Abstract
Constitutively activated forms of the transmembrane receptor tyrosine kinase c-KIT have been associated with systemic mast cell disease, acute myeloid leukemia, and gastrointestinal stromal tumors. Reports of the resistance of the kinase domain mutation D816V to the adenosine triphosphate (ATP)-competitive kinase inhibitor imatinib mesylate prompted us to characterize 14 c-KIT mutations reported in association with human hematologic malignancies for transforming activity in the murine hematopoietic cell line Ba/F3 and for sensitivity to the tyrosine kinase inhibitor PKC412. Ten of 14 c-KIT mutations conferred interleukin 3 (IL-3)-independent growth. c-KIT D816Y and D816V transformed cells were sensitive to PKC412 despite resistance to imatinib mesylate. In these cells, PKC412, but not imatinib mesylate, inhibited autophosphorylation of c-KIT and activation of downstream effectors signal transducer and transcriptional activator 5 (Stat5) and Stat3. Variable sensitivities to PKC412 or imatinib mesylate were observed among other mutants. These findings suggest that PKC412 may be a useful therapeutic agent for c-KIT-positive malignancies harboring the imatinib mesylate-resistant D816V or D816Y activation mutations. (Blood. 2005;106:721-724)
Introduction
c-KIT is a member of the type 3 subclass of transmembrane receptor tyrosine kinases, characterized by 5 immunoglobulin-like domains in the extracellular region, a negative regulatory juxtamembrane (JM) domain and a split adenosine triphosphate-binding and phosphotransferase tyrosine kinase domain (reviewed in Broudy1 ). c-KIT activation by stem cell factor (SCF) promotes dimerization and transphosphorylation at tyrosine residues, resulting in downstream signaling events leading to cell growth. Mutations of c-KIT have been associated with hematopoietic and nonhematopoietic tumors, including systemic mast cell disease (SMCD),2-4 acute myeloid leukemia (AML),5-8 and gastrointestinal stromal tumors (GISTs).9-12
The tyrosine kinase inhibitor imatinib mesylate (Gleevec; Novartis Pharma AG, Basel, Switzerland) is efficacious in the majority of patients with GIST harboring c-KIT mutations.9,13 c-KIT is most commonly activated in GIST tumors by small deletions in the JM that are thought to constitutively activate the tyrosine kinase by loss of autoinhibitory function.14 All c-KIT JM deletion mutants tested thus far are sensitive to inhibition by imatinib mesylate. These observations have suggested that imatinib mesylate might be of value in the treatment of SMCD or AML that also have activating mutations in c-KIT. However, the most common activating alleles in the context of SMCD and AML are the D816V and D816Y mutations in the c-KIT activation loop, and these mutations have been shown to be highly resistant to imatinib mesylate in vivo15 and in vitro.16-19
PKC412 is a novel staurosporine-derived tyrosine kinase inhibitor that targets protein kinase C (PKC), kinase insert domain-containing receptor (KDR), vascular endothelial growth factor receptor-2 (VEGF-R2), platelet-derived growth factor receptor α (PDGFRα), Fms-like tyrosine kinase 3 (FLT3), and c-KIT.20 PKC412 has previously been shown to be effective against the fusion protein Fip1-like 1 (FIP1L1)-PDGFRα with mutations in the kinase domain that are resistant to imatinib mesylate.21 Since inhibitors of D816V/Y c-KIT mutations would potentially have therapeutic activity in SMCD and AML, we have evaluated the effectiveness of PKC412 against a panel of c-KIT mutations identified in patients with SMCD, GIST, and AML.
Study design
Cell culture
A 2.9-kB XhoI cDNA fragment encoding human (hu)-c-KIT (lacking amino acids 510-513, GNNK) was cloned into the XhoI site of pCDNA3. c-KIT point mutations (Table 1) were generated (Transformer Site-Directed Mutagenesis Kit; Clontech, Palo Alto, CA) and subcloned into the XhoI site of MSCV-IRES-GFP and MSCV-PGK-NEO. The IL-3-dependent mouse pre-B-cell line Ba/F3 was maintained, and retrovirus was prepared, as previously described.22 Ba/F3 cells were transduced with viral supernatant, expanded for 2 passages (with 10 ng/mL rmIL-3), then selected for IL-3-independent growth. Ba/F3 cells transduced with Neo-containing vectors were first selected in IL-3 with 100 mg/mL G418. IL-3-independent Ba/F3 cells transduced with wild-type (WT) c-KIT were selected with 20 ng/mL rhSCF.
Imatinib mesylate and PKC412 IC50s of transforming c-KIT mutations
Cell line/c-KIT mutation . | Reference . | Location* . | Transforming . | Imatinib mesylate IC50, nM† . | PKC412 IC50, nM†‡ . |
|---|---|---|---|---|---|
| Ba/F3 | — | — | — | 7 274 | 304 |
| kit WT and rhSCF, without IL-3 | — | NA | — | 237 | 138 |
| kit WT and rhSCF, with IL-3 | — | NA | — | 24 360 | 345 |
| delTYD(417-419) and 1 | Gari et al, 19998 | EC | Yes | 226 | 217 |
| delTYD(417-419) and RG | Gari et al, 19998 | EC | Yes | 45 | 95 |
| A502Y503dup | Lux et al, 200010 | EC | Yes | 196 | 206 |
| V5301 | Gari et al, 19998 | TM | No | ND§ | ND§ |
| V559D | Hirota et al, 199811 | JM | Yes | 11 | 265 |
| V560G | Furitsu et al, 19932 | JM | Yes | 53 | 82 |
| delV559V560 | Hirota et al, 199811 | JM | Yes | 15 | 146 |
| R634W | Current study | K | Yes | 198 | 85 |
| K642E | Lux et al, 200010 | K | No | ND§ | ND§ |
| D816Y | Tsujimura et al. 19953 | K | Yes | 840 | 33 |
| D816V | Furitsu et al, 19932 | K | Yes | 10 651 | 44 |
| D820G | Pignon et al, 19974 | K | No | ND§ | ND§ |
| N822K | Beghini et al, 20027 | K | Yes | 139 | 59 |
| E839K | Longley et al, 199912 | K | No | ND§ | ND§ |
Cell line/c-KIT mutation . | Reference . | Location* . | Transforming . | Imatinib mesylate IC50, nM† . | PKC412 IC50, nM†‡ . |
|---|---|---|---|---|---|
| Ba/F3 | — | — | — | 7 274 | 304 |
| kit WT and rhSCF, without IL-3 | — | NA | — | 237 | 138 |
| kit WT and rhSCF, with IL-3 | — | NA | — | 24 360 | 345 |
| delTYD(417-419) and 1 | Gari et al, 19998 | EC | Yes | 226 | 217 |
| delTYD(417-419) and RG | Gari et al, 19998 | EC | Yes | 45 | 95 |
| A502Y503dup | Lux et al, 200010 | EC | Yes | 196 | 206 |
| V5301 | Gari et al, 19998 | TM | No | ND§ | ND§ |
| V559D | Hirota et al, 199811 | JM | Yes | 11 | 265 |
| V560G | Furitsu et al, 19932 | JM | Yes | 53 | 82 |
| delV559V560 | Hirota et al, 199811 | JM | Yes | 15 | 146 |
| R634W | Current study | K | Yes | 198 | 85 |
| K642E | Lux et al, 200010 | K | No | ND§ | ND§ |
| D816Y | Tsujimura et al. 19953 | K | Yes | 840 | 33 |
| D816V | Furitsu et al, 19932 | K | Yes | 10 651 | 44 |
| D820G | Pignon et al, 19974 | K | No | ND§ | ND§ |
| N822K | Beghini et al, 20027 | K | Yes | 139 | 59 |
| E839K | Longley et al, 199912 | K | No | ND§ | ND§ |
IC50 indicates inhibitory concentration 50%; WT, wild type; rhSCF, recombinant human stem cell factor; IL-3, interleukin 3; NA, not applicable; EC, extracellular domain; TM, transmembrane domain; ND, not determined; JM; juxtamembrane, K, kinase domain.
Domain of c-KIT containing mutation.
Data are representative of 2 or more experiments performed in triplicate.
L-3 rescued PKC412 growth inhibition (data not shown).
The indicated mutants did not transform Ba/F3 cells to factor independence. Thus, we were unable to test the ability of kinase inhibitors to inhibit growth in the absence of IL-3.
Patient samples were collected following informed consent in accordance with the institutional review board (IRB)-approved Dana-Farber Cancer Institute protocol 01-206.
Growth inhibition
Cells (1 × 104) in 100 μL medium with or without IL-3 were incubated with various concentrations of imatinib mesylate or PKC412 in 96-well plates for 48 hours in triplicate. 3H-thymidine (1 μCi [0.037 MBq]) was added, and cells were incubated for 4 hours. Cells were harvested, and 3H-thymidine incorporation was determined. Growth inhibition was plotted as the ratio of the average 3H-thymidine incorporation in drug-treated wells relative to no-drug controls. IC50s for imatinib mesylate or PKC412 were calculated by sigmoidal curve fitting software (OriginLab, Northampton, MA).
Immunoprecipitations
Cell lysates, immunoprecipitations, and immunoblotting were performed as previously described.22 c-KIT was precipitated with 5 μg anti-human c-KIT antibody (Assay Design Systems, Ann Arbor, MI), separated on a 7.5% denaturing polyacrylamide gel and transferred to nitrocellulose. Blots were probed with mouse anti-phosphotyrosine antibody 4G10 (Upstate, Charlottesville, VA), stripped, and reprobed with mouse anti-human c-KIT (E1): SC-17806 (Santa Cruz Biotechnology, Santa Cruz, CA). Whole-cell lysates (1/10th volume immunoprecipitate [IP] input) were similarly separated and probed with rabbit anti-phospho-Stat5 (signal transducer and transcriptional activator 5) or rabbit anti-phospho-Stat3, stripped, and reprobed with rabbit anti-Stat5a or rabbit anti-Stat3 (Cell Signaling Technology, Beverly, MA).
To assess constitutive tyrosine kinase autophosphorylation, cells were grown in RPMI plus 10% fetal calf serum (FCS), and 4 mg cell lysate was immunoprecipitated. To assess PKC412 inhibition of c-KIT autophosphorylation, cells were washed 3 times in RPMI without FCS or IL-3, then incubated with or without IL-3 and PKC412 (0-500 nM) for 4 hours. WT Ba/F3 cells were starved of IL-3 and serum for 4 hours prior to addition of IL-3 and PKC412. Cell equivalents (1 × 107) of cell lysate were immunoprecipitated.
Results and discussion
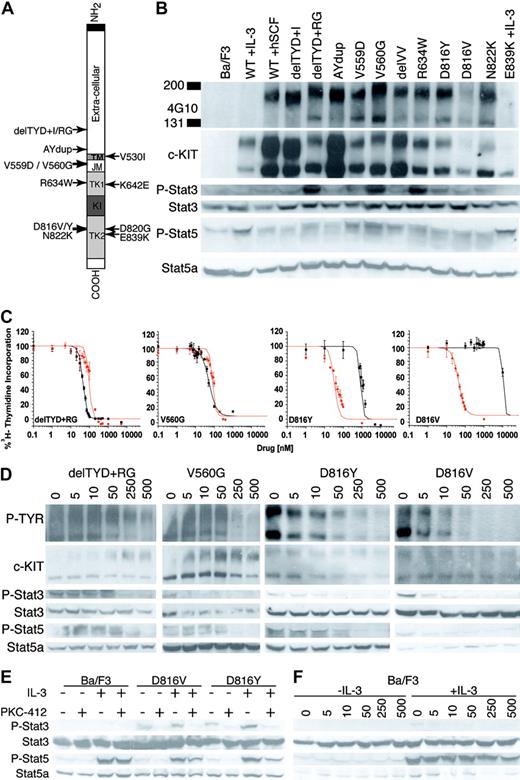
The IL-3-dependent murine pre-B-cell line Ba/F3 was transduced with retroviruses expressing WT c-KIT or 1 of 14 c-KIT mutants and selected for IL-3-independent growth (Table 1; Figure 1A). Ten of 14 mutant forms of c-KIT transformed Ba/F3 cells to IL-3-independent growth in the absence of rhSCF. Transforming mutations mapped to the extracellular domain (delTYD (417-419) + I,8 delTYD (417-419) + RG,8 and A502Y503dup (AYdup)10 ), the juxtamembrane domain (V559D,11 V560G,2 and delV559V560 (delVV)11 ), and the kinase domain (R634W, D816Y,3 D816V,2 and N822K7 ). This is the first report of the kinase domain mutation R634W, identified from a patient with chronic myelomonocytic leukemia. Consistent with a report that E839K inactivates the kinase domain,12 E839K did not transform Ba/F3 cells to IL-3-independent growth. K642E has been shown to be constitutively phosphorylated in a GIST-derived cell line10,23 but did not transform Ba/F3 cells in this study. D820G4 and V530I,8 each identified in a single patient, also did not transform Ba/F3 cells.
Immunoprecipitated c-KIT was constitutively phosphorylated in all transformed cell lines and Ba/F3 cells expressing WT c-KIT grown with 20 ng/mL rhSCF (Figure 1B). We observed differential phosphorylation of 2 c-KIT bands of approximately 160 and 145 kDa, representing the fully glycosylated cell surface receptor, and incompletely processed internalized forms of c-KIT, respectively. Our findings confirm reports that Stat3 is aberrantly phosphorylated by D816V and other c-KIT-activating mutations.24,25 Stat5 was constitutively phosphorylated by all c-KIT-activating mutations grown in the absence of IL-3.
Imatinib mesylate resistant c-Kit mutations are sensitive to PKC412. (A) Schematic of the c-KIT protein indicating the relative location of structural and functional domains, as well as location of 14 c-KIT mutations evaluated. The JM delVV mutation occurs at V559V560. ▪ indicates amino terminal signal peptide. Other domains of c-KIT are indicated as follows: □, extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain; TK1, nucleotide binding subdomain of split kinase domain; KI, kinase insert domain; and TK2, catalytic subdomain of split kinase domain. (B) Ba/F3-transforming c-KIT mutations are constitutively phosphorylated and phosphorylate Stat3 and Stat5. Blots from top to bottom: antiphosphotyrosine Western blot of Ba/F3 lysates immunoprecipitated with anti-c-KIT; anti-c-KIT Western blot of immunoprecipitates in top blot; anti-phospho-Stat3 Western blot of whole-cell lysates (WCLs) from IP in top blot; anti-Stat3 Western blot of same WCLs; anti-phospho-Stat5 Western blot of WCLs from IP in top blot; and anti-Stat5a Western blot of the same WCLs. (C) Representative growth curves of delTYD + RG, V560G, D816Y, and D816V transduced Ba/F3 cell lines. Plotted is the percentage ± SD of 3H-thymidine incorporation of drug-treated cells relative to no-drug controls. Cells were treated with imatinib mesylate (black) or PKC412 (red). Cells were grown in the absence of IL-3 or rhSCF. (D) Dose response of PKC412 inhibition of c-KIT, Stat5, and Stat3 phosphorylation. Shown are data from delTYD + RG, V560G, D816Y, and D816V transformed Ba/F3 cells. Blots are as in panel B. Cells were grown in the absence of IL-3 and the indicated concentration of PKC412 (nM) for 4 hours. (E) PKC412 does not inhibit IL-3-induced Stat5 phosphorylation. Blots from top to bottom: anti-phospho-Stat3 Western blot of WCLs from indicated cell lines with or without IL-3 (10 ng/mL) and PKC412 (250 nM/mL), for which Ba/F3 cells were starved of IL-3 and serum for 4 hours prior to addition of IL-3 and PKC412; anti-Stat3 Western blot of WCLs in top blot; anti-phospho-Stat5 Western blot of WCLs as in top blot; and anti-Stat5a Western blot of same WCLs. (F) Dose response of Stat5 phosphorylation to PKC412 in Ba/F3 cells. Blots are as for panel E. Ba/F3 cells were starved of IL-3 and FCS for 4 hours, then incubated with or without IL-3 (10 ng/mL) in the presence of the indicated concentration of PKC412 for 4 hours.
Imatinib mesylate resistant c-Kit mutations are sensitive to PKC412. (A) Schematic of the c-KIT protein indicating the relative location of structural and functional domains, as well as location of 14 c-KIT mutations evaluated. The JM delVV mutation occurs at V559V560. ▪ indicates amino terminal signal peptide. Other domains of c-KIT are indicated as follows: □, extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain; TK1, nucleotide binding subdomain of split kinase domain; KI, kinase insert domain; and TK2, catalytic subdomain of split kinase domain. (B) Ba/F3-transforming c-KIT mutations are constitutively phosphorylated and phosphorylate Stat3 and Stat5. Blots from top to bottom: antiphosphotyrosine Western blot of Ba/F3 lysates immunoprecipitated with anti-c-KIT; anti-c-KIT Western blot of immunoprecipitates in top blot; anti-phospho-Stat3 Western blot of whole-cell lysates (WCLs) from IP in top blot; anti-Stat3 Western blot of same WCLs; anti-phospho-Stat5 Western blot of WCLs from IP in top blot; and anti-Stat5a Western blot of the same WCLs. (C) Representative growth curves of delTYD + RG, V560G, D816Y, and D816V transduced Ba/F3 cell lines. Plotted is the percentage ± SD of 3H-thymidine incorporation of drug-treated cells relative to no-drug controls. Cells were treated with imatinib mesylate (black) or PKC412 (red). Cells were grown in the absence of IL-3 or rhSCF. (D) Dose response of PKC412 inhibition of c-KIT, Stat5, and Stat3 phosphorylation. Shown are data from delTYD + RG, V560G, D816Y, and D816V transformed Ba/F3 cells. Blots are as in panel B. Cells were grown in the absence of IL-3 and the indicated concentration of PKC412 (nM) for 4 hours. (E) PKC412 does not inhibit IL-3-induced Stat5 phosphorylation. Blots from top to bottom: anti-phospho-Stat3 Western blot of WCLs from indicated cell lines with or without IL-3 (10 ng/mL) and PKC412 (250 nM/mL), for which Ba/F3 cells were starved of IL-3 and serum for 4 hours prior to addition of IL-3 and PKC412; anti-Stat3 Western blot of WCLs in top blot; anti-phospho-Stat5 Western blot of WCLs as in top blot; and anti-Stat5a Western blot of same WCLs. (F) Dose response of Stat5 phosphorylation to PKC412 in Ba/F3 cells. Blots are as for panel E. Ba/F3 cells were starved of IL-3 and FCS for 4 hours, then incubated with or without IL-3 (10 ng/mL) in the presence of the indicated concentration of PKC412 for 4 hours.
The effect of imatinib mesylate or PKC412 on cell growth of c-KIT-expressing cell lines was evaluated in a 3H-thymidine incorporation assay (Table 1; Figure 1C; data not shown). Consistent with previous reports, D816V was resistant to imatinib mesylate (IC50, > 10 000 nM).16-19 D816Y was also observed to be resistant to imatinib mesylate (IC50, > 800 nM). Four mutations, delTYD + I, AYdup, R634W, and N822K, showed IC50s for imatinib mesylate similar to Ba/F3-WT-c-KIT + 20 ng/mL rhSCF (100-300 nM). As previously reported, V560G19 was hypersensitive to imatinib mesylate, as were the mutations delTYD + RG, V559D, and delVV (IC50s < 100 nM).
PKC412 inhibited growth of all c-KIT-transformed Ba/F3 lines, with IC50s segregating into 2 groups. The imatinib mesylate-resistant D816V and D816Y mutants, as well as delTYD + RG, V560G, R634W, and N822K, were more sensitive to PKC412 (IC50s, 33-95 nM) than was the wild-type receptor stimulated with rhSCF (IC50, 138 nM). The remaining mutations had higher IC50s for PKC412 (146-265 nM).
The level of tyrosine autophosphorylation of the imatinib mesylate-resistant D816V and D816Y c-KIT mutants, as well as phosphorylation of Stat3 and Stat5, was dose dependent on PKC412 over a wide concentration range (5-500nM) and correlated with inhibition of cell growth (Table 1; Figure 1D). Similar findings were observed for representative imatinib mesylate-sensitive extracellular (del-TYD + RG) and JM (V560G) mutants. Although Stat5 phosphorylation by D816V and D816Y was inhibited by PKC412 (Figure 1D-E), an increased level of Stat5 phosphorylation induced by stimulation with IL-3 induction was not inhibited in these cells (Figure 1E). IL-3 did not rescue PKC412 inhibition of phosphorylation of Stat3 (Figure 1E) and did not inhibit IL-3-induced Stat5 phosphorylation in WT Ba/F3 Cells (Figure 1E-F).
These data indicate that among the reported c-KIT mutations in human hematologic malignancies, 10 of 14 confer factor independent growth to Ba/F3 cells. There is variable sensitivity of these mutations to inhibition by imatinib mesylate or PKC412. Notably, however, the most common mutations D816V and D816Y are highly resistant to imatinib mesylate but sensitive to inhibition by PKC412. These findings suggest that PKC412 may be an effective therapeutic agent for treatment of patients with certain imatinib mesylate-resistant c-Kit mutations.
Prepublished online as Blood First Edition Paper, March 24, 2005; DOI 10.1182/blood-2004-12-4617.
Supported in part by Postdoctoral Fellowship Grant from the American Cancer Society (PF-02-133-01-LIB) (J. D. Growney), by the National Institutes of Health (grants DK50564, CA-66996), and by the Leukemia and Lymphoma Society (D.G.G. and J. D. Griffin).
One of the authors (J. D. Griffin) has declared a financial interest in Novartis Pharma AG, whose potential product, PKC412, was studied in the present work. One of the authors (D.F.) is employed by a company (Norvartis Pharma AG) whose potential product was studied in the present work.
J. D. Growney performed all of the molecular cloning, growth inhibition, and signaling experiments; analyzed the data; and wrote the manuscript. J.J.C. sequenced the patient samples that identified the novel mutation. J.A. provided technical assistance with cell culture and Western blotting. R.S. was the treating physician for the patient with the novel mutation and participated in discussions of experimental design and interpretation of data. D.F. provided the pharmacologic reagents for this study and contributed to writing of the manuscript and experimental design. J. D. Griffin contributed to writing the manuscript and discussions of experimental design. D.G.G. participated in the design and analysis of the experiments and writing of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
D.G.G is an investigator of the Howard Hughes Medical Institute.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal