Abstract
Animals lacking Src homology 2 domain-containing inositol 5-phosphatase (SHIP) display a reduction in lymphopoiesis and a corresponding enhancement of myelopoiesis. These effects are mediated at least in part by elevated levels of interleukin 6 (IL-6). Here, we show the lymphopoiesis block in SHIP–/– mice is due to suppression of the lymphoid lineage choice by uncommitted progenitors. The suppression can be reproduced in vitro with recombinant IL-6, and IL-6 acts directly on hematopoietic progenitors. The block is partially overcome in SHIP–/– IL-6–/– double-deficient animals. IL-6 does not suppress but actually enhances proliferation of lymphoid-committed progenitors, indicating the IL-6 target cells are hematopoietic stem cells or multipotent progenitors. The findings suggest a mechanism for the lymphopenia that accompanies proinflammatory diseases.
Introduction
Interleukin 6 (IL-6) is a multifunctional cytokine that is produced during inflammation. Administration of IL-6 to patients1 or animals2 following transplantation and irradiation accelerates recovery of the myeloid compartment. Thus, IL-6 supports myeloid development and function in vivo. However, IL-6–/– mice have normal numbers of peripheral myeloid3 and lymphoid4 cells. This indicates that although IL-6 is not required for terminal differentiation of these cell types, IL-6 elevation can contribute to myeloid development.
Deregulation of IL-6 production is implicated in the pathology of several disease processes. IL-6 supports the growth of myelomas5 and IL-6 transgenic mice develop a fatal plasmacytosis.6 Indeed, IL-6–/– mice are completely resistant to the induction of plasmacytomas by pristane,7 although normal B cells are capable of developing into plasma cells without IL-6.8 Furthermore, IL-6–/– animals fail to develop collagen- or antigen-induced arthritis,9,10 indicating an important role for IL-6 in the development of the autoimmune features of the disease. Increased IL-6 levels are found in several autoimmune diseases (for a review, see Ishihara and Hirano11 ), including rheumatoid arthritis.12 Such patients also exhibit an unexplained lymphopenia that correlates with disease severity.13 Naive B-cell lymphopenia and elevated plasma cell levels occur in patients with active autoimmune disease,14 features consistent with the IL-6 transgenic animal strains mentioned. Likewise, the B-cell lymphopenia in rheumatoid arthritis was associated with proinflammatory features, including elevated acute-phase proteins.15 Neutrophilia and lymphopenia are similarly associated with inflammation that accompanies bacterial sepsis16,17 and viral infections.18-20 Thus, the clinical data on IL-6 and the animal models of IL-6 transgenics and knockouts suggest that IL-6 plays an important role in the pathogenesis of autoimmune and other proinflammatory diseases.
Src homology 2 domain-containing inositol 5-phosphatase (SHIP) is expressed in hematopoietic cells and is a critical negative regulator of phosphatidylinositol 3-kinase. Accordingly, SHIP maintains the balance of positive and negative signals in lymphocytes and myeloid cells. SHIP-deficient animals show a myeloproliferative syndrome, characterized by splenomegaly and a massive infiltration of myeloid cells into lungs.21 Our previous work showed that bone marrow of SHIP–/– mice exhibited a progressive, age-dependent defect in B lymphopoiesis and a corresponding increase in myelopoiesis.22 Using an in vitro system, we found that bone marrow–derived progenitors from SHIP–/– mice failed to develop into B cells and showed a pronounced bias to become macrophages. The developmental defects in SHIP–/– mice were associated at least in part with increased IL-6 production and could be overcome in culture by neutralizing anti–IL-6 antibodies. In the mouse and in culture, B-cell development was arrested at an early progenitor stage but the precise stage of the block was unclear. Likewise, the mechanism by which IL-6 has these affects on hematopoiesis was not understood.
All blood cells arise from uncommitted hematopoietic stem cells. Stem cells are found within a mixed population of bone marrow cells lacking all lineage markers (Lin–) and expressing high levels of the c-Kit receptor (c-Kithi), and the surface marker Sca-1.23 Uncommitted hematopoietic progenitors give rise to 2 separate lineages. One lineage has the capacity to form myeloid, erythroid, and megakaryocyte cell types; the other is capable of forming lymphoid and natural killer cells. Lymphoid-committed progenitors (prolymphocytes) can be detected using sorted Lin– populations that express low levels of c-Kit, Sca-1, and the α chain of the IL-7 receptor (IL-7R+Sca-1+c-Kitlo).24 The more primitive early lymphoid-committed progenitors (ELPs) diverge from cells present in the Lin–c-KithiSca-1+ population that also contains progenitors for myeloid lineages and stem cells.25 The commitment step from multipotent progenitors to myeloid or lymphoid lineages is a critical but poorly understood developmental decision.
Earlier,22 we assigned the B lymphopoiesis defect in SHIP–/– mice to the ELP-to-prolymphocyte transition because absolute numbers of prolymphocytes in SHIP–/– marrow were diminished, whereas ELP numbers appeared similar to wild-type. However, the issue was complicated because these early progenitors cannot be unequivocally resolved by surface markers alone. Recent experiments revealed that ELPs, present within the Lin–c-KithiSca-1+ mixed lineage population, can be resolved from other populations expressing similar surface markers by expression of the recombination activating gene 1 (RAG1).26 Transgenic mice having green fluorescent protein (GFP) within a single allele of the rag1 locus (RAG1-GFP knock-in mice27 ) mark the transcription of the rag1 gene during hematopoietic cell development. GFP+ cells present within the Lin–c-KithiSca-1+ population were enriched for lymphoid progenitors, whereas the GFP– population contained stem cells and myeloid progenitors.26
Here, we have identified the stage of the lymphopoiesis defect in SHIP–/– mice using the RAG1-GFP knock-in animals. The defect corresponds to the critical lymphoid or myeloid lineage choice of uncommitted progenitor cells, the first and most significant lineage decision of these hematopoietic progenitors. The lymphopoiesis defect is partially corrected in SHIP and IL-6 double-deficient mice. We also report in vitro experiments indicating that IL-6 at levels found in SHIP–/– mice can alter the lineage decision made by progenitors, promote their expansion, and bias their choice to the myeloid lineage. However, IL-6 did not alter survival or the lineage commitment of purified ELPs. Our findings indicate that the uncommitted progenitor cells are the direct target of IL-6. We propose that IL-6 blocks lymphopoiesis by 2 mechanisms: elevating the expansion of uncommitted progenitors and suppressing the lymphoid option. The data might account for the lymphopenia described in clinical situations of inflammation.
Materials and methods
Mice
RAG1-GFP knock-in mice have been described previously.27 SHIP–/– mice on the C57Bl/6 background were obtained from Dr G. Krystal (Terry Fox Laboratory, BC Cancer Agency, Vancouver, BC, Canada). IL-6–/– mice on the C57Bl/6 background were purchased from Jackson Laboratory (Bar Harbor, ME). SHIP–/–, RAG1-GFP, and SHIP–/–IL-6–/– mice were obtained by interbreeding.
Antibodies and lineage depletion
Anti-CD3 (145-2C11), anti–Mac-1/CD11b (M1/70), anti–CD16/32 (2.4G2), anti–B220/CD45R (RA3/6B2), anti–Gr1/Ly6G (RB6-8C5), and anti–TER-119/Ly76 were used as purified antibodies. Fluorochrome- or biotin-conjugated antibodies (anti-CD2 [RM2-5], anti-CD3, anti-CD4 [GK1.5], anti-CD8 [53-6.7], anti-CD19 [1D3], anti–CD127/IL-7Rα [SB/199], anti-B220, anti–Mac-1, anti–Sca-1, anti–c-Kit, anti-Gr1, anti–VCAM-1, and anti–TER-119) were purchased from BD PharMingen (San Diego, CA). Dead cells were eliminated by staining with 7-amino-actinomycin D (7-AAD; BD Biosciences, Mountain View, CA) in the final step. Phycoerythrin–Texas red tandem-conjugated streptavidin was purchased from Caltag (Burlingame, CA). Lineage depletion was accomplished by incubating marrow cells from femurs and tibias with anti–Mac-1, anti-B220, anti-CD3, anti-Gr1, and anti–TER-119 followed by goat anti–rat IgG magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). All cells were stained with fluorochrome-conjugated antibodies to Sca-1, c-Kit, and a mixture of lineage markers (CD2, CD3, CD8, CD19, B220, Mac-1, Gr1, TER-119). Stained cells were sorted by the Moflo cell sorter (Cytomation, Fort Collins, CO) or FACSAria (Becton Dickinson, San Jose, CA), analyzed with FlowJo software (Tree Star, Ashland, OR).
Stromal cell-free, serum-free culture
Stromal cell-free, serum-free culture of progenitor cells was described previously.22,28 Sorted cells were cultured for 14 days at 2000 cells/well with recombinant mouse stem cell factor (SCF; 20 ng/mL), Flt-3 ligand (FL; 100 ng/mL), IL-7 (2 ng/mL), or IL-6 (2 ng/mL) purchased from R&D Systems (Minneapolis, MN). The medium was changed every 3 days and expanded to 24-well plates for the last 7 days. For single-cell culture, the same culture conditions as described were done, except that single cells were sorted into 96-well plates.
Stromal cell coculture
Sorted cells were cocultured with OP9 stromal cells (2000 cells/well) in 24-well plates in medium containing recombinant cytokines (SCF, 20 ng/mL; FL, 100 ng/mL; IL-7, 2 ng/mL) for 12 days and phenotyped by flow cytometry. As described, cells were sorted into 96-well plates for single-cell culture. The potential contamination of OP9 stroma cells and dead cells was eliminated from the analysis by using biotinylated anti–vascular cell adhesion molecule 1 (anti–VCAM-1) in combination with phycoerythrin–Texas red tandem-conjugated streptavidin and 7-AAD at the same FL3 channel of FACScalibur.
Results
Site of lymphopoiesis block in SHIP–/– mice
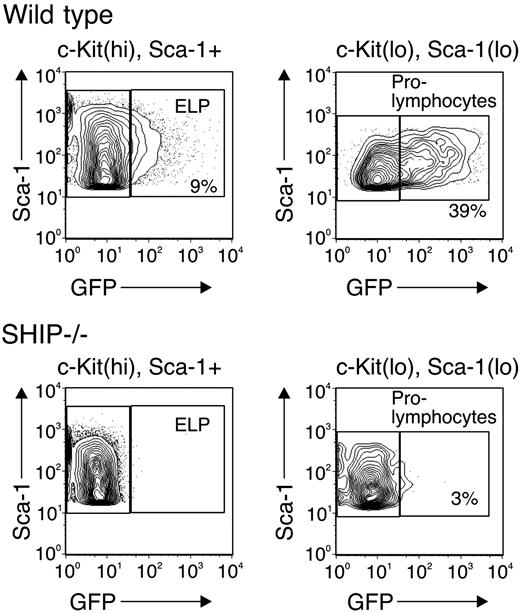
To address the stage of the lymphopoiesis defect of SHIP-deficient mice, we generated SHIP–/–RAG1-GFP knock-in mice by interbreeding. The lymphoid-committed progenitors of the RAG1-GFP knock-in animals coexpress RAG1 and GFP can be identified by the GFP marker. The cells from this animal have been used to identify ELPs present within the Lin–c-KithiSca-1+ fraction that also contains hematopoietic stem cells and myeloid progenitors bearing the same surface markers.26 For these experiments, bone marrow cells from SHIP–/–RAG1-GFP knock-in mice were depleted of lineage-expressing cells and analyzed for levels of c-Kit, Sca-1, and GFP. The results are shown in Figure 1 and are representative of 3 similar experiments. We found that the Lin–c-KithiSca-1+ fraction of wild-type mice contained 9% GFP-expressing cells, corresponding to the ELP fraction. In contrast, we found no detectable ELPs in the same fraction when the cells were derived from SHIP–/– marrow. Similarly, we found a 10-fold decrease in the Lin–c-KitloSca-1loGFP+ subpopulation, corresponding to the prolymphocyte fraction in SHIP-deficient marrow (39% versus 3%). Thus, hematopoietic stem cells in SHIP–/– mice generate myeloid progenitors,22 but fail to produce lymphoid progenitors. These findings indicate that the lymphopoiesis defect in SHIP–/– mice maps to the lineage choice decision made by uncommitted hematopoietic progenitors.
The suppression of lymphopoiesis in SHIP–/– animals is partially overcome in an IL-6–/– background
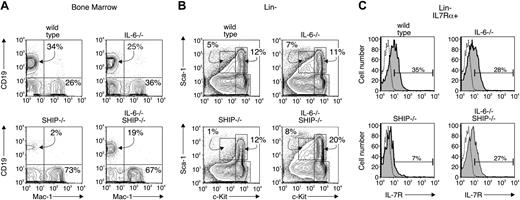
Our earlier report indicated a dominant role for IL-6 in the lymphopoiesis defect of SHIP–/– mice. We measured IL-6 levels in serum of wild-type and SHIP–/– animals and found no detectable amount in the wild-type animals at any age. SHIP–/– mice likewise show no detectable IL-6 at birth, but the level gradually rises to about 2 ng/mL sera by 4 weeks of age (not shown). We hypothesized22 that these high IL-6 levels block lymphopoiesis and elevate myelopoiesis. To test this notion, we crossed SHIP–/– mice with IL-6–/– mice and examined the presence or absence of CD19+ lymphoid- or Mac-1+ myeloid-committed progenitor cells. We found (Figure 2A; Table 1) that bone marrow of wild-type animals displayed 34% CD19+ cells and 26% Mac-1+ cells. The percentages of CD19+ and Mac-1+ cells of IL-6–deficient mice were similar to wild-type, but marrow cells of SHIP–/– had very few CD19+ (2%) and a corresponding increase in Mac-1+ cells (73%). Bone marrow cells of IL-6–/–SHIP–/– mice showed a partial reconstitution of the CD19+ compartment (19%), but no significant change in the levels of Mac-1+ cells (67%). We also examined the relative numbers of progenitor cells by staining for levels of c-Kit and Sca-1. We found (Figure 2B) that the percentage of c-kithi, Sca-1+ progenitor cells, a fraction that contains hematopoietic stem cells, from IL-6–/– and SHIP–/– were essentially identical to wild-type, but the same fraction in IL-6–/–SHIP–/– was increased 2-fold. The prolymphocyte fraction, defined as c-KitloSca-1lo IL7Rα+ (Figure 2C; Table 1), was decreased 10-fold in SHIP-deficient mice. The percentage of the prolymphocyte fraction in the IL-6–/–SHIP–/– was slightly less than wild type (35% versus 27%; Figure 2C), and the total cell number was about 80% to 90% of wild-type (Table 1), indicating this fraction is largely reconstituted in the double knockout mice. These findings are consistent with the notion that the lymphopoiesis defects in SHIP–/– mice are due at least in part to elevated levels of IL-6.
Populations of bone marrow cells or Lin– of wild-type, IL-6–/–, SHIP–/–, and IL-6–/– SHIP–/– mice
. | Wild-type . | IL-6-/- . | SHIP-/- . | SHIP-/- IL-6-/- . |
|---|---|---|---|---|
| Bone marrow cells, × 106 | ||||
| CD19+ | 58 ± 19 | 35 ± 11 | 3 ± 2 | 18 ± 9 |
| Mac-1+ | 56 ± 18 | 51 ± 10 | 78 ± 12 | 78 ± 6 |
| CD3+ | 3 ± 2 | 4 ± 1 | 1 ± 0.5 | 1.5 ± 0.5 |
| Lin-, × 106 | 2 ± 0.5 | 2 ± 0.1 | 2 ± 0.5 | 2 ± 0.4 |
| c-KithiSca-1+, × 104 | 24 ± 10 | 19 ± 3 | 21 ± 2 | 45 ± 4 |
| c-KitloSca-1lo IL7R+, × 104 | 5 ± 1 | 5 ± 1 | 0.3 ± 0.02 | 4 ± 1 |
. | Wild-type . | IL-6-/- . | SHIP-/- . | SHIP-/- IL-6-/- . |
|---|---|---|---|---|
| Bone marrow cells, × 106 | ||||
| CD19+ | 58 ± 19 | 35 ± 11 | 3 ± 2 | 18 ± 9 |
| Mac-1+ | 56 ± 18 | 51 ± 10 | 78 ± 12 | 78 ± 6 |
| CD3+ | 3 ± 2 | 4 ± 1 | 1 ± 0.5 | 1.5 ± 0.5 |
| Lin-, × 106 | 2 ± 0.5 | 2 ± 0.1 | 2 ± 0.5 | 2 ± 0.4 |
| c-KithiSca-1+, × 104 | 24 ± 10 | 19 ± 3 | 21 ± 2 | 45 ± 4 |
| c-KitloSca-1lo IL7R+, × 104 | 5 ± 1 | 5 ± 1 | 0.3 ± 0.02 | 4 ± 1 |
Each population in the total bone marrow cells was analyzed by flow cytometry (see “Materials and methods”). The data were obtained from five 4-week-old mice and are shown as average number per mouse and SE.
Hematopoietic progenitor populations in wild-type and SHIP–/–mice. Bone marrow cells from wild-type (top row) and SHIP–/– (bottom row) mice, both of which were RAG1-GFP knock-in background, were depleted of Lin+ (CD2, CD3, CD8, CD19, B220, Mac-1, Gr1, TER-119) cells. The Lin– fraction was analyzed for c-Kit, Sca-1, and GFP expression. Contaminating Lin+ cells were gated out, and the Lin– population was separated into c-KithiSca-1+ (left column) and c-KitloSca-1lo (right column) for GFP analysis. The number in each panel indicates the percentage of GFP+ cells.
Hematopoietic progenitor populations in wild-type and SHIP–/–mice. Bone marrow cells from wild-type (top row) and SHIP–/– (bottom row) mice, both of which were RAG1-GFP knock-in background, were depleted of Lin+ (CD2, CD3, CD8, CD19, B220, Mac-1, Gr1, TER-119) cells. The Lin– fraction was analyzed for c-Kit, Sca-1, and GFP expression. Contaminating Lin+ cells were gated out, and the Lin– population was separated into c-KithiSca-1+ (left column) and c-KitloSca-1lo (right column) for GFP analysis. The number in each panel indicates the percentage of GFP+ cells.
IL-6 gene-targeting restores lymphopoiesis in SHIP–/–mice. Bone marrow from wild-type, IL-6–/–, SHIP–/–, and IL-6–/– SHIP–/– mice were analyzed by flow cytometry and the results are representative of 5 separate animals. (A) B lymphocytes and myeloid cells from marrow of the various indicated animal strains were detected with anti-CD19 and anti–Mac-1 antibodies, respectively. (B) Bone marrow cells from indicated animals were depleted of Lin+ (CD2, CD3, CD8, CD19, B220, Mac-1, Gr1, TER-119) cells. The Lin– fraction was analyzed for levels of c-kit and Sca-1 expression. The gates for c-kithiSca-1+, c-kithiSca-1–, and c-kitloSca-1lo fraction are shown in each panel. The number indicates the percentage of cells falling within the indicated gate. (C) The Lin– fraction was gated on the fraction positive for reactivity with anti–IL-7Rα antibody. IL-7Rα+ cells are plotted for c-kit and Sca-1 expression. The absolute cell numbers from 5 animals are summarized in Table 1. Dotted line indicates isotype control; shaded curve, IL-7Rα.
IL-6 gene-targeting restores lymphopoiesis in SHIP–/–mice. Bone marrow from wild-type, IL-6–/–, SHIP–/–, and IL-6–/– SHIP–/– mice were analyzed by flow cytometry and the results are representative of 5 separate animals. (A) B lymphocytes and myeloid cells from marrow of the various indicated animal strains were detected with anti-CD19 and anti–Mac-1 antibodies, respectively. (B) Bone marrow cells from indicated animals were depleted of Lin+ (CD2, CD3, CD8, CD19, B220, Mac-1, Gr1, TER-119) cells. The Lin– fraction was analyzed for levels of c-kit and Sca-1 expression. The gates for c-kithiSca-1+, c-kithiSca-1–, and c-kitloSca-1lo fraction are shown in each panel. The number indicates the percentage of cells falling within the indicated gate. (C) The Lin– fraction was gated on the fraction positive for reactivity with anti–IL-7Rα antibody. IL-7Rα+ cells are plotted for c-kit and Sca-1 expression. The absolute cell numbers from 5 animals are summarized in Table 1. Dotted line indicates isotype control; shaded curve, IL-7Rα.
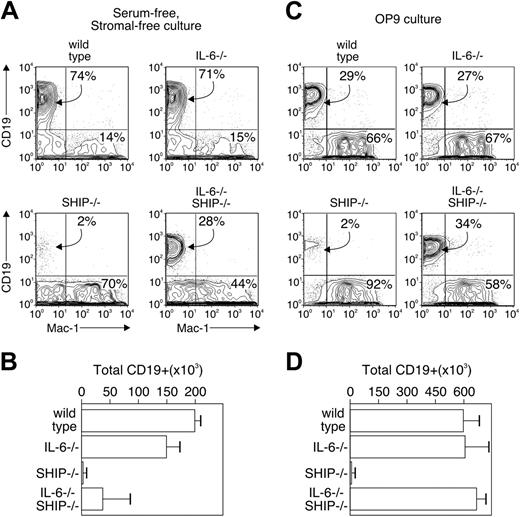
As an additional test of the IL-6 contribution to the lymphopoiesis defect in SHIP–/– mice, we isolated Lin–c-KithiSca-1+, IL-7Rα– progenitors, a fraction that includes functional ELPs from bone marrow of the same animals. The cells were cultured in a serum-free, stromal cell-free system that supports lymphoid development to the prolymphocyte stage,22,28 or with the stromal cell line OP9, derived from op/op mice and supporting in vitro development of hematopoietic stem cells.29 After 2 weeks, the percentages of cells expressing CD19 and Mac-1 were evaluated. We found (Figure 3) that progenitors from wild-type animals produced 74% CD19+ and 14% Mac-1+ cells in the stromal-free condition and 29% and 66%, respectively, in the OP9 coculture. Production of CD19+ and Mac-1+ cells from progenitors of IL-6–deficient mice were similar to wild type in both types of culture, whereas progenitors of SHIP–/– mice failed to make CD19+ cells and showed an elevation in Mac-1+ cells in both culture types (70% and 92% Mac-1+, respectively). Progenitors from double-deficient animals were able to produce CD19+ prolymphocytes (28% in the serum-free and 34% in the OP9 culture), indicating that lymphopoiesis was at least partially restored when SHIP-deficient progenitors are unable to make IL-6. The production of Mac-1+ myeloid cells was correspondingly reduced to 44% and 58%. Together, these findings are consistent with our hypothesis that the spontaneous production of IL-6 by progenitors of SHIP–/– mice blocks lymphopoiesis in the animals and by extension in patients with proinflammatory diseases. However, other lymphopoiesis defects in the SHIP–/– marrow cells could be present, because the total number of CD19+ B lymphocytes in marrow (Table 1), and production of prolymphocytes in vitro (Figure 3D), of the double-knockout animals or cultured cells was still less than that of the wild-type.
Surface phenotype of single-cell cultures of progenitors. c-KithiSca-++ IL-7Rα– fraction containing uncommitted progenitors was isolated from wild-type, IL-6–/–, SHIP–/–, and IL-6–/– SHIP–/– animals. The sorted cells were cultured with serum-free, stromal cell-free condition (A-B) or with OP9 stromal cell (C-D). The results in panels C and D are the average and SD of 2 independent experiments, with each experiment having 3 individual wells. (A-B) The sorted cells were cultured with SCF, IL-7, and FL as previously described.22 The phenotype of colonies that emerged were examined by staining with anti-CD19 and anti–Mac-1 antibodies (A). Total CD19+ cells number after culture is shown in panel B. (C-D) The sorted cells were in the presence of OP9 stromal cells with SCF, IL-7, and FL for 12 days. The phenotype of colonies that emerged were examined by staining with anti-CD19 and anti–Mac-1 antibodies (C). Total CD19+ cell numbers after culture are shown in panel D. Numbers in panels A and C indicate the percentage of cells falling within the indicated gates. Bars in panels B and D represent the average and standard error of 4 replicate samples.
Surface phenotype of single-cell cultures of progenitors. c-KithiSca-++ IL-7Rα– fraction containing uncommitted progenitors was isolated from wild-type, IL-6–/–, SHIP–/–, and IL-6–/– SHIP–/– animals. The sorted cells were cultured with serum-free, stromal cell-free condition (A-B) or with OP9 stromal cell (C-D). The results in panels C and D are the average and SD of 2 independent experiments, with each experiment having 3 individual wells. (A-B) The sorted cells were cultured with SCF, IL-7, and FL as previously described.22 The phenotype of colonies that emerged were examined by staining with anti-CD19 and anti–Mac-1 antibodies (A). Total CD19+ cells number after culture is shown in panel B. (C-D) The sorted cells were in the presence of OP9 stromal cells with SCF, IL-7, and FL for 12 days. The phenotype of colonies that emerged were examined by staining with anti-CD19 and anti–Mac-1 antibodies (C). Total CD19+ cell numbers after culture are shown in panel D. Numbers in panels A and C indicate the percentage of cells falling within the indicated gates. Bars in panels B and D represent the average and standard error of 4 replicate samples.
Uncommitted but not lymphoid-committed hematopoietic progenitors are the direct targets of IL-6
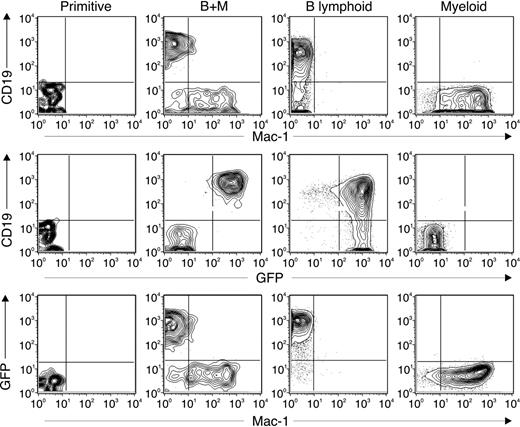
To more rigorously test if IL-6 affects the differentiation of uncommitted progenitors, we used the RAG1-GFP knock-in model and performed single-cell cultures with sorted Lin–GFP–c-Kithi Sca-1+ progenitors. We measured the cloning efficiency and the lineage outcome of cells within individual wells. As described, we applied 2 types of culture systems: the serum-free, stromal-free model that uses recombinant cytokines or the OP9 stromal cell coculture. We added recombinant IL-6 to 2 ng/mL, a level similar to that found in sera of SHIP-deficient mice. The single-cell cultures were maintained for 10 or 14 days and analyzed for CD19, Mac-1, and GFP expression. We found 4 distinct phenotypes resulting from these cultures (Figure 4). Some cultures maintained a primitive phenotype and expressed neither CD19 nor Mac-1 and were GFP–. Some wells contained both myeloid and lymphoid progenitors, marked by Mac-1 and CD19 expression, respectively. The CD19+ cells were GFP+, whereas the Mac-1+ cells were GFP–. Other cultures showed only B-lymphoid or myeloid progenitors and solely expressed CD19 or Mac-1, respectively.
Using these markers, we counted the percentages of wells having the phenotypes of primitive, B lymphoid, myeloid, or mixed B+ myeloid cells. The results are shown in Table 2 and are representative of 2 identical experiments. We found that the cloning efficiency was higher in the OP9 coculture system than the serum-free, stromal-free cultures, probably because OP9 stroma generate additional and unidentified cytokines that better support hematopoiesis in vitro. However, in both types of cultures the addition of IL-6 markedly suppressed the development of the B-lymphoid phenotype and increased the number of myeloid-only cultures. The lymphopoiesis defect of the uncommitted progenitors seen in the stromal-free culture containing IL-6 argues that the IL-6 target is the uncommitted progenitors themselves, rather than a marrow stromal cell. Thus, IL-6 prevents the formation of cells expressing RAG1, the earliest known marker of lymphoid lineage-committed cells,26 from otherwise uncommitted progenitors. We further noted that IL-6 increased the number of cultures with a primitive phenotype, suggesting that IL-6 expands the progenitor population. The data indicate that IL-6 suppresses the lymphoid lineage choice by directly acting on hematopoietic progenitors.
IL-6 alters the lineage decision of hematopoietic stem cells
Culture and genotype . | IL-6 added . | Primitive, % wells . | Myeloid, % wells . | B lymphoid, % wells . | Myeloid + B lymphoid, % wells . | No. positive colonies/no. plated wells . |
|---|---|---|---|---|---|---|
| OP9 culture | ||||||
| Wild-type; RAG1-GFP | No | 6.9 | 31.0 | 20.7 | 41.4 | 29/120 |
| Wild-type; RAG1-GFP | Yes | 15.4 | 76.9 | 2.6 | 5.1 | 39/120 |
| SHIP-/-; RAG1-GFP | No | 4.3 | 52.2 | 17.4 | 26.1 | 23/120 |
| SHIP-/-; RAG1-GFP | Yes | 7.1 | 92.9 | 0.0 | 0.0 | 28/120 |
| Serum-free, stroma-free culture | ||||||
| Wild-type; RAG1-GFP | No | 0 | 20.0 | 20.0 | 40.0 | 5/240* |
| Wild-type; RAG1-GFP | Yes | 5.7 | 94.3 | 0.0 | 0.0 | 35/240 |
| SHIP-/-; RAG1-GFP | No | 22.2 | 77.8 | 0.0 | 0.0 | 18/120 |
| SHIP-/-; RAG1-GFP | Yes | 11.4 | 88.6 | 0.0 | 0.0 | 44/120 |
Culture and genotype . | IL-6 added . | Primitive, % wells . | Myeloid, % wells . | B lymphoid, % wells . | Myeloid + B lymphoid, % wells . | No. positive colonies/no. plated wells . |
|---|---|---|---|---|---|---|
| OP9 culture | ||||||
| Wild-type; RAG1-GFP | No | 6.9 | 31.0 | 20.7 | 41.4 | 29/120 |
| Wild-type; RAG1-GFP | Yes | 15.4 | 76.9 | 2.6 | 5.1 | 39/120 |
| SHIP-/-; RAG1-GFP | No | 4.3 | 52.2 | 17.4 | 26.1 | 23/120 |
| SHIP-/-; RAG1-GFP | Yes | 7.1 | 92.9 | 0.0 | 0.0 | 28/120 |
| Serum-free, stroma-free culture | ||||||
| Wild-type; RAG1-GFP | No | 0 | 20.0 | 20.0 | 40.0 | 5/240* |
| Wild-type; RAG1-GFP | Yes | 5.7 | 94.3 | 0.0 | 0.0 | 35/240 |
| SHIP-/-; RAG1-GFP | No | 22.2 | 77.8 | 0.0 | 0.0 | 18/120 |
| SHIP-/-; RAG1-GFP | Yes | 11.4 | 88.6 | 0.0 | 0.0 | 44/120 |
Sorted Lin-c-kithiSca-1+GFP- progenitors were cultured with OP9 stromal cells or in serum-free, stromal-free conditions in media containing cytokines (SCF, 20 ng/mL; FL, 100 ng/mL; IL-7, 2 ng/mL). IL-6 was added to 2 ng/mL as indicated. After 10 days (OP9 cultures) or 14 days (cytokine cultures), the individual wells were analyzed for CD19, Mac-1, and GFP by flow cytometry. The identity of the clone (primitive, myeloid, B lymphoid, or mixed) was determined as shown in Figure 4. Shown are the percentage of wells having the indicated phenotype. The experiment is representative of 2 identical trials.
One of the 5 colonies had a phenotype that could not be determined.
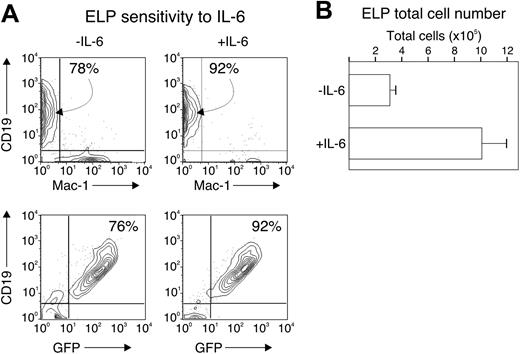
An additional mechanistic possibility is that IL-6 prevents the survival and expansion of committed lymphoid progenitors, but not their formation. To test this possibility, we sorted ELP (defined as c-KithiSca-1+GFP+) from wild-type, RAG1-GFP knock-in mice and cultured 2000 cells under conditions described in Table 2 for 14 days and in the presence or absence of IL-6. We found that the ELP progeny maintained their commitment to the lymphoid fate, acquiring CD19 at the end of the culture period (78% and 92%, respectively), and remaining GFP+ (76% and 92%, respectively), regardless of the presence of IL-6 (Figure 5A). The total number of progeny of the ELP culture increased about 300-fold without IL-6 and nearly 1000-fold with IL-6 (Figure 5B), accompanied by an increase in cell viability (not shown).
Surface phenotype of single-cell cultures of uncommitted progenitors. Lin–c-kithiSca-1+GFP– fraction containing uncommitted progenitors was isolated from RAG1-GFP knock-in background, wild-type, and SHIP–/– mice. The cells were cultured in the presence of the OP9 stromal cell or with SCF, IL-7, and FL as previously described.22,28 IL-6 (2 ng/mL) was added as indicated and the cells were cultured for 10 to 14 days. The phenotype of colonies that emerged were examined by staining with anti-CD19 and anti–Mac-1 antibodies and by GFP expression. The profiles shown were used to define the phenotype of colonies summarized in Table 2.
Surface phenotype of single-cell cultures of uncommitted progenitors. Lin–c-kithiSca-1+GFP– fraction containing uncommitted progenitors was isolated from RAG1-GFP knock-in background, wild-type, and SHIP–/– mice. The cells were cultured in the presence of the OP9 stromal cell or with SCF, IL-7, and FL as previously described.22,28 IL-6 (2 ng/mL) was added as indicated and the cells were cultured for 10 to 14 days. The phenotype of colonies that emerged were examined by staining with anti-CD19 and anti–Mac-1 antibodies and by GFP expression. The profiles shown were used to define the phenotype of colonies summarized in Table 2.
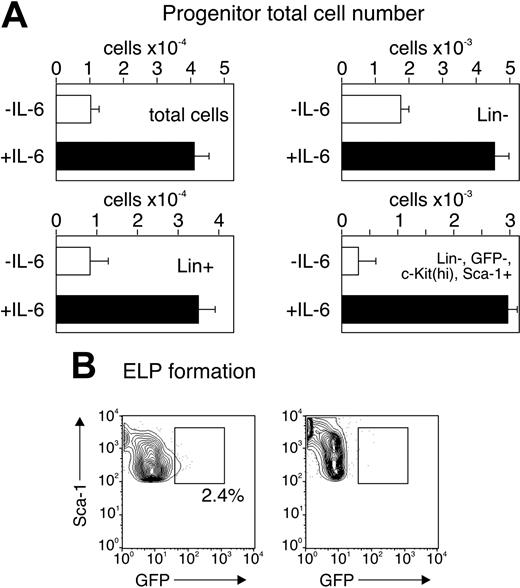
We also tested the effect of IL-6 on progenitor cell survival and expansion. For these experiments, we measured the total cells derived from 3-day bulk cultures of 5000 sorted (Lin–c-kithi Sca-1+GFP–) uncommitted progenitors in the serum-free, stromal-free conditions and in the presence or absence of IL-6. Progenitors do not express demonstrable lineage-assigned surface markers in this time period28,30 but can express GFP. Thus, results at days 2 to 3 reflect the development programs of the input populations. We found (Figure 6A) that all subpopulations expanded with IL-6, but especially the Lin–GFP–c-KithiSca-1+ cells, the heterogeneous population that contains hematopoietic stem cells. Secondly, we measured expression of GFP in uncommitted progenitors cultured for only 3 days in cytokines, with or without IL-6. We found (Figure 6B) that a small fraction of the cells became GFP+ within 3 days without IL-6, but we found no detectable GFP expression in cells that were cultured with IL-6. These findings indicate that, at least in vitro, IL-6 does not block survival of lymphoid-committed or uncommitted progenitors, but actually promotes their expansion. The data are consistent with our interpretation that the stage of the lymphopoiesis suppression is very early and prior to the development of ELPs. Together, the data suggest that the effect of IL-6 is to expand proliferation and survival of uncommitted progenitors as well as suppress their ability to choose a lymphoid lineage fate.
IL-6 does not alter ELP commitment. Two thousand cells from the c-kithiSca-1+GFP+ fraction of wild-type, RAG1-GFP animals were cultured in serum-free, stroma cell-free conditions with or without 2 ng/mL IL-6 for 14 days. The progeny were analyzed for CD19 and Mac-1 expression by flow cytometry (A) and counted for total cell number (B). Numbers in panel A indicate the percentage of cells falling within the indicated gate. Bars in panel B represent the average and standard error of duplicate wells from 2 separate experiments.
IL-6 does not alter ELP commitment. Two thousand cells from the c-kithiSca-1+GFP+ fraction of wild-type, RAG1-GFP animals were cultured in serum-free, stroma cell-free conditions with or without 2 ng/mL IL-6 for 14 days. The progeny were analyzed for CD19 and Mac-1 expression by flow cytometry (A) and counted for total cell number (B). Numbers in panel A indicate the percentage of cells falling within the indicated gate. Bars in panel B represent the average and standard error of duplicate wells from 2 separate experiments.
Discussion
Our results show that the lymphopoiesis defect in SHIP-deficient mice maps to the point at which hematopoietic stem cells make a lineage decision, to either the myeloid or lymphoid fates. The lymphopoiesis defect could be reproduced with recombinant IL-6 and was at least partially overcome in IL-6–/– mice. We further showed that the IL-6 target population are within the Lin–GFP– c-KithiSca-1+ progenitor population that contains hematopoietic stem cells. Lymphoid-committed ELPs maintained their lymphoid commitment and were able to survive in the presence of IL-6. In fact, the ELP fraction displayed an elevation in proliferation and in viability in the presence of IL-6. Single-cell cultures in a serum-free, stromal-free environment of the marrow fraction containing hematopoietic stem cells showed suppression of the lymphoid option, further indicating the target of IL-6 is the uncommitted hematopoietic stem cell.
IL-6 accelerates proliferation of uncommitted progenitors. Five thousand cells from the sorted c-kithiSca-1+GFP– fraction from wild-type, RAG1-GFP animals were cultured as in Figure 5 and total cell numbers were counted at day 2 (A). The cells were analyzed for expression of lineage markers (CD2, CD3, CD8, CD19, B220, Mac-1, Gr1) and were designated as either Lin+ or Lin–. The Lin– fraction was further analyzed for Sca-1 expression and for levels of c-Kit. The cell number after culture is shown as absolute numbers of duplicate wells (± SD) when cultured without (□) or with (▪) IL-6. The results are representative of 2 separate experiments. (B) Sorted c-kithiSca-1+GFP– fraction from wild-type, RAG1-GFP animals was cultured as in panel A for 72 hours, gated on Lin–, c-Kithi, and analyzed for levels of Sca-1 and GFP. The results are representative of 2 separate experiments.
IL-6 accelerates proliferation of uncommitted progenitors. Five thousand cells from the sorted c-kithiSca-1+GFP– fraction from wild-type, RAG1-GFP animals were cultured as in Figure 5 and total cell numbers were counted at day 2 (A). The cells were analyzed for expression of lineage markers (CD2, CD3, CD8, CD19, B220, Mac-1, Gr1) and were designated as either Lin+ or Lin–. The Lin– fraction was further analyzed for Sca-1 expression and for levels of c-Kit. The cell number after culture is shown as absolute numbers of duplicate wells (± SD) when cultured without (□) or with (▪) IL-6. The results are representative of 2 separate experiments. (B) Sorted c-kithiSca-1+GFP– fraction from wild-type, RAG1-GFP animals was cultured as in panel A for 72 hours, gated on Lin–, c-Kithi, and analyzed for levels of Sca-1 and GFP. The results are representative of 2 separate experiments.
Although the effect of IL-6 on lymphopoiesis is clear, not all the lymphopoiesis defects in SHIP–/– mice appear to be due to IL-6. The Lin–c-KitloSca-1loIL-7R+ prolymphocyte population was completely reconstituted in the SHIP and IL-6 double-knockout mice, but CD19+ cells were only partially restored. The observation suggests that IL-6 targets lymphopoiesis events prior to the formation of IL-7R+ prolymphocytes. However, there appears to be an additional need for SHIP that is independent of IL-6 and is beyond the prolymphocyte stage but prior to differentiation to CD19+ immature B cells. The potential for additional hematopoiesis defects is likely because the development of uncommitted progenitors is driven by cytokine receptors (c-Kit,31 Flt3 receptor,32 and the IL-7R33 ) that use phosphatidylinositol 3-kinase in signal transduction. SHIP regulates events driven by phosphatidylinositol 3-kinase; hence, the loss of SHIP can alter the ability or the extent of progenitors responding to these cytokines in stages beyond ELPs.
The mechanism of action of IL-6 in blocking the lymphoid option of progenitors is still speculative. However, it is apparent from our in vitro results that IL-6 does not promote death of cells that have made a lymphoid lineage commitment. Likewise, IL-6 did not reprogram committed ELPs, but actually increased ELP expansion. Therefore, the influence of IL-6 on lymphopoiesis is not to prevent survival or expansion of lymphoid progenitors and appears rather to suppress the lymphoid option of stem cells. Although the biochemical basis of the suppression requires further study, the suppression may be due to alterations in transcription factor expression, which determine hematopoietic cell fate (for a review, see Hirose et al34 ). The transcription factors are induced by cytokine receptors that are, in turn, influenced by phosphatidylinositol 3-kinase and SHIP.
We found that IL-6 also expands the primitive progenitor population, defined as Lin–Sca-1+c-Kithi, as described earlier.35 Nevertheless, IL-6 is not absolutely required for hematopoiesis because it occurs in IL-6–deficient mice, albeit with decreased numbers of progenitors.36 Animals having both IL-6 and soluble IL-6 receptors as transgenes show elevated numbers of hematopoietic progenitors, defined as Lin–Sca-1+c-Kit+, in liver and spleen.37 IL-6 was reported to act directly on hematopoietic stem cells to induce their expansion,38,39 which would suggest that IL-6 receptors are expressed on hematopoietic stem cells. To our knowledge, this issue has not been directly addressed, although the most primitive progenitors from human cord blood were shown to express the IL-6 receptor.40 Likewise, the IL-6 receptor appeared in expression arrays of nonhuman primate bone marrow progenitors.41 These data are consistent with our finding that IL-6 acts directly on the primitive precursors (Table 2).
The most simple explanation of these findings is that IL-6 blocks lymphopoiesis by 2 mechanisms. First, IL-6 expands the proliferation and survival of uncommitted progenitors and helps maintain them in a primitive state. This conclusion is evident from the data in Table 2, showing an increase in the number of cells having a primitive phenotype when in the presence of IL-6. It is also apparent from the data in Figure 6, showing an increase in the number of Lin–GFP–c-KithiSca-1+ population that contains hematopoietic stem cells. Second, IL-6 closes the lymphoid option of the expanding uncommitted progenitors. This notion is evident from the phenotype of adult SHIP–/– mice, which have high circulating IL-6 levels, and from the data in Table 2, which shows that in the presence of IL-6 the number of clones with a lymphoid phenotype decreased to nil. We propose that, with the lymphoid option closed, the expanding stem cells are forced to take the myeloid option, thereby leading to an elevation in myelopoiesis.
Similar alterations in hematopoiesis occur in clinical situations in which IL-6 is elevated, such as autoimmune diseases, acute infection, or in sepsis. IL-6 is not required for the terminal differentiation of any hematopoietic cell type and thus IL-6–deficient animals have a normal compliment of hematopoietic cells. Nevertheless, such animals are not subject to experimentally induced arthritis9,10 or certain features of sepsis.42 Our results are the first to link elevations in IL-6 to lymphopenia. Furthermore, our findings show how a cytokine can discriminate primitive hematopoietic cells present at the critical threshold of the lymphoid/myeloid lineage decision.
Prepublished online as Blood First Edition Paper, April 14, 2005; DOI 10.1182/blood-2005-02-0456.
Supported by National Institutes of Health grants AI49264 and CA64268.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal