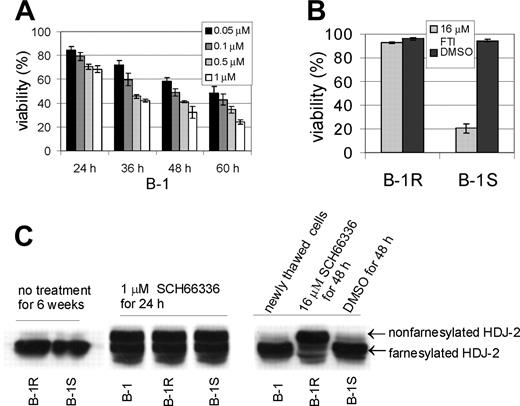
We cultured pro-B-leukemic cells (named B-1) from a P190 BCR/ABL transgenic mouse on mitotically inactivated fibroblast feeder layers.11 B-1 was characterized in more detail with respect to sensitivity to the FTI SCH66336. As shown in Figure 1A, the cells were initially very sensitive to this drug. After a 48-hour incubation in 0.1 μM SCH6636, about 50% of the cells were killed. An aliquot of these cells was subsequently exposed to progressively increasing concentrations of the FTI, up to 16 μM, over the course of about 3 months, yielding B-1R cells. A parallel culture (B-1S) was grown in the presence of vehicle (DMSO) during the same period of time. To test the stability of the drug-resistant phenotype, we removed SCH66336 from the medium of the B-1R cells and cultured both B-1S and B-1R cells for an additional 6 weeks before reexposure to 16 μM FTI for 48 hours. As shown in Figure 1B, the B-1S cells markedly declined in viability, whereas the B-1R cells remained fully resistant to 16 μM drug, indicating the phenotype was stable.
Stable drug-resistant phenotype of B-1R cells is not caused by inactivation of the FT. (A) B-1 cells were treated with SCH66336 as indicated. Viability is expressed as the percentage of trypan blue-excluding cells of the total number of cells present. The error bars indicate the standard deviation. (B) B-1R or B-1S cells were cultured for 6 weeks without SCH66336, then treated de novo with 16 μM SCH66336 or with vehicle DMSO for 48 hours and viability was determined. One of 2 experiments with similar results is shown. The error bars in panels A and B indicate standard deviation. (C) Western blot analysis with an anti-HDJ-2 antibody, diagnostic for FT activity. B-1R or B-1S cells not treated with SCH66336 show only the farnesylated form of HDJ-2 (left), indicating that the FT is active. After 24 hours of treatment with 1 μM SCH66336, the original B-1 cells, the FTI-resistant B-1R cells, and the drug-sensitive B-1S cells all exhibit the appearance of the more slowly migrating, nonfarnesylated HDJ-2 protein (middle), showing that all 3 contain an FT that is inhibited by the FTI. Treatment of B-1R cells with 16 μM SCH66336 (right) caused the appearance of very large amounts of nonfarnesylated HDJ-2 protein.
Stable drug-resistant phenotype of B-1R cells is not caused by inactivation of the FT. (A) B-1 cells were treated with SCH66336 as indicated. Viability is expressed as the percentage of trypan blue-excluding cells of the total number of cells present. The error bars indicate the standard deviation. (B) B-1R or B-1S cells were cultured for 6 weeks without SCH66336, then treated de novo with 16 μM SCH66336 or with vehicle DMSO for 48 hours and viability was determined. One of 2 experiments with similar results is shown. The error bars in panels A and B indicate standard deviation. (C) Western blot analysis with an anti-HDJ-2 antibody, diagnostic for FT activity. B-1R or B-1S cells not treated with SCH66336 show only the farnesylated form of HDJ-2 (left), indicating that the FT is active. After 24 hours of treatment with 1 μM SCH66336, the original B-1 cells, the FTI-resistant B-1R cells, and the drug-sensitive B-1S cells all exhibit the appearance of the more slowly migrating, nonfarnesylated HDJ-2 protein (middle), showing that all 3 contain an FT that is inhibited by the FTI. Treatment of B-1R cells with 16 μM SCH66336 (right) caused the appearance of very large amounts of nonfarnesylated HDJ-2 protein.
Drug resistance against the FTI could have developed because of acquired mutations in the farnesyl transferase enzyme, which would make the FTI unable to inhibit it.30 We investigated this possibility using Western blot analysis and an antibody diagnostic for FT activity.31 B-1R or B-1S cells not exposed to FTI only contained the fully processed, faster migrating farnesylated form of the HDJ-2 protein (Figure 1C, left). This showed that the FT enzyme was active. Because treatment with 16 μM SCH66336 killed most of the B-1S cells, we used a lower dose of 1 μM and a short 24-hour treatment to compare the inhibition of the FT in B-1, B-1S, and B-1R cells. As shown in Figure 1C, middle, the amount of non-farnesylated HDJ-2 protein, which has a retarded mobility, was indistinguishable in the B-1R, B-1S, and B-1 cells. Exposure of the B-1R cells to 16 μM SCH66336 caused an almost complete block of the farnesylation of the HDJ-2 protein (Figure 1C, right). This result shows that the FT was still inhibited by the drug in the B-1R cells.
Del Villar et al30,32 reported that resistance to FTIs can be generated and the substrate specificity of the FT enzyme can be changed by point mutations in specific residues of the FTβ subunit. To investigate whether the substrate specificity of the FT could have been modified in the B-1R cells because of point mutations, we sequenced exons 5 and 11 of the FTβ gene in the B-1R cells. However, no mutations were found.
Microarray analysis on RNA isolated from the B-1R and B-1S cells showed that expression of the leukocyte ABCA1 member of the ABC transporter family, to which the multidrug resistance protein multidrug resistance 1 (MDR1)/ABCB1 also belongs33 was significantly decreased in the B-1R cells. RT-PCR and real-time RT-PCR confirmed this and showed that the B-1R cells had 50-fold lower ABCA1 mRNA than B-1S cells (Figure 2C, left).
Expression of ATP11a mRNA in FTI-resistant lymphoblasts. (A) RT-PCR using primers for murine ATP11a (top) and ABCA1 (middle) or actin (bottom) on the mouse RNAs indicated above the panel. B-1 represents the original cells in short-term culture. (B) Northern blot analysis of ATP11a mRNA expression in pro-B-leukemia cells. The blot was hybridized to a mouse ATP11a probe and washed to a stringency of 0.01 × SSC, 65°C. The positions of 28 and 18S rRNA are as indicated. (Right) Ethidium bromide-stained gel; (left) autoradiogram. (C) Real-time RT-PCR using primers for ABCA1 (left) or ATP11a (right). Values are relative to those of actin. B-1R cells expressed 140-fold more mRNA for ATP11a and 50-fold lower levels of ABCA1 than B-1S cells. Primers used are described in “Materials and methods.” Error bars indicate standard deviation.
Expression of ATP11a mRNA in FTI-resistant lymphoblasts. (A) RT-PCR using primers for murine ATP11a (top) and ABCA1 (middle) or actin (bottom) on the mouse RNAs indicated above the panel. B-1 represents the original cells in short-term culture. (B) Northern blot analysis of ATP11a mRNA expression in pro-B-leukemia cells. The blot was hybridized to a mouse ATP11a probe and washed to a stringency of 0.01 × SSC, 65°C. The positions of 28 and 18S rRNA are as indicated. (Right) Ethidium bromide-stained gel; (left) autoradiogram. (C) Real-time RT-PCR using primers for ABCA1 (left) or ATP11a (right). Values are relative to those of actin. B-1R cells expressed 140-fold more mRNA for ATP11a and 50-fold lower levels of ABCA1 than B-1S cells. Primers used are described in “Materials and methods.” Error bars indicate standard deviation.
However, the gene of which the expression differed the most between the drug resistant and sensitive cells (almost 100-fold increased in B-1R) was an unknown product. This was annotated (Affymetrix U74Av2 GeneChip; Affymetrix, Santa Clara, CA) as being a “rat UA20 homologous” gene, a short mRNA putatively encoding a protein of 104-amino acid residues. Subsequent database searches and analysis of the genomic DNA in mouse and humans containing this putative gene suggested to us that instead, it perhaps could be the alternatively spliced 3′ untranslated region of an upstream locus (not shown). That locus encodes a product, which, based on DNA homology database searches, was previously identified as a possible member of the extended ABC transporter family.34 We therefore performed RT-PCR and real-time quantitative RT-PCR analysis using primers derived from this gene, called ATP11a. This analysis showed that the B-1R cells indeed expressed 70- to 160-fold more ATP11a mRNA than the original B-1 cells and the B-1S cells, both of which are FTI sensitive (Figure 2C, right). Even after nonexposure of the B-1R cells to FTI for 6 weeks, these high ATP11a mRNA levels remained (not shown). Northern blot analysis confirmed that the ATP11a mRNA, of around 8 kb, was overexpressed in the B-1R cells (Figure 2B). Southern blot analysis of the ATP11A gene in the B-1R DNA showed that this locus was not amplified (not shown).
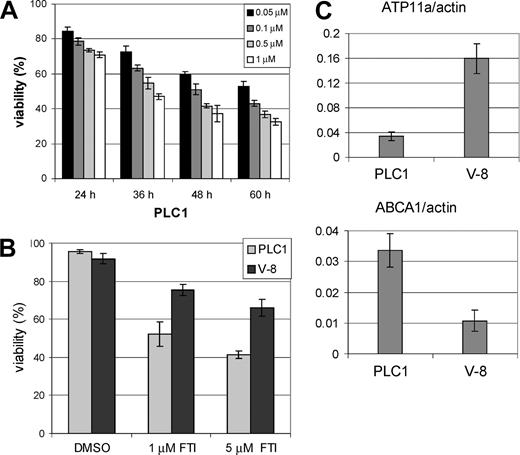
A second independently generated pro-B-leukemia cell line, called PLC1, showed a similar sensitivity as the B-1 cells to SCH66336 (Figure 3A). In other experiments, these cells had been grown in a nude mouse model for several weeks, while the mice had not received any treatment with drugs11 and were subsequently put in culture again. This passage appeared to have spontaneously generated cells (called V-8) that were more resistant to SCH66336 (Figure 3B). Whereas the original PLC1 cells had levels of ATP11a similar to those in B-1S (ATP11a/actin values of 0.03 and 0.03, respectively), the V-8 cells had higher expression levels of ATP11a (ATP11a/actin = 0.16) as well as lower ABCA1 levels (Figure 3C). This suggests a correlation between relative resistance to SCH66336 and ATP11a levels.
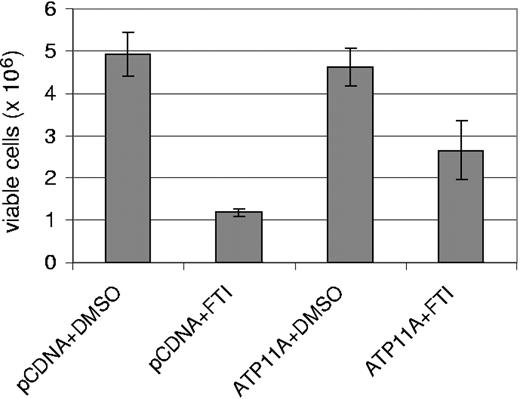
Does ATP11a overexpression confer resistance to SCH66336? To investigate this, we obtained a full-length human ATP11A cDNA clone and transfected it into human embryonic kidney 293 cells, which are sensitive to treatment with 20 μM SCH66336. In cells transfected with pcDNA vector, FTI treatment for 36 hours resulted in an increased accumulation of cells in G2/M, consistent with the reported effect of SCH66336 in causing a G2/M pause in different cell lines,35 including cells transfected with BCR/ABL.12 The cells transfected with ATP11A did not show an effect of the drug at 36 hours (Table 1). Moreover, we found that transient transfection of ATP11A increased the overall viability of the cells 2- to 3-fold as compared with cells that expressed vector only, when the cells were exposed to 20 μM SCH66336 for 48 hours (Figure 4). These experiments show that increased levels of ATP11A cause increased resistance to the effects of FTI SCH66336.
Effect of ATP11A on SCH6636-induced G2/M block
. | 24 h . | . | 36 h . | . | ||
|---|---|---|---|---|---|---|
| Transfected/treated . | G1, % . | G2/M, % . | G1, % . | G2/M, % . | ||
| pcDNA/DMSO | 61.5 | 18.6 | 58.3 | 23.2 | ||
| pcDNA/20 μM SCH66336 | 62.4 | 20.6 | 49.4 | 34.5 | ||
| ATP11A/DMSO | 53.7 | 31.5 | 62.0 | 22.2 | ||
| ATP11A/20 μM SCH66336 | 57.1 | 26.3 | 55.6 | 26.1 | ||
. | 24 h . | . | 36 h . | . | ||
|---|---|---|---|---|---|---|
| Transfected/treated . | G1, % . | G2/M, % . | G1, % . | G2/M, % . | ||
| pcDNA/DMSO | 61.5 | 18.6 | 58.3 | 23.2 | ||
| pcDNA/20 μM SCH66336 | 62.4 | 20.6 | 49.4 | 34.5 | ||
| ATP11A/DMSO | 53.7 | 31.5 | 62.0 | 22.2 | ||
| ATP11A/20 μM SCH66336 | 57.1 | 26.3 | 55.6 | 26.1 | ||
293 cells were transfected with either pcDNA plus EGFP or ATP11A plus EGFP. Twenty-four hours after transfection, treatment with either DMSO or 20 μM SCH66336 in DMSO was initiated. EGFP-positive cells were analyzed for DNA content using propidium iodide at the indicated time points. The percentage of cells in specific phases of the cell cycle is indicated.
PLC1 and derived V-8 pro-B-leukemia cells show correlation between relative sensitivity to SCH66336 and relative ATP11a levels. (A) PLC1 cells were treated with the indicated concentrations of SCH66336 for the indicated amount of time. Viability is expressed as the percentage of trypan blue-excluding cells of the total number of cells present. This experiment was performed in parallel to the one shown in Figure 1A. (B) Viability of parental PLC1 cells was compared with that of PLC1 cells that had been passaged in a nude mouse (V-8 cells); the amount of SCH66336 is as indicated. Viability was measured after 48 hours of drug treatment. (C) Real-time RT-PCR analysis of ABCA1 and ATP11a expression in PLC1 and V-8 cells. Values are relative to those of actin. Note the average values of ATP11a/actin were 0.06 for B-1, 0.03 for B-1S, 4.3 for B-1R (Figure 2C), 0.03 for PLC1, 0.16 for V-8, and 18.1 for NIH3T3 fibroblasts. Error bars indicate the standard deviation.
PLC1 and derived V-8 pro-B-leukemia cells show correlation between relative sensitivity to SCH66336 and relative ATP11a levels. (A) PLC1 cells were treated with the indicated concentrations of SCH66336 for the indicated amount of time. Viability is expressed as the percentage of trypan blue-excluding cells of the total number of cells present. This experiment was performed in parallel to the one shown in Figure 1A. (B) Viability of parental PLC1 cells was compared with that of PLC1 cells that had been passaged in a nude mouse (V-8 cells); the amount of SCH66336 is as indicated. Viability was measured after 48 hours of drug treatment. (C) Real-time RT-PCR analysis of ABCA1 and ATP11a expression in PLC1 and V-8 cells. Values are relative to those of actin. Note the average values of ATP11a/actin were 0.06 for B-1, 0.03 for B-1S, 4.3 for B-1R (Figure 2C), 0.03 for PLC1, 0.16 for V-8, and 18.1 for NIH3T3 fibroblasts. Error bars indicate the standard deviation.
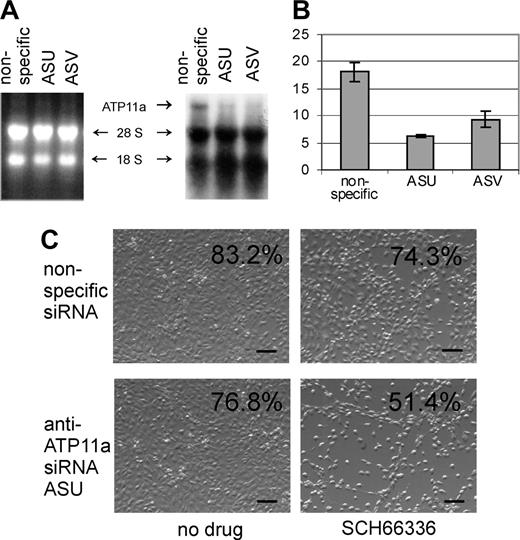
If increased levels of ATP11a confer protection against SCH66336, decreasing its expression should make the cells more sensitive to it. To address this, we selected a cell line, mouse NIH3T3 fibroblasts, that are relatively resistant against SCH66336, but can be killed by high drug doses of 50 μM. Using real-time RT-PCR, we measured about 300-fold more ATP11a mRNA in the NIH3T3 cells than in the B-1 cells (ATP11a/actin 18.1 and 0.06; Figure 5B). We transfected these cells with 2 siRNAs specific for murine ATP11a and with a nonspecific siRNA. As shown in Figure 5A, Northern blot analysis showed that NIH3T3 cells transfected with the nonspecific siRNA clearly express ATP11a mRNA and that ATP11a expression was decreased by the ATP11a-specific siRNAs. Real-time RT-PCR further confirmed a substantial reduction in ATP11a mRNA levels by the specific siRNAs 36 hours after transfection (Figure 5B). Next, we treated cells transfected with the nonspecific and the ASU anti-ATP11a siRNA with 50 μM SCH66336 24 hours after transfection and measured cell viability 48 hours later. As shown in Figure 5C, the transfectants that expressed the ASU siRNA were more sensitive to SCH66336 than the cells transfected with the nonspecific siRNA.
Increased ATP11A expression provides protection against SCH66336. 293 HEK cells were cotransfected with EGFP and ATP11A or with EGFP and pcDNA. After 24 hours, 20 μM SCH66336 was added to the medium, and a viable cell count was performed 48 hours later. The result shown is 1 of 2 independently performed experiments with similar results. Error bars indicate the standard deviation.
Increased ATP11A expression provides protection against SCH66336. 293 HEK cells were cotransfected with EGFP and ATP11A or with EGFP and pcDNA. After 24 hours, 20 μM SCH66336 was added to the medium, and a viable cell count was performed 48 hours later. The result shown is 1 of 2 independently performed experiments with similar results. Error bars indicate the standard deviation.
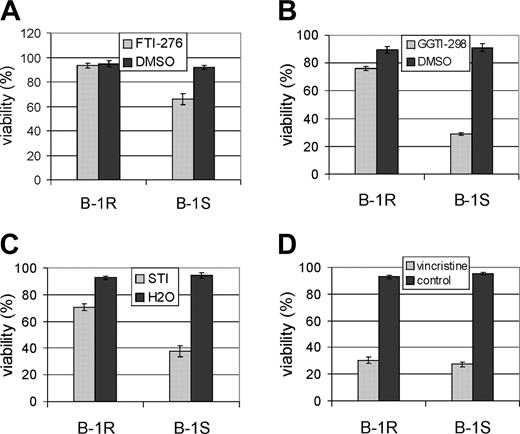
On the basis of its sequence, ATP11a belongs to an extended family of ABC transporters that includes proteins conferring the multidrug resistance phenotype to cancer cells.33 We therefore asked whether the overexpression of this putative ABC transporter would also be correlated with resistance to other drugs. SCH66336 is a tricyclic FTI.36 Therefore, we tested FTI-276, a nonpeptidomimetic FTI similar to SCH66336 but with a different ring structure. As shown in Figure 6A, the B-1R cells were also resistant to treatment with 10 μM FTI-276 for 48 hours as compared with the B-1S cells.
Farnesyltransferase inhibitors are directed against an enzyme that attaches a farnesyl lipid moiety to a number of proteins. A different posttranslational modification involving a geranylgeranyl moiety is catalyzed by the geranylgeranyl transferase (GGT). A number of important signal transduction regulators, including proteins implicated in cancer, are geranyl-geranylated.37 Because inhibitors of GGT (GGTIs) are also being considered for the treatment of leukemia and lymphoma,38,39 we tested GGTI-298, a compound structurally similar to FTI SCH66336. As shown in Figure 6B, the B-1R cells, which are resistant to SCH66336, showed very little decrease in viability when exposed to 20 μM GGTI-298, whereas around 70% of the B-1S cells were killed. We also tested the viability of the B-1R and B-1S cells in the presence of imatinib mesylate, a small molecule inhibitor of the Bcr/Abl tyrosine kinase. Surprisingly, although imatinib mesylate/STI571 is structurally different from the FTIs and GGTI tested here, we found that the B-1R cells showed around 70% viability when treated with 5 μM STI571, whereas only 40% of B-1S cells remained viable after 48 hours of drug treatment.
Knockdown of ATP11a expression increases sensitivity to SCH66336. (A) Northern blot analysis of NIH3T3 cells transfected with nonspecific siRNA, or with 2 different siRNAs specific for murine ATP11a designated ASU and ASV. An arrow points to the 8-kb ATP11a mRNA. (Left) Ethidium bromide-stained gel; (right) autoradiogram. (B) Real-time RT-PCR to determine levels of ATP11a after transfection of cells with siRNAs. RNAs were isolated 36 hours after transfection. Values are relative to those of actin. Error bars indicate standard deviation. (C) Phase-contrast images of cells transfected with ASU or nonspecific siRNAs for 24 hours and subsequently exposed to 50 μM SCH66336 for 48 hours. Bars indicate 100 μm. The percentage of viable cells is indicated in the upper right-hand corners. The result shown is 1 of 2 independently performed experiments with similar results.
Knockdown of ATP11a expression increases sensitivity to SCH66336. (A) Northern blot analysis of NIH3T3 cells transfected with nonspecific siRNA, or with 2 different siRNAs specific for murine ATP11a designated ASU and ASV. An arrow points to the 8-kb ATP11a mRNA. (Left) Ethidium bromide-stained gel; (right) autoradiogram. (B) Real-time RT-PCR to determine levels of ATP11a after transfection of cells with siRNAs. RNAs were isolated 36 hours after transfection. Values are relative to those of actin. Error bars indicate standard deviation. (C) Phase-contrast images of cells transfected with ASU or nonspecific siRNAs for 24 hours and subsequently exposed to 50 μM SCH66336 for 48 hours. Bars indicate 100 μm. The percentage of viable cells is indicated in the upper right-hand corners. The result shown is 1 of 2 independently performed experiments with similar results.
We next compared the sensitivity of the B-1S and B-1R cells to the vinca alkaloid vincristine, commonly used for the treatment of acute lymphoblastic leukemia40 and also containing hydrocarbon ring structures. However, both B-1R and B-1S cells showed equal sensitivity to this compound, retaining only 30% viability after 48 hours of treatment with 0.5 μM of this drug (Figure 6D).
Discussion
In the current study we intentionally made leukemic lymphoblasts drug resistant by exposing them to gradually increased concentrations of a relatively new drug that is currently being tested in human clinical trials for a variety of cancers. During induction of drug resistance, cells were kept in contact with stroma to model the interactions occurring in the bone marrow of a patient during drug treatment. We found that, although the cells initially were very sensitive to 0.1 μM SCH66336, it was possible to derive progeny from them that proliferated in the presence of 16 μM, a 160-fold increased concentration.
The one other study that examined FTI resistance, against tipifarnib (R115777; Zarnestra), in an established human colon cancer cell line, did not report the mechanism that would cause the resistance.41 They suggested but did not investigate the possibility that an acquired mutation in the farnesyltransferase enzyme30 could have caused this phenotype. This was clearly not the cause of FTI resistance in the current study; instead, we found significantly decreased expression of the ABC transporter ABCA1 and markedly increased up-regulation of a novel protein also belonging to the ABC transporter family, ATP11a.
ABCA1 is critical for cholesterol, aminophospholipid, and bile salt transport, and it is mutated in Tangier disease42 but is not known to be involved in the transport of drugs. Because ABCA1 expression is down-regulated when cells are exposed to geranylgeranyl phosphatase, an inhibitor of the geranylgeranyl transferase (GGT) enzyme,43 the decreased expression of ABCA1 found in the current study may relate to a possible altered balance between FT and GGT activity, or the products of these enzymes, in cells exposed to an inhibitor of FT. Alternatively, because SCH66336 inhibits the function of multidrug resistance proteins MDR1/ABCB1 and multidrug resistance protein 1 (MRP1)/ABCC1 and MRP2/ABCC2,44 the drug could affect the activity of multiple members of the ABC transporter family via an unknown common mechanism. Therefore, the significance of the decreased levels of ABCA1 on the drug resistant phenotype, if any, is currently not clear.
Cells resistant to SCH66336 are also resistant to other small molecule inhibitors but not to vincristine. The viability of cells was determined after treatment for 24 hours with (A) 10 μm FTI-276, (B) 20 μM GGTI-298, (C) 5 μM STI571, or (D) 0.5 μM vincristine. The result shown is 1 of 2 independently performed experiments with similar results. The data points show the mean ± SEM of triplicate measurements.
Cells resistant to SCH66336 are also resistant to other small molecule inhibitors but not to vincristine. The viability of cells was determined after treatment for 24 hours with (A) 10 μm FTI-276, (B) 20 μM GGTI-298, (C) 5 μM STI571, or (D) 0.5 μM vincristine. The result shown is 1 of 2 independently performed experiments with similar results. The data points show the mean ± SEM of triplicate measurements.
We found that the levels of ATP11a mRNA were increased up to 100-fold in the cells intentionally made SCH66336 resistant. The ATP11a gene product belongs to an extended family of P-type ATPases. P-type ATPases use the energy of ATP hydrolysis to transport compounds across membranes. Two of 3 subfamilies identified transport heavy metals or H, Na, K; the third subfamily transports substances other than metal ions. However, for most of the latter subfamily members, the real physiologic substrate(s) have not been identified. On the basis of sequence similarity, Halleck et al34 identified about 16 genes belonging to this subfamily of putative aminophospholipid-amphipath transporters. This family includes the familial intrahepatic cholestasis type 1 (FIC 1; ATP8B1) gene product. FIC 1 is involved in primary bile formation.45 On the basis of its primary structure and homology to domains in other family members, ATP11a is predicted to have 10 transmembrane domains and 1 ATP-binding site. Because ATP11a (also designated as ATPase Class VI, type 11A) is expressed in many different types of mouse tissues such as heart, brain, muscle, and liver34 as well as in human blood and colon (UniGene Hs. 508757),46 if it is a transporter, it is likely to have a transport function more general than that in, for example, primary bile formation.
We found that modulation of ATP11a levels through either overexpression or siRNA knockdown caused cells to be less sensitive or more sensitive to SCH66336. One explanation for these findings is that ATP11a is able to directly transport SCH66336 out of cells. However, if ATP11a is indeed a drug transporter, the question arises why its effect in the transfected 293 and 3T3 cells was relatively modest. A number of factors could contribute to these moderate responses. To begin with, drugs such as SCH66336 have a much less acute cytotoxic effect on cells in general, in comparison with very cytotoxic drugs such as vincristine. This is clearly seen in vivo in animal models13 or in human patients.47 Other investigators, working with SCH66336 in vitro, have also described that the drug slows proliferation12 or is cytostatic for some melanoma cells.48 Second, these experiments used immortal established cell lines, which were inherently 200- to 500-fold more resistant to SCH66336 than the leukemic lymphoblasts: 20 μM (293 cells) or 50 μM (NIH3T3 cells) drug was needed to kill substantial numbers of cells, whereas the B-1 lymphoblasts had a 50% reduction in viability after 48 hours of exposure to only 0.1 μM drug. Finally, cells were transiently transfected, which causes only transient expression of either anti-ATP11a siRNA or of the ATP11a cDNA.
Quantitatively, there appeared to be some correlation between the levels of ATP11a expression and degree of resistance against SCH66336 in the leukemia cells: B-1 and B-1R cells both maintained 80% viability in the presence of an amount of drug that differed 320-fold (0.05 μM and 16 μM SCH66336, respectively; Figure 1A-B) and the levels of ATP11a mRNA in the 2 populations differed about 72-fold. However, we cannot formally exclude the possibility that the overexpression of ATP11a indirectly causes the drug resistance, for example, by altering the levels of other drug transporters.
We conclude that overexpression of ATP11a confers on malignant lymphoblasts, either directly or indirectly, the increased ability to survive in the presence of small molecule inhibitors. Although the normal cellular substrates of ATP11a are not known, the gene appears to be ubiquitously expressed, including alternatively spliced forms that potentially encode transporters with different substrate specificities (see “Materials and methods”). Also, we found that the levels of ATP11a were spontaneously increased in lymphoblastic leukemia cells that had been passaged in a nude mouse without exposure to SCH66336. Therefore, some types of cancer may naturally express high levels of ATP11A and could be already partly resistant against small molecule inhibitor-type drugs before treatment is started. Also, our study indicates that elevated levels of ATP11a gene expression could be induced in some types of cancer because of treatment with drugs such as SCH66336. It will be therefore of interest to investigate how this gene is regulated on a transcriptional level and whether it is possible to silence its expression in leukemic cells. In addition, ATP11a levels may have prognostic value in assessing the likelihood of success before initiation of treatment with certain small molecule inhibitors in cancer drug therapy.
Prepublished online as Blood First Edition Paper, April 28, 2005; DOI 10.1182/blood-2004-09-3655.
Supported by the Public Health Service (PHS) (grant CA90321) (N.H.), the Kenneth T. and Eileen L. Norris Foundation (B.Z. and J.G.), and the T.J. Martell Foundation (J.G. and N.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Michael Anderson and his laboratory for access to and help with real-time RT-PCR and Suparna Mishra for the PLC1 cells.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal