Abstract
Although c-Maf is crucial for Th2 differentiation and production of interleukin 4 (IL-4), its regulation is poorly understood. We report that Vav1–/– CD4+ T cells display deficient T-cell receptor (TCR)/CD28-induced IL-4 and c-Maf expression and, conversely, enhanced interferon γ (IFN-γ) production and T-bet expression (even when cultured under Th2-polarizing conditions), but intact expression of other Th2 cytokines and GATA-3. Up-regulation of c-Maf was dependent on Ca2+/nuclear factor of activated T cell (NFAT) and, together with IL-4 production, could be rescued in Vav1–/– T cells by Ca2+ ionophore. Deficient IL-4 production was restored by retrovirus-mediated Vav1 expression, but only partially by retroviral c-Maf expression. Similar IL-4 → IFN-γ skewing was observed in intact, antigen-primed Vav1–/– mice. Thus, Vav1 is selectively required for IL-4 and c-Maf expression, a requirement reflecting, at least in part, the dependence of c-Maf expression on Ca2+/NFAT signaling.
Introduction
T helper (Th) cells play a central role in the immune response via direct cell-cell contact or secretion of multiple immunoregulatory cytokines. The division of Th cells into 2 subsets based on their pattern of cytokine production is associated with discrete cytokine production profiles among CD4+ T cells.1-3 Th1 cells secrete interleukin 2 (IL-2), interferon γ (IFN-γ), and lymphotoxin (LT), whereas Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13. In addition to T-cell receptor (TCR) signals, the differentiation and maintenance of Th1/Th2 subsets is regulated by cytokines and costimulatory signals.2,4 Cytokines also mediate cross-regulation between Th1 and Th2 cells, in which differentiation and activation of one subset inhibits development and function of the reciprocal subset.1,5
Considerable progress has been made in recent years in characterization of transcription factors that dictate the development of Th1 or Th2 subsets.6,7 GATA-3, which binds to the IL5 but not the IL4 proximal promoter,8-10 is a critical regulator of Th2 development.9 On the other hand, the Th1-specific transcription factor, T-bet, plays a central role in Th1 development.11 c-Maf was identified as the first Th2-specific transcription factor that binds to the IL4 proximal promoter.12 In contrast to the rapid induction of GATA-3 and T-bet by cytokines, the induction of c-Maf by TCR signaling is slower under Th2-skewing conditions.13 Transgenic expression of c-Maf diminishes IFN-γ production,14 and c-Maf– deficient mice display a severe impairment of IL-4, but not other Th2 cytokine, production.15 Taken together, these results demonstrate that c-Maf is an IL4 gene-specific transactivator and that c-Maf and GATA-3 promote the differentiation of Th2 cells by distinct but complementary mechanisms.
Earlier studies described differences between Th1 and Th2 cells in TCR-induced protein tyrosine kinase (PTK) activation, tyrosine phosphorylation profiles, and Ca2+ signaling.16-19 Recent studies pointed to the importance of mitogen-activated protein kinases (MAPKs) in Th1/Th2 differentiation and cytokine production.20,21 Thus, c-Jun N-terminal kinases (JNK), p38 kinase, and MAPK kinase 3 (MKK3) are required for Th1 differentiation and IFN-γ production.22-25 Conversely, Ras, mitogen-activated protein (MAP)/extracellular regulated kinase (ERK) kinase (MEK), and ERK are required for Th2 differentiation.26 Nuclear factor of activated T-cell (NFAT) proteins, especially NFATc1 (NFAT2), are critical for IL-4 expression and Th2 differentiation.27,28 Th2 development is also severely impaired in the absence of Itk, a relatively TCR-proximal Tec family PTK.29,30 We recently provided additional evidence that SWAP-70–like adapter of T cells (SLAT) promotes Th2 differentiation via its association with the ZAP-70 kinase and inhibition of its function at a TCR-proximal signaling step.31 However, despite this progress, little is known regarding early TCR-proximal signaling events that regulate Th1/Th2 differentiation. In particular, the regulation of c-Maf expression, which is mediated by TCR, but not cytokine, signals,13 is poorly understood.
Vav1 represents a critical enzyme and adaptor protein in TCR signaling pathways. Analysis of Vav1-deficient mice indicated that Vav1 is required for T-cell development and antigen receptor-mediated T- or B-lymphocyte activation32-34 as well as for TCR clustering and actin cytoskeleton reorganization.35,36 Proper Vav1 function is also necessary for receptor-induced activation of the MAP kinase ERK and the transcription factors NFAT and nuclear factor-κB (NF-κB), and for intact Ca2+ mobilization.35-37 Consistent with these findings, we and other groups showed that Vav1 overexpression in T cells enhances activation of transcriptional elements in the IL2 gene,38-40 in particular NFAT. However, although some studies pointed to the importance of Vav1 in IL-4 production,41,42 it is unknown if Vav1 plays a role in the differentiation or function of Th1/Th2 cells.43
In this study, we used Vav1–/– mice to demonstrate a novel role for this signal transducer in the development of IL-4–producing Th2 cells. We show that IL-4 production and c-Maf expression are selectively impaired and Th1 development is enhanced in Vav1-deficient T cells as assessed in vitro and in vivo. Furthermore, we demonstrate that intact Ca2+ signaling and NFAT activation are required for inducing c-Maf expression in response to TCR or costimulatory signals or both. These results support a connection between TCR/CD28 engagement and c-Maf expression mediating Th2 differentiation and IL-4 expression at the level of Vav1. Thus, Vav1 plays an important role in TCR/CD28-initiated signaling pathways leading to c-Maf and IL-4 expression.
Materials and methods
Mice and reagents
Vav1-deficient mice37,44 were a gift from Dr V. Tybulewicz (National Institute for Medical Research, London, United Kingdom). Female C57BL/6 and Vav1-deficient C57BL/6 mice were bred and maintained at our pathogen-free animal facility. Eight- to 12-week-old mice were used in all experiments. All animal studies were approved by the Animal Care Committee of the La Jolla Institute for Allergy and Immunology, San Diego, CA. Recombinant IL-2, IL-4, and IL-12 were purchased from PeproTech (Rocky Hill, NJ). Neutralizing monoclonal antibodies (mAbs) specific for IL-4 (11B11), IL-12 (C17.8), and IFN-γ (H22) were purchased from PharMingen (San Diego, CA). Monoclonal anti–T-bet (39D) and anti–GATA-3 (HG3-31), and polyclonal anti–c-Maf (M157), anti-Grb2 (C23), and anti-Jak1 (Q19) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antiphospho-signal transducer and activator of transcription 6 (STAT6; Tyr641; 5A4) and polyclonal antiphospho-Jak1 (Tyr1022/1023) antibodies were purchased from Cell Signaling Technology (Beverly, MA), and an antiactin polyclonal antibody was from ICN Biomedical (Costa Mesa, CA). An anti-Vav1 polyclonal antibody was obtained from Upstate Biotechnology (Lake Placid, NY). Anti-CD3 (2C11) and anti-CD28 (37.51) mAbs were purified from culture supernatants of the corresponding hybridomas. Other reagents were obtained from Sigma (St Louis, MO).
Antigens and immunization
Keyhole limpet hemocyanin (KLH) was obtained from Calbiochem (San Diego, CA). Mice were immunized subcutaneously in the tail base with 50 μg KLH emulsified in incomplete Freund adjuvant (IFA) or complete Freund adjuvant (CFA; Fischer Scientific, Pittsburgh, PA). Draining periaortic plus inguinal lymph nodes or spleens were removed 10 days after immunization.
Cell culture and stimulation
Mouse naive CD4+ T cells were purified as described.31 Briefly, lymph node and spleen cells were passed through a T-cell enrichment column (R&D Systems, Minneapolis, MN). CD4+ T cells were enriched by negative selection using a magnetic-activated cell sorting (MACS) system with rat anti–mouse CD8 and B220 antibodies (PharMingen) followed by incubation with goat anti–rat immunoglobulin-coated magnetic beads (Miltenyi Biotech, Auburn, CA). Residual antigen-presenting cells (APCs) and in vivo activated T cells were removed by isolating high-density cells spun through a Percoll (Sigma) step gradient. The purified T-cell populations of both wild-type and Vav1–/– mice contained similarly low levels of contaminating CD3+NK1.1+ (NKT) cells (∼0.5%) and about 2% to 5% CD44highCD62low T cells (data not shown). These cells were cultured in anti-CD3/CD28–coated 24-well plates (2 × 106 cells/well) in a total volume of 2 mL in the presence of 10 U/mL recombinant IL-12 and 10 μg/mL anti–IL-4 antibody to promote Th1 development, or in the presence of 100 U/mL recombinant IL-4 and 10 μg/mL anti–IL-12 antibody to promote Th2 differentiation. Recombinant IL-2 (20 U/mL) was added to the cultures on day 2 and, in some experiments, on day 0. In some experiments, the cells were cultured in the presence of anti–IFN-γ antibody (10 μg/mL). After 7 days, cells were washed and restimulated using anti-CD3/CD28 antibody–coated plates for intracellular cytokine staining (ICCS). In some experiments, draining periaortic plus inguinal lymph node T cells were cultured in 96-well plates (5 × 105 cells/well) in a total volume of 0.2 mL with the indicated KLH concentrations. Proliferation was determined after 72 hours by 3HTdR uptake in cells labeled for the final 16 hours. Levels of IFN-γ, IL-2, IL-4, IL-5, or IL-13 in 48 hours-stimulated culture supernatants were quantified by enzyme-linked immunosorbent assay (ELISA; PharMingen).
ICCS
ICCS was performed as described.31 Briefly, T cells restimulated with plate-bound anti-CD3/CD28 antibodies for 8 hours were cultured in the presence of 10 μg/mL brefeldin A (Sigma) for the final 2 hours of culture. Cells were fixed in 3.7% paraformaldehyde for 10 minutes, permeabilized with phosphate-buffered saline (PBS) containing 0.5% saponin plus 1% bovine serum albumin (BSA), and stained with fluorescein isothiocyanate (FITC)–conjugated anti–IFN-γ and phycoerythrin (PE)–conjugated anti– IL-4 antibodies (PharMingen) for 30 minutes. The stained cells were washed twice with PBS containing 0.5% saponin and 1% BSA, resuspended in 1% BSA in PBS, and analyzed using fluorescence-activated cell sorting (FACSCalibur; BD Biosciences, San Jose, CA).
Quantitative transcript analysis
Total RNA was isolated from naive or differentiated Th cells using an RNeasy Kit (Qiagen, Valencia, CA). First-strand cDNAs were synthesized using the SuperScript Preamplification System (Invitrogen, Carlsbad, CA). Reverse transcription-polymerase chain reaction (RT-PCR) was performed using the SYBR Green real-time PCR assay (PE Applied Biosystems, Boston, MA). Sequences of primers used were: c-Maf, AGCAGTTGGTGACCATGTCG (5′) and TGGAGATCTCCTGCTTGAGG (3′); T-bet, CAACAACCCCTTTGCCAAAG (5′) and TCCCCCAAGCAGTTGACAGT (3′); GATA-3, GAAGGCATCCAGACCCGAAAC (5′) and ACCCATGGCGGTGACCATGC (3′); IL-4, CGAAGAACACCACAGAGAGTGAGCT (5′) and GACTCATTCATGGTGCAGCTTATCG (3′); IFN-γ, GGATGCATTCATGAGTATTGC (5′) and CCTTTTCCGCTTCCTGAGG (3′); IL-5, CGCTCACCGAGCTCTGTTG (5′) and CCAATGCATAGCTGGTGATTTTT (3′); hypoxanthine guanine phosphoribosyltransferase (HPRT), CTGGTGAAAAGGACCTCTCG (5′) and TGAAGTACTCATTATAGTCAAGGGCA (3′). Cycling conditions were 2 minutes at 50°C and 10 minutes at 95°C followed by 45 cycles of 15 seconds at 95°C and 1 minute at 60°C. Analysis used sequence detection software supplied with the instrument. mRNA expression was normalized to HPRT abundance. Subsequently, all data were expressed relative to the expression level in unstimulated wild-type Th2 cells, which was set as 1.
Retroviral constructs and transduction
The murine Vav1 cDNA was subcloned into the BglII and XhoI sites of the retroviral vector pMIG containing a green fluorescent protein (GFP) marker gene. The c-Maf-RV and GFP-RV vectors (a gift of Dr I.-C. Ho at Brigham and Women's Hospital, Boston, MA) were described.45 Retroviral transduction was performed as described.31 Briefly, platinum-E packaging cells (0.75 × 106)46 were plated on 60-mm dishes in 3 mL Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). Following overnight incubation, the cells were transfected with 3 μg retroviral plasmid DNA using FuGENE 6 transfection reagent (Roche, Indianapolis, IN). After 20 hours, the FuGENE 6-containing medium was replaced with 3 mL DMEM plus 10% FBS. Cultures were maintained for 24 hours, the retroviral supernatant was harvested, supplemented with 5 μg/mL Polybrene, and used to infect Vav1-deficient CD4+ T cells that had been preactivated with anti-CD3, anti-CD28, and 100 U/mL recombinant IL-2 for 18 hours. Plates were centrifuged for 1 hour (800g, 33°C) incubated for 8 hours at 33°C and for 16 hours at 37°C, followed by 2 additional retroviral infections at daily intervals. The medium was exchanged with RPMI 1640 medium supplemented with 10% FBS plus 20 U/mL recombinant IL-2. Transduction efficiency in surviving T cells was assessed on day 4 (ie, 1 day after the last retroviral infection) by GFP fluorescence, and cytokine-producing cells were enumerated by ICCS as described (see “ICCS”).
Results
Intrinsically impaired IL-4 production by Vav1-deficient CD4+ T cells
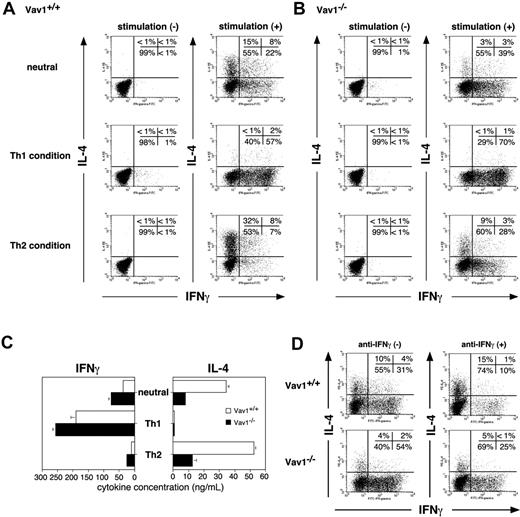
To study the potential role of Vav1 in Th1/Th2 development, we primed naive CD4+ C57BL/6 T cells with anti-CD3/CD28 antibodies in the absence of added cytokines and, after 7 days, washed and restimulated the cells with the same antireceptor antibodies. We then enumerated cytokine-producing cells by ICCS and FACS analysis. Under these neutral conditions, wild-type T cells differentiated almost equally well toward either IFN-γ– or IL-4–producing cells (Figure 1A). Unexpectedly, Vav1-deficient CD4+ T cells were severely impaired in their ability to generate IL-4–producing cells under the same conditions (15% versus 3% in wild-type or Vav1–/– T cells, respectively). On the other hand, the Vav1 mutation enhanced the development of IFN-γ–producing cells (22% versus 39% in wild-type or Vav1–/– cells; Figure 1A-B).
Priming cells in the presence of exogenous IL-4 and neutralizing anti–IL-12 antibody (Th2-inducing conditions) further revealed the extent of the deficit in Vav1-deficient CD4+ T cells. Although the number of IL-4–producing Vav1–/– cells increased from 3% to 9% under these conditions, it still remained substantially lower than that generated from Vav1-expressing T cells cultured even under neutral conditions (15%). Moreover, under Th2-inducing conditions, the Vav1 mutation significantly enhanced the proportion of IFN-γ–producing T cells (7% versus 28% in wild-type or Vav1–/– T cells, respectively). Thus, Vav1-deficient CD4+ T cells are severely impaired in their ability to develop into IL-4– producing cells and, instead, they preferentially develop into IFN-γ–producing cells. In contrast to Th2-inducing conditions, the absence of Vav1 did not affect, and perhaps even slightly enhanced (from 57% to 70%), the development of IFN-γ–producing cells under Th1-inducing conditions (Figure 1A-B). Collectively, these data indicate that TCR/CD28 signaling in the absence of Vav1 fails to support development of IL-4–producing effector cells, and enhances the development of IFN-γ–producing cells. Quantitation of cytokine levels in culture supernatants by an ELISA revealed similar results (Figure 1C) and confirmed that the defective IL-4 production by Vav1–/– T cells was not overcome even when recombinant IL-2 was added to the cultures at initiation instead of day 2 (data not shown). These findings are supported by IL4 and IFNγ gene reporter assays in transfected Vav1-deficient (J.Vav1) Jurkat T cells47 (data not shown).
Given the fact that IFN-γ can inhibit the development of IL-4–producing Th2 cells,1,5 the impaired IL-4 production by Vav1–/– T cells under Th2-inducing conditions could reflect a secondary effect of the outgrowth of IFN-γ–producing cells rather than an intrinsic failure to generate IL-4–producing cells. To address this possibility, we cultured CD4+ T cells under standard Th2-inducing conditions in the presence of an added neutralizing anti–IFN-γ antibody, thereby excluding the potential effect of IFN-γ secreted by the Vav1-deficient T cells. As expected, the addition anti–IFN-γ to cultures of wild-type T cells induced skewing in favor of Th2 development reflected by a change in the ratio of IL-4–producing T cells to IFN-γ–producing T cells from 1:3 to 1.5:1 in the absence or presence of anti–IFN-γ, respectively (Figure 1D). In cultures of Vav1–/– T cells, the anti–IFN-γ antibody reduced the proportion of IFN-γ–producing cells by about 50% (as expected); however, it (or anti–IL-12; data not shown) did not increase the number of IL-4–producing cells, which remained very low (4%-5%) relative to the proportion of IFN-γ–producing T cells. Thus, although secreted IFN-γ may partially account for the increased proportion of IFN-γ–producing Vav1–/– T cells generated under Th2-inducing conditions, it cannot account for the markedly impaired IL-4 expression by the same cells. Therefore, the impaired IL-4 production represents a primary defect resulting from the Vav1 mutation. Nevertheless, to rule out any potential contribution of secreted IFN-γ to the observed effects, we routinely included a neutralizing anti–IFN-γ antibody in all subsequent experiments.
Effector Th cell development in the absence of Vav1. Naive CD4+ T cells from wild-type and Vav1–/– mice were primed by stimulation with anti-CD3/CD28– coated tissue culture plates under Th1, Th2, or neutral conditions. After 7 days, the cells were washed, counted, and restimulated with plate-bound anti-CD3/CD28 antibodies. (A-B) Cytokine-producing cells were enumerated by ICCS after 8 hours of stimulation. (C) Levels of IFN-γ or IL-4 in 48-hour stimulated culture supernatants were quantified by ELISA. The data shown are representative of 5 independent experiments. Data are expressed as the mean value of triplicate determinations ± standard error (SE). (D) T cells primed under Th2-inducing conditions in the absence or presence of a neutralizing anti–IFN-γ antibody were restimulated in anti-CD3/CD28 antibodies– coated tissue culture plates, and IL-4+ versus IFN-γ+ cells were enumerated by ICCS.
Effector Th cell development in the absence of Vav1. Naive CD4+ T cells from wild-type and Vav1–/– mice were primed by stimulation with anti-CD3/CD28– coated tissue culture plates under Th1, Th2, or neutral conditions. After 7 days, the cells were washed, counted, and restimulated with plate-bound anti-CD3/CD28 antibodies. (A-B) Cytokine-producing cells were enumerated by ICCS after 8 hours of stimulation. (C) Levels of IFN-γ or IL-4 in 48-hour stimulated culture supernatants were quantified by ELISA. The data shown are representative of 5 independent experiments. Data are expressed as the mean value of triplicate determinations ± standard error (SE). (D) T cells primed under Th2-inducing conditions in the absence or presence of a neutralizing anti–IFN-γ antibody were restimulated in anti-CD3/CD28 antibodies– coated tissue culture plates, and IL-4+ versus IFN-γ+ cells were enumerated by ICCS.
Selectivity of the Vav1 mutation-associated defect for IL-4
To determine whether the Vav1 mutation affects other Th2 cytokines besides IL-4, we extended the analysis to measurements of IL-5 and IL-13 levels in polarized Th2 cells restimulated with an anti-CD3 antibody. In addition, we also assessed proliferation of the same cells. Although the anti-TCR–induced proliferation of naive Vav1–/– T cells is known to be impaired,33-35,37 we found that proliferation of restimulated, previously primed T cells was comparable in wild-type versus Vav1–/– T cells, despite the fact that the deficient T cells failed to produce detectable levels of IL-2 (Figure 2). Consistent with our earlier findings (Figure 1), the Vav1–/– effector T cells secreted more IFN-γ and less IL-4 than their wild-type counterparts. Of importance, however, the production of 2 other Th2 cytokines, IL-5 and IL-13, by the Vav1–/– T cells remained intact (Figure 2).
Defective c-Maf expression by Vav1–/– T cells
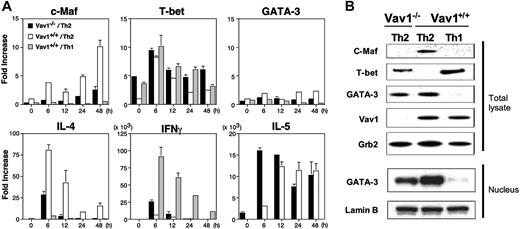
To address the molecular mechanisms that account for the impaired IL-4 expression and enhanced IFN-γ production by Vav1–/– CD4+ T cells cultured under Th2-inducing conditions, we used real-time RT-PCR as well as immunoblotting to analyze the expression of several transcription factors and cytokines, which are known to influence Th1/Th2 cell differentiation, in polarized and restimulated Th2 cells. Consistent with our earlier results (Figures 1, 2), Vav1–/– T cells cultured under Th2-inducing conditions expressed lower levels of IL4 mRNA than their wild-type counterparts; in addition, the expression of cMaf mRNA was markedly impaired in Vav1–/– Th2 cells at all time points examined. In contrast, we did not observe a consistent reduction in the expression of IL5 mRNA level in the same cells; IL5 mRNA expression in Vav1–/– Th2 cells was higher than in the wild-type Th2 cells at an early time point (6 hours), but not later (Figure 3A). The significance of these possible differences is unclear given the intact IL-5 production by the same cells (Figure 2). By comparison, Vav1+/+ Th1 cells displayed very low or undetectable levels of IL4 or IL5 mRNA but, as expected, much higher levels of IFNγ mRNA than Th2 cells from either Vav1–/– or wild-type mice. The reduced IL4 and cMaf mRNA expression was paralleled by undetectable expression of c-Maf protein by Vav1–/– Th2 cells comparable to that in wild-type Th1 cells (Figure 3B). The expression of another Th2-specific transcription factor, GATA-3, was somewhat lower in Vav1–/– Th2 cells by comparison with their wild-type counterparts both at the mRNA (at 24-48 hours; Figure 3A) and protein (Figure 3B) levels. However, the significance of this reduction is questionable given that IL5 mRNA (Figure 3A) or protein (Figure 2) expression were intact in Vav1–/– Th2 cells.
IL-4 production is selectively impaired in Vav1–/– Th2 cells. Naive CD4+ T cells from Vav1–/– or wild-type mice were primed in anti-CD3/CD28–coated plates under Th2-inducing conditions in the presence of an anti–IFN-γ antibody. After 7 days, the cells were washed, counted, and restimulated at 2 × 105 cells/well with plate-bound anti-CD3 antibody. Proliferation was determined after 72 hours of stimulation by 3HTdR uptake. Levels of IFN-γ, IL-2, IL-4, IL-5, or IL-13 in 48-hour stimulated culture supernatants were quantified by ELISA. All data are expressed as the mean value of triplicate determinations ± SE. The data shown are representative of 3 independent experiments.
IL-4 production is selectively impaired in Vav1–/– Th2 cells. Naive CD4+ T cells from Vav1–/– or wild-type mice were primed in anti-CD3/CD28–coated plates under Th2-inducing conditions in the presence of an anti–IFN-γ antibody. After 7 days, the cells were washed, counted, and restimulated at 2 × 105 cells/well with plate-bound anti-CD3 antibody. Proliferation was determined after 72 hours of stimulation by 3HTdR uptake. Levels of IFN-γ, IL-2, IL-4, IL-5, or IL-13 in 48-hour stimulated culture supernatants were quantified by ELISA. All data are expressed as the mean value of triplicate determinations ± SE. The data shown are representative of 3 independent experiments.
The mRNA expression of IFNγ was significantly enhanced (at 6-12 hours) and that of the Th1-inducing transcription factor, T-bet,11 was also increased (at 24-48 hours) in Vav1–/– Th2 cells relative to their wild-type counterparts (Figure 3A). Vav1–/– Th2 cells also expressed a significantly higher level of T-bet protein than wild-type Th2 cells, and this level approached that found in wild-type Th1 cells (Figure 3B). As expected, wild-type Th1 cells expressed very low amounts of cMaf or GATA3 mRNA (Figure 3A) and undetectable levels of the corresponding proteins (Figure 3B). Similarly, we did not detect c-Maf expression in Vav1–/– T cells cultured under Th1-inducing conditions (data not shown). Taken together, these results indicate that the Vav1 mutation greatly impairs the expression of c-Maf and, conversely, enhances the expression of T-bet in CD4+ T cells cultured under Th2-polarizing conditions.
Dependence of c-Maf expression on Ca2+/NFAT signaling
Vav1-deficient CD4+ T cells fail to generate a sustained calcium flux following TCR engagement,32,33,37,43 and this defect is associated with impaired nuclear translocation,36 but intact up-regulation,35 of NFATc1, which is important for IL-4 expression and Th2 differentiation.27,28 Additionally, activation of the MAPK, ERK, which also appears to play an important role in the generation of effector Th2 cells,20,21,26 was found to be impaired in Vav1–/– T cells.37 We confirmed that Vav1–/– T cells cultured under Th2-inducing conditions displayed similar defects in NFAT and ERK (but not JNK or p38) activation; as a control, no differences in the up-regulation of JunB, which has been implicated in Th2 differentiation,48 were observed between the wild-type and Vav1–/– T cells (data not shown).
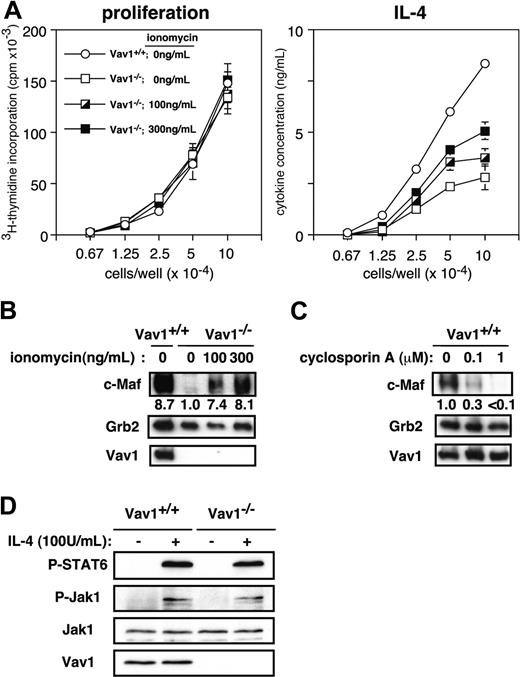
Relatively little is known about the signals that regulate c-Maf expression in differentiating Th2 cells and, in particular it is not known whether c-Maf expression is functionally linked to Ca2+ or NFAT signaling. Therefore, we used several independent approaches to determine whether the impaired NFAT activation and c-Maf expression are causally related or independent of each other. First, to restore the impaired Ca2+/calcineurin/NFAT pathway, we stimulated Vav1–/– CD4+ T cells with anti-CD3/CD28 antibodies under Th2-inducing conditions in the presence of ionomycin and assessed in parallel proliferation and IL-4 secretion. Although addition of ionomycin to the cultures did not affect the proliferation of the cells, it partially restored the ability of Vav1-deficient cells to produce IL-4 (Figure 4A). The effect of higher ionomycin concentrations (∼1 μg/mL) could not be evaluated reliably because they were toxic to the cells and inhibited proliferation (data not shown). Second, a similar addition of ionomycin also induced a marked increase in c-Maf expression in Vav1–/– T cells stimulated under Th2-inducing conditions; in fact, the expression of c-Maf in Vav1–/– T cells treated with the highest ionomycin concentration (300 ng/mL) was similar to that in control, wild-type Th2 cells that were not stimulated with ionomycin (Figure 4B). Third, when wild-type CD4+ T cells were stimulated under similar conditions, addition of cyclosporin A, which blocks Ca2+ signaling and NFAT activation,49 significantly suppressed the up-regulation of c-Maf in a dose-dependent manner (Figure 4C). Together, these experiments reveal that the impaired Ca2+ signaling and NFAT activation are responsible, at least in part, for the defective up-regulation of c-Maf in Vav1–/– T cells.
Defective expression of c-Maf by Vav1-deficient T cells. CD4+ T cells from Vav1–/– or wild-type mice were stimulated with plate-bound anti-CD3/CD28 antibodies under Th2- or Th1-inducing conditions. After 5 days, the cells were washed and restimulated in anti-CD3–coated plates. (A) RNA expression of c-Maf, T-bet, GATA-3, IL-4, IFN-γ, and IL-5 was analyzed at the indicated times by real-time RT-PCR. Transcript levels were normalized to the expression level of HPRT transcripts in the same sample and are represented as transcript abundance relative to unstimulated wild-type Th2 cells. The data shown are representative of 3 independent experiments. (B) Total lysates or nuclear extracts prepared 48 hours after restimulation were analyzed by immunoblotting with the indicated antibodies. Grb2 expression was analyzed as a control for equal protein loading. All data are expressed as the mean value of triplicate determinations ± SE.
Defective expression of c-Maf by Vav1-deficient T cells. CD4+ T cells from Vav1–/– or wild-type mice were stimulated with plate-bound anti-CD3/CD28 antibodies under Th2- or Th1-inducing conditions. After 5 days, the cells were washed and restimulated in anti-CD3–coated plates. (A) RNA expression of c-Maf, T-bet, GATA-3, IL-4, IFN-γ, and IL-5 was analyzed at the indicated times by real-time RT-PCR. Transcript levels were normalized to the expression level of HPRT transcripts in the same sample and are represented as transcript abundance relative to unstimulated wild-type Th2 cells. The data shown are representative of 3 independent experiments. (B) Total lysates or nuclear extracts prepared 48 hours after restimulation were analyzed by immunoblotting with the indicated antibodies. Grb2 expression was analyzed as a control for equal protein loading. All data are expressed as the mean value of triplicate determinations ± SE.
To determine whether initial IL-4 receptor signaling is responsible for the impaired c-Maf expression and differentiation of IL-4–producing cells in Vav1-deficient mice, we investigated both proximal (Jak1 activation) and more distal (STAT6 phosphorylation) signaling events mediated by the IL-4 receptor in IL-4–stimulated primary CD4+ T cells. Both of these responses were comparable between wild-type and Vav1–/– T cells (Figure 4D). Thus, the Vav1 mutation does not appear to affect IL-4 signaling.
Restoration of IL-4 production by ectopic Vav1, but not c-Maf, expression
To establish more directly the important role of Vav1 in IL-4 expression, we analyzed the effect of retrovirus-mediated ectopic Vav1 expression in Vav1–/– T cells. We generated a retroviral murine Vav1 expression vector and used it (or a control “empty” retrovirus) to infect anti-CD3/CD28-primed Vav1–/– CD4+ T cells. After 4 days of culture under Th2-polarizing conditions, we analyzed IL-4 production by ICCS. Under these conditions, we achieved a transduction efficiency of 40% to 50%, and immunoblot analysis confirmed the ectopic expression of Vav1 in the infected (GFP+) Vav1–/– T cells (data not shown). Fewer than 1% IL-4+ cells were found in the unstimulated T-cell population (data not shown). In a representative experiment (Figure 5A), transduction of primary Vav1–/– CD4+ T cells with Vav1 increased the fraction of IL-4–producing cells in the restimulated GFP+ population from about 19.2% to 40.6%, a level exceeding the proportion of IL-4+ cells in a control population of wild-type Th2 cells (Figure 5A). This result clearly demonstrates that Vav1 itself is fully capable of restoring IL-4 production in stimulated Vav1–/– T cells. We also examined the effect of retroviral Vav1 transduction on IFN-γ– producing cells in the same cultures but found no consistent effects due to failure to achieve a clear separation between the IFN-γ– and IFN-γ+ subpopulations within the GFP+ population (data not shown).
To determine whether the deficient c-Maf expression associated with the Vav1 mutation can in itself account for the impaired IL-4 production, we also transduced Vav1–/– T cells primed under Th2-inducing conditions with a c-Maf–expressing retrovirus. c-Maf expression in the retrovirus-infected Vav1–/– T cells greatly exceeded the endogenous level of c-Maf in wild-type Th2 cells (data not shown). Nevertheless, although ectopic c-Maf expression increased the fraction of IL-4–producing cells in the GFP+ population from about 19.8% to 24.1%, this level was still significantly lower than the proportion of IL-4+ T cells in the control population of wild-type Th2 cells (34.2%; Figure 5B). Thus, unlike Vav1, c-Maf only partially restores IL-4 production in stimulated Vav1–/– T cells, suggesting that Vav1 regulates IL-4 production via additional mechanisms that are independent of c-Maf.
Impaired c-Maf expression in Vav1–/– Th2 cells is linked to deficient Ca2+ signaling. (A) T cells were stimulated under Th2-inducing conditions in the presence or absence of the indicated concentrations of ionomycin. After 7 days, the cells were washed, counted, and restimulated in anti-CD3–coated tissue culture plates. Proliferation (left) and cytokine-producing cells (right) were determined as in Figure 2. Data are expressed as the mean value of triplicate determinations ± SE. (B) Naive CD4+ T cells were cultured under Th2- or Th1-inducing conditions in the presence or absence of the indicated ionomycin concentrations. Lysates prepared 48 hours after restimulation were analyzed by immunoblotting. Numbers indicate the intensity of c-Maf signal relative to Vav1–/– Th2 cells cultured without ionomycin (= 1) after normalization to the Grb2 signal. (C) Naive CD4+ T cells from wild-type mice were cultured under Th2-inducing conditions in the presence or absence of indicated doses of cyclosporin A. Lysates prepared 48 hours after restimulation were analyzed by immunoblotting. The c-Maf signal was quantitated as in panel B. (D) Primary CD4+ T cells were stimulated with IL-4 (100 U/mL) for 5 minutes and cell lysates resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were immunoblotted with the indicated antibodies.
Impaired c-Maf expression in Vav1–/– Th2 cells is linked to deficient Ca2+ signaling. (A) T cells were stimulated under Th2-inducing conditions in the presence or absence of the indicated concentrations of ionomycin. After 7 days, the cells were washed, counted, and restimulated in anti-CD3–coated tissue culture plates. Proliferation (left) and cytokine-producing cells (right) were determined as in Figure 2. Data are expressed as the mean value of triplicate determinations ± SE. (B) Naive CD4+ T cells were cultured under Th2- or Th1-inducing conditions in the presence or absence of the indicated ionomycin concentrations. Lysates prepared 48 hours after restimulation were analyzed by immunoblotting. Numbers indicate the intensity of c-Maf signal relative to Vav1–/– Th2 cells cultured without ionomycin (= 1) after normalization to the Grb2 signal. (C) Naive CD4+ T cells from wild-type mice were cultured under Th2-inducing conditions in the presence or absence of indicated doses of cyclosporin A. Lysates prepared 48 hours after restimulation were analyzed by immunoblotting. The c-Maf signal was quantitated as in panel B. (D) Primary CD4+ T cells were stimulated with IL-4 (100 U/mL) for 5 minutes and cell lysates resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were immunoblotted with the indicated antibodies.
Impaired IL-4 production and enhanced Th1 development of Vav1–/– T cells in vivo
To assess the effect of the Vav1 mutation on Th1/Th2 development in vivo, we took advantage of findings that antigen priming in CFA favors the production of Th1 cytokines, whereas priming with IFA favors a Th2 response.50 Thus, we primed wild-type or Vav1–/– mice with KLH in either of these 2 adjuvants and assessed the proliferative and cytokine recall responses of their draining lymph node T cells 10 days later (Figure 6).
Antigen priming in IFA generated a KLH-specific T-cell proliferative response and IL-2 production level that were substantially lower in the Vav1–/– cells by comparison with their wild-type counterparts (Figure 6A). Thus, antigen-stimulated Vav1–/– T cells were clearly hyporeactive when primed in vivo under conditions that favor Th2 development. These results are similar to those obtained in earlier studies with anti-CD3/CD28-stimulated Vav1–/– T cells cultured under neutral conditions.34 Of importance, we also found that Vav1–/– T cells produced a much lower (< 20% of wild-type) level of IL-4 when the mice were primed with IFA (Figure 6A). Unexpectedly, and at variance with the results obtained with in vitro differentiated Th2 cells (Figure 2), under the same conditions the Vav1–/– T cells produced significantly higher level of IL-5 and, conversely, somewhat lower levels of IL-13 than the wild-type T cells (Figure 6A). Undefined host factors may account for this difference between the in vitro and in vivo findings. Consequently, the dissociation of Th2 cytokine production between IL-4 and IL-5/IL-13 was also observed in T cells from antigen-primed Vav1–/– mice.
When Vav1–/– mice were primed with KLH in CFA, their T cells proliferated as well as similarly primed wild-type T cells, even though IL-2 production by these cells was still substantially reduced (Figure 6B). Although this result seems surprising, it is consistent with a previous study demonstrating intact antiviral responses in Vav1–/– mice,51 most likely reflecting a compensating influence of Vav3, which is expressed in T cells and plays a partially redundant positive role in their responsiveness.52,53 Furthermore, Vav1–/– T cells produced significantly higher level of IFN-γ (> 5-fold) and IL-5, but lower levels of IL-4 and IL-13 than their wild-type counterparts (Figure 6B). We confirmed this skewed Th1 differentiation by ICCS analysis of the same KLH/CFA-immunized mice (Figure 6C). This immunization protocol increased the proportion of antigen-induced IFN-γ–producing CD4+ T cells in Vav1–/– mice from 0.03% (wild-type) to 0.14%, that is, by about 5-fold. Thus, Vav1-deficient mice demonstrate a propensity toward the development of Th1 cells. Taken together with the earlier results (Figures 1, 2), our findings clearly support the conclusion that the Vav1 mutation causes a severe and relatively selective impairment IL-4 production and, conversely, enhanced Th1 (IFN-γ+) differentiation.
Reconstitution of IL-4 production by retroviral Vav1, but not c-Maf, expression. Naive CD4+ T cells from Vav1–/– or wild-type mice were infected with a Vav1-expressing (A) or a c-Maf–expressing (B) and control (GFP) retrovirus 18 hours after primary activation by anti-CD3 plus anti-CD28 antibodies plus IL-2 under Th2-inducing conditions. Cells were harvested on day 4, restimulated with anti-CD3 plus anti-CD28 antibodies, and IL-4–producing cells were enumerated by ICCS 8 hours later. Data are displayed as dot plots showing GFP (horizontal axis) versus intracellular IL-4 (vertical axis) expression. The numbers show percentages of IL-4+ and IL-4– T cells within the GFP+ population (= 100%). The data shown are representative of 3 independent experiments.
Reconstitution of IL-4 production by retroviral Vav1, but not c-Maf, expression. Naive CD4+ T cells from Vav1–/– or wild-type mice were infected with a Vav1-expressing (A) or a c-Maf–expressing (B) and control (GFP) retrovirus 18 hours after primary activation by anti-CD3 plus anti-CD28 antibodies plus IL-2 under Th2-inducing conditions. Cells were harvested on day 4, restimulated with anti-CD3 plus anti-CD28 antibodies, and IL-4–producing cells were enumerated by ICCS 8 hours later. Data are displayed as dot plots showing GFP (horizontal axis) versus intracellular IL-4 (vertical axis) expression. The numbers show percentages of IL-4+ and IL-4– T cells within the GFP+ population (= 100%). The data shown are representative of 3 independent experiments.
In vivo Th development in Vav1–/– mice. (A-B) Groups of 3 wild-type mice (○) or 3 Vav1–/– mice (•) were primed subcutaneously with 100 μg KLH given either in IFA (A) or in CFA (B). On day 10, the draining periaortic and inguinal lymph node cells were stimulated in vitro with the indicated KLH concentrations in triplicate. Proliferation and cytokine production were determined as in Figure 2. All data are expressed as the mean value of triplicate determinations ± SE. (C) Spleen cells from mice immunized as in panel B were stimulated with 1 μg/mL KLH, and IFN-γ– producing cells were enumerated by ICCS 16 hours later. The data shown are representative of 3 independent experiments.
In vivo Th development in Vav1–/– mice. (A-B) Groups of 3 wild-type mice (○) or 3 Vav1–/– mice (•) were primed subcutaneously with 100 μg KLH given either in IFA (A) or in CFA (B). On day 10, the draining periaortic and inguinal lymph node cells were stimulated in vitro with the indicated KLH concentrations in triplicate. Proliferation and cytokine production were determined as in Figure 2. All data are expressed as the mean value of triplicate determinations ± SE. (C) Spleen cells from mice immunized as in panel B were stimulated with 1 μg/mL KLH, and IFN-γ– producing cells were enumerated by ICCS 16 hours later. The data shown are representative of 3 independent experiments.
Discussion
Vav1 plays important roles in T-cell development and in peripheral TCR-induced proliferation and IL-2 production.32-37,43,54,55 However, its potential role in Th1/Th2 differentiation has not been previously addressed.43 Although an earlier study implicated a role for Vav1 in activation of an IL-4–luciferase reporter gene in transfected Jurkat T cells,42 the biologic relevance of this work remains unclear. In the present study, we used Vav1–/– mice to conduct a detailed analysis of TCR/CD28-induced differentiation and activation of Th1 and Th2 cells in vitro and in vivo. Our findings provide novel insights regarding the role of Vav1 and Ca2+/NFAT signaling in Th2 differentiation by demonstrating, first, that Vav1 plays an important role in the expression of IL-4 but not other Th2 cytokines such as IL-5 or IL-13; second, that this role reflects a critical dependence of c-Maf expression on Vav1; third, that the Vav1 mutation is associated with abnormally high T-bet and IFN-γ expression in T cells cultured under normally Th2-inducing conditions; and, fourth, that optimal up-regulation of c-Maf in differentiating Th2 cells depends, at least in part, on intact Ca2+/NFAT signaling. The selectively impaired Th2 differentiation of Vav1–/– T cells was not secondary to the established IL-2 deficiency of these cells because addition of exogenous IL-2 to the cultures even on day 0 (data not shown) did not rescue the deficient IL-4 production. Furthermore, IL-2 is required not only for IL-4 priming, but also for IFN-γ priming,56 and our results clearly demonstrate that IFN-γ production by Vav1–/– T cells was not reduced but, rather, enhanced.
Our findings that the Vav1 mutation reduced the production of IL-4 but not that of 2 other Th2 cytokines, IL-5 and IL-13, indicates that Vav1 does not globally regulate the Th2 locus but, rather, expression of the IL4 gene in a more selective manner. Consistent with this conclusion, the Vav1 mutation also impaired the up-regulation of c-Maf, a transcription factor that selectively promotes IL-4 expression.13 Thus, CD4+ T cells and NKT cells from c-Maf–/– mice are markedly deficient in IL-4 production, but produce normal levels of IL-13 and IgE, and, when differentiated in the presence of exogenous IL-4, these T cells produced approximately normal levels of other Th2 cytokines.15 In contrast to its dramatic effect on c-Maf expression, the absence of Vav1 only slightly reduced the expression of GATA-3, another Th2-specific transcription factor, which, unlike c-Maf, regulates the Th2 cytokine locus in a global manner and promotes production of several Th2 cytokines in addition to IL-4.8-10,57,58 The selective regulation of IL-4, but not IL-5 or IL-13 expression, by Vav1 is also consistent with the finding that the expression of Th2 cytokines at the single-cell level can be mutually exclusive and that the expression of IL-13 is regulated by a mechanism distinct from that regulating the expression of IL-4.58 The selective impairment in IL-4 and c-Maf expression by Vav1–/– T cells is highly reminiscent of the phenotype of ICOS-deficient mice,45 raising the possibility that an ICOS → Vav → c-Maf → IL-4 axis may operate in Th2 cells, consistent with a recent report demonstrating functional coupling of Vav to ICOS costimulation.59 However, Vav1–/– T cells did not display any impairment in their ability to up-regulate ICOS expression when stimulated and cultured under Th2-inducing conditions (data not shown).
Of interest, restimulated Vav1–/– T cells primed under Th2-inducing conditions also expressed an abnormally high level of the Th1-inducing transcription factor, T-bet,11 which was, in fact, quite similar to T-bet expression in wild-type T cells cultured under Th1-inducing conditions. Thus, the abnormal up-regulation of T-bet may account, to a large degree, for the increased IFN-γ production and, conversely, decreased IL-4 expression, by Vav1–/– CD4+ T cells cultured under Th2-inducing conditions. However, based on an earlier study,11 enhanced T-bet expression would be expected to also reduce IL-5 expression, which clearly was not the case in the Vav1–/– T cells. Little is known about the intracellular signals that regulate T-bet expression, and TCR signals, STAT and IFN-γ can all contribute to T-bet up-regulation.60,61 It remains to be determined whether enhanced T-bet expression and, conversely, impaired c-Maf expression caused by the Vav1 mutation are coregulated or independent of each other. In addition to increased T-bet expression, the impaired c-Maf expression and the resulting deficiency in IL-4 production are also likely to contribute to the enhanced IFN-γ production by Vav1–/– T cells cultured under Th2-inducing conditions, because both IL-462 and c-Maf14 can inhibit Th1 differentiation.
Activation of T cells under Th2-inducing conditions up-regulates expression of a c-Maf in a relatively slow manner. The mechanisms that regulate c-Maf expression are not clear, but it has been reported that TCR or costimulatory signals (or both), rather than cytokine (IL-4) signals, up-regulate its expression in differentiating T cells.13 This conclusion is consistent with findings that chromatin structural changes initiated by the TCR and maintained by subsequent cytokine-driven signaling pathways are required for differentiation of Th cells.63 Our findings that initial events of IL-4 receptor signaling, that is, Jak1 activation and STAT6 tyrosine phosphorylation, were intact in Vav1–/– T cells despite the impaired c-Maf expression is consistent with the idea that secreted IL-4 and subsequent signaling through its receptor do not play a major role in promoting c-Maf expression, at least initially. Together, these findings support the notion that TCR or costimulatory signals, rather than cytokines, likely regulate c-Maf expression associated with IL-4 production during Th2 differentiation via Vav1 signaling pathways. Even more important, our findings begin to shed light on an important but unresolved question, that is, which TCR/CD28-initiated signaling pathways mediate c-Maf expression. Thus, we demonstrate, first, that Vav1 is required for inducing c-Maf expression in differentiating Th2 cells and, second, that this requirement reflects, to a large degree, the importance of Ca2+ signaling and NFAT activation in c-Maf expression. Such a role for Ca2+ signals or NFAT in c-Maf expression has not been previously established, although ionomycin stimulation can induce transcription and expression of the IL4 gene.18,29
Our findings (data not shown) extend previous reports that were based on analysis of primary Vav1–/– T cells by demonstrating that similar defects in NFATc1 expression and nuclear translocation are also evident when Vav1–/– T cells are cultured under Th2-polarizing conditions. Therefore, one mechanism through which Vav1 may regulate IL-4 expression is by regulating Ca2+ signaling pathways, because the Vav1 mutation results in deficient TCR-induced Ca2+ mobilization and NFAT activation.32,33,37,43 The role of Ca2+ signals and NFAT in Th2 differentiation is complex and Th2 activation requires a critical balance among NFAT family members. Thus, NFATc1 promotes Th2 differentiation and IL-4 expression,27,28 whereas NFATc2 and NFATc3 inhibit Th2 differentiation.64,65
Of interest, Itk–/– mice show a similar phenotype to that of Vav1–/– mice, that is, defects in development of Th2-dependent responses in vitro and in vivo,29,30,66 reduced Ca2+ influx and NFATc1 nuclear translocation in vitro, and restoration of IL-4 production by ionomycin.29 More recently, it was shown that, although stimulation of Itk–/– CD4+ T cells in the presence of skewing cytokines leads to efficient Th1 or Th2 lineage cell differentiation, stimulation of the same cells with low-avidity TCR ligands in the absence of skewing cytokines (which normally leads to GATA-3 up-regulation and Th2 development), results in enhanced T-bet expression and Th1 diferentiation.67 Therefore, a shared Vav-Itk signaling pathway may explain these similarities. Indeed, Vav1 can transduce TCR signals leading to activation of Itk, which then promotes Ca2+ signaling and, hence, NFAT activation via phosphorylation and activation of phospholipase C γ1 (PLC-γ1).43 Thus, suppression of T-bet or NFAT activation by both Vav1 and Itk, perhaps in a linked pathway, may explain the similarities between Vav1–/– and Itk–/– T cells. However, there are also important differences between Vav1–/– and Itk–/– T cells. Thus, we did not observe in Vav1–/– T cells the reduced IL-5 and IL-13 production found in Itk–/– T cells. Furthermore, Vav1–/–, but not Itk–/–29 or NFATc1–/–27,28 T cells cultured under Th2-inducing conditions display enhanced IFN-γ production both in vitro and in vivo. Recent findings that PKC-θ –/– mice display selectively impaired Th2 responses68,69 are also intriguing given the demonstrated functional link between Vav1 and PKC-θ.42,70 However, it remains to be determined whether PKC-θ –/– T cells manifest similar molecular abnormalities to those we observed in Vav1–/– T cells, that is, impaired c-Maf and excessive T-bet expression under Th2-polarizing culture conditions.
The differences between Vav1–/– and Itk–/– T cells, as well as our findings that ionomycin or ectopic c-Maf expression did not fully restore IL-4 production by Vav1–/– T cells suggest that impaired Ca2+ signaling and c-Maf expression are not likely to be the only mechanisms accounting for the defective IL-4 expression in Vav1–/– T cells. This notion is also supported by findings that, unlike Vav1–/– T cells, which display enhanced IFN-γ expression, c-Maf–/– T cells do not show a similar increase.15 Another potential mechanism accounting for the defective c-Maf/IL-4 expression is implicated by the selective defect in TCR/CD28-induced ERK activation in Vav1–/– T cells cultured under Th2-inducing conditions (data not shown), given the reported importance of ERK in generating CD4+ Th2 effector cells.20,21,26 This result, which was obtained by using CD4+ T cells cultured under Th2-polarizing conditions, confirms and extends the previous report of deficient ERK activation in primary Vav1–/– T cells cultured under neutral conditions.37 The requirement of ERK for optimal Th2 differentiation may reflect the importance of this MAPK in induction or activation of activator protein 1 (AP-1), which binds to the IL4 gene promoter.71 A more recent study revealed that JunB, but not other Jun family members, was selectively induced in Th2 cells and not in Th1 cells during differentiation; furthermore, JunB bound to the IL4 promoter and synergized with c-Maf to activate an IL4 luciferase reporter gene.48 Thus, it would be interesting to determine whether ERK and AP-1 are also required for optimal c-Maf expression.
In addition, the guanine nucleotide exchange factor (GEF) activity of Vav1 toward small Rac-family guanosine triphosphatases (GTPases), which resides in its Dbl-homology (DH) domain, may also play an important role in IL-4 production and Th2 differentiation. In this regard, a precedent exists for a differential role of Rac2 in Th1 versus Th2 differentiation.72 Studies to determine whether these or other mechanisms are involved in the impaired differentiation of Vav1–/– CD4+ T cells are in progress.
In summary, our findings indicate that Vav1 plays an important role in TCR/CD28-mediated signal transduction pathways leading to IL-4 expression in differentiating Th2 cells and that this role reflects, at least in part, a requirement for Vav1 and NFAT in up-regulation of c-Maf expression and a role for Vav1 in suppressing T-bet expression. However, the Vav1 requirement reflects its important role in additional signaling pathways apart from c-Maf expression, which are also critical for Th2 development and expansion. Our findings begin to address the molecular basis of TCR and costimulatory signaling pathways that regulate c-Maf expression, an important and unresolved question. Future work should be aimed at defining the precise mechanisms through which Vav1 selectively regulates the expression of c-Maf and, hence, IL-4 production.
Prepublished online as Blood First Edition Paper, April 21, 2005; DOI 10.1182/blood-2004-10-4074.
Supported by National Institutes of Health grants GM50819 and CA3299 (A.A.) and by an Uehara Memorial Foundation grant (Y.T.). This is publication no. 640 from the La Jolla Institute for Allergy and Immunology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank I.-C. Ho, T. Kitamura, Y.-C. Liu, C. Staib, and V. Tybulewicz for providing mice, cells, and plasmids and for helpful advice, and N. Weaver for manuscript preparation.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal