Abstract
In this study we report that R-etodolac (SDX-101), at clinically relevant concentrations, induces potent cytotoxicity in drug-sensitive multiple myeloma (MM) cell lines, as well as in dexamethasone (MM.1R)-, doxorubicin (Dox40/RPMI8226)-, and bortezomib (DHL4)-resistant cell lines. Immunoblot analysis demonstrates that R-etodolac induces apoptosis characterized by caspase-8, -9, and -3 and PARP (poly-ADP [adenosine diphosphate]-ribose polymerase) cleavage and down-regulation of cyclin D1 expression. Subcytotoxic doses of R-etodolac up-regulate myeloid cell leukemia-1 proapoptotic variant (Mcl-1S), while enhancing dexamethasone (Dex)-induced caspase activation and apoptosis. The combination of R-etodolac with Dex results in a highly synergistic cytotoxic effect. R-etodolac also induces apoptosis against primary cells isolated from patients with MM refractory to chemotherapy. Although interleukin 6 (IL-6) and insulin-like growth factor-1 (IGF-1) abrogate Dex-induced MM cell cytotoxicity, neither IL-6 nor IGF-1 protects against R-etodolac-induced cytotoxicity in MM cells. R-etodolac also inhibits viability of MM cells adherent to bone marrow stromal cells (BMSCs), thereby overcoming a mechanism of drug resistance commonly observed with other conventional chemotherapeutic agents. Our data, therefore, indicate that R-etodolac circumvents drug resistance in MM cells at clinically relevant concentrations, targets Mcl-1, and can be synergistically combined with Dex. (Blood. 2005;106:706-712)
Introduction
Multiple myeloma (MM) remains an incurable plasma cell malignancy, despite treatment with alkylating agents, anthracyclines, corticosteroids,1,2 as well as high-dose chemotherapy combined with stem cell transplantation,3-5 because of, at least in part, both intrinsic and acquired drug resistance.6-8 Although initial treatment with conventional drugs such as dexamethasone (Dex) effectively induces death in MM cells, prolonged drug exposure results in the development of drug resistance. Furthermore, the bone marrow (BM) microenvironment confers drug resistance in MM cells via at least 2 different mechanisms: (1) cell adhesion-mediated drug resistance (CAM-DR) through adhesion of MM cells to fibronectin found on BM stromal cells (BMSCs) and (2) activation of phosphatidylinositol 3-kinase (PI3-K)/a PI3-K target (Akt) and/or Janus kinase 2 (JAK2)/signal transducers and activators of transcription 3 (STAT3) signaling because of high levels of cytokines found in the BM milieu (such as interleukin-6 [IL-6] and insulin-like growth factor-1 [IGF-1]).9-12 Rationally designed treatments targeting the BM microenvironment, as well as the MM cell, can overcome drug resistance in both preclinical and early clinical studies.13-16
Etodolac (1,8-diethyl-1,3,4,9-tetrahydropyrano-[3,4-b] indole-1-acetic acid) is a nonsteroidal anti-inflammatory drug (NSAID) that inhibits both cyclooxygenase 1 (COX-1) and -2, and is approved for treatment of degenerative joint disease and rheumatoid arthritis.17,18 Etodolac exists as a racemic mixture of the R- and S-enantiomers, and, unlike all other chiral NSAIDs, the 2 enantiomers of etodolac are not metabolically interconvertible. The R-etodolac isoform (SDX-101) lacks significant COX inhibitory activity and, thereby, has a more favorable safety profile than the racemic etodolac because of lack of COX-dependent side effects, such as gastrointestinal toxicity.
R-etodolac was recently demonstrated to inhibit transcription of a β-catenin-dependent T-cell- and lymphoid-enhancing transcription factor (TCF/LEF) receptor gene in HEK293 cells and to diminish the in vitro survival of chronic lymphocytic leukemia (CLL) cells.19 R-etodolac also has in vivo prostate cancer antitumor activity via its direct effect on the peroxisome proliferator-activated receptor-γ (PPAR-γ) and retinoid X receptor-α (RXR-α) pathways.20,21
R-etodolac (SDX-101) is currently being tested (Salmedix, San Diego, CA) in phase 2 clinical trials for treatment of refractory CLL. Reduction in average lymphocyte counts have been observed in patients with CLL administered 1000 to 2400 mg R-etodolac twice daily, at steady-state blood levels of approximately 300 to 600 μM.22
In this report, we analyzed, at clinically achievable drug concentrations, the activity and mechanism of action of R-etodolac in both in vitro and ex vivo MM models. Our results suggest that R-etodolac has proapoptotic activity in MM cells, including drug-resistant lines as well as in primary cells obtained from patients. R-etodolac targets myeloid cell leukemia-1 (Mcl-1), increasing the levels of its proapoptotic variant Mcl-1s and resulting in the functional inhibition of the full-length antiapoptotic variant Mcl-1L. Finally, synergistic cytotoxic results are obtained for R-etodolac in combination with Dex, providing the framework for clinical trials of this novel agent to improve patient outcome in MM.
Materials and methods
Reagents
R-etodolac was provided by Salmedix (San Diego, CA). It was dissolved in dimethyl sulfoxide (DMSO; 250 mM) and stored at -20°C until use, avoiding multiple freeze-thaw cycles. IL-6 and IGF-1 were purchased from R&D Systems (Minneapolis, MN). Pan caspase inhibitor Z-Val-Ala-Asp(oMe)-monofluoroketone (Z-VAD-FMK) (Calbiochem, San Diego, CA) was dissolved in DMSO, stored at -20°C, and used at 25 μM. Dex was used as in our prior studies.23
Cell culture
Dex-sensitive (MM.1S) and resistant (MM.1R) human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). RPMI8226 and U266 human MM cell lines were obtained from American Type Culture Collection (Manassas, VA). Doxorubicin (Dox)-resistant (RPMI-Dox40) cells were kindly provided by Dr William Dalton (Lee Moffitt Cancer Center, Tampa, FL). OPM1 plasma cell leukemia cells24 were kindly provided by Dr Edward Thompson (University of Texas Medical Branch, Galveston). IL-6-dependent human plasma cell line INA-6 was obtained from Dr Martin Gramatzki (University of Erlangen-Nuernberg, Erlangen, Germany).25 Human SUDHL4 (DHL4) lymphoma cells were kindly provided by Dr Margaret Shipp (Dana-Farber Cancer Institute, Boston, MA). Each of these cell lines were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS; Sigma Chemical, St Louis, MO, USA), 2 μM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO, Grand Island, NY), with the exception of INA-6 cells, which were cultured in medium that additionally contained 1 ng/mL IL-6. To evaluate cell viability, cells were mixed with the same volume of 0.4% trypan blue solution and evaluated for dye exclusion using light microscopy, as previously described.26
Primary MM cells and BMSCs from patients with MM
Tumor cells (> 90% CD138+) were purified from the BM of patients with MM using the RosetteSep negative selection system (StemCell Technologies, Vancouver, BC, Canada), as described previously.27 BM mononuclear cells separated by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) were cultured in Dulbecco modified Eagle medium (DMEM; Sigma Chemical) supplemented with 20% heat-inactivated feral bovine serum, 2 μM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin for 3 to 6 weeks to generate BM stromal cells (SCs).28 Approval for these studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki protocol.
Growth inhibition assay
The growth inhibitory effect of R-etodolac or Dex on growth of MM cell lines, peripheral blood mononuclear cells (PBMCs), and BMSCs was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT; Chemicon International, Temecula, CA) dye absorbance, as previously described.27
Immunoblotting
MM cells were cultured with R-etodolac, in the presence or absence of caspase inhibitors; harvested; washed; and lysed using lysis buffer: 50 mM Tris (tris(hydroxymethyl)aminomethane)-HCl (pH 7.4), 150 mM NaCl, 1% NP-40 (Nonidet P-40, [Octylphenoxy]polyethoxyethanol), 10 mM sodium pyrophosphate, 5 mM EDTA (ethylenediaminetetraacetic acid), 1 mM EGTA (ethylene glycol tetraacetic acid), 2 mM Na3VO4, 5 mM NaF, 1 mM PMSF (phenylmethylsulfonyl fluoride), 5 μg/mL leupeptin, and 5 μg/mL aprotinin, as described previously.29 The whole-cell lysates (40 μg per lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) separation, transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA), and immunoblotted with anti-poly-ADP (adenosine diphosphate)-ribose polymerase (PARP), -caspase-8, -caspase-3, -caspase-9, -Bcl-2 (B-cell lymphoma-2)-associated X protein (BAX), and cyclin D1 antibodies (Abs) (Cell Signaling, Beverly, MA), as well as with anti-MCl-1, -Bcl-2, and -α-tubulin Abs (Santa Cruz Biotechnology, Santa Cruz, CA).
Cell-cycle profiling
MM.1S cells were treated with either R-etodolac or DMSO control prior to culture for 24 hours. Cell-cycle profiling was assessed by flow cytometry, as in prior studies.27
Effect of R-etodolac on MM cell growth in the BM
To evaluate the effect of drug on growth of MM cells adherent to BMSCs, MM.1S cells (3 × 104 cells/well) were cultured for 24 hours in BMSC-coated 96-well plates (Costar, Cambridge, MA), in the presence or absence of R-etodolac. DNA synthesis was measured by [3H]-thymidine (Perkin Elmer, Boston, MA) uptake, with [3H]-thymidine (0.5 μCi/well [0.0185 MBq]) added during the last 8 hours of 24-hour cultures. All experiments were performed in quadruplicate.
Statistical analysis
Statistical significance of differences observed in R-etodolac-treated compared with control cultures was determined using a Student t test. The minimal level of significance was P less than .01.
Isobologram analysis
The interaction between R-etodolac and Dex was analyzed using CalcuSyn software program (Biosoft, Ferguson, MO) to determine whether the combination was additive or synergistic, as described previously,30,31 This program is based on the Chou-Talalay method, which calculates a combination index (CI). Analysis is performed according to the following equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2 + (D)1(D)2/(Dx)1(Dx)2, where (D)1 and (D)2 are the doses of drug 1 and drug 2 that have x effect when used in combination; whereas (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have the same x effect when used alone. When the CI approximates 1, this represents the conservation isobologram and indicates additive effects. CI approximately less than 0.7 indicates synergism.
Results
R-etodolac induces cytotoxicity in MM cell lines
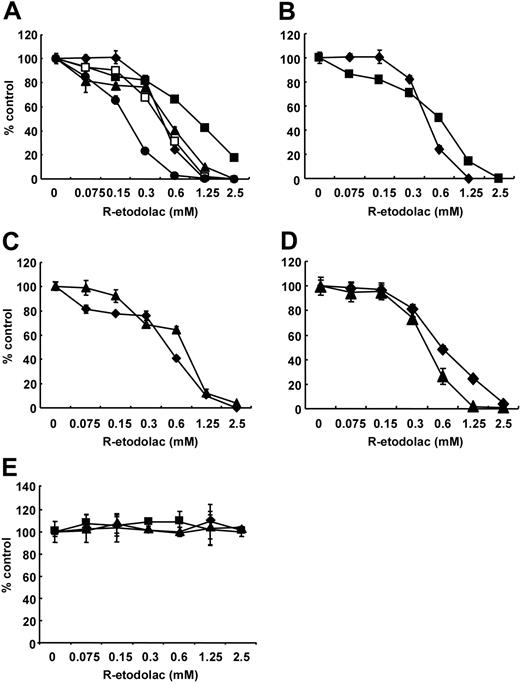
We first examined the growth inhibitory effect of R-etodolac (0-2.5 mM) at 48 hours in MM cell lines (MM.1S, U266, RPMI8226, INA-6, and OPM1) using MTT assays. R-etodolac induced significant cytotoxicity in MM.1S, U266, RPMI8226, INA-6, and OPM1 MM cell lines with IC50 (inhibitory concentration 50%) of 0.49, 1.06, 0.54, 0.22, and 0.47 mM, respectively (Figure 1A). R-etodolac also triggers cytotoxicity, with IC50 of 0.62, 0.76 and 0.62 mM, respectively, in Dex-resistant MM.1R (Figure 1B), Dox-resistant RPMI-Dox40 (Figure 1C), and bortezomib-resistant DHL4 cells32 (Figure 1D). Importantly, R-etodolac does not induce cytotoxicity in PBMCs from 3 healthy volunteers with concentrations as high as 2.5 mM (Figure 1E). These results indicate that R-etodolac selectively induces cytotoxicity in MM cells, even those resistant to conventional and novel chemotherapy, at concentrations that are not appreciably cytotoxic to normal PBMCs.
R-etodolac induces growth inhibition in MM cell lines. (A) MM.1S (♦), U266 (▪), RPMI8226 (▴), INA-6 (•), and OPM1 (□) MM cells; (B) Dex-sensitive MM.1S (♦) and DEX-resistant MM.1R (▪) MM cells; (C) RPMI8226 (♦) and doxorubicin-resistant RPMI-Dox40 (▴) MM cells; (D) DHL4 (♦) and MM.1S (▴) cells; as well as (E) normal peripheral mononuclear cells from 3 healthy volunteers, no. 1 (♦), no. 2 (▪), and no. 3 (▴) were cultured for 48 hours in the presence of R-etodolac (0-2.5 mM). Cell growth was assessed by MTT assays, and data represent mean (± SD) of quadruplicate cultures.
R-etodolac induces growth inhibition in MM cell lines. (A) MM.1S (♦), U266 (▪), RPMI8226 (▴), INA-6 (•), and OPM1 (□) MM cells; (B) Dex-sensitive MM.1S (♦) and DEX-resistant MM.1R (▪) MM cells; (C) RPMI8226 (♦) and doxorubicin-resistant RPMI-Dox40 (▴) MM cells; (D) DHL4 (♦) and MM.1S (▴) cells; as well as (E) normal peripheral mononuclear cells from 3 healthy volunteers, no. 1 (♦), no. 2 (▪), and no. 3 (▴) were cultured for 48 hours in the presence of R-etodolac (0-2.5 mM). Cell growth was assessed by MTT assays, and data represent mean (± SD) of quadruplicate cultures.
R-etodolac induces apoptosis via caspase-8, -9, and -3 and PARP cleavage
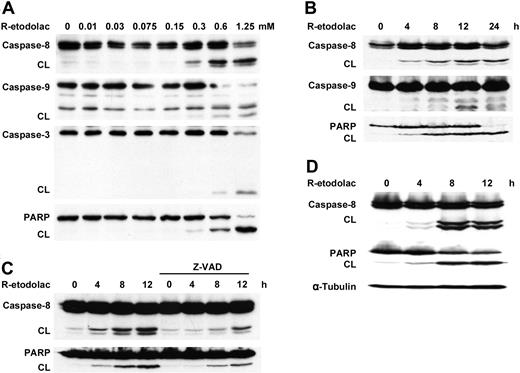
The mechanism of R-etodolac-induced apoptosis in MM cells was studied by immunoblot analysis. As can be seen in Figure 2A-B, treatment of MM.1S cells with R-etodolac induces cleavage of caspase-8, -9, and -3 and PARP, a hallmark of apoptosis, in a dose- and a time-dependent fashion (Figure 2A-B). Conversely, the pan-caspase inhibitor Z-VAD-FMK blocks R-etodolac-induced caspase-8 and PARP cleavage in MM.1S cells (Figure 2C). R-etodolac also induces caspase activation and PARP cleavage in RPMI8226 cells (Figure 2D). These results indicate that cytotoxicity triggered by R-etodolac, as is the case with bortezomib,33 As2O329 , and 2-Methoxyestradiol (2ME-2),34 is mediated via caspase-8, -9, and -3 activation and apoptosis.
R-etodolac induces down-regulation of cyclin D1 expression
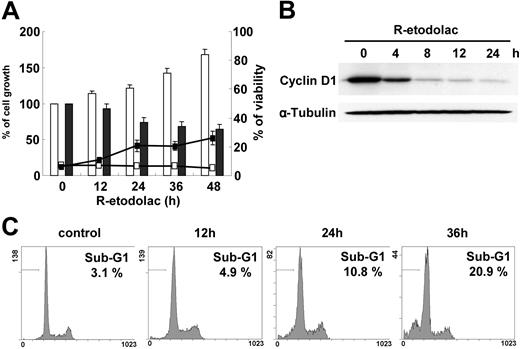
We and others have reported that expression of cyclin D1 is important for MM cell growth and prognosis.12,35 Since recent reports show that R-etodolac down-regulates cyclin D1 expression and induces growth inhibition in CLL19 and prostate cancer,20 we next investigated whether R-etodolac down-regulates cyclin D1 in MM cells. In U266 cells, which are less sensitive to R-etodolac compared with other MM cells (Figure 1A), 1 mM R-etodolac induces growth inhibition but only minimal cell death (Figure 3A). Immunoblotting shows that 1 mM R-etodolac significantly down-regulates cyclin D1 at 4 hours (Figure 3B) without significant change of cell-cycle profile. Sub-G1 cells gradually increase to 20.9% of total at 36 hours (Figure 3C). These data suggest that R-etodolac down-regulates expression of cyclin D1 in MM cells and results in accumulation in an apoptotic population by cell-cycle analysis.36
R-etodolac induces caspase cleavage and activation of apoptosis. MM.1S cells were cultured (A) for 24 hours with R-etodolac (0-1.25 mM) or (B) with R-etodolac (0.6 mM) for the indicated times. CL indicates cleaved band. (C) MM.1S cells were preincubated with Z-VAD-FMK (25 μM) for 30 minutes prior to treatment with R-etodolac (0.6 mM) for the indicated times. (D) RPMI8226 cells were also cultured with or without R-etodolac (0.6 mM) for indicated times. Total cell lysates were subjected to Western blotting using anti-caspase-8, -caspase-9, and -caspase-3, -PARP, and -α-tubulin Abs.
R-etodolac induces caspase cleavage and activation of apoptosis. MM.1S cells were cultured (A) for 24 hours with R-etodolac (0-1.25 mM) or (B) with R-etodolac (0.6 mM) for the indicated times. CL indicates cleaved band. (C) MM.1S cells were preincubated with Z-VAD-FMK (25 μM) for 30 minutes prior to treatment with R-etodolac (0.6 mM) for the indicated times. (D) RPMI8226 cells were also cultured with or without R-etodolac (0.6 mM) for indicated times. Total cell lysates were subjected to Western blotting using anti-caspase-8, -caspase-9, and -caspase-3, -PARP, and -α-tubulin Abs.
R-etodolac synergistically enhances dexamethasone cytotoxicity against MM cells
As we have previously shown that novel chemotherapeutic agents can augment cytotoxicity of dexamethasone (Dex),27,29,34,37 we further examined whether R-etodolac enhances the growth inhibitory effect of Dex. MM.1S cells were cultured for 24 hours with Dex (0.5 or 1.0 μM) in media or with sub-IC50 concentrations of R-etodolac (0.15-0.6 mM). R-etodolac enhances growth inhibition mediated by Dex, as analyzed by thymidine uptake (Figure 4A) and MTT assay (Figure 4B). While 0.3 mM R-etodolac or 1.0 μM Dex alone triggered 9.0% or 27.7% cytotoxicity in MTT assays, respectively, combining R-etodolac with Dex at the same concentrations induced 84.3% cytotoxicity. To determine whether the combination therapy of R-etodolac with Dex has additive or synergistic cytotoxicity, we analyzed the interaction between Dex and R-etodolac by isobologram analysis using the CalcuSyn software program.30 On the basis of the Chou-Talalay method, to calculate CIs, we generated dose-effect curves for varying concentrations of Dex and R-etodolac (Figure 4C).31 At doses ranging from 0.25 to 2 μM Dex combined with 75 to 600 μM R-etodolac, CI ranged from 0 to 0.035, suggesting that this combination is strongly synergistic (Table 1).
Cls calculated at increasing concentrations of R-etodolac and Dex
R-etodolac, μM . | Dex, μM . | Fraction affected . | Cl . |
|---|---|---|---|
| 75 | 0.25 | 0.55 | 0.035 |
| 150 | 0.5 | 0.75 | 0.005 |
| 300 | 1 | 0.91 | 0.001 |
| 600 | 2 | 0.95 | 0.000 |
R-etodolac, μM . | Dex, μM . | Fraction affected . | Cl . |
|---|---|---|---|
| 75 | 0.25 | 0.55 | 0.035 |
| 150 | 0.5 | 0.75 | 0.005 |
| 300 | 1 | 0.91 | 0.001 |
| 600 | 2 | 0.95 | 0.000 |
R-etodolac induces down-regulation of cyclin D1 expression. (A) U266 cells were cultured with control media (□) or 1.0 mM R-etodolac (▪) at the indicated times. Assessed by trypan blue exclusion, the percentage of cell growth is shown in the bar graph; and the percentage of cell viability is depicted by line. Data represent mean (± SD) of quadruplicate cultures. (B) U266 cells were cultured with or without R-etodolac (1.0 mM) for the indicated times. Total cell lysates were subjected to Western blotting using anti-cyclin D1 and -α-tubulin Abs. (C) Cell-cycle profile was assessed by propidium iodide (PI) staining and flow cytometric analysis. The percentage on each panel indicates the percentage of cells in the sub-G1 region (horizontal bar).
R-etodolac induces down-regulation of cyclin D1 expression. (A) U266 cells were cultured with control media (□) or 1.0 mM R-etodolac (▪) at the indicated times. Assessed by trypan blue exclusion, the percentage of cell growth is shown in the bar graph; and the percentage of cell viability is depicted by line. Data represent mean (± SD) of quadruplicate cultures. (B) U266 cells were cultured with or without R-etodolac (1.0 mM) for the indicated times. Total cell lysates were subjected to Western blotting using anti-cyclin D1 and -α-tubulin Abs. (C) Cell-cycle profile was assessed by propidium iodide (PI) staining and flow cytometric analysis. The percentage on each panel indicates the percentage of cells in the sub-G1 region (horizontal bar).
Subtoxic doses of R-etodolac induce up-regulation of Mcl-1s and Dex-induced apoptosis in MM cells
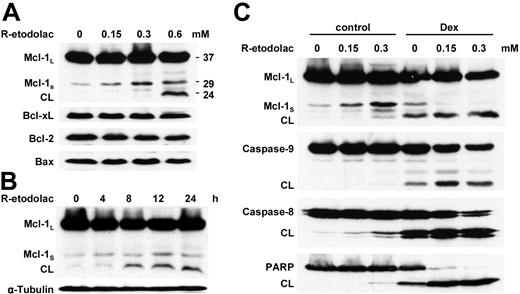
Since Mcl-1 plays an important role in proliferation and inhibition of apoptosis,38-43 as well as the induction of drug resistance,44-46 we next assessed the level of Mcl-1 protein expression as modulated by R-etodolac. Immunoblotting shows that subcytotoxic concentrations of R-etodolac (0.15 or 0.3 mM) induce up-regulation of 29 kDa Mcl-1S, which is a short-splicing variant of the MCL1 gene with antagonistic potential against 37-kDa Mcl-1L.47,48 Although 24-hour treatment with a toxic dose of R-etodolac (0.6 mM) does not significantly change expression of Bcl-2 family proteins such as Bcl-xL, BAX, and Bcl-2, cleavage of full-length Mcl-1L to 24-kDa Mcl-1 is induced, with a probable loss of its antiapoptotic activity49,50 (Figure 5A). Cleavage of Mcl-1L triggered by cytotoxic concentrations of R-etodolac (0.6 mM) is detected as early as 8 hours (Figure 5B). These results suggest that the concomitant up-regulation of Mcl-1S and cleavage of Mcl-1L may be key events involved in the apoptotic activity of R-etodolac.
R-etodolac synergistically enhances Dex-induced cytotoxicity against MM cells. MM.1S cells were cultured for 24 hours with control media (□) and with 0.5 μM Dex (▦) or 1.0 μM Dex (▪) in the presence or absence of R-etodolac (0.15-0.6 mM). Cell growth was assessed by [3H]-thymidine uptake (A) and MTT assays (B). CPM indicates counts per minute. (C) Dose dependency for inhibition of proliferation of MM1.S cells at 24 hours was analyzed by MTT assays, after culture with 18 to 600 μM R-etodolac alone (♦), 0.06 to 2 μM Dex alone (□), or both (▴). Data represent mean (± SD) of quadruplicate cultures.
R-etodolac synergistically enhances Dex-induced cytotoxicity against MM cells. MM.1S cells were cultured for 24 hours with control media (□) and with 0.5 μM Dex (▦) or 1.0 μM Dex (▪) in the presence or absence of R-etodolac (0.15-0.6 mM). Cell growth was assessed by [3H]-thymidine uptake (A) and MTT assays (B). CPM indicates counts per minute. (C) Dose dependency for inhibition of proliferation of MM1.S cells at 24 hours was analyzed by MTT assays, after culture with 18 to 600 μM R-etodolac alone (♦), 0.06 to 2 μM Dex alone (□), or both (▴). Data represent mean (± SD) of quadruplicate cultures.
To delineate mechanisms underlying the synergistic anti-MM activity of combined R-etodolac and Dex, we then assessed Mcl-1 expression and activation of apoptotic signaling in MM.1S cells cultured with either media alone or media containing 1.0 μM Dex, in the presence of subtoxic dose of R-etodolac (0.15 or 0.3 mM). Immunoblotting shows that subtoxic doses of R-etodolac induce up-regulation of Mcl-1S and significantly enhance Dex-induced cleavage of Mcl-1L, caspase-8, caspase-9, and PARP (Figure 5C). These results suggest that Mcl-1S induced by R-etodolac may potentiate Dex-mediated apoptosis.
R-etodolac induces apoptosis in primary MM patient cells
R-etodolac induced dose-dependent cytotoxicity in CD138+ BM cells (IC50 of 0.30 and 0.89 mM), isolated from 2 patients with MM whose disease was refractory to multiple prior therapies, including dexamethasone, melphalan, thalidomide, or bortezomib (Figure 6A). It also induced apoptosis, evidenced by caspase-8 activation and PARP cleavage on immunoblotting (Figure 6B). These results demonstrate that R-etodolac also has activity against cells of patients with refractory MM at clinically achievable plasma concentrations, as illustrated in previously reported studies in patients with CLL.22
Low doses of R-etodolac induce up-regulation of Mcl-1s and Dex-induced apoptosis in MM cells. (A) MM.1S cells were cultured with R-etodolac at the indicated doses for 24 hours. (B) MM.1S cells were cultured with R-etodolac (0.6 mM) for the indicated times. (C) MM.1S cells were cultured for 24 hours with control media or 1.0 μM Dex, in the presence or absence of R-etodolac (0.15 or 0.3 mM). Total cell lysates were subjected to Western blotting using anti-caspase-8, -caspase-9, -PARP, -Bax, -Bcl-2, -Bcl-xL, -Mcl-1, and -α-tubulin Abs.
Low doses of R-etodolac induce up-regulation of Mcl-1s and Dex-induced apoptosis in MM cells. (A) MM.1S cells were cultured with R-etodolac at the indicated doses for 24 hours. (B) MM.1S cells were cultured with R-etodolac (0.6 mM) for the indicated times. (C) MM.1S cells were cultured for 24 hours with control media or 1.0 μM Dex, in the presence or absence of R-etodolac (0.15 or 0.3 mM). Total cell lysates were subjected to Western blotting using anti-caspase-8, -caspase-9, -PARP, -Bax, -Bcl-2, -Bcl-xL, -Mcl-1, and -α-tubulin Abs.
R-etodolac overcomes the stimulatory effects of IL-6 and IGF-1
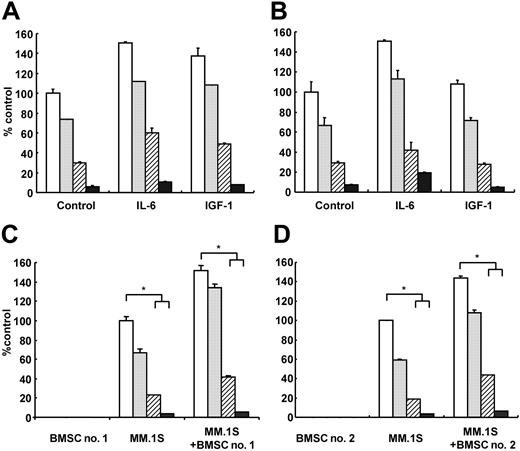
Since we and others have demonstrated that IL-639,51-54 and IGF-155,56 mediate both growth and inhibition of apoptosis in MM cells, we next examined whether R-etodolac can overcome the effects of exogenous IL-6 and IGF-1. Although IL-6 (25 ng/mL) and IGF-1 (50 ng/mL) trigger increased MM.1S and RPMI8226 cell growth relative to media alone, neither IL-6 nor IGF-1 protected against R-etodolac-induced anti-MM activity (Figure 7A-B).
R-etodolac overcomes the growth stimulatory effects of IL-6, IGF-1, and BMSCs. MM.1S (A) and RPMI8226 (B) MM cells were cultured for 24 hours with control media (□) and with 0.3 mM (▦), 0.6 mM (▨), or 1.25 mM (▪) R-etodolac, in the presence or absence of IL-6 (25 ng/mL) and IGF-1 (50 ng/mL). MM.1S cells, 2 different patient BMSCs (BMSC no. 1 [C] and BMSC no. 2 [D]), or both MM.1S cells and BMSCs were cultured for 24 hours with control media (□), and with 0.3 mM (▦), 0.6 mM (▨), or 1.25 mM (▪) R-etodolac. Cell growth was assessed by [3H]-thymidine uptake, and data represent mean (± SD) of quadruplicate cultures. *P < .01.
R-etodolac overcomes the growth stimulatory effects of IL-6, IGF-1, and BMSCs. MM.1S (A) and RPMI8226 (B) MM cells were cultured for 24 hours with control media (□) and with 0.3 mM (▦), 0.6 mM (▨), or 1.25 mM (▪) R-etodolac, in the presence or absence of IL-6 (25 ng/mL) and IGF-1 (50 ng/mL). MM.1S cells, 2 different patient BMSCs (BMSC no. 1 [C] and BMSC no. 2 [D]), or both MM.1S cells and BMSCs were cultured for 24 hours with control media (□), and with 0.3 mM (▦), 0.6 mM (▨), or 1.25 mM (▪) R-etodolac. Cell growth was assessed by [3H]-thymidine uptake, and data represent mean (± SD) of quadruplicate cultures. *P < .01.
R-etodolac inhibits MM cell growth triggered by tumor cell binding to BMSCs
Since we previously showed that the BM microenvironment confers growth-stimulating and drug-resistance effects in MM cells,23,28,57-59 we next studied the antagonistic effect of R-etodolac on paracrine MM cell growth in the BM milieu. We first examined the direct cytotoxicity of R-etodolac on patient BMSCs using MTT assay; no significant growth inhibition in BMSCs was triggered by R-etodolac treatment (data not shown). MM.1S cells were next cultured with or without BMSCs from 2 patients, in the presence or absence of R-etodolac. Tumor cell adherence to BMSCs triggered increased [3H]-thymidine uptake in MM.1S cells (Figure 7C-D), and R-etodolac inhibited this up-regulation of DNA synthesis in a dose-dependent fashion. These data suggest that R-etodolac also ablates the growth stimulatory effect of the BM microenvironment on MM cells.
R-etodolac induces apoptosis in cells of patients with MM. CD138+ cells were isolated from BM of patients with MM (MM no. 1 and MM no. 2) who had relapsed and were refractory to conventional therapies. (A) MM no. 1 (♦), MM no. 2 (▪), and MM.1S cell line (▴) were cultured for 48 hours in the presence of R-etodolac (0-2.5 mM). Cell growth was assessed by MTT assays, and data represent mean (± SD) of triplicate cultures. (B) MM no. 1 and MM no. 2 cells were cultured with R-etodolac (0.6 mM) for 24 hours. Total cell lysates were subjected to immunoblotting using anti-caspase-8 and anti-PARP Abs.
R-etodolac induces apoptosis in cells of patients with MM. CD138+ cells were isolated from BM of patients with MM (MM no. 1 and MM no. 2) who had relapsed and were refractory to conventional therapies. (A) MM no. 1 (♦), MM no. 2 (▪), and MM.1S cell line (▴) were cultured for 48 hours in the presence of R-etodolac (0-2.5 mM). Cell growth was assessed by MTT assays, and data represent mean (± SD) of triplicate cultures. (B) MM no. 1 and MM no. 2 cells were cultured with R-etodolac (0.6 mM) for 24 hours. Total cell lysates were subjected to immunoblotting using anti-caspase-8 and anti-PARP Abs.
Discussion
Although MM is incurable with conventional and high-dose therapies, novel rationally based treatment strategies both directly targeting MM cells as well as the BM microenvironment offer great promise to improve patient outcome since these approaches have the potential to overcome drug resistance as illustrated in both preclinical and clinical studies.13,16 In this report, we demonstrate that R-etodolac (SDX-101) induces cytotoxicity in drug-sensitive and drug-resistant, including dexamethasone-, doxorubicin-, and bortezomib-resistant, MM cell lines and in primary patient MM cells, with IC50 of 0.22 to 1.06 mM. Importantly, we observe no cytotoxicity in PBMCs at drug concentrations of 0 to 2.5 mM, suggesting selective cytotoxicity against MM tumor cells. Since reductions in average lymphocyte counts have been observed in patients with CLL administered R-etodolac at steady-state blood levels of approximately 300 to 600 μM,22 the cytotoxicity observed in vitro against MM cell lines and primary patient samples at concentrations of 1 mM is encouraging.
Recently, attention has been focused on the antiproliferative activity of NSAIDs and their potential utility as chemopreventive and antitumor agents.60,61 To date, however, the mechanisms underlying the effects of these drugs on cancer cells are undefined. Although inhibition of COX with subsequent decrease in prostaglandin production may explain part of the antitumor activity of certain NSAIDs, some of these drugs exert anti-inflammatory and antitumor activity in COX-independent manners.62,63 For example, NSAIDs might inhibit nuclear factor-κ B (NF-κB) signaling64-68 or p42/44 mitogen-activated protein kinase (MAPK)69 or modulate PPAR-γ or -δ.70 The fact that R-etodolac, an enantiomer of the commercially available NSAID lacking COX inhibitory activity, has cytotoxic activity against chronic lymphocytic leukemia,19 prostate cancer,20,21 and MM supports the hypothesis that COX-independent mechanisms may be important in the antitumor activity of other NSAIDs.
As is true in the MM cells treated with thalidomide/IMiDs (immunomodulatory derivatives of thalidomide),37 bortezomib,23 and lysophosphatidic acyltransferase-β inhibitor,27 R-etodolac strongly enhances Dex-induced cytotoxicity in MM.1S cells, suggesting differential apoptotic signaling cascades for these agents versus Dex. While Dex induces caspase-9 activation via a cytochrome-c-independent, Smac (second mitochondrial-derived activator of caspase)-dependent, pathway,71 R-etodolac triggers synergistic augmentation of Dex-induced cytotoxicity, confirmed by isobologram analysis, via caspase-8 and -9 activation, PARP cleavage, and modulation of Mcl-1. Importantly, subcytotoxic concentrations of R-etodolac induce up-regulation of Mcl-1s, a short splicing variant that lacks BH (Bcl2 homology) 1 and 2 and the transmembrane domain due to the splicing out of exon 2 during mRNA processing.47,48 Unlike the full-length Mcl-1L protein, Mcl-1s is a proapoptotic protein that heterodimerizes with Mcl-1L, consequently acting as an antagonist.47
Inactivation of Mcl-1L in myeloma cells using antisense oligonucleotide has been shown to decrease proliferation and trigger mitochondrial-mediated apoptosis, suggesting that Mcl-1 plays an important role in MM.40,42 R-etodolac-induced up-regulation of Mcl-1s may, therefore, result in the functional inhibition of Mcl-1L, rendering the cells more sensitive to Dex-induced intrinsic mitochondrial-mediated apoptosis. A similar model was proposed recently for the compound clerodane in prostate cancer cells.72 Studies are currently ongoing to expand our understanding of the R-etodolac effect on the expression and function of Mcl-1s and its role in apoptosis of MM cells. These results will provide a rational framework for clinical use of R-etodolac in combination with dexamethasone, as well as with other conventional or novel chemotherapeutic agents.
We and others have previously reported that IL-6 triggers proliferation of MM cells via activation of the Ras (p21ras)/Raf/MAPK kinase/p42/44 MAPK signaling cascade,57,73,74 and survival of MM cells via JAK2/STAT3 activation.38,75 IGF-1 also promotes MM cell proliferation and survival via Ras/Raf/MAPK kinase/p42/44 MAPK and JAK2/STAT3 signaling cascades.54 IL-6 protects against Dex-induced apoptosis via PI3-K/Akt signaling.76 Our results suggest that neither IL-6 nor IGF-1 protects against R-etodolac-induced cytotoxicity. Since we have previously shown that the BMSCs confer growth stimulation and drug resistance effects in MM cells in the BM milieu,23,58,77 we further studied the effect of R-etodolac on MM cell division in the BM milieu. R-etodolac inhibits thymidine incorporation even of MM.1S adherent to BMSCs, indicating that R-etodolac, in contrast to conventional therapies, can overcome the mitotic-stimulating effect of the BM microenvironment on MM cells.
In summary, this is the first report to demonstrate that R-etodolac has antitumor activity against MM cells. R-etodolac can induce cytotoxicity and apoptosis in drug-sensitive and drug-resistant MM cell lines, as well as in primary patient MM cells within the BM milieu, at concentrations that are without cytotoxicity on normal PBMCs and at clinically achievable plasma levels. Low concentrations of R-etodolac can trigger synergic enhancement of Dex-mediated apoptosis with attendant up-regulation of Mcl-1s. R-etodolac is currently being tested in phase 2 clinical trials for treatment of refractory CLL, and our results provide the preclinical framework for clinical trials of this drug alone or in combination with Dex, to improve patient outcome in MM.
Prepublished online as Blood First Edition Paper, March 31, 2005; DOI 10.1182/blood-2005-02-0838.
Supported by National Institutes of Health Specialized Programs of Research Excellence (SPORE) (grants IP50 CA10070-01, PO-1 CA78378, and RO-1 CA50947), the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.), the Multiple Myeloma Research Foundation (T.H., D.C., K.P., Y.T.T.), and the Cure for Myeloma Fund (K.C.A.).
L.M.L., S.K., and G.E. are employees of Salmedix Inc. SDX-101 is a proprietary product of Salmedix Inc.
H.Y. and M.H. equally contributed to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 4. R-etodolac synergistically enhances Dex-induced cytotoxicity against MM cells. MM.1S cells were cultured for 24 hours with control media (□) and with 0.5 μM Dex (▦) or 1.0 μM Dex (▪) in the presence or absence of R-etodolac (0.15-0.6 mM). Cell growth was assessed by [3H]-thymidine uptake (A) and MTT assays (B). CPM indicates counts per minute. (C) Dose dependency for inhibition of proliferation of MM1.S cells at 24 hours was analyzed by MTT assays, after culture with 18 to 600 μM R-etodolac alone (♦), 0.06 to 2 μM Dex alone (□), or both (▴). Data represent mean (± SD) of quadruplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2005-02-0838/6/m_zh80140581360004.jpeg?Expires=1769154851&Signature=nveRwfKHcWBXZJmgujHcxCkJig~ORWUQ-aI0fmbg3HT-xEDJj7nrUEJv1sks6M7V22Gr1V5Y2hgg3LiYkXL2BvJFALlFbOzKfgrR7mJiWqyZtUjQ0k4bPiFodEUK3xk4fIi1DgwQLT-XEilM9TvIccoQzDJ9ETbg0TauVopKaVBcW6aDHimLgZehrpe3OHdJw60NqR6QT-drlRjN6HiPO2tyPMwwwfCO2D6TsMy5~BrC3uho8TQ35ilNgZgXyOwI5RGhvPyaQdmvO6MwS~e6UvSYr4Pu4mwku9hqrafte7afF0IBEC3wC9VmBn-J664ixSFmAOcCB97eA0XIBrkesQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. R-etodolac overcomes the growth stimulatory effects of IL-6, IGF-1, and BMSCs. MM.1S (A) and RPMI8226 (B) MM cells were cultured for 24 hours with control media (□) and with 0.3 mM (▦), 0.6 mM (▨), or 1.25 mM (▪) R-etodolac, in the presence or absence of IL-6 (25 ng/mL) and IGF-1 (50 ng/mL). MM.1S cells, 2 different patient BMSCs (BMSC no. 1 [C] and BMSC no. 2 [D]), or both MM.1S cells and BMSCs were cultured for 24 hours with control media (□), and with 0.3 mM (▦), 0.6 mM (▨), or 1.25 mM (▪) R-etodolac. Cell growth was assessed by [3H]-thymidine uptake, and data represent mean (± SD) of quadruplicate cultures. *P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2005-02-0838/6/m_zh80140581360006.jpeg?Expires=1769154851&Signature=G7KTymoSy2KbSUsXOzWE6oijAkZtIwV4GLLvZKwsu-J7akF7Qi3a-ljxcyWHCpbzCBaoQiMvFjsOZJbveqH3flop5iwM1oaOsoQRSGJPsTda3qTbCr4lSzOUvW8fiixSMdPFhsg35qHAya7wDvNskOWj1WuIbJNvIywkAUSO3bKrdPay0G7iBO0hEL2N3o04VCPYCdXeBlCW8HGMR~pTUGBDY~liP6RHmqQ4QXFKFo-i3vUbpm0crV9RcheNb-eZ8iEuBOdZLrW~vc43Y1pfVaaKUqhTHpxpUnSp7W3dZ0120wfPLaoGurZe6KKYOoTgjWWiRDzmAYqWSzUqSyqWpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal