Abstract
Ingenol 3-angelate (PEP005) is a selective small molecule activator of protein kinase C (PKC) extracted from the plant Euphorbia peplus, whose sap has been used as a traditional medicine for the treatment of skin conditions including warts and cancer. We report here that PEP005 also has potent antileukemic effects, inducing apoptosis in myeloid leukemia cell lines and primary acute myeloid leukemia (AML) cells at nanomolar concentrations. Of importance, PEP005 did not induce apoptosis in normal CD34+ cord blood myeloblasts at up to 2-log concentrations higher than those required to induce cell death in primary AML cells. The effects of PEP005 were PKC dependent, and PEP005 efficacy correlated with expression of PKC-delta. The delta isoform of PKC plays a key role in apoptosis and is therefore a rational potential target for antileukemic therapies. Transfection of KG1a leukemia cells, which did not express PKC-delta or respond to PEP005, with enhanced green fluorescent protein (EGFP)-PKC-delta restored sensitivity to induction of apoptosis by PEP005. Our data therefore suggest that activation of PKC-delta provides a novel approach for treatment of acute myeloid leukemia and that screening for PKC-delta expression may identify patients for potential responsiveness to PEP005. (Blood. 2005;106:1362-1368)
Introduction
Improvements in drug therapy and in patient care have progressively improved survival among younger patients with acute myeloid leukemia (AML). However, the risk of AML increases with age and success rates in older patients remain very poor.1 As a result, the overall outcome remains very poor. In a study of 214 unselected AML patients in Sweden in whom the median age of patients was 69.5 years, median survival was 5.8 months and probable survival at 5 years was calculated as 9.3%.2 The clinical problem of AML is exacerbated by the shifting population demographics in developed countries toward a more elderly population. Current estimates suggest that by the year 2050 approximately 40% of the population of Europe and North America will be older than 60 years,3 and the impact of this statistic on AML and indeed other cancer incidence, and consequently on health care systems, is clear.
The factors underlying the poorer responses of elderly AML patients to chemotherapy are complex. However, they include the fact that AML occurring in older patients is less responsive to myelosuppressive chemotherapy and that older patients are intrinsically less able to tolerate this form of therapy. Circumvention of these obstacles will require the development of adjunctive therapies that improve tumor responses while not exacerbating the systemic toxicities of established chemotherapeutics.
A number of approaches can be used to find new drugs with possible application to AML and other cancers, including high throughput screening of small molecule libraries. An example of this is the identification of the Bcr-Abl selective protein kinase inhibitor imatinib (Glivec), which has been successfully exploited in the treatment of chronic myelogenous leukemia (CML).4 However, a second and potentially invaluable approach to identifying therapeutically useful small molecules is to revisit remedies established in folklore.
A study in Brisbane demonstrated that significant numbers of the Australian public believed in the efficacy of sap from the common plant Euphorbia peplus, also known as radium weed or petty spurge, as a curative agent against skin cancers and solar keratoses.5 Clinical studies in the 1970s provided preliminary evidence to support this belief, with a biopsy-verified case study demonstrating remission of a basal cell carcinoma after home treatment with crude E peplus sap.6 The active agent in E peplus sap has now been identified as a hydrophobic diterpene ester, ingenol 3-angelate (PEP005), which has been shown to have topical antitumor activity against human cancer cell lines grown as subcutaneous tumors in mice.7
PEP005 is a potent activator of protein kinase C (PKC),8 a family of signaling isoenzymes that regulate many cell processes including proliferation, differentiation, and apoptosis.9,10 This signaling pathway has already been the target of several novel anticancer agents,11-13 and the potential for therapeutic activity of PEP005 beyond skin cancer therefore seemed likely.
Here we have investigated the in vitro antileukemic activity of PEP005 against both AML cell lines and primary AML blast cells and determined more precisely its mode of action. Our data show that PEP005 was able to induce apoptosis in cell lines and primary AML blasts, whereas, in contrast, nonmalignant myeloid blasts were resistant to PEP005-induced apoptosis but were induced to partially differentiate. Importantly, not all myeloid cell lines were equally sensitive to PEP005, and we noted that the resistance displayed by KG1a cells was associated with the failure to express PKC-δ. However, transfection of KG1a cells with enhanced green fluorescent protein (EGFP)-PKC-δ restored not only PKC-δ expression but also an apoptotic response to PEP005. Analyses using selective PKC inhibitors and nuclear relocation further indicated that the antineoplastic action of PEP005 against AML is mediated via activation of PKC-δ. Together our findings provide a compelling case for the targeted activation of PKC-δ as a rational therapeutic intervention in acute myeloid leukemia and, by extension, other cancers in which tumor progression has not involved suppression of this PKC isoenzyme.
Patients, materials, and methods
Cell cultures
The myeloid leukemic cell lines HL60, NB4, U937, K562, and KG1a were all grown in RPMI 1640 medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum (Sera Laboratories International, Crawley, United Kingdom), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma Aldrich, Poole, United Kingdom) at 37°C and 5% CO2. AML blasts were isolated from patient bone marrow aspirates and peripheral blood of 8 patients at diagnosis or relapse. Patients were not receiving AML therapy at time of sampling. Blasts were isolated by Ficoll density centrifugation and were cultured in serum-free insulin transferrin selenium-positive (ITS+) medium (Life Technologies) containing 300 ng/mL stem cell factor (SCF) and 10 ng/mL interleukin 3 (IL3; R and D Systems, Abingdon, United Kingdom) and antibiotics. Blast purity ranged from 80% to 95%, as assessed by expression of CD34. Normal myeloblasts were isolated from cord blood or adult mobilized peripheral blood. Briefly, CD34+ cells were indirectly selected from the mononuclear cell preparations using antibody-coated magnetic beads (Miltenyi Biotec, United Kingdom). The study was approved by the ethics committee of the Birmingham University Hospital National Health Service (NHS) trust, and all patients gave written informed consent in accordance with the Declaration of Helsinki.
Assays for apoptosis and differentiation in AML cells
To determine the effects of ingenol 3-angelate (PEP005), cells were incubated for up to 5 days with medium alone or PEP005 at a range of concentrations from 0.2 nM to 20 μM. PEP005 was extracted from Euphorbia peplus and supplied as a 98.5% pure preparation by Peplin (Brisbane, Australia) as a dry pellet and was made up to a stock of 20 μg/mL in acetone on a weekly basis. Stocks were stored at 4°C and diluted into medium when required. Final acetone concentration was never more than 0.1%. Apoptosis and differentiation were assessed after 1 to 5 days. Apoptosis was determined by several methods: cells were fixed and stained with propidium iodide and DNA content was revealed by flow cytometry, with apoptotic cells forming a sub-G0/G1 peak14 ; the presence of activated caspase-3 was determined using a commercial kit based upon the cleavage of a fluorescent caspase-3 peptide substrate (Oncoimmunin, Columbus, OH); or the methyl-thiazol tetrazolium (MTT) assay was used as a marker of cell viability.15
Cell differentiation was determined by staining of cells with anti-CD11b antibody as a general early marker of myeloid differentiation. In studies with HL60 cells, PEP005-treated and 20 nM phorbol myristate (PMA)-treated (Sigma Aldrich) cells were also assessed for their ability to phagocytose complement-coated yeast, a marker of a fully differentiated myeloid cell, as previously described.16 The ingestion of 3 or more yeasts was taken as a positive result. In normal cord blood or adult bone marrow myeloblasts, loss of CD34 was taken as a marker of differentiation. Isotype-matched controls were used and all antibodies were from DAKO (Cambridge, United Kingdom). Differentiation was also induced by incubation of cells with 10 nM all-trans retinoic acid (ATRA; Sigma Aldrich). Cells were also used to prepare cytospins, which were then differentially stained using a commercial May-Grünwald-Giemsa stain (Diff-Kwik; Gamidor, Abingdon, United Kingdom) to identify cells with a blast or differentiated or apoptotic morphology. Slides were examined using a Zeiss III RS brightfield microscope equipped with a 40 ×/0.75 water-immersion objective (Carl Zeiss, Jena, Germany). Images were captured with a SPOT2 camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed using Image-Pro 4.0 software (Media Cybernetics, Silver Spring, MD).
Protein kinase C activation and expression assays
PKC isoenzyme expression in leukemic cell lines was determined by Western blotting. Cells (0.5 × 106) were lysed in lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 7.4, containing 150 mM NaCl, 0.5 mM EDTA [ethylenediaminetetraacetic acid], 1 mM dithiothreitol, 1 mM phenymethylsulfonylfluoride, 10 μg/mL of aprotinin, leupeptin, and pepstatin A, and 1% Triton-X-100). The lysate was spun at 1000g for 10 minutes to isolate nuclei and was then combined 1:1 with sodium dodecyl sulfate (SDS) sample buffer and boiled for 5 minutes. The extracts were then analyzed by Western blotting using antibodies to the major isoenzymes found in myeloid cells, namely PKC-α, PKC-β, PKC-δ, and PKC-ζ (all purchased from Santa Cruz Biotechnology, Santa Cruz, CA). Blots were developed using an enhanced chemiluminescence (ECL) method (Amersham Pharmacia, Buckinghamshire, United Kingdom).
PKC translocates from the soluble to the particulate fraction of cells when activated, and this method was used to assess activation of PKC-δ by PEP005 and PMA. Briefly, cells were incubated for 10 minutes in medium alone or 20 nM PEP005 or 20 nM PMA, washed twice in phosphate-buffered saline (PBS), and then lysed by homogenization in hypotonic lysis buffer in the absence of detergent. Lysis was checked using trypan blue uptake. Nuclei were isolated by centrifugation at 1000g for 10 minutes. Centrifugation at 100 000g for 45 minutes at 4°C was used to isolate the cytosol (supernatant) and cell membrane (pellet) fractions. All were taken up in SDS sample buffer and analyzed for PKC-δ by Western blotting.
Caspase-3 phosphorylation
To determine if PEP005 induced caspase-3 phosphorylation, HL60 cells were incubated with 1.5 × 107 Bq/mL 32PO4 (Amersham Pharmacia) in phosphate-free medium for 3 hours prior to addition of 20 nM PEP005 or PEP005 and 5 μM rottlerin (Calbiochem, Nottingham, United Kingdom). Cells were then lysed and caspase-3 was immunoprecipitated with an anti-caspase-3 antibody (BD Pharmingen, Oxford, United Kingdom) or an irrelevant isotype-matched control antibody (DAKO), and the immunoprecipitate was isolated using an anti-mouse immunoglobulin G (IgG) antibody (DAKO) and protein G-coated magnetic beads (microbeads; Miltenyi Biotec). The isolate was taken up in SDS sample buffer and run on a 10% SDS-polyacrylamide gel electrophoresis (PAGE) gel and the labeled proteins were visualized using a phosphorimager.
PKC transfection studies
KG1a cells were transiently transfected with EGFP-tagged mouse PKC-δ subcloned into pEGFP-N1 plasmid (kindly provided by P. Blumberg, National Cancer Institute [NCI], Bethesda, MD) using an Amaxa nucleofection apparatus (Amaxa, Koeln, Germany). Transfection efficiency was approximately 35% as judged by fluorescence-activated cell sorter (FACS) analysis, and cells were treated with PEP005 (0.2 μM-20 μM) 24 hours after transfection. Cell viability in EGFP-positive cells was assessed by analysis of cell morphology (forward scatter and side scatter profile) by FACS and loss of viability confirmed in the total cell culture by MTT assay after 3 days. Briefly, 24 hours after transfection, 2 × 104 cells were plated in 5 wells in 96-well plates and exposed to 0, 0.2, 2, and 20 μM PEP005. At 72 hours, 20 μL MTT substrate at 5 mg/mL was added and plates were incubated at 37°C. After 3 hours, 150 μL media was removed and replaced with 200 μL dimethyl sulfoxide (DMSO). Absorbance at an optical density (OD) of 550 nm was read on a Biotech plate reader (Amersham Pharmacia) and corrected for absorbance obtained from blank media controls.
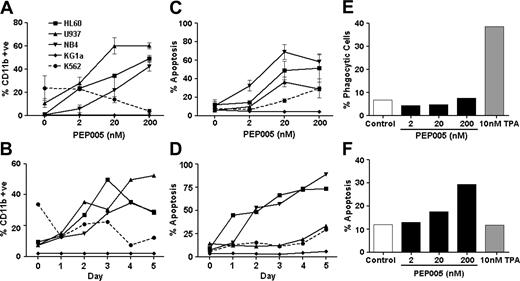
PEP005 induces apoptosis and differentiation in leukemic cell lines. There were 5 leukemic cell lines treated with PEP005 for 5 days. (A-B) Differentiation was assessed by expression of CD11b and (C-D) apoptosis was assessed by entry of cells into a subdiploid phase. Differentiation and apoptosis were also measured in response to PEP005 and PMA after 3 days by assessing phagocytosis of yeast (E) and caspase-3 activity (F). Data in panels A and E are expressed as mean ± standard deviation.
PEP005 induces apoptosis and differentiation in leukemic cell lines. There were 5 leukemic cell lines treated with PEP005 for 5 days. (A-B) Differentiation was assessed by expression of CD11b and (C-D) apoptosis was assessed by entry of cells into a subdiploid phase. Differentiation and apoptosis were also measured in response to PEP005 and PMA after 3 days by assessing phagocytosis of yeast (E) and caspase-3 activity (F). Data in panels A and E are expressed as mean ± standard deviation.
Immunofluorescence imaging of PKC-δ-GFP activation
KG1a cells were transfected with PKC-δ-EGFP or pEGFP-N1 (vector control). At 24 hours after transfection, cells were treated with 0, 0.2, 2, and 20 μM PEP005 and cytospins prepared after 15 minutes. Slides were fixed with 2% paraformaldehyde in PBS for 20 minutes, rinsed briefly in PBS, and mounted in Vectashield containing DAPI (4,6 diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA). Slides were examined using a Leica Fluorescence microscope (Leica, Heidelberg, Germany) fitted with a × 60 oil immersion objective. Images were captured using a Hamamatsu C4742-95 camera (Grafter Imaging, Austin, TX) and analyzed using OpenLab 3.1 software (Improvision, Coventry, United Kingdom).
Statistics
Data presented here represent a minimum of 3 experiments and, where appropriate, data are expressed as means plus or minus SD. Statistical significance was assessed by Student t test, and a P value less than .05 was taken as a significantly different value.
Results
PEP005 has antileukemic effects against cell lines and primary AML blasts
PEP005 is a small molecule activator of the 8 classical and novel PKC isoenzymes.8 PEP005 has already been shown to have antineoplastic potential against skin cancers, and an initial screen of the cytotoxic effects of PEP005 against other cancer cell types revealed potent effects on leukemic cell lines (data not shown). The antileukemic potential of PEP005 was therefore investigated further.
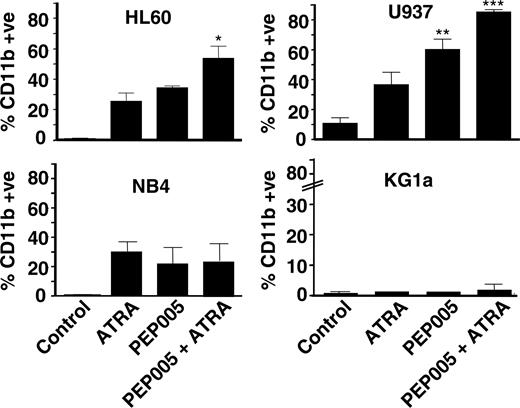
PEP005 synergizes with ATRA to induce CD11b expression. Of the lines, 4 were treated with 10 nM ATRA and 20 nM PEP005, alone or in combination, and differentiation was determined after 5 days. Data are mean ± SD of 3 experiments. *P < .05; **P < .01; and ***P < .001 for data compared with ATRA alone.
PEP005 synergizes with ATRA to induce CD11b expression. Of the lines, 4 were treated with 10 nM ATRA and 20 nM PEP005, alone or in combination, and differentiation was determined after 5 days. Data are mean ± SD of 3 experiments. *P < .05; **P < .01; and ***P < .001 for data compared with ATRA alone.
There were 5 myeloid leukemia cell lines treated with PEP005, and differentiation and apoptosis were determined. Differentiation was initially assessed by gain of CD11b expression (Figure 1A). Apoptosis was measured by FACS analysis of sub-G1 DNA (Figure 1C) and caspase-3 activation (data not shown). There were 3 cell lines (HL60, NB4, and U937) induced to express CD11b (Figure 1A) and enter apoptosis (Figure 1C) in response to nanomolar PEP005 concentrations, with optimal effects at 20 nM. K562 cells also responded to PEP005 but were less sensitive, with the maximal response seen with 2 μM PEP005; they only entered apoptosis and did not increase expression of CD11b (Figure 1A-B). KG1a cells were resistant to PEP005 and did not enter apoptosis or show CD11b expression even at 20 μM PEP005.
Kinetic studies (Figure 1B,D) suggested that some cell cultures, such as NB4 and HL60, showed a mixed response, with up to half of the cells entering apoptosis within the first 1 to 2 days of treatment and the remainder expressing CD11b. Other lines such as U937 expressed CD11b as the predominant response before entering apoptosis. The differentiation data gained using CD11b as a marker were therefore difficult to interpret. However, gain of CD11b is an early step in the differentiation process and to determine if PEP005 differentiated promyeloid cells into functional end cells (monocytes or neutrophils), we treated HL60 cells with PEP005 or PMA and measured their ability to phagocytose yeast after 3 days of treatment. While PMA induced differentiation of HL60 cells toward phagocytes (Figure 1E) with no increase in apoptosis (Figure 1F), PEP005 treatment did not lead to production of phagocytic cells and apoptosis was the dominant effect. We conclude that induction of apoptosis was the predominant effect of PEP005.
We noted that the PEP005-responsive cell lines were those lines that could also be induced to differentiate by all-trans retinoic acid (ATRA), an agent used in differentiation therapy of acute promyelocytic leukemia (APL). To determine if the responses to PEP005 and ATRA were interrelated, we treated cells with ATRA and PEP005 alone and in combination and measured the differentiation response. PEP005 was able to synergize with ATRA in 2 of the cell lines (HL60 and U937) but not in NB4 (Figure 2) or K562 (data not shown) cells. KG1a did not respond to ATRA or PEP005.
Blasts isolated from the bone marrow of 8 patients diagnosed with AML were also treated with PEP005, and 7 of these were induced to enter apoptosis. Apoptosis was measured by FACS analysis of sub-G1 DNA and caspase-3 activation (Figure 3A). After 48 hours of treatment with 20 nM PEP005, few viable AML blast cells remained, and the shrunken size and condensed nuclear morphology characteristic of apoptosis were predominant (Figure 3C, top panels). All 7 responsive AML samples showed similar sensitivity to PEP005, with apoptosis induced at concentrations as low as 2 nM and with maximal effect seen at 10 to 20 nM PEP005 (Figure 3A-B). The major difference in response seen between the AML samples was in the level of apoptosis induced, and at 20 nM PEP005 the values for induction of apoptosis ranged from 56% to 95%. Normal CD34+ myeloblasts isolated from cord blood (Figure 3C) and from adult marrow acquired after stem cell mobilization therapy (data not shown) were also exposed to PEP005. Induction of apoptosis was not seen at concentrations of PEP005 that were effective against cell lines and AML blast cells, and cytotoxicity did not occur even at 200 nM PEP005 (Figure 3C, top panels). Interestingly, the normal myeloblasts were induced to differentiate, as indicated by loss of CD34 and progression to promyelocyte- and myelocyte-like morphologies, in response to PEP005, and this effect was seen at doses of 20 nM to 2.0 μM PEP005 (Figure 3C lower panel). These observations indicate a potentially broad therapeutic window for PEP005 that spans at least 2-log concentrations.
PEP005 induces apoptosis in primary AML marrow blasts but not in normal myeloblasts. (A) PEP005 induced apoptosis in a primary AML cell culture. Apoptosis was determined by appearance of a subdiploid peak (▪) or active caspase-3 (•). (B) Meaned data for apoptosis induction by PEP005 in 7 primary AML samples. Data are mean ± SD. *P < .01 compared with non-parallel-treated controls. (C) Morphology on cytospins of example AML and nonmalignant CD34+ myeloblasts from cord blood after treatment with 20 nM PEP005 and 200 nM PEP005, respectively. Differential staining showed that normal myeloblasts did not enter apoptosis, but when exposed to higher concentrations of PEP005 had a more differentiated phenotype. (D) An example of a FACS plot of nonmalignant myeloblasts treated with PEP005, showing reduced CD34 staining indicative of differentiation.
PEP005 induces apoptosis in primary AML marrow blasts but not in normal myeloblasts. (A) PEP005 induced apoptosis in a primary AML cell culture. Apoptosis was determined by appearance of a subdiploid peak (▪) or active caspase-3 (•). (B) Meaned data for apoptosis induction by PEP005 in 7 primary AML samples. Data are mean ± SD. *P < .01 compared with non-parallel-treated controls. (C) Morphology on cytospins of example AML and nonmalignant CD34+ myeloblasts from cord blood after treatment with 20 nM PEP005 and 200 nM PEP005, respectively. Differential staining showed that normal myeloblasts did not enter apoptosis, but when exposed to higher concentrations of PEP005 had a more differentiated phenotype. (D) An example of a FACS plot of nonmalignant myeloblasts treated with PEP005, showing reduced CD34 staining indicative of differentiation.
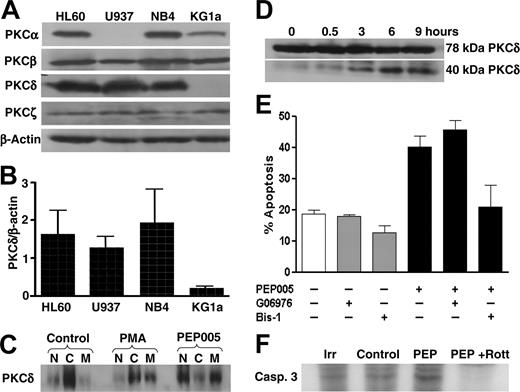
PEP005 actions are PKC-δ dependent. (A) Analysis by Western blotting of PKC-α,-β,-δ, and -ζ expression in 4 leukemic cell lines. β-actin was assessed as a loading control. (B) Expression of PKC-δ correlated with PEP005 responsiveness. PKC-δ expression was assessed by Western blotting in 4 leukemic cell lines and measured by densitometry. Data are expressed as PKC-δ expression relative to β-actin in the same sample. (C) Activation of PKC-δ by PEP005 (20 nM) and PMA (20 nM) was determined by measuring PKC-δ levels in the cytosolic (C), nuclear (N), and cell membrane (M) fractions of HL60 cells. (D) HL60 cells were incubated with 20 nM PEP005 for up to 9 hours and the presence of full-length (78-kDa) and the cleaved (40-kDa) fragment of PKC-δ was detected by Western blotting. (E) HL60 cells were incubated with 20 nM PEP005, 1 μM bisindolylmaleimide 1 (Bis-1), 20 nM Go6976, or PEP005 in combination with either Bis-1 or Go6976. Apoptosis was determined by caspase-3 activation. (F) HL60 cells were radiolabeled with 32PO4 prior to treatment with 20 nM PEP005 in the absence or presence of rottlerin and immunoprecipitation of caspase-3. An isotype-matched antibody (Irr) was used as a control. Blots in panels A, C, and D are representative of 3 separate experiments, and data in panels B and E are mean ± SD of 3 experiments.
PEP005 actions are PKC-δ dependent. (A) Analysis by Western blotting of PKC-α,-β,-δ, and -ζ expression in 4 leukemic cell lines. β-actin was assessed as a loading control. (B) Expression of PKC-δ correlated with PEP005 responsiveness. PKC-δ expression was assessed by Western blotting in 4 leukemic cell lines and measured by densitometry. Data are expressed as PKC-δ expression relative to β-actin in the same sample. (C) Activation of PKC-δ by PEP005 (20 nM) and PMA (20 nM) was determined by measuring PKC-δ levels in the cytosolic (C), nuclear (N), and cell membrane (M) fractions of HL60 cells. (D) HL60 cells were incubated with 20 nM PEP005 for up to 9 hours and the presence of full-length (78-kDa) and the cleaved (40-kDa) fragment of PKC-δ was detected by Western blotting. (E) HL60 cells were incubated with 20 nM PEP005, 1 μM bisindolylmaleimide 1 (Bis-1), 20 nM Go6976, or PEP005 in combination with either Bis-1 or Go6976. Apoptosis was determined by caspase-3 activation. (F) HL60 cells were radiolabeled with 32PO4 prior to treatment with 20 nM PEP005 in the absence or presence of rottlerin and immunoprecipitation of caspase-3. An isotype-matched antibody (Irr) was used as a control. Blots in panels A, C, and D are representative of 3 separate experiments, and data in panels B and E are mean ± SD of 3 experiments.
PEP005 responsiveness correlates with PKC-δ expression
Recent studies have already established that PEP005 is an activator of PKC signaling pathways.8 PKC is a multi-isoenzyme family with differential tissue expression and involvement in cell proliferation, differentiation, and apoptosis.9,17 We therefore determined if the varied responsiveness of leukemic cell lines to PEP005 was related to differential expression of PKC isoenzymes. We measured expression of the 4 isoenzymes that predominate in myeloid cells, namely PKC-α, -β, -δ, and -ζ. There was no consistent association between PEP005 responsiveness and expression of PKC-α, PKC-β, or PKC-ζ (Figure 4A). However, expression of PKC-δ did correlate with PEP005 sensitivity, with good levels of this isoenzyme expressed in HL60, U937, and NB4 and no significant expression detected in KG1a (Figure 4B). In addition, PEP005 induced activation of PKC-δ in HL60 cells, detected by translocation of the enzyme from the cytosol to the membrane fraction of cells (Figure 4C). Interestingly, initial translocation of PKC-δ was to the nuclear and cell membranes following treatment with PEP005, whereas PMA induced translocation only to the cell membrane (Figure 4C). This differential effect of PEP005 and PMA on PKC-δ subcellular location was also reported in COS cells transfected with PKC-δ.8 PKC-δ is known to undergo proteolysis by caspase-3 to release a proapoptotic 40-kDa catalytic fragment, and we therefore determined if PEP005 treatment resulted in the production of the 40-kDa fragment of PKC-δ. Figure 4D shows that the 40-kDa fragment was produced rapidly in HL60 cells. The likelihood that the proapoptotic activity of PEP005 was mediated through activation of PKC-δ was further supported by the observation that PEP005-induced apoptosis of HL60 cells was inhibited by the broad range PKC inhibitor bisindolylmaleimide 1 (Bis-1, Figure 4E), but not by Go6976, which is an inhibitor of the classical PKC isoenzymes PKC-α and -β.18 Interestingly, the differentiating actions of ATRA on leukemic cells have been shown to be in part mediated via activation of PKC-δ and phosphorylation of retinoid acid receptor α (RARα).19 This may explain why PEP005-induced partial differentiation was seen only in the ATRA-responsive cell lines. To investigate the possible proapoptotic mode of action of PKC-δ, we determined the effect of PEP005 treatment on caspase-3 phosphorylation. A very recent publication reported that PKC-δ was able to phosphorylate and activate caspase-3 in monocytic cells.20 Figure 4F shows that PEP005 was able to induce caspase-3 phosphorylation and that this was ablated by the PKC-δ inhibitor rottlerin.
Expression of PKC-δ in KG1a cells confers responsiveness to PEP005
To confirm the key role played by PKC-δ in mediating the effects of PEP005, KG1a cells were transfected with EGFP-tagged PKC-δ and 24 hours later were treated with PEP005 for 3 days. Transfection was confirmed by FACS analysis and transfection efficiency was approximately 35%. Expression of PKC-δ at the protein level was confirmed by Western blotting 24 hours after transfection (Figure 5A). Importantly, exposure of EGFP-positive PKC-δ-expressing KG1a cells to PEP005 resulted in apoptosis, assessed by increased forward scatter and decreased side scatter by FACS, at doses commensurate with those inducing apoptosis in HL60, U937, and NB4 cells. In contrast, EGFP-positive cells transfected with control vector were resistant to PEP005-induced apoptosis even at 20 μM PEP005 (Figure 5B). Loss of viability was confirmed in the total cell population by MTT assay (data not shown).
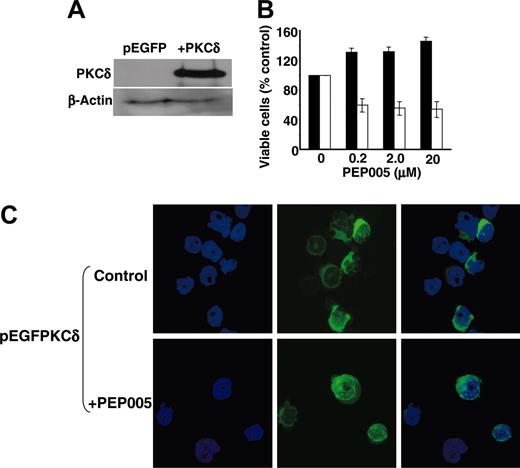
Transfection of cells with GFP-PKC-δ reinstates PEP005 responsiveness. (A) KG1a cells were transiently transfected with EGFP-tagged PKC-δ, and expression of PKC-δ was confirmed by Western blotting after 24 hours. (B) PEP005 was added to cultures 24 hours after transfection and apoptosis was determined after 3 days in PEP005-treated EGFP-positive KG1a cells transfected with pEGFP empty vector (▪) or pEGFP-PKC-δ (□) using analysis of forward and side scatter profiles by FACS. Data are mean ± SD of 6 experiments. (C) Translocation of EGFP-tagged PKC-δ following treatment of KG1a cells with 200 nM PEP005 was monitored by confocal microscopy and shows translocation to the nuclear region of the cells within 5 minutes of treatment. Nuclei are counterstained with DAPI.
Transfection of cells with GFP-PKC-δ reinstates PEP005 responsiveness. (A) KG1a cells were transiently transfected with EGFP-tagged PKC-δ, and expression of PKC-δ was confirmed by Western blotting after 24 hours. (B) PEP005 was added to cultures 24 hours after transfection and apoptosis was determined after 3 days in PEP005-treated EGFP-positive KG1a cells transfected with pEGFP empty vector (▪) or pEGFP-PKC-δ (□) using analysis of forward and side scatter profiles by FACS. Data are mean ± SD of 6 experiments. (C) Translocation of EGFP-tagged PKC-δ following treatment of KG1a cells with 200 nM PEP005 was monitored by confocal microscopy and shows translocation to the nuclear region of the cells within 5 minutes of treatment. Nuclei are counterstained with DAPI.
Finally, fluorescence microscopy confirmed that PEP005-induced apoptosis of EGFP-PKC-δ-expressing KG1a cells was preceded by the activation of PKC-δ. Figure 5C shows that prior to exposure to PEP005, EGFP-PKC-δ was located predominantly in the cytosol of KG1a cells but that PEP005 treatment induced its translocation to the nucleus and perinuclear region. Furthermore, we have shown previously that translocation of PKC-δ to the nucleus is associated with induction of apoptosis in human neutrophils and T cells21 and that key substrates of this isoenzyme involved in the induction of apoptosis include nuclear lamin B.22
We conclude therefore that PEP005 mediates selective and potent antineoplastic actions against AML cells that are mediated via the activation of PKCδ and its translocation to the nucleus.
Discussion
The key role of the PKC family of isoenzymes in the regulation of cell proliferation, differentiation, and apoptosis has identified them as important targets for drug design.23 In particular, 2 isoenzymes, PKC-α and PKC-δ, appear to play specific roles in tumor promotion and suppression. PKC-α promotes EGF-transforming activity24 and is generally antiapoptotic,25 whereas PKC-δ is antiproliferative,26 promotes retinoid-induced differentiation,19 and mediates proapoptotic signals.27-29
The screening of small molecule libraries is a topical and rational approach to the discovery of novel drugs that target these important signaling pathways, but our data illustrate that traditional medicines represent another powerful and relatively untapped source of therapeutic small molecular compounds that are not readily synthesized chemically.
Although PKC-δ selective activators30 and inhibitors31 have been isolated from natural sources, they have not been used clinically to date, possibly due to their acute toxicity or the lack of suitability for large-scale production. Consequently, targeting of PKC isoenzymes therapeutically has, to date, been predominantly via broad specificity agents. Bryostatin 1, a functional PKC antagonist, has been in clinical trial as an anticancer agent,12 as has the PKC-activating phorbol ester PMA.13 Ingenol derivatives, which are structurally closely related to phorbols, have received much less attention. Ingenol 3-angelate (PEP005) extracted from Euphorbia peplus has recently been shown to have activity against human skin tumors grafted on to mice7 and is also a potent activator of PKC.8 There are, however, key differences between the effects of PMA and PEP005 on promyeloid leukemic cells: most notably, PEP005 does not induce complete differentiation and apoptosis is the predominant effect, whereas PMA induced full differentiation,32 with no effect on cell viability.
The differential effects of PMA and PEP005 may derive from their differential activation of PKC isoenzymes in whole cells or the site of activation within the cell.33 PKC is a family of 11 isoenzymes, 8 of which are responsive to PEP005 and PMA in vitro8 by their binding to the C1 domain in the classical (α, βI, βII, γ) and novel (δ, ϵ, η, θ) PKCs. While PEP005 shows little selectivity in its activation of PKC isoenzymes in vitro, in vivo it induces a distinct pattern of translocation to that seen with PMA. Most notably, 10 nM PEP005 induced a rapid translocation of PKC-δ from the cytosol to the nuclear membrane and perinuclear region in COS cells transfected with GFP-tagged PKCs.8 In contrast, 10 nM PMA had little effect and 100 nM PMA was required to give translocation of PKC-δ, and this was initially to the plasma membrane and not the nucleus or perinuclear region.8 In the studies reported here, we also showed that PEP005 induced a rapid nuclear translocation of PKC-δ in HL60 cells, whereas PMA induced translocation to the cell membrane. In KG1a cells transfected with PKC-δ, PEP005 also induced translocation to the nucleus. The direction of translocation of PKC is important, as it will dictate where the enzyme is active within the cell and hence its substrate availability.
A role for PKC-δ in the nuclear events of apoptosis has been known for several years. Kufe's group (Ghayur et al27 and Emoto et al28 ) showed that PKC-δ was cleaved and activated by caspase-3 during apoptosis in cell lines, and overexpression of the catalytic fragment of PKC-δ was sufficient to induce apoptosis. Previous studies in a leukemic cell line TUR have also shown that defects in the PKC-δ signaling pathway were responsible for resistance to apoptosis in these cells.34 Our own studies have shown that PKC-δ translocates to the nucleus and is required for apoptosis in normal T cells and neutrophils.35,36 PKC-δ is also involved in producing several of the characteristics of apoptosis, including activation of scramblase to produce flipping out of phosphatidylserine on the cell surface,37 inhibition of DNA repair enzymes,38 and dissolution of the nuclear lamina.22 The translocation of PKC-δ to the nucleus by PEP005 is therefore consistent with the proapoptotic effect of the compound on leukemic cells. More recently, PKC-δ has been shown to be able to phosphorylate and activate caspase-3, identifying another proapoptotic substrate for this PKC isoenzyme.20 We show here that PEP005 treatment also results in PKC-δ-dependent phosphorylation of caspase-3.
Growth inhibition and differentiation have been reported previously when PKC-δ was overexpressed,39 but the mechanism for induction of the differentiated phenotype was not defined. In our studies, we noted that responsiveness to PEP005 also corresponded with the ability to differentiate in response to ATRA. HL60, U937, and NB4, each of which are ATRA responsive, were all PEP005 responsive, whereas KG1a, which is unresponsive to ATRA, was not PEP005 sensitive. ATRA is a potent inducer of cell differentiation in malignant cells and its use in acute promyelocytic leukemia (APL) has greatly improved the prognosis for these patients.40 Retinoids mediate their biologic effects by binding to nuclear receptors of the RAR or retinoid X receptor (RXR) family. ATRA binds to RARs and induces formation of RAR:RXR dimers, which bind to retinoic acid response elements (RAREs) in DNA and initiate the transcription of antiproliferative and prodifferentiating genes. Retinoids also regulate the activation of various signaling pathways including mitogen-activated protein (MAP) kinase and PKC, and these are also involved in mediating the effects of retinoids.19,41 Platanias' group (Kambhampati et al19 ) has shown that ATRA treatment can induce association of PKC-δ with RARα in leukemic cell lines NB4 and HL60, leading to activation of RAR transcriptional activity and induction of the differentiation gene STAT1. Importantly, ATRA therapy has reaped benefit only in APL and has had little impact in other forms of AML. This relates to the fact that APL cells bear RARα fusion protein mutations not found in other AMLs. However, we observed additive differentiation effects of ATRA and PEP005 in HL60 and U937 cells, which are not APL cells, but not in NB4 cells, which are APL cells and carry characteristic RARα fusion proteins. These data may indicate that PEP005 and ATRA may be useful in combination differentiation therapy of non-APL AML. Moreover, preliminary toxicology data show that PEP005 can be given systemically at doses that were effective against the AML isolates.
In summary, PEP005 represents a novel small molecule PKC activator isolated from a plant source used in traditional medicine for treatment of skin cancer. Although it can activate a broad range of PKC isoenzymes in vitro, its ability to induce nuclear translocation of PKC-δ endows it with potent antileukemic effects. The fact that cells not expressing PKC-δ were resistant to PEP005 is also useful clinically as it provides a simple test for potential responsiveness to treatment with PEP005.
Prepublished online as Blood First Edition Paper, April 21, 2005; DOI 10.1182/blood-2004-10-4117.
Supported by funds from Peplin Ltd, the European Commission (LSHB-CT-2004-503467), and The Leukaemia Research Fund. C.M.B. is a Leukaemia Research Fund Senior Lecturer, and A.M. was supported by a Medical Research Council postgraduate studentship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal