Abstract
Mast cells are found in tissues throughout the body where they play important roles in the regulation of inflammatory responses. One characteristic feature of mast cells is their longevity. Although it is well established that mast cell survival is dependent on stem cell factor (SCF), it has not been described how this process is regulated. Herein, we report that SCF promotes mast cell survival through inactivation of the Forkhead transcription factor FOXO3a (forkhead box, class O3A) and down-regulation and phosphorylation of its target Bim (Bcl-2 [B-cell lymphoma-2] interacting modulator of cell death), a Bcl-2 homology 3 (BH3)–only proapoptotic protein. SCF induced a rapid and transient phosphorylation of Akt (protein kinase B) and FOXO3a. SCF treatment prevented up-regulation of Bim protein expression and led to increased Bim phosphorylation. Bim phosphorylation was inhibited by PD98059 and LY294002 treatment, suggesting the involvement of mitogen-activated protein kinase kinase/mitogen-activated protein kinase (MEK/MAPK) and phosphatidylinositol 3 (PI3)–kinase pathways in this process. Overexpression of phosphorylation-deficient FOXO3a caused an up-regulation of Bim and induced mast cell apoptosis even in the presence of SCF. Mast cell apoptosis induced by the phosphorylation-deficient FOXO3a was attenuated in bim–/– mast cells. Because apoptosis is abnormally reduced in bim–/– mast cells, these data provide evidence that Akt-mediated inhibition of FOXO3a and its transcription target Bim provides an important mechanism by which SCF acts to prevent apoptosis in mast cells.
Introduction
Mast cells are long-lived multifunctional effector cells of the immune system originating from the hematopoietic CD34+ stem cells found in the bone marrow.1 From the bone marrow, mast cell precursors enter the circulation where they are recruited into peripheral tissues to mature and express their final phenotype under the influence of stem cell factor (SCF) and other locally produced cytokines.2 Although best known for their role in allergic reactions, mast cells are now also recognized as cells of importance in both innate immunity and in the onset and severity of chronic inflammations.3,4 The versatile effector mechanisms mast cells have been endowed with can be deduced from their capability to release a wide variety of inflammatory mediators such as histamine, proteases, and cytokines that are preformed and stored in granules and prostaglandins, leukotrienes, and cytokines that are secreted upon activation.5
The number of tissue mast cells is normally relatively constant, but during an acute or chronic inflammation the number can increase substantially.6 The regulation of mast cell numbers is most likely regulated by proliferation, migration, and apoptosis or survival. The mechanisms that regulate the viability of mature mast cells or promote mast cell apoptosis are poorly investigated. SCF is a cardinal growth factor in mast cell biology, regulating mast cell growth, differentiation, adhesion, migration, and survival.7 The number of tissue mast cells is at least in part regulated by SCF produced by resident stromal cells. SCF rescues mast cells from spontaneous apoptosis in vitro, whereas inhibition of SCF synthesis in vivo leads to mast cell apoptosis.8-10 Although it is accepted that SCF is a prosurvival factor for mast cells, it remains largely unclear how SCF promotes survival in these cells.
The B-cell lymphoma-2 (Bcl-2) family, which contains both prosurvival and proapoptotic proteins, are essential regulators of cell survival and apoptosis.11 The levels and interactions of prosurvival versus proapoptotic Bcl-2 family proteins determine whether a cell survives or will undergo apoptosis. During apoptosis induced by proapoptotic Bcl-2 family members, cytochrome c is released from the mitochondria and a caspase cascade is activated that induces DNA fragmentation.12,13 The prosurvival Bcl-2 family members include Bcl-2, Bcl-XL, Bcl-w, Mcl-1 (myeloid cell leukemia-1), and A1/Bfl-1, while the proapoptotic family members include Bax (Bcl-2–associated X protein), Bak (Bcl-2 homologue antagonist/killer), and Bok (Bcl-2–related ovarian killer), which share 3 regions of homology with their prosurvival relatives, and the Bcl-2 homology 3 (BH3)–only protein group, including Bad (Bcl-2 antagonist of cell death), Hrk (Harakiri), Bim (Bcl-2–interacting modulator of cell death), Bid (BH3-interacting domain death agonist), Puma (p53 up-regulated modulator of apoptosis), Noxa, and Bmf (Bcl-2–modifying factor), which share with the family only the short (9-16 amino acid [aa]) BH3 domain. Experiments with transfected cells have indicated that the BH3- only proteins probably act upstream of Bax and Bak.14,15
Treatment of mast cells with SCF has been shown to increase the levels of the prosurvival Bcl-2 family members Bcl-2 and Bcl-XL.16,17 SCF does not, however, affect the expression of A1/Bfl-1, a prosurvival protein shown to be crucial for crystallizable Fc ϵ receptor I (FcϵRI) activation–induced mast cell survival.18 SCF acts via its receptor Kit that has intrinsic tyrosine kinase activity. One signaling pathway downstream of Kit known to be involved in the regulation of cell survival involves activation of phosphatidylinositol 3-kinase (PI3-K) and its downstream target protein kinase B, also referred to as Akt. Akt has been suggested to promote cell survival through phosphorylation-mediated inactivation of the BH3-only protein Bad,19-21 but the relevance of this process for the survival of hematopoietic cells remains unclear because bad–/– mice have normal numbers of these cells.22 Another target of Akt involved in the control of apoptosis is the family of Forkhead transcription factors.23,24 When the 3 mammalian Forkhead members, forkhead transcription factor/forkhead box O1A (FKHR/FOXO1a), FKHR-like protein 1/forkhead box O3A (FKHRL1/FOXO3a), and atypical fibroxanthoma (AFX)/FOXO4, are phosphorylated by activated Akt, they are exported from the nucleus and the transcription of their target genes is thereby inhibited.23,25 One of the genes transcriptionally regulated by FOXO3a is the BH3-only protein Bim.26
In the present study, we show that SCF prevents mast cell apoptosis induced by growth factor withdrawal by actively preventing Bim expression via inactivation/phosphorylation of the Forkhead transcription factors FOXO1a and FOXO3a. SCF also promotes phosphorylation of Bim by PI3-K and mitogen-activated protein kinase/extracellular regulated protein kinase (MEK/ERK)–dependent pathways. Phosphorylation through the MEK/ERK pathway has recently been described to promote proteosome-dependent degradation of Bim,27,28 and thereby represents an additional mechanism by which SCF promotes protection from Bim-mediated apoptosis in mast cells.
Materials and methods
Reagents
Murine recombinant stem cell factor was supplied from PeproTech (Rocky Hill, NJ). Horseradish peroxidase (HRP)–conjugated antimouse antibody and [32P]-orthophosphate (PBS43) were obtained from Amersham Biosciences (Uppsala, Sweden). LumiGLO, HRP antirabbit antibody, antibodies against phospho-Akt(Ser473), phospho-Akt(Thr308), Akt, and phospho-FOXO1(Ser256) were all purchased from Cell Signaling Technology (Beverly, MA). Antibodies against phospho-FOXO3a(Ser253), phospho-FOXO3a(Thr32), and FOXO1 were purchased from Upstate Biotechnology (Lake Placid, NY). Anti-Bim antibody was bought from Affinity BioReagents (Golden, CO). Alkaline phosphatase (AP) was bought from Roche (Mannheim, Germany). LY294002 and PD98059 were supplied from Calbiochem Novabiochem (La Jolla, CA). An Annexin V–staining kit was purchased from R&D Systems (Minneapolis, MN). Other reagents were purchased from Sigma Chemicals (St Louis, MO).
Mast cell cultures
Bone marrow–derived cultured mouse mast cells (BMCMCs) were obtained by culturing mouse bone marrow from 3- to 4-month-old C57BL/6J mice (Bommice, Ry, Denmark) for 4 to 5 weeks in 15% WEHI-3–enriched conditioned RPMI 1640 medium (containing interleukin 3 [IL-3]), supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer, 1 × Minimum Essential Media (MEM) nonessential amino acid solution, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 100 IU/mL penicillin G, and 50 μg/mL streptomycin. The cell medium was changed once a week. Mast cell differentiation was confirmed by toluidine blue staining.
Bim–/– BMCMCs were cultured from the bim-deficient mice strain (266/266del) backcrossed for more than 12 generations onto inbred C57BL/6J mice.29 Wild-type (wt) and bim-deficient bone marrow was collected and cultured for 4 to 5 weeks in RPMI 1640 supplemented with 10% FBS, 5 ng/mL IL-3, 25 ng/mL SCF, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 50 μg/mL penicillin G, and 25 μg/mL streptomycin. Animals were housed at the Rudbeck Animal Facility and cared for by the staff according to local regulations. All experiments were approved by the Ethical Committee of Uppsala, Sweden.
In experiments investigating SCF-mediated effects, BMCMCs were suspended at 1 × 106 cells/mL in starvation medium (RPMI 1640 medium supplemented with either 0.1% FBS or 0.2% bovine serum albumin [BSA] and 2 mM l-glutamine, 100 IU/mL penicillin G, and 50 μg/mL streptomycin). SCF (50 ng/mL) was added, and the mast cells were incubated at 37°C, 5% CO2 for the indicated time periods.
Western blotting
To analyze phosphorylation of Akt and Forkhead family members, BMCMCs were suspended at 1 × 106 cells/mL in starvation medium for 15 to 20 hours prior to addition of SCF (50 ng/mL). The mast cells were incubated at 37°C for the indicated periods of time. To investigate the effect of SCF on the expression of Bim, SCF (50 ng/mL) was added to BMCMCs at the same time as the cells were deprived of IL-3 and serum and incubated for 24 hours in 37°C, 5% CO2. To determine diminished Bim expression, BMCMCs were suspended at 1 × 106 cells/mL in starvation medium for 24 hours prior to addition of SCF (50 ng/mL) for the indicated time periods.
After activation the mast cells were lysed in sodium dodecyl sulfate (SDS) lysis buffer (62.5 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 6.8, 2% wt/vol SDS, 10% glycerol, 50 mM DTT [dithiothreitol], 0.01% wt/vol bromphenol blue), and sonicated on ice. The phosphorylation and/or the total amount of the proteins of interest were studied by Western immunoblotting using a NuPAGE Bis-Tris Western gel (NOVEX Electrophoresis GmbH, Frankfurt, Germany). After electrophoresis the proteins were electroblotted onto nitrocellulose membranes (Hybond ECL [enhanced chemiluminescence]; Amersham Biosciences). After transfer filters were blocked in Tris-buffered saline containing 5% wt/vol nonfat dry milk and 0.1% Tween 20. Membranes were then incubated with primary antibody overnight at 4°C and with HRP-conjugated secondary antibody for 1 hour at room temperature. The proteins were visualized using the ECL system (LumiGLO, New England Biolabs, Beverly, MA) and exposure to a Hybond ECL film (Amersham Biosciences).
Treatment with inhibitors and phosphatases
For dephosphorylation, lysates were incubated for 1 hour at 40°C in dephosphorylation buffer and AP before Western immunoblot analysis. When investigating the effect on Akt and FOXO3a phosphorylation, LY294002 (50 μM) was added 30 minutes before activation with SCF for 5 minutes. When studying the effect of LY294002 (50 μM) or PD98059 (5, 20, or 40 μM) on Bim phosphorylation, the inhibitors were added directly together with SCF and incubated for 24 hours before analysis by Western immunoblot.
[32P]-orthophosphate labeling and immunoprecipitation
To demonstrate enhanced phosphorylation of BimEL, BMCMCs were labeled with [32P]-orthophosphate. BMCMCs were suspended in starvation medium for 24 hours prior to incubation with 2 mCi/mL (74 MBq/mL) [32P]-orthophosphate for 3 hours at 37°C. The labeled mast cells were either left untreated or treated with 50 ng/mL SCF for 10 minutes. After treatment the mast cells were lysed in lysis buffer (Cell Signaling Technology), and Bim was immunoprecipitated from the cell lysates for 2 hours on ice. The immunoprecipitates were collected on protein-A–Sepharose beads (Amersham Biosciences) and were washed 3 times with cell lysis buffer. The samples were boiled for 5 minutes at 95°C in reducing SDS sample buffer, separated on 4% to 12% gradient Bis-Tris Western gels (NOVEX Electrophoresis GmbH), and electrophoretically transferred onto nitrocellulose membranes (Amersham Biosciences). Phosphorylated Bim was visualized by using a phosphoimaging device.
Retroviral vectors and infections
The retroviral infection of BMCMCs was performed as previously described.30 Phoenix-Eco cells were transfected with either pBabe-puro containing 4-hydroxy tamoxifen (4-OHT)–inducible mutated human FOXO3(A3) (pBabe-Puro/FOXO3(A3):ER) (kindly provided by Dr P. Coffer, Utrecht, the Netherlands)31 or pBabe-puro only. Twenty-four hours later the supernatants were harvested, filtered, and supplemented with protamine sulfate at 4 μg/mL. These supernatants were used for transduction of GP+E86 cells to establish a polyclonal producer cell line. BMCMCs were then cultured with filtered supernatant from the producer cell line in the presence of 4 μg/mL protamine sulfate followed by selection in puromycin, at 2 μg/mL for 3 days and at 1 μg/mL for 5 days.
Detection of apoptosis by fluorescence-activated cell sorting (FACS) analysis
Apoptosis of BMCMCs was studied by propidium iodine staining or using an Annexin V–staining kit according to manufacturers' protocol followed by flow cytometric analysis on a FACSCalibur (Becton Dickinson, San Jose, CA).
Results
SCF induces phosphorylation of Akt and its downstream target Forkhead in mast cells
SCF is the most potent survival factor for mast cells, but it is not known how SCF promotes mast cell survival. Because Akt plays a decisive role in cell survival,32 we first investigated by Western blotting whether SCF treatment of BMCMCs induced phosphorylation of Akt at residues Ser473 and Thr308. We found that Akt was rapidly phosphorylated upon stimulation with SCF, reaching maximum phosphorylation after 5 minutes at both Ser473 and Thr308 (Figure 1A). The phosphorylation of Ser473 was more prominent and sustained compared with the phosphorylation of Thr308.
The Forkhead transcription factor family members FOXO1a and FOXO3a are downstream targets of Akt23,24,33-35 that are known to regulate genes involved in the control of the cell cycle and apoptosis. Phosphorylation of these transcription factors by Akt results in their cytoplasmic retention and inactivation, thereby inhibiting transcription of their target genes. To determine whether FOXO1a and FOXO3a are phosphorylated upon treatment with SCF, we investigated the effect of SCF treatment on the phosphorylation status of these 2 Forkhead family members in BMCMCs by Western blotting. As shown in Figure 1B, FOXO1a(Ser256), FOXO3a(Ser253), and FOXO3a(Thr32) were maximally phosphorylated after 5 minutes of treatment with SCF. This phosphorylation was sustained over the 2-hour time period that was investigated.
Phosphorylation of Akt and Forkhead transcription factors upon treatment of BMCMCs with SCF. BMCMCs were treated for the indicated time periods with SCF, and the phosphorylation of (A) Akt(Ser473) and Akt(Thr308) or of (B) FOXO1a(Ser256), FOXO3a(Ser253), and FOXO3a(Thr32) was analyzed by Western blotting. Arrow indicates band corresponding to FOX01a. Comparable results were obtained in at least 3 separate experiments.
Phosphorylation of Akt and Forkhead transcription factors upon treatment of BMCMCs with SCF. BMCMCs were treated for the indicated time periods with SCF, and the phosphorylation of (A) Akt(Ser473) and Akt(Thr308) or of (B) FOXO1a(Ser256), FOXO3a(Ser253), and FOXO3a(Thr32) was analyzed by Western blotting. Arrow indicates band corresponding to FOX01a. Comparable results were obtained in at least 3 separate experiments.
SCF prevents up-regulation of the Bim protein. BMCMCs, deprived of growth factors and serum, were either left untreated (–) for 0 or 24 hours or treated with SCF (+) for 24 hours. BimEL and BimL protein expression was analyzed by Western blotting. Comparable results were obtained in at least 3 separate experiments.
SCF prevents up-regulation of the Bim protein. BMCMCs, deprived of growth factors and serum, were either left untreated (–) for 0 or 24 hours or treated with SCF (+) for 24 hours. BimEL and BimL protein expression was analyzed by Western blotting. Comparable results were obtained in at least 3 separate experiments.
SCF activation represses induction of Bim
FOXO3a has been reported to transcriptionally regulate the expression of the BH3-only proapoptotic Bcl-2 family member Bim.26 Multiple isoforms of Bim have been characterized, the most prominent ones being BimEL (196 aa), BimL (149 aa), and BimS (110 aa).36 To study the expression levels of Bim in mast cells, BMCMCs were deprived of IL-3 and serum for 24 hours to induce apoptosis. Cytokine withdrawal resulted in increased expression of BimEL and BimL compared with nonstarved cells (Figure 2). Treatment of the cells with SCF prevented the induction of Bim after cytokine and serum withdrawal (Figure 2). Thus, SCF could counteract the induction of Bim after cytokine and serum deprivation of IL-3–dependent BMCMCs.
Phosphorylation-deficient FOXO3 induces Bim expression
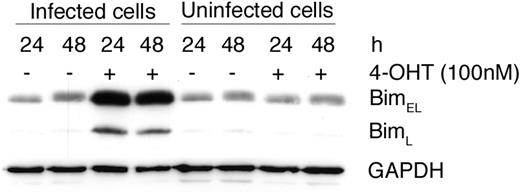
To prove that FOXO3a is involved in the regulation of Bim expression, we infected BMCMCs with a retrovirus encoding an inducible human FOXO3–estrogen receptor (ER) fusion protein, FOXO3(A3):ER.31 Upon addition of 4-OHT, this protein is released from its chaperone heat shock protein 90 (HSP90), transported into the nucleus where it can induce transcription of target genes, such as Bim. To ensure that FOXO3 is not inactivated by Akt-mediated phosphorylation, the 3 identified Akt phosphorylation sites (Thr32, Ser253, and Ser315) were converted to alanines. Treatment with 4-OHT induced a strong expression of Bim in FOXO3(A3):ER-infected BMCMCs but not in control cells (Figure 3). Thus, FOXO3a is involved in regulating the transcription of Bim in mast cells.
Bim is phosphorylated upon SCF treatment
In our studies on the effect of SCF on Bim expression, we found that there was not only a decrease in the levels of Bim but also that there was a shift in the migration of the BimEL protein compared with the migration of BimEL from cells starved for 24 hours (Figure 2). In other hematopoietic cells a band shift of Bim has previously been reported to be due to phosphorylation of Bim.37,38 To investigate whether the band shift upon SCF treatment was due to phosphorylation, lysates from SCF-stimulated and unstimulated BMCMCs were treated with AP. Upon treatment with AP, the intensity of the upper band was reduced and the lower band became more condensed, indicating that the upper band represents phosphorylated Bim (Figure 4A). DMSO was added as a control to prove that the AP solvent did not have an effect on the status of Bim in BMCMCs. Furthermore, immunoprecipitation of Bim from [32P]-orthophosphate–labeled mast cells that had been starved for 24 hours before being treated with SCF revealed an enhanced phosphorylation of Bim (Figure 4B). These results demonstrate that SCF stimulation of BMCMCs causes phosphorylation of BimEL.
Expression of a dominant-active mutant of FOXO3 results in up-regulation of Bim. BMCMCs were infected with a retrovirus encoding FOXO3(A3):ER and were left untreated (–) or incubated with 4-OHT (+) in the presence of SCF for the indicated time periods. The level of Bim was analyzed by Western blotting. As a control for possible toxic effects, uninfected cells were also treated with 4-OHT. Comparable results were obtained in at least 3 separate experiments. GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase.
Expression of a dominant-active mutant of FOXO3 results in up-regulation of Bim. BMCMCs were infected with a retrovirus encoding FOXO3(A3):ER and were left untreated (–) or incubated with 4-OHT (+) in the presence of SCF for the indicated time periods. The level of Bim was analyzed by Western blotting. As a control for possible toxic effects, uninfected cells were also treated with 4-OHT. Comparable results were obtained in at least 3 separate experiments. GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase.
We also performed a kinetic study on the effect of SCF on Bim expression and phosphorylation in BMCMCs starved for 24 hours prior to addition of SCF. As shown in Figure 4C, a mobility shift of Bim already could be observed 10 minutes after the addition of SCF. A decrease in the expression level of Bim was determined 1 to 2 hours after SCF treatment.
SCF-induced phosphorylation of Bim is regulated via both the MEK/mitogen-activated protein kinase (MAPK) and the PI3-kinase pathways
To explore the signaling pathways that regulate the phosphorylation of Bim in mast cells, we pretreated these cells with LY294002, a PI3-K inhibitor, or PD98059, a MEK1/2 inhibitor, before SCF stimulation. We found that SCF-induced phosphorylation of Akt-(Ser473) was blocked by pretreatment with LY294002 (Figure 5A). Inhibition of PI3-kinase also led to decreased phosphorylation of FOXO3a(Ser253) and inhibition of Bim phosphorylation in SCF-treated BMCMCs (Figure 5A).
SCF induces phosphorylation and down-regulation of Bim protein. (A) BMCMCs were left untreated (–) or treated with SCF (+). Indicated lysates were treated with AP. The SCF-induced mobility shift of Bim was vanished by AP treatment. DMSO indicates dimethyl sulfoxide. (B) [32P]-orthophosphate–labeled, starved BMC-MCs were left untreated (–) or treated with SCF (+) for 10 minutes. Bim was isolated by immunoprecipitation and separated by SDS–polyacrylamide gel electrophoresis (PAGE). The phosphorylation of Bim was visualized by phosphoimaging. (C) BMCMCs were starved for 24 hours prior to SCF treatment for indicated time periods. Both the levels and duration of phosphorylation and expression of Bim were analyzed by Western blot. Data represent comparable results from 2 to 3 separate experiments.
SCF induces phosphorylation and down-regulation of Bim protein. (A) BMCMCs were left untreated (–) or treated with SCF (+). Indicated lysates were treated with AP. The SCF-induced mobility shift of Bim was vanished by AP treatment. DMSO indicates dimethyl sulfoxide. (B) [32P]-orthophosphate–labeled, starved BMC-MCs were left untreated (–) or treated with SCF (+) for 10 minutes. Bim was isolated by immunoprecipitation and separated by SDS–polyacrylamide gel electrophoresis (PAGE). The phosphorylation of Bim was visualized by phosphoimaging. (C) BMCMCs were starved for 24 hours prior to SCF treatment for indicated time periods. Both the levels and duration of phosphorylation and expression of Bim were analyzed by Western blot. Data represent comparable results from 2 to 3 separate experiments.
Bim is regulated by the PI3-K and MEK/MAPK signaling pathways. BMCMCs were left untreated or were treated with SCF alone or with SCF in combination with inhibitors. (A) Cells were treated with the PI3-K inhibitor LY294002, and the levels of phosphorylation of Akt(Ser473), FOXO3a(Ser253), and Bim were analyzed by Western blotting. (B) Cells were treated with the MEK/MAPK inhibitor PD98059, and the levels of phosphorylation of Akt(Ser473), ERK1/2, and Bim were analyzed by Western blotting. Comparable results were obtained in at least 3 separate experiments.
Bim is regulated by the PI3-K and MEK/MAPK signaling pathways. BMCMCs were left untreated or were treated with SCF alone or with SCF in combination with inhibitors. (A) Cells were treated with the PI3-K inhibitor LY294002, and the levels of phosphorylation of Akt(Ser473), FOXO3a(Ser253), and Bim were analyzed by Western blotting. (B) Cells were treated with the MEK/MAPK inhibitor PD98059, and the levels of phosphorylation of Akt(Ser473), ERK1/2, and Bim were analyzed by Western blotting. Comparable results were obtained in at least 3 separate experiments.
It has been shown that the phosphorylation of Bim in neuronal PC12 cells is only regulated by the MEK/MAPK pathway and not by the PI3-K pathway.39 Therefore, we also investigated the effect of the MEK1/2 inhibitor PD98059 on Bim phosphorylation in BMCMCs after treatment with SCF. PD98059 blocked the SCF-induced phosphorylation of ERK1/2 and Bim in a dose-dependent manner (Figure 5B), whereas the inhibitor had no effect on the phosphorylation of Akt(Ser473) (Figure 5B). Thus, in contrast to neurons, both the PI3-K and MEK pathways are involved in the regulation of Bim phosphorylation in BMCMCs.
Phosphorylation-deficient FOXO3 triggers apoptosis in BMCMCs
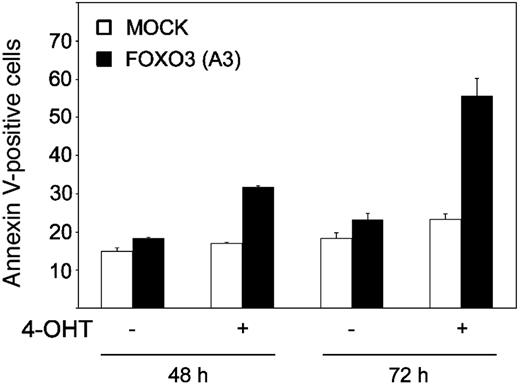
To prove that FOXO3a phosphorylation is critical for SCF-induced mast cell survival, we investigated the effect of FOXO3(A3):ER on cultured BMCMCs. Upon 48-hour treatment of BMCMCs with 4-OHT, the amount of apoptotic cells increased from 18.2% (no 4-OHT) to 31.7%. After 72 hours the percentage of apoptotic cells increased to 55.6% (Figure 6). Mock-infected cells were not affected by 4-OHT treatment, indicating that the effect observed was solely dependent on the enforced activation of the FOXO3 mutant. We also observed that induction of FOXO3(A3):ER led to a block in the cell-cycle progression of mast cells and up-regulation of p27Kip1 (data not shown). Our results thus show that FOXO3a regulates SCF-mediated survival in mast cells.
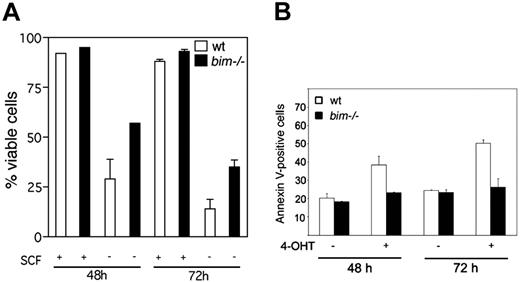
Mutated nonphosphorylatable FOXO3 induces apoptosis in wild-type but not bim–/– BMCMCs
To investigate whether Bim is involved in growth factor withdrawal–induced mast cell death we compared the survival of BMCMCs derived from wt and bim–/– mice. We could not observe any difference in viability between wt and bim–/– BMCMCs when cultured in SCF-supplemented medium (Figure 7A). However, upon 48 or 72 hours of SCF withdrawal, bim–/– BMCMCs demonstrated increased viability compared with wt cells, demonstrating that Bim is a critical factor in the regulation of mast cell apoptosis (Figure 7A). Next, we infected BMCMCs from both wt and bim–/– mice with either pBabe-puro or pBabe:FOXO3(A3):ER and compared the viability before and after induction with 4-OHT. Apoptosis induced by FOXO3(A3) was prohibited in BMCMCs from bim–/– mice (Figure 7B). The percentage of apoptotic cells after 48 and 72 hours of 4-OHT treatment was 23.2% and 26.3%, respectively, in bim–/– BMCMCs. In contrast, the number of apoptotic BMCMCs from wt mice as measured by Annexin V staining gradually increased from 38.4% after 48 hours to 50.3% after 72 hours (Figure 7B). As a control, BMCMCs from wt mice or bim–/– mice were mock-infected and then treated with 4-OHT without any effects on growth or survival (data not shown).
Expression of FOXO3(A3) suppresses SCF-mediated mast cell survival. BMCMCs were infected with a retrovirus encoding a dominant-active mutant of FOXO3(A3):ER (▪) and were then either left untreated (–) or incubated with 4-OHT (+) in the presence of SCF for 48 or 72 hours, respectively. Cell viability was analyzed by annexin V staining and flow cytometry. As a control, mock-infected cells (□) carrying the puromycin gene only were treated with 4-OHT. The experiment was repeated 3 times, and the data shown correspond to 1 representative experiment in duplicate (mean ± SEM).
Expression of FOXO3(A3) suppresses SCF-mediated mast cell survival. BMCMCs were infected with a retrovirus encoding a dominant-active mutant of FOXO3(A3):ER (▪) and were then either left untreated (–) or incubated with 4-OHT (+) in the presence of SCF for 48 or 72 hours, respectively. Cell viability was analyzed by annexin V staining and flow cytometry. As a control, mock-infected cells (□) carrying the puromycin gene only were treated with 4-OHT. The experiment was repeated 3 times, and the data shown correspond to 1 representative experiment in duplicate (mean ± SEM).
Discussion
The survival of mast cells is dependent on the presence of continued expression of SCF by stromal cells within the tissues.10,40 Although SCF plays a major role in supporting the survival of mast cells, the mechanisms by which this occurs are only partially understood. In this study we show that SCF treatment of BMCMCs leads to (1) inhibition of FOXO3a-mediated transcription of proapoptotic Bim and (2) phosphorylation of Bim. Thus, SCF actively represses Bim activity in mast cells and this contributes to the survival-promoting activity by SCF.
The Bcl-2 family of prosurvival and proapoptotic proteins are critical regulators of cell survival, and the balance between the 2 subgroups determines whether a cell will survive or undergo apoptosis.41 The role of Bcl-2 family members in the regulation of mast cell survival and apoptosis has not been studied in any greater detail, although there are reports that Bcl-2 and Bcl-XL are expressed in mast cells.16,42-46 In addition, we have previously shown that the Bcl-2 family member A1 is critical for the prosurvival effect triggered by crosslinking of the high-affinity immunoglobulin E (IgE) receptor, FcϵRI.18
It has been reported that SCF can up-regulate the expression of Bcl-2 and Bcl-XL in mast cells16 and that mast cells with a mutated and intrinsically activated Kit receptor have increased expression of Bcl-2 and Bcl-XL.17,46 Systematic studies, however, on the mechanism of SCF-regulated mast cell survival are still missing. With the exception of progenitors for human natural killer cells, SCF-mediated cell survival does not appear to be dependent on prosurvival Bcl-2 family members.47-49 We have recently found that SCF mediates survival in myeloid cells through a PI3-K–dependent pathway but not through induction of antiapoptotic Bcl-2 family members.50 Furthermore, SCF treatment of hematopoietic cells induces phosphorylation of Forkhead transcription factors that are known to be involved in the regulation of cell survival.30,51 These findings suggest that SCF might regulate mast cell survival via a PI3-K–dependent pathway that represses the expression of proapoptotic proteins that are under the transcriptional control of Forkhead.
Akt is a downstream target of PI3-K52 that is involved in the regulation of several cellular functions, including survival (reviewed in Hemmings53 and Coffer et al54 ). In response to a range of growth factors, Akt is activated by phosphorylation at Ser473 and/or Thr308.55 Accordingly, we found that Akt became rapidly phosphorylated at Ser473 and Thr308 upon treatment of BMCMCs with SCF (Figure 1A). Ser473 and Thr308 were both rapidly phosphorylated, but only phosphorylation of Ser473 persisted for several hours (data not shown). Akt regulates the activity of several pathways known to be involved in the control of cell survival, including the transactivation activity of Forkhead transcription factors.32 Similarly to the phosphorylation of Akt, we found that FOXO3a was rapidly phosphorylated at both Thr32 and Ser253 upon SCF stimulation of BMCMCs. The phosphorylation of both Akt and FOXO3a was diminished after treatment of the cells with the PI3-K inhibitor LY294002.
Previous studies of the Forkhead transcription factors have shown that they regulate the expression of cell death–inducing proteins Bim and Fas ligand (FasL) as well as proteins involved in regulating the cell cycle.25,26,56-58 There are 2 major pathways to apoptosis that can be divided into the death receptor–induced pathway (eg, Fas) and the mitochondrial-regulated pathway where the Bcl-2 family members are involved. These 2 pathways both result in activation of caspases that cause cell demolition.12 Because it has been reported that apoptosis of mast cells induced by growth factor withdrawal is independent of Fas,44 we found Bim rather than FasL a likely candidate to be further investigated.
FOXO3(A3) induces apoptosis in wild-type but not bim–/– mast cells. (A) SCF withdrawal–induced mast cell death is reduced by loss of Bim. BMCMCs from wt or bim–/– mice were either deprived of SCF (–) or grown in SCF + IL-3 (+) for the indicated times. Cell viability was measured by propidium iodide staining and FACS analysis. (B) BMCMCs from wt or bim–/– mice were infected with a retrovirus encoding a dominant-active mutant of FOXO3(A3):ER and treated with SCF in the presence or absence of 4-OHT. The experiment was repeated 3 times. The results are presented as the percentage of apoptotic cells determined by annexin V staining of 1 representative experiment in duplicate (mean ± SEM).
FOXO3(A3) induces apoptosis in wild-type but not bim–/– mast cells. (A) SCF withdrawal–induced mast cell death is reduced by loss of Bim. BMCMCs from wt or bim–/– mice were either deprived of SCF (–) or grown in SCF + IL-3 (+) for the indicated times. Cell viability was measured by propidium iodide staining and FACS analysis. (B) BMCMCs from wt or bim–/– mice were infected with a retrovirus encoding a dominant-active mutant of FOXO3(A3):ER and treated with SCF in the presence or absence of 4-OHT. The experiment was repeated 3 times. The results are presented as the percentage of apoptotic cells determined by annexin V staining of 1 representative experiment in duplicate (mean ± SEM).
Bim has been reported to be involved in the regulation of apoptosis in many different cell types, including B and T lymphocytes, monocytes, granulocytes, and neurons.29,59-61 It is now widely accepted that increased expression of BimEL is a common response to withdrawal of survival factors in a variety of cell types.
SCF treatment of growth factor–deprived mast cells caused significant repression of Bim-protein expression. In addition, by Western blot analysis we observed a shift in Bim mobility (Figures 2 and 4C). Treatment of cell extracts with phosphatase and labeling of BMCMCs with [32P]-orthophosphate demonstrated that Bim is indeed phosphorylated upon SCF treatment. This is in accordance with other studies which reported that Bim is phosphorylated in cytokine-stimulated cells.27,28,37-39,62-64 However, phosphorylation of Bim upon SCF treatment has not been reported before. It is suggested that Bim is phosphorylated on serine residues (Ser69 and Ser109 or Ser55, Ser65, and Ser 100) upon growth factor stimulation38,64 and that this targets Bim for degradation by the proteosome machinery.27,28
The biologic importance of the PI3-K and MEK/MAPK pathways in cell survival after growth factor withdrawal in hematopoietic cells has been controversial. Some conflicting findings exist on the signaling pathways involved in the regulation of Bim-induced cell death.37,39,65 Although the induction of Bim expression appears to be a common response to withdrawal of growth factors in many different cells, the signaling pathways regulating Bim expression and phosphorylation seem to differ depending on the cell type and/or stimuli. Using synthetic inhibitors, our results indicate that both the PI3-K and the MEK/MAPK pathways are involved in regulating the phosphorylation of Bim in mast cells. The PI3-K inhibitor LY294002 diminished phosphorylation of Akt, FOXO3a, and Bim in healthy cells (Figure 5), and the MEK/MAPK inhibitor PD98059 totally blocked phosphorylation of BimEL and ERK1/2 but, as expected, not Akt (Figure 5). This may indicate that growth factor stimulation causes Bim phosphorylation through 2 pathways, a PI3-K–dependent and a PI3-K–independent one that signals through ERK1/2.
Our analysis of the function of Forkhead transcription factors and Bim in mast cell survival was facilitated by the use of inducible expression of a phosphorylation-deficient dominant-active mutant of human FOXO3.26 To prove that FOXO3a is involved in regulating SCF-dependent mast cell survival, FOXO3(A3):ER was induced by 4-OHT, an approach that has previously been used by us and others in hematopoietic progenitor cells.30 If FOXO3a is involved in the regulation of Bim expression and mast cell survival, one would expect 4-OHT–induced FOXO3(A3):ER fusion protein activation to kill BMCMCs even in the presence of SCF. Indeed, we found that FOXO3(A3):ER activation induced Bim expression and mast cell apoptosis. This indicates that inactivation of FOXO3a is essential for the ability of SCF to promote mast cell survival. Furthermore, activation of the phosphorylation-deficient FOXO3(A3):ER failed to kill bim–/– BMCMCs. Because apoptosis of bim–/– mast cells was attenuated after growth factor deprivation, these results collectively suggest that Bim is a predominant activator of apoptosis in cytokine-deprived mast cells and that SCF promotes cell survival via inactivation of FOXO3a to down-regulate Bim activity.
In conclusion, in this article we describe a mechanism for SCF-regulated mast cell survival. We found that the PI3-K and MEK/MAPK pathways regulate the phosphorylation and thereby degradation of Bim, while FOXO3a regulates the transcription of Bim. These findings provide new insights into the regulation of mast cell life span and function that may have implications for the treatment of diseases in which these cells play a major role.
Prepublished online as Blood First Edition Paper, April 26, 2005; DOI 10.1182/blood-2004-12-4792.
Supported by grants from the Swedish Research Council-Medicine, the Swedish Cancer Foundation, the Children's Cancer Foundation of Sweden, the HKH Kronprinsessan Lovisas Förening För Barnasjukvård, the Swedish Heart Lung Foundation, the Göran Gustafsson's Foundation, the Ollie and Elof Ericsson's Foundation, the King Gustaf V's 80-Years Foundation, and the Lilly and Ragnar Åkerhamn's Foundation.
C.M. and J.A. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Erik Ullerås for helpful discussions and Dr Andreas Strasser for critical reading of the manuscript. We also thank Dr Paul Coffer for providing FOXO3(A3):ER and Dr Philippe Bouillet and Dr Jerry Adams for the bim–/– mice.



![Figure 4. SCF induces phosphorylation and down-regulation of Bim protein. (A) BMCMCs were left untreated (–) or treated with SCF (+). Indicated lysates were treated with AP. The SCF-induced mobility shift of Bim was vanished by AP treatment. DMSO indicates dimethyl sulfoxide. (B) [32P]-orthophosphate–labeled, starved BMC-MCs were left untreated (–) or treated with SCF (+) for 10 minutes. Bim was isolated by immunoprecipitation and separated by SDS–polyacrylamide gel electrophoresis (PAGE). The phosphorylation of Bim was visualized by phosphoimaging. (C) BMCMCs were starved for 24 hours prior to SCF treatment for indicated time periods. Both the levels and duration of phosphorylation and expression of Bim were analyzed by Western blot. Data represent comparable results from 2 to 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/4/10.1182_blood-2004-12-4792/7/m_zh80160582660004.jpeg?Expires=1767832331&Signature=i2UojMVKPfARtz2eqiFmMnZ-uUGa79pdkYE~uJPjPgsFxpdDxoyF2~aHTTfbb3JrGlgizNSTY-0VKGQFRxIfk5BixKThW3rQw7dKXI84htpyqeOgOnaIqsXAAthMlbWg9cSdI1OVWHVzVKdflgar78zRq7cVu1KDr0wNuJr6aSO7c-zRtWNQnlaN2pOFyhqr0CQ3ZzGC-OF5W0uOyWEthwqJmvB-vnRevchS7IPFvXB3q~jbxevybpMt11V9~7WHjgCX4L19Mj8HpLk0GPvsqZFTYHtSmoSxYbkAZ6bhJBtL4n4RhrcLhpUUBN~9UEPpRdjMdEIv2Vlt0BfGwLZZOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal