Abstract
Natural killer (NK) cells must be able to eliminate infected and transformed cells while remaining tolerant of normal cells. NK-cell self-tolerance is thought to be maintained by self-major histocompatibility complex (MHC) class I recognition; however, there are examples where NK cells are not regulated by MHC class I and yet remain self-tolerant. Here, we show that 2B4 (CD244) and CD48 represent a second system for murine NK-cell self-recognition. 2B4 and MHC class I receptors act nonredundantly to inhibit NK lysis of syngeneic tumor cells. NK cells from β2 microglobulin (β2m)-deficient mice and NK cells that lack expression of self-MHC-binding inhibitory receptors are inhibited by 2B4. Moreover, we provide the first in vivo evidence for MHC-independent NK self-recognition in a bone marrow rejection assay. These data suggest that NK-cell self-tolerance can be mediated by molecules other than MHC. (Blood. 2005;106:1337-1340)
Introduction
B and T lymphocytes from healthy individuals are tolerant of self-antigens and are activated by foreign antigens. In contrast, although natural killer (NK) cells are also tolerant of normal cells that express self-ligands, NK cells are activated by cells that lack self-ligands. As described in the “missing-self” hypothesis, NK cells monitor for self-major histocompatibility complex (MHC) class I expression and eliminate tumor cells and virally infected cells that lack self-MHC class I.1-3 Consequently, healthy allogeneic cells are also targets for NK cells because they lack self-MHC. For self- or non-self-discrimination, NK cells express inhibitory receptors that recognize MHC class I, including members of the human killer cell immunoglobulin (Ig)-like receptor family, murine Ly49 family, and the conserved CD94/NK-cell group 2 (NKG2) receptors.4 MHC-binding receptors are on overlapping subsets of NK cells; it is thought that all NK cells must express at least one self-MHC-binding inhibitory receptor to achieve self-tolerance.5
2B4 (CD244) is expressed by all NK cells, and the ligand for 2B4, CD48, is expressed broadly on hematopoietic cells.6 Human 2B4 is predominantly activating, although it is inhibitory on immature NK cells and those from patients with X-linked lympho-proliferative disorder.6,7 Surprisingly, NK cells from 2B4-deficient mice exhibit enhanced killing of CD48+ targets, indicating that murine 2B4 acts as an inhibitory receptor.8 Differences in 2B4 signaling may stem from contextual influences of signaling lymphocytic activation molecule-associated molecule (src homology 2 domain protein 1A).9
There are examples when self-MHC class I inhibition is absent, yet NK cells remain self-tolerant. Humans and mice that have low MHC class I expression due to transporter-associated with antigen processing 2 (TAP-2) protein or β2 microglobulin (β2m) deficiency, have self-tolerant NK cells.10 NK cells from MHC class I-deficient individuals may have decreased activating receptor function to account for self-tolerance,11,12 although other studies disagree,13,14 so the mechanism of tolerance remains unknown. Some NK cells in MHC class I-sufficient hosts lack any known self-MHC class I inhibitory receptors; in C57BL/6 H-2b mice, 10% of NK cells lack expression of the H-2b-binding inhibitory receptors, Ly49C, Ly49I, and CD94/NKG2A.12 Furthermore, there is a window during NK development in which MHC-binding inhibitory receptors are absent, but some effector functions are present, giving rise to a pool of seemingly unregulated immature NK cells.15-18
Self- or non-self-discrimination is often manifest in transplantation settings, wherein T cells recognize allogeneic antigens as foreign, and NK cells recognize allogeneic MHC in terms of missing-self. However, even healthy, autologous cells must meet certain criteria to avoid elimination by immune cells, namely by not expressing foreign antigen and maintaining expression of self-MHC. Herein, we demonstrate that 2B4 represents a second system for murine NK-cell self-recognition in which the widely expressed ligand, CD48, acts as an autonomous marker of normal cells. Previously, we demonstrated that 2B4 inhibits NK-cell responses to tumor cells in vitro and in vivo.8 We also found that 2B4 inhibits killing of nontransformed allogeneic and syngeneic cells in vitro.8 The current study extends these observations by (1) comparing the relative contributions of MHC class I and CD48 to NK-cell self-tolerance, (2) determining whether this inhibitory system accounts for the self-tolerance of NK cells that are not regulated by MHC class I, and (3) demonstrating that 2B4 regulates NK-cell tolerance to normal, nontransformed cells in vivo.
Study design
Mice, cell lines, and NK-cell preparation
C57BL/6 (H-2b, B6, wild-type [WT]) and Rag-1-/- mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and β2m-/- mice from Taconic (Germantown, NY). 2B4-/- mice were generated on the C57BL/6 background as described.19 CD48- and CD48+ RMA-S (H-2b low, thymoma cell line) were generated as described previously.8 Briefly, CD48- RMA-S cell line, which is a spontaneous variant of the RMA-S cell line, was stably transduced with either empty SAMEN vector, or vector containing CD48, by retroviral spin-infection and selected with neomycin. RMA cells (H-2b+, CD48+) and RMA-S cells were maintained as previously described.8 Interleukin 2 (IL-2)-stimulated NK cells were derived as described previously.8 Polyinosinic:polycytidylic acid (PolyI:C; Sigma, Saint Louis, MO)-stimulated NK cells were obtained from Rag-1-/- mice given 200 μg polyI:C intraperitoneally 30 hours prior to harvesting the spleens.
In vitro cytotoxicity assay
Chromium51-labeled targets were incubated at the indicated ratios with NK cells for 4 hours as described previously.8
Bone marrow rejection assay
For transplantation, bone marrow from WT B6 donor mice was dyed with 2 μM PKH26 (Sigma) for 5 minutes at room temperature, and β2m-/- bone marrow was dyed with 5 μM CFSE (5,6-carboxyfluorescein diacetate, succinimidyl ester; Molecular Probes, Eugene, OR) for 10 minutes at 37°C. WT and β2m-/- cells (10 × 106 each) were coinjected intravenously into WT and 2B4-/- mice given 8.5 Gy irradiation the day prior. Mice were depleted of NK cells using 10 μL anti-asialo-GM1 (Wako Chemicals, Richmond, VA) intraperitoneally on days -3 and -1 prior to transplantation. After 48 hours, splenocytes were harvested and analyzed by flow cytometry.
Results and discussion
Given that both 2B4 and Ly49 molecules can inhibit NK cells, we wanted to determine whether these two act redundantly to inhibit NK cells. If the systems are redundant self-markers, then one alone would be sufficient to inhibit NK cells. Conversely, if the two systems are nonredundant, then a cell would require both for protection from NK cells. To test this, NK cells from B6 mice were tested for lysis of RMA variants. RMA cells are H-2b+ and CD48+; RMA-S cells are TAP-deficient RMA cells, and thus are H-2blow and CD48- or CD48+. WT NK cells exhibited the lowest cytotoxicity against CD48+ RMA (H-2b+) targets (Figure 1A). Absence of MHC relieved some inhibition as seen with CD48+ RMA-S (H-2b-) targets. However, maximal cytotoxicity was seen only against the CD48-H-2b- targets. In comparison to WT, 2B4-/- NK cells demonstrated greater killing of RMA cells (Figure 1A). Removal of MHC class I lead to maximal 2B4-/- NK lytic activity, but CD48 had no effect. Over all, these data demonstrate that CD48 and MHC class I provide additive, nonredundant layers of protection from NK cells.
CD48 inhibition is nonredundant with MHC class I and regulates NK cells from MHC class I-deficient mice and NK cells that lack self-MHC class I inhibitory receptors. (A) WT and 2B4-/- NK cells activated with IL-2 were tested for lysis of CD48-H-2b- RMA-S, CD48+H-2b- RMA-S, and CD48+H-2b+ RMA target cells. (B) IL-2-stimulated NK cells from WT and β2m-/- mice were tested for lysis of CD48-H-2b- RMA-S, CD48+H-2b- RMA-S, and CD48+H-2b+ RMA target cells. (C) Ly49C/I+ NKG2A/C/E+ and Ly49C/I- NKG2A/C/E- NK cells from polyI:C-treated B6 mice were sorted by MoFlo (DakoCytomation, Fort Collins, CO) for DX5-phycoerythrin-positive (PE+) and either Ly49C/I(clone 5E6)/NKG2A/C/E(clone 20d5)-fluorescein isothiocyanate (FITC)-positive or -negative NK populations resulting in 99% purity. PolyI:C stimulation in vivo was used instead of IL-2 stimulation in vitro, because IL-2 may reverse a potential hyporesponsive phenotype of these populations.12 NK-cell populations were tested for lysis of CD48+ and CD48- RMA-S targets at an effector-target (E/T) ratio of 20:1. Error bars in all experiments indicate standard deviation from triplicate wells within 1 experiment. Data shown are representative of at least 3 independent experiments.
CD48 inhibition is nonredundant with MHC class I and regulates NK cells from MHC class I-deficient mice and NK cells that lack self-MHC class I inhibitory receptors. (A) WT and 2B4-/- NK cells activated with IL-2 were tested for lysis of CD48-H-2b- RMA-S, CD48+H-2b- RMA-S, and CD48+H-2b+ RMA target cells. (B) IL-2-stimulated NK cells from WT and β2m-/- mice were tested for lysis of CD48-H-2b- RMA-S, CD48+H-2b- RMA-S, and CD48+H-2b+ RMA target cells. (C) Ly49C/I+ NKG2A/C/E+ and Ly49C/I- NKG2A/C/E- NK cells from polyI:C-treated B6 mice were sorted by MoFlo (DakoCytomation, Fort Collins, CO) for DX5-phycoerythrin-positive (PE+) and either Ly49C/I(clone 5E6)/NKG2A/C/E(clone 20d5)-fluorescein isothiocyanate (FITC)-positive or -negative NK populations resulting in 99% purity. PolyI:C stimulation in vivo was used instead of IL-2 stimulation in vitro, because IL-2 may reverse a potential hyporesponsive phenotype of these populations.12 NK-cell populations were tested for lysis of CD48+ and CD48- RMA-S targets at an effector-target (E/T) ratio of 20:1. Error bars in all experiments indicate standard deviation from triplicate wells within 1 experiment. Data shown are representative of at least 3 independent experiments.
β2M-deficient mice have low MHC class I expression, yet NK cells from β2m-/- mice are tolerant of autologous MHC class Ilow cells.20,21 NK cells of β2m-/- mice express higher levels of Ly49 receptors and thus may be inhibited by the low levels of MHC class I found in such mice.22-24 However, increased sensitivity to low levels of residual MHC is unlikely to account for NK self-tolerance, because even in the absence of all MHC class I (as in β2m-/-TAP-1-/- mice), NK cells remain self-tolerant.24 It has been proposed by some groups that β2m-/- NK cells are self-tolerant because they are hypofunctional11,12 and by others that β2m-/- NK cells recognize non-MHC inhibitory self-ligands.13 Therefore, we determined whether 2B4 is the inhibitory receptor that maintains NK-cell tolerance in β2m-deficient mice. IL-2-stimulated β2m-/- NK cells were tested for lysis of RMA variants. Like WT, β2m-/- NK cells had the lowest lysis of CD48+ RMA (H-2b+) (Figure 1B). In comparison, the CD48+H-2b- RMA-S targets were more susceptible to β2m-/- NK lysis. But removal of CD48 inhibition permitted substantially more lytic activity by the β2m-/- NK cells. This finding suggests that in the absence of MHC class I, 2B4-CD48 interactions play a dominant role in tolerance.
Recent studies indicate that a proportion of NK cells in C57BL/6 mice lack known class I inhibitory receptors.12 To determine whether 2B4 regulates NK cells lacking self-MHC-binding inhibitory receptors, Ly49C/I- NKG2A/C/E- NK cells were sorted from polyI:C-stimulated mice and tested for 2B4 function. If Ly49C/I/NKG2A/C/E- NK cells are hypofunctional, they would be expected to have low killing of class I-low (CD48- RMA-S) targets. However, both Ly49C/I/NKG2A/C/E- and Ly49C/I/NKG2A/C/E+ cells lysed CD48- RMA-S cells, indicating that NK cells lacking known inhibitory receptors for H-2b, and thus potentially self-reactive, are not hypofunctional (Figure 1C). Importantly, Ly49C/I- NKG2A/C/E- cells were inhibited when the target expressed CD48. This finding is consistent with the hypothesis that 2B4-CD48 interaction contributes to the self-inhibition of NK-cell subsets that may be minimally regulated by MHC class I signals.
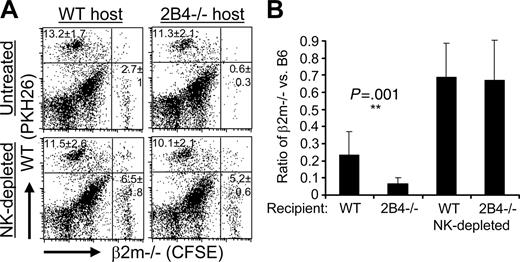
Both 2B4 and MHC class I inhibitory receptors inhibit NK-cell clearance of syngeneic bone marrow in vivo. CFSE-labeled β2m-/- B6 bone marrow cells and PKH26-labeled WT B6 bone marrow cells were coinjected into lethally irradiated untreated or NK-depleted WT and 2B4-/- mice. After 48 hours, splenocytes were analyzed by flow cytometry for remaining donor cells. (A) Representative dot plots. The percentage of cells and standard deviations within the PKH26+ or CFSE+ quadrant are indicated for that quadrant. Five mice per experimental group were analyzed for each experiment, and the data shown are from 1 of 2 independent experiments. P = .003 Student t test comparing β2m-/- donor cells remaining in untreated WT and 2B4-/- hosts. (B) Rejection of β2m-/- bone marrow is depicted as the ratio of the percentage of β2m-/- (CFSE+) cells divided by the percentage of WT control (PKH26+) cells recovered in each individual recipient from flow cytometry analysis as shown in panel A. Data shown represent the mean of pooled data from 2 independent experiments, each experiment had 5 mice per experimental group. Error bars represent standard deviations of the pooled data. **P = .001 Student t test comparing the β2m-/- donor-cell rejection ratio in untreated WT and 2B4-/- hosts.
Both 2B4 and MHC class I inhibitory receptors inhibit NK-cell clearance of syngeneic bone marrow in vivo. CFSE-labeled β2m-/- B6 bone marrow cells and PKH26-labeled WT B6 bone marrow cells were coinjected into lethally irradiated untreated or NK-depleted WT and 2B4-/- mice. After 48 hours, splenocytes were analyzed by flow cytometry for remaining donor cells. (A) Representative dot plots. The percentage of cells and standard deviations within the PKH26+ or CFSE+ quadrant are indicated for that quadrant. Five mice per experimental group were analyzed for each experiment, and the data shown are from 1 of 2 independent experiments. P = .003 Student t test comparing β2m-/- donor cells remaining in untreated WT and 2B4-/- hosts. (B) Rejection of β2m-/- bone marrow is depicted as the ratio of the percentage of β2m-/- (CFSE+) cells divided by the percentage of WT control (PKH26+) cells recovered in each individual recipient from flow cytometry analysis as shown in panel A. Data shown represent the mean of pooled data from 2 independent experiments, each experiment had 5 mice per experimental group. Error bars represent standard deviations of the pooled data. **P = .001 Student t test comparing the β2m-/- donor-cell rejection ratio in untreated WT and 2B4-/- hosts.
To investigate whether 2B4 inhibits NK-cell elimination of syngeneic cells in vivo, WT and 2B4-deficient mice were tested for rejection of bone marrow transplants. Mice were injected with CFSE-dyed β2m-deficient B6 bone marrow and an equal number of PKH26-dyed B6 WT bone marrow. Two days later, the spleens were harvested from WT and 2B4-/- recipients, and remaining donor cells were detected. Representative dot plots are shown from one experiment (Figure 2A) as well as the ratio of β2m-/- versus WT donor cells recovered (Figure 2B). WT NK cells rejected
β2m-/- bone marrow, as the ratio of β2m-/- versus control WT bone marrow retained in unmanipulated hosts is significantly lower (0.23 ± 0.13) than the corresponding NK-depleted recipients (0.7 ± 0.20; Figure 2B). That this difference is due to NK-cell-mediated rejection of β2m-/- cells is supported by the fact that, in NK-depleted mice, the ratio of β2m-/- versus WT cells recovered approached the ratio injected. Although, ideally, the ratio of β2m-/- and WT bone marrow cells retained in NK-depleted mice should be 1.0, small differences in counting of cells injected can readily influence this measurement. What is important to note is that the number of β2m-/- bone marrow cells retained in NK-depleted WT and 2B4-/- recipients (6.5% ± 1.8% versus 5.2% ± 0.6%, Figure 2A) is not significantly different.
2B4-/- mice exhibited significantly greater rejection of the β2m-/- cells as compared with WT mice (compare WT rejection ratio of 0.23 ± 0.13 with 2B4-/- rejection ratio of 0.06 ± 0.04, P = .001; Figure 2B). These results indicate that the 2B4-CD48 interaction protects autologous cells from NK-cell rejection in the absence of self-MHC class I.
In vivo we did not find evidence for killing of MHC class I+ syngeneic bone marrow by 2B4-/- mice (Figure 2A; 13.2% ± 1.7% WT cells recovered in WT hosts compared with 11.3% ± 2.1% recovered in 2B4-/- hosts). This may be due to compensation in 2B4-/- mice preventing in vivo autoreactivity, a phenomenon seen for the inhibitory receptor signal regulatory protein alpha (SIRPα).25 Indeed, it has been shown that anti-CD48 treatment of WT mice prevents engraftment of syngeneic bone marrow.26
In summary, we found that NK cells insufficiently regulated by MHC are inhibited from killing syngeneic cells by 2B4-CD48 interaction. Most notably, our results demonstrate the first evidence for non-MHC-mediated NK tolerance in vivo. Because CD48, the ligand for 2B4, is widely expressed on hematopoietic cells, these findings have implications for NK-mediated rejection of normal and malignant hematopoietic cells.
Prepublished online as Blood First Edition Paper, May 3, 2005; DOI 10.1182/blood-2005-01-0357.
Supported by National Institutes of Health (grant RO1AI20451) (V.K.) and the Medical Scientist Training Program (grant GM07281) (M.E.M.).
M.E.M. designed the research, performed the research, analyzed the data, and wrote the paper; D.G. performed the research and analyzed data; V.K. designed the research, analyzed the data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
IL-2 was obtained through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), NIH.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal